Abstract
Background
Neonatal Encephalopathy (NE) is a prominent cause of infant mortality and neurodevelopmental disability. Hypothermia is an effective neuroprotective therapy for newborns with encephalopathy. Post-hypothermia functional-anatomical correlation between neonatal neurobehavioral abnormalities and brain injury findings on MRI in encephalopathic newborns has not been previously described.
Aim
To evaluate the relationship between neonatal neurobehavioral abnormalities and brain injury on magnetic resonance imaging (MRI) in encephalopathic newborns treated with therapeutic hypothermia.
Study Design
Neonates with hypoxic ischemic encephalopathy (HIE) referred for therapeutic hypothermia were prospectively enrolled in this observational study. Neurobehavioral functioning was assessed with the NICU Network Neurobehavioral Scale (NNNS) performed at target age 14 days. Brain injury was assessed by MRI at target age 7–10 days. NNNS scores were compared between infants with and without severe MRI injury.
Subjects & Outcome Measures
Sixty-eight term newborns (62% males) with moderate to severe encephalopathy underwent MRI at median 8 days (range 5–16) and NNNS at median 12 days of life (range 5–20). Fifteen (22%) had severe injury on MRI.
Results
Overall Total Motor Abnormality Score and individual summary scores for Nonoptimal Reflexes and Asymmetry were higher, while Total NNNS Z-score across cognitive/behavioral domains was lower (reflecting poorer performance) in infants with compared to those without severe MRI injury (p<0.05).
Conclusions
Neonatal neurobehavioral abnormalities identified by the NNNS are associated with MRI brain injury in encephalopathic newborns post-hypothermia. The NNNS can provide an early functional assessment of structural brain injury in newborns, which may guide rehabilitative therapies in infants after perinatal brain injury.
Neonatal Encephalopathy (NE) is a prominent cause of infant mortality and neurodevelopmental disability.1–2 Hypothermia has emerged as the only proven effective neuroprotective therapy for newborns with encephalopathy. However, despite its success, infants with moderate to severe encephalopathy continue to have a 30–70% risk of death or significant disability.3–6 It is critical that areas of deficit are systematically quantified in order to gauge treatment effects and guide rehabilitative therapies. Brain injury findings on MRI in encephalopathic newborns have been published from two large multicenter randomized controlled trials of whole body hypothermia (NICHD7 and TOBY8 trials). However, post-hypothermia functional-anatomical correlation between neonatal neurobehavioral abnormalities and brain injury findings on MRI in encephalopathic newborns has not been previously described. Early assessment of infants at risk for functional impairment after perinatal brain injury is essential to inform planning of developmentally supportive care and guide referrals to early intervention services for this high-risk population.
The NICU Network Neurobehavioral Scale (NNNS) is a strong candidate for use in documenting neurobehavioral status post therapeutic hypothermia. The NNNS is a comprehensive standardized assessment designed to measure processes of biobehavioral organization in neonates. The NNNS was developed by Lester and Tronick as a quantitative assessment of neurological integrity and behavioral functioning in high-risk infants under the auspices of the National Institute of Child Health and Human Development Neonatal Research Network.9 The examination consists of 45 administration items and 70 observation items that are scored and transformed into 13 summary scores based on conceptual and statistical grouping of items.10 These scores offer quantitative measures of individual neurobehavioral domains including: Habituation, Attention, Handling, Quality of Movement, Regulation, Nonoptimal Reflexes, Asymmetrical Reflexes, Stress/Abstinence, Arousal, Hypertonicity, Hypotonicity, Excitability, and Lethargy. Normative data is available11 and the instrument has adequate psychometric properties.12 Additionally, the NNNS has been related to later developmental outcome in other high risk neonatal populations (i.e. substance exposed13–14 and preterm infants15). Certification to administer the NNNS is achieved after formal instruction and reliability testing for both administration and scoring.
As current and future neuroprotective therapies become available for newborns presenting with encephalopathy after birth, reliable early neurobehavioral assessment can serve a critical role in both confirming the functional impact of anatomical injury diagnosed by MRI and providing detailed assessment of affected domains in order to guide rehabilitative therapies. The present study was undertaken to evaluate if the NNNS can serve as a systematic evaluation of neurobehavioral functioning in this highrisk population. We hypothesized that HIE infants with severe MRI brain injury would have poorer performance on the NNNS compared to those with mild injury or normal MRI.
