Abstract
Background
An emerging body of evidence suggests that ambient levels of air pollution during pregnancy are associated with preterm birth.
Methods
To further investigate these relationships we used vital record data to construct a retrospective cohort of 476,489 births occurring between 1994 and 2004 in five central counties of metropolitan Atlanta. Using a time-series approach, we examined aggregated daily counts of preterm birth in relation to ambient levels of carbon monoxide, nitrogen dioxide, sulfur dioxide, ozone, particulate matter < 10 μm in diameter (PM10), particulate matter < 2.5 μm in diameter (PM2.5) and speciated PM measurements. Daily pollutant levels in five-county Atlanta were characterized using a population-weighted spatial average of air quality monitors in the study area. We also examined ambient concentrations at individual monitors in analyses limited to mothers with residential geocodes within four miles of each monitor. Relationships between average pollution levels during three gestational windows of interest were modeled using Poisson generalized linear models. Results were adjusted for seasonal and long-term time trends.
Results
Although most results were null, there were three positive associations between ambient pollution levels and preterm birth in the four-mile capture-area analyses. Daily preterm birth rates were associated with average NO2 concentrations in the preceding six weeks and with average PM2.5 sulfate and PM2.5 water-soluble metal concentrations in the preceding week.
Conclusions
Results provide limited support for late-pregnancy effects of ambient air pollution on preterm birth.
Preterm birth (before 37 weeks of gestation) is a leading cause of infant morbidity and mortality, affecting 13% of births in the United States in 2005.1 An emerging body of evidence suggests that ambient levels of air pollution may play a role in the incidence of preterm birth.2–4 However, the gestational window of susceptibility has not been consistent across studies, with associations most commonly reported for exposures during early pregnancy (the first month or first trimester)5–9 or in late pregnancy (the third trimester, the last 6 weeks, the last month, the last week).5,7,8,10–14 Previous studies have also been inconsistent regarding the specific pollutants associated with preterm birth, although most studies suggest associations with ambient measures of particulate matter (PM).5–8,11–14 Sulfur dioxide (SO2), as well as traffic related pollutants such as nitrogen dioxide (NO2) and carbon monoxide (CO), have also been associated with preterm birth in several studies.5,8–16
Although the pathophysiology of preterm birth remains poorly understood, evidence suggests a role for inflammatory pathways as well as implantation errors in early pregnancy.17 Both of these pathways offer plausible mechanisms by which air pollution could increase the risk of preterm birth. Air pollution levels in the weeks following conception could disrupt implantation and placentation and increase the risk of preterm birth through suboptimal placental function. In late pregnancy, high levels of air pollution could activate either an acute or sustained inflammatory response leading to the initiation of early labor.
To investigate the relationship between ambient air pollution during gestation and the incidence of preterm birth, we conducted a time-series analysis in the central five counties of metropolitan Atlanta during 1994–2004. In addition to the US Environmental Protection Agency (EPA) criteria pollutants (ozone [O3], SO2, NO2, CO, PM10, PM2.5), we investigated speciated particle measurements that are rarely available on a daily basis and have not been previously assessed in relation to preterm birth. We focused on three gestational windows of exposure based on findings from previous air pollution studies, as well as current hypotheses about biological mechanisms leading to preterm birth: the first month of gestation, the final week of gestation and the final six weeks of gestation.