METHODS
Participants
All patients referred to our Level IIIC neonatal intensive care unit (NICU) over a 4-year period (May 2008–June 2012) for therapeutic hypothermia were approached for enrollment in this prospective observational study. Participants were treated with whole-body hypothermia according to the NICHD Neonatal Research Network protocol.4 Therapeutic hypothermia was offered based on established NICHD inclusion criteria (i.e. infants were greater than 36 weeks gestational age, greater than 1800 grams at birth, demonstrated metabolic acidosis and/or low Apgar scores, and exhibited signs of moderate to severe clinical encephalopathy). Infants with suspected chromosomal abnormalities or major congenital anomalies were excluded. The study was approved by the Institutional Review Board at Children’s National Medical Center. Written informed consent and Health Insurance Portability and Accountability Act Authorization were obtained from the parent(s) of each participant.
Data Collection
Magnetic Resonance Imaging
MRI was performed at target 7–10 days of life on a 1.5 Tesla scanner (Signa, General Electric, Milwaukee, USA). Standard sequences included sagittal and axial spin echo (SE) T1, dual echo axial SE proton density (PD) and T2 images, coronal fast spin echo (FSE) T2 and axial diffusion weighted images (DWI). Images were reviewed by 2 neuroradiologists (N.K. & G.V.) who were masked to the clinical characteristics and NNNS scores of the participants. Images were scored according to Barkovich16 with deep nuclear gray injury assigned a basal ganglia (BG) score ranging from 0–4 and cortical/white matter injury assigned a watershed (WS) score ranging from 0–5. White matter injury (WMI) was also scored according to Miller as mild, moderate or severe17. Discrepancies in scoring were resolved by consensus. Participants were classified as having severe MRI injury if BG score was ≥3, WS score was ≥4, or severe WMI was present. Dichotomization of MRI outcome was done to facilitate clinical interpretation of results and based on previous studies using similar methodologies evaluating qualitative MRI interpretation in this population.8, 18–19
Neurobehavioral Assessment
The NNNS was performed in study participants at target age 14 days by a certified examiner. NNNS summary scores were grouped into 2 categories: 1) those that reflected motor performance and 2) those that reflected cognitive/behavioral functioning. Motor scores that were comprised of counts of abnormal items in a given domain were summated to derive a Total Motor Abnormality Score as an overall measure of motor performance across domains. Cognitive/behavioral summary scores, which were comprised of a calculated mean of requisite items, were converted to z-scores normalized to published values for healthy term newborns,11 with positive scores reflecting better performance on a given domain compared to these norms. This enabled calculation of a Total NNNS Z-score (summation of the 6 cognitive/behavioral functioning z-scores) that represented an individual participant’s cognitive/ behavioral performance across domains. Quality of movement was assessed separately as a measure of motor maturity scored as a calculated mean of requisite items. Description of the grouping and clinical interpretation of each summary score is presented in Table 1.20 For comparison, standard neurological assessment with the Amiel-Tison Neurological Assessment at Term21 was performed by a pediatric neurologist on the same day the NNNS was completed.
Table 1.
NNNS Summary Score Descriptions20
| Summary Score | Clinical Interpretation |
|---|---|
| Motor Performance Domains | |
| Hypertonia | Measure of increased muscle tone in arms, legs, trunk, neck and shoulders |
| Hypotonia | Measure of decreased or low muscle tone in arms, legs, trunk, neck and shoulders |
| Asymmetry | Measure of times that reflexes on one side of the body are stronger or weaker than the other side |
| Reflexes | Non-optimal responses to assessment of newborn reflexes (reflects presence and strength of response) |
| Excitability | Measure of high levels of motor and physiologic reactivity |
| Lethargy | Measure of low levels of motor and physiologic reactivity |
| Quality of movement | Overall measure of motor maturity |
| Cognitive/Behavioral Functioning Domains | |
| Habituation | Capacity of infant’s ability to “protect” sleep by progressively inhibiting response to stimuli |
| Handling | Indicates amount of external input from examiner required to elicit infant’s attention |
| Attention | Measure of sustained alertness and threshold for stimulation/distractibility |
| Arousal | Measure of how quickly the infant becomes irritable or highly active when handled or left alone |
| Regulation | Indicates infant’s ability to regulate state and soothe when upset |
| Stress/Abstinence | Overall measure of stress response to manipulation |
Statistical Analysis
Descriptive statistics are presented as a mean +/− standard deviation or median (range) as appropriate. Independent samples t and Fisher’s Exact Tests were used to evaluate differences between groups for continuous and categorical variables respectively. Mann Whitney U tests were used to evaluate differences in non-parametric variables such as pH and Apgar scores. Multiple regression models were also used to evaluate the relationship between NNNS and severe MRI injury. Covariates included in the models were selected from baseline and clinical characteristics (i.e. birthweight, gestational age, gender, age at NNNS, and age at MRI) that differed between outcome groups by univariable analyses. Statistical analyses were performed with SPSS 13.0 (SPSS Inc., Chicago, IL).
RESULTS
A total of 94 term encephalopathic newborns were enrolled in the study. Fifteen patients (16%) died prior to target age for NNNS and MRI. NNNS was not performed in 11/79 (14%) eligible surviving infants due to either clinical instability precluding exam at target age (n=4) or exam missed due to unavailability of the examiner at time of discharge (n=7). These infants all had moderate encephalopathy and were similar to the final study cohort who were assessed by the NNNS with regards to demographic and presenting characteristics (p>0.05). Data was therefore available for 68 participants who underwent NNNS examination at median 12 days of life (range 5–20). Complete NNNS data with requisite number of items to calculate a Total NNNS Z-score was available for 51/68 (75%) participants. Infants with incomplete NNNS data had higher frequency of seizures and severe encephalopathy (p<0.05), reflecting severity of illness that precluded administration/scoring of all requisite items. Clinical characteristics of the study population are presented in Table 2.
TABLE 2.
Characteristics of the Study Population
| Overall Cohort (n=68) |
Complete NNNS (n=51) |
|
|---|---|---|
| Birthweight* (Kilograms) | 3.4 ± 0.7 | 3.3 ± 0.7 |
| Gestational Age* (weeks) | 38.8 ± 1.9 | 38.7 ± 1.8 |
| Gender, n (%male) | 42 (62) | 32 (63) |
| Apgar | ||
| 1 minute | 2 (0–6) a | 2 (0–6) |
| 5 minute | 4 (0–9) a | 4 (0–9) |
| 10 minute | 5 (0–9) b | 5 (0–8) e |
| Presenting pH | 6.97 (6.5–7.35) | 7 (6.5–7.34) f |
| Base Deficit | 18 (8–36) d | 17 (8–36) g |
| Clinical Seizure, n (%) | 20 (29) | 11 (22)** |
| Encephalopathy Grade | ||
| Moderate | 60 (88) | 48 (94)** |
| Severe | 8 (12) | 3 (6) |
| DOL NNNS | 12 (5–20) | 12 (5–19) |
| DOL MRI | 8 (5–16) | 9 (5–16) |
Data presented as median (range) except where indicated,
mean ± SD
Significant difference between groups with and without complete NNNS (p<0.05)
Data available for
67,
56,
66,
60 of 68 patients;
42,
50,
46 of 51 patients
MRI was performed in all enrolled infants at a median age of 8 days (range 5–16 days). Severe injury on MRI was observed in 15 (22%) of infants. The majority (n=9) of infants had severe BG injury. The remaining infants had severe WS injury (n=4), global injury (n=1) or severe WMI (n=1). Twenty-five (37%) infants had normal MRI, while the remainder had mild WS (n=5), BG (n=2) or WMI (n=21). Infants with incomplete NNNS were more likely to have severe MRI injury compared to patients with complete NNNS (7/17 [41%] vs 8/51 [16%], p=0.043), reflecting higher risk of injury when functional status precluded administration/scoring of all requisite items. Infants with severe MRI injury trended to be of older gestational age (Severe Injury 39.5± 1.9 vs. No Severe Injury 38.5 ± 1.8 weeks, p=0.09) and older at age of NNNS assessment (Severe Injury 14 ± 2 vs. No Severe Injury 11 ± 3 days, p=0.005). Otherwise baseline characteristics were similar between infants with and without severe MRI injury (p>0.05).