METHODS
Study Population
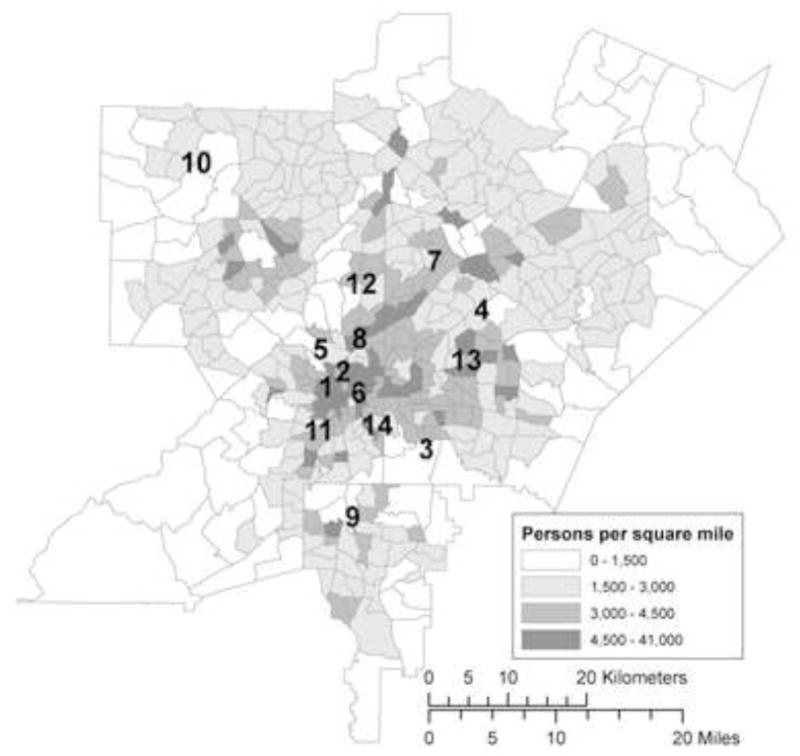
We obtained vital record data for births to mothers residing in five-county metropolitan Atlanta (Cobb, Clayton, DeKalb, Fulton and Gwinnett) from the Office of Health Research and Policy, Georgia Division of Public Health. The study area, shown in Figure 1, included 1752 square miles (4538 km2), an area with a radius 16 miles (26 km) at its narrowest and 32 miles (52 km) at its widest. The cohort included singleton births without major structural birth defects between 1 January 1994 and 31 December 2004 who reached at least 20 weeks of gestation. We further restricted inclusion to Hispanic, non-Hispanic black, non-Hispanic white, or Asian infants with complete data on maternal marital status and education. After exclusions, 476,489 out of 509,776 births (93%) were eligible for analysis. There were 387,123 eligible births after 1 January 1996, when daily PM10 monitoring data began, and 293,688 eligible births after 1 August 1998, when PM2.5 and speciated PM monitoring began.
Figure 1.
Five-county metropolitan Atlanta, population density according to the 2000 Census and location of ambient air quality monitoring stations
1. Indicates Jefferson St. monitoring station (CO, NO2, SO2, O3, PM10, PM2.5, PM components monitored); 2, Georgia Tech (NO2, SO2, PM10); 3, South DeKalb (NO2, O3, PM2.5); 4, Tucker (NO2, PM2.5); 5, Fire Station 8 (PM10, PM2.5); 6, Fulton Health Dept (PM10); 7, Doraville Health Center (PM10, PM2.5); 8, East Rivers School (PM10, PM2.5); 9, Forest Park (PM2.5); 10, Kennesaw (PM2.5); 11, Fort McPherson (PM2.5); 12, Roswell Road (CO); 13, DeKalb Tech (CO); 14, Confederate Ave (SO2, O3).
Outcome Definition
Preterm birth was defined as a live birth before gestational week 37; the earliest live births were recorded at 20 weeks. For 98.5% of the cohort, gestational age was calculated using the reported date of the last menstrual period (LMP). For women whose LMP date was missing or implausible (<20 or >44 weeks), the clinical estimate of gestational age was substituted (1.4% of births). For the remaining 0.1% of records without a valid LMP date or clinical estimate, we used the gestational age estimated by the Georgia Division of Public Health based on infant birth weight. Because medically indicated preterm birth and spontaneous preterm birth share many risk factors, we included induced preterm births in our primary analysis.18,19 However, we also conducted sensitivity analyses excluding inductions in order to assess the robustness of results.
The outcome definition differed slightly depending on the air pollution exposure window being investigated. For the late pregnancy windows, the population was limited to infants who reached at least 29 weeks’ gestation. This was based on our a priori hypothesis that the acute effects of air pollution would be unlikely to induce extreme preterm birth at less than 29 weeks. For the first month of gestation exposure window assessment, all preterm births between 20 and 36 weeks were included, based on the hypothesis that disruption of the implantation and placentation process early in pregnancy could increase vulnerability to both extreme and moderate prematurity.
Counts of preterm birth were determined for each day, aggregated either by conception date or birth date depending on the exposure window being investigated. The daily counts of preterm birth (numerator) were