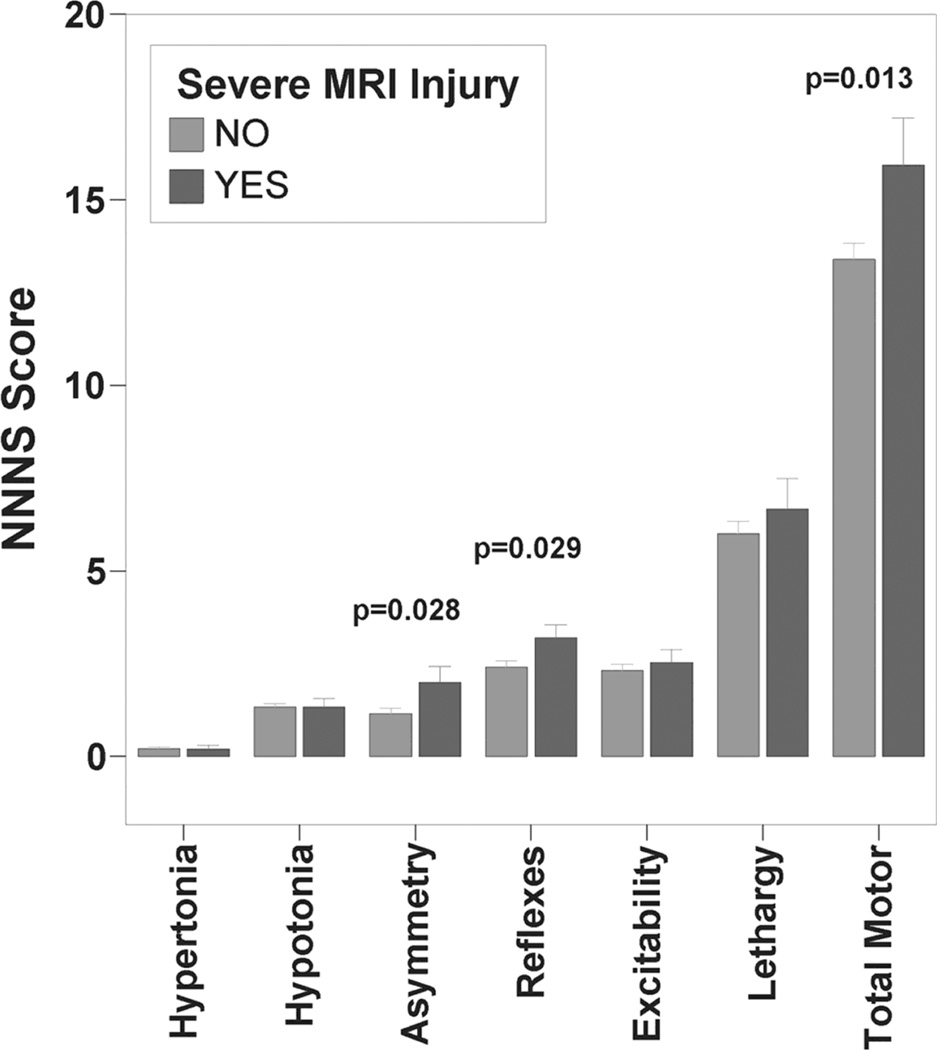
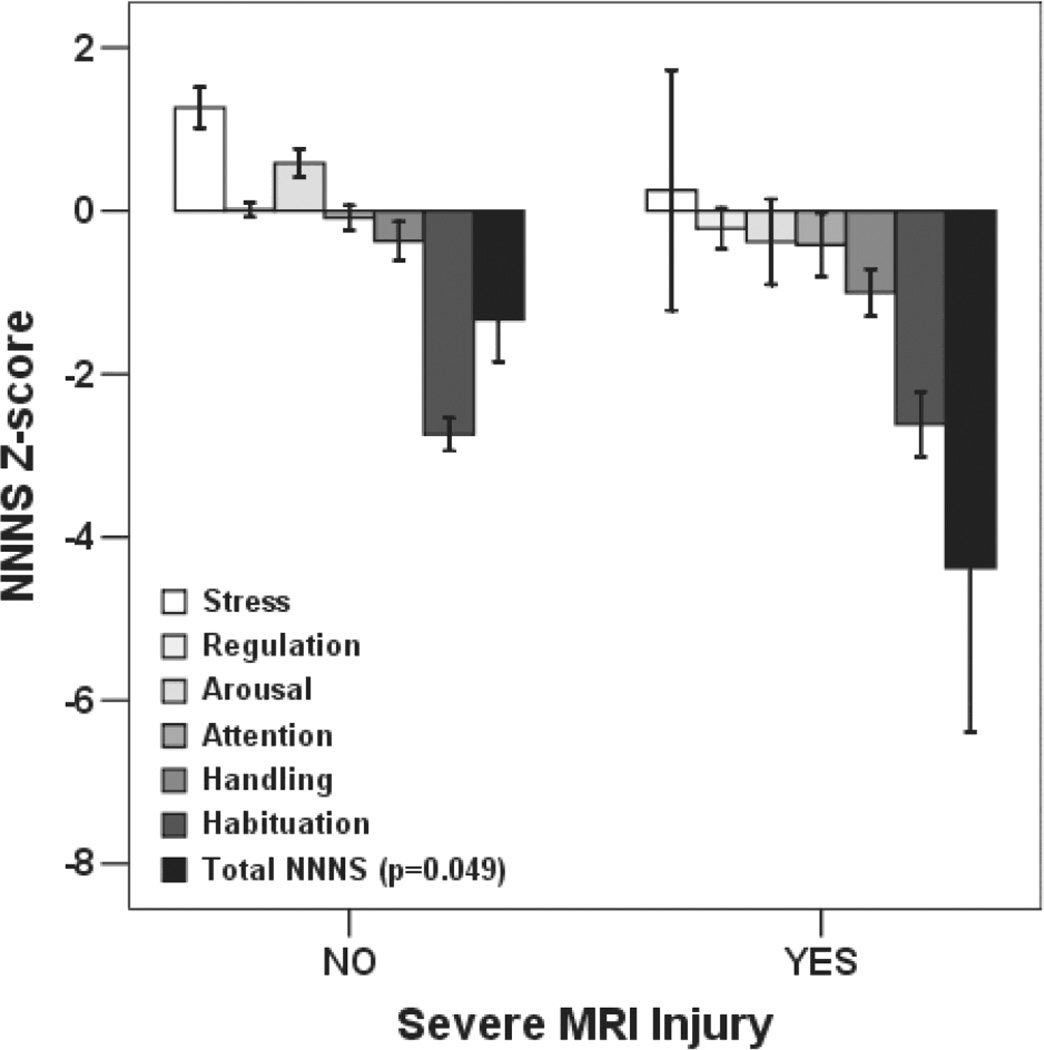
NNNS Total Motor Abnormality Score and individual summary scores for Asymmetry and Non-optimal Reflexes were higher in infants with severe MRI injury compared to those without severe injury (Figure 1). Except for Habituation score, mean cognitive/behavioral functioning Z-scores were lower across domains (reflecting suboptimal performance) in infants with severe MRI injury (Figure 2), but this difference was only statistically significant for Total NNNS Z-Score (p=0.049). Quality of movement score did not differ between groups (p>0.05). Mean NNNS scores and severity classification by clinical neurological assessments are presented in Table 3 for comparison. While initial encephalopathy grade did not differentiate between groups with and without severe MRI injury, both neurological and neurobehavioral exam performed after hypothermia were associated with MRI outcome group. After controlling for gestational age and age at NNNS in a multiple regression model, Total Motor Abnormality, Non-optimal Reflexes, and Asymmetry scores remained significantly associated with severe MRI injury, while the association between lower Total NNNS Z-score and severe MRI injury was no longer statistically significant (Table 4).
Figure 1.
NNNS motor summary scores by MRI severity. Bars represent mean score ± standard error of the mean.
Figure 2.
NNNS cognitive/behavioral functioning Z-scores by MRI severity. Positive scores represent optimal performance on any given domain. Bars represent mean score ± standard error of the mean.
Table 3.
Neurological and NNNS Examination by MRI Outcome Category
| No/Mild MRI Injury (n=53) |
Severe MRI Injury (n=15) |
P Value | |
|---|---|---|---|
| Clinical Neurological Exam, n (%) | |||
| • Encephalopathy Grade at Presentation4, 22 | |||
| – Moderate | 29 (92) | 11 (73) | 0.287 |
| – Severe | 4 (8) | 4 (27) | |
| • Neurological Exam21 at 14 days | |||
| – Normal | 24 (45) | 0 (0) | <0.001 |
| – Minor/Moderate | 29 (55) | 11 (73) | |
| – Severe | 0 (0) | 4 (27) | |
| NNNS Exam9 at 14 days | |||
| • Total NNNS Z-Score* | −1.33 ± 3.4 | −4.38 ± 5.3 | 0.049 |
| • Total Motor Abnormality Score | 13 ± 3 | 16 ± 5 | 0.013 |
For patients with complete NNNS (No/Mild Injury n=44 vs Severe Injury n=7)
Table 4.
Summary of Multiple Regression Models
| Dependent Variable | B | SE | 95% CI | P |
|---|---|---|---|---|
| Total Motor | 3.221 | 1.077 | 1.069–5.373 | 0.004 |
| Abnormality Score | ||||
| Non-optimal | 1.000 | 0.361 | 0.279–1.721 | 0.007 |
| Reflexes | ||||
| Asymmetry | 0.934 | 0.380 | 0.175–1.694 | 0.017 |
| Total NNNS | −2.633 | 1.462 | −5.576–0.311 | 0.078 |
| Z Score |
B = regression coefficient for severe MRI Injury, SE = standard error, 95% CI = 95% confidence interval Covariates included in final model = gestational age (weeks), age at NNNS (days)
DISCUSSION
In the present study, vulnerabilities in several neurobehavioral domains were identified using the NNNS in encephalopathic newborns after therapeutic hypothermia. Clinical signs of neurobehavioral dysfunction in motor domains were associated with MRI evidence of brain injury Identifying neurobehavioral abnormalities and understanding the association between functional performance and structural damage is critical for guiding treatment and improving outcome after perinatal brain injury. Assessment instruments that are valid, reliable, and practical for use in this high-risk population are needed. The current study supports that the NNNS may be useful in this capacity and deserves further evaluation in this population.
Standard neurological exam and classification of encephalopathy by Sarnat staging have been traditionally used to document clinical neurological status in babies with HIE.22 Recently, the initial clinical exam has been demonstrated to be less useful as a predictor of outcome in infants who are treated with hypothermia,23whereas serial examination or examination after rewarming had improved predictive abilities. 23–24 In the present study, only 4/8 (50%) of infants with severe encephalopathy at presentation had severe MRI injury post hypothermia. Conversely 11/60 (18%) of patients initially presenting with moderate encephalopathy had severe injury on MRI. These results further support that initial clinical assessment of encephalopathy grade, while important for early risk-stratification to guide therapeutic decision making, is not an absolute indicator of later developmental outcome. Clinical assessment after hypothermia is therefore an important aspect of care that can help further risk stratify patients for additional interventions (e.g. longer cooling, other future adjuvant therapies) or reparative/rehabilitative therapies (e.g. stem cell therapies, directed early intervention services), as well as offer prognostic information for families. An instrument such as the NNNS, that provides detailed continuous measures rather than normal versus abnormal classifications, may allow for detection of subtle but significant functional impairment after perinatal brain injury. It should be noted that the NNNS is currently largely utilized in the research setting for quantification of abnormalities, possibly related to the training and certification requirement for reliability. Further study is needed to assess if the NNNS provides more accurate prediction of outcome compared to standard neurological exam performed post-recovery from hypothermia. Additionally, these future studies evaluating the ability of the NNNS to predict later developmental impairment will need to establish cut-points for NNNS scores before it can be translated into more widespread clinical application.
While MRI remains the ‘gold standard’ for the subacute diagnosis of perinatal brain injury,25,26 prediction of later functional impairment remains imprecise.16,17 This may be due to microstructural injury below anatomical resolution of MRI in cases where impairment manifests in the setting of normal imaging. Conversely, intact outcome observed in the setting of diagnosed anatomical injury may be due to the inherent plasticity and reparative capacity of the newborn brain. Thus, clinical assessment of the functional impact of anatomical injury remains important in the care of these high-risk infants. It is possible that independent assessment of brain structure and function provide additive and/or corroborating information about the neurological status of the infant. Such complementary information is important when making treatment decisions and counseling families.
Neurobehavioral abnormalities detected by the NNNS may represent early manifestations of later neurodevelopmental impairment, as the NNNS has been demonstrated to be predictive of outcome in other at-risk groups. NNNS performance has been correlated with behavioral problems in school-aged children exposed to drugs in utero,13,14 with motor outcome at 2 years of age in children born pre-term,15 and with medical and behavioral problems through age 4 years, 6 months in very pre-term infants.14 NNNS correlation with long-term developmental outcome is needed in infants with HIE and is currently underway.
There are limitations to the present study. That all surviving eligible patients did not undergo NNNS evaluation may introduce selection bias. However, given this was a random and relatively rare occurrence, and that missed infants did not have distinguishing clinical or demographic characteristics from the study population evaluated, concern for biased results is somewhat mitigated. That some infants were not evaluated due to clinical instability at 2 weeks of life, may in itself be an indicator of functional status since infants who had incomplete assessments were at higher risk for severe MRI injury. Sample size limitations precluded more robust statistical analysis. Inclusion of all potential covariates was not feasible in this dataset, thus the statistical approach to minimize included variables via preliminary univariable analysis was used. We included age at NNNS exam as a covariate that significantly differed between MRI outcome groups. The impact of postnatal age on the NNNS is unclear as the exam is described to be valid from the first day of life through 46 to 48 weeks post conceptual age.27 Although we targeted a specific day of life for NNNS assessment, remaining variability was accounted for by inclusion of this factor in the regression analyses. We also included gestational age, which demonstrated a trend towards difference between MRI outcome groups. Gestational age is a known important and immutable factor that has a prominent relationship with both developmental outcome and NNNS profiles.13 It is acknowledged that selected covariates included in the regression analyses may not represent all significant variables that could affect the relationship between NNNS scores and MRI injury. Finally, although the final sample size included would allow for detection of a small to medium effect size (f2=0.12–16) according to post-hoc power analyses,28 it is possible that sample size limitations could have affected detection of a more subtle but significant relationship between Total NNNS Z-score and MRI outcome. We consider these analyses hypothesis generating. Further study is needed, and underway, to evaluate the relationship between NNNS scores, MRI injury and later developmental outcome.
CONCLUSIONS
Subtle alterations in the neurobehavior of encephalopathic newborns following therapeutic hypothermia can be identified by NNNS assessment. Abnormalities in motor domains are associated with evidence of injury on MRI. Further investigation is warranted to evaluate the potential role of the NNNS as an early assessment of injury severity and predictor of later outcome for encephalopathic newborns treated with hypothermia.
ACKNOWLEDGEMENTS
This project was supported by Award Numbers UL1TR000075, KL2TR000076 and K12RR17613 from the NIH National Center for Research Resources. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
The authors acknowledge Jennifer Teng, M.S. for her assistance with data collection and management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
None declared
REFERENCES
- 1.Dilenge ME, Majnemer A, Shevell MI. Long-term developmental outcome of asphyxiated term neonates. J Child Neurol. 2001;16:781–792. doi: 10.1177/08830738010160110201. [DOI] [PubMed] [Google Scholar]
- 2.Shankaran S, Woldt E, Koepke T, Bedard MP, Nandyal R. Acute neonatal morbidity and long-term central nervous system sequelae of perinatal asphyxia in term infants. Early Hum Dev. 1991;25:135–148. doi: 10.1016/0378-3782(91)90191-5. [DOI] [PubMed] [Google Scholar]
- 3.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomized trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 4.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 5.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–1358. doi: 10.1056/NEJMoa0900854. Erratum N Engl J Med 2010;362:1056-1056. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs SE, Morley CJ, Inder TE, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 165:692–700. doi: 10.1001/archpediatrics.2011.43. [DOI] [PubMed] [Google Scholar]
- 7.Shankaran S, Barnes PD, Hintz SR, et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2012;97:F398–F404. doi: 10.1136/archdischild-2011-301524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutherford M, Ramenghi LA, Edwards AD, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010 Jan;9(1):39–45. doi: 10.1016/S1474-4422(09)70295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lester BM, Tronick EZ. History and description of the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics. 2004;113:634–640. [PubMed] [Google Scholar]
- 10.Lester BM, Tronick EZ. Appendix 3: Summary Score calculations. Pediatrics. 2004;113:695–699. [Google Scholar]
- 11.Tronick EZ, Olson K, Rosenberg R, Bohne L, Lu J, Lester BM. Normative neurobehavioral performance of healthy infants on the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics. 2004;113:676–678. [PubMed] [Google Scholar]
- 12.Lester BM, Tronick EZ, LaGasse L, et al. Summary statistics of Neonatal Intensive Care Unit Network Neurobehavioral Scale scores from the Maternal Lifestyle Study: a quasi-normative sample. Pediatrics. 2004;113:668–675. [PubMed] [Google Scholar]
- 13.Lester BM, Bagner DM, Liu J, et al. Infant neurobehavioral dysregulation: behavior problems in children with prenatal substance exposure. Pediatrics. 2009;124(5):1355–1362. doi: 10.1542/peds.2008-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Bann C, Lester B, et al. Neonatal neurobehavior predicts medical and behavioral outcome. Pediatrics. 2010;125:e90–e98. doi: 10.1542/peds.2009-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephens BE, Liu J, Lester B, et al. Neurobehavioral assessment predicts motor outcome in preterm infants. J Pediatr. 2010;156:366–371. doi: 10.1016/j.jpeds.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barkovich AJ, Hajnal BL, Vigneron D, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR. 1998;19:143–149. [PMC free article] [PubMed] [Google Scholar]
- 17.Miller SP, Ramaswamy V, Michelson D, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr. 2005;146:453–460. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 18.Massaro AN, Chang T, Kadom N, et al. Biomarkers of brain injury in neonatal encephalopathy treated with hypothermia. J Pediatr. 2012;161:434–440. doi: 10.1016/j.jpeds.2012.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glass HC, Nash KB, Bonifacio SL, et al. Seizures and magnetic resonance imaging-detected brain injury in newborns cooled for hypoxic-ischemic encephalopathy. J Pediatr. 2011;159:731–735. doi: 10.1016/j.jpeds.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boukydis CFZ, Bigsby R, Lester BM. Clinical use of the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics. 2004;113:679–689. Appendix 2:694. [PubMed] [Google Scholar]
- 21.Amiel-Tison C. Update of the Amiel-Tison neurologic assessment for the term neonate or at 40 weeks corrected age. Pediatr Neurol. 2002;27:196–212. doi: 10.1016/s0887-8994(02)00436-8. [DOI] [PubMed] [Google Scholar]
- 22.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. Arch Neurol. 1976;33:696–675. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- 23.Gunn AJ, Wyatt JS, Whitelaw A, et al. Therapeutic hypothermia changes the prognostic value of clinical evaluation of neonatal encephalopathy. J Pediatr. 2008;152:55–58. doi: 10.1016/j.jpeds.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Shankaran S, Laptook AR, Tyson JE, et al. Evolution of encephalopathy during whole-body hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr. 2012;160:567–572. doi: 10.1016/j.jpeds.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ment LR, Bada HS, Barnes P, et al. Practice parameter: neuroimaging of the neonate: report of the Quality Standards Subcomittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;58:1726–1738. doi: 10.1212/wnl.58.12.1726. [DOI] [PubMed] [Google Scholar]
- 26.van Laerhoven H, de Haan TR, Offringa M, et al. Prognostic tests in term neonates with hypoxic-ischemic encephalopathy: a systematic review. Pediatrics. 2013;131:88–98. doi: 10.1542/peds.2012-1297. [DOI] [PubMed] [Google Scholar]
- 27.Lester BM, Tronick EZ. Using the NNNS. In: Lester BM, Tronick EZ, editors. NICU Network Neurobehavioral Scale (NNNS) Manual. Baltimore, MD: Paul H. Brookes Pub. Co.; 2004. pp. 13–19. [Google Scholar]
- 28.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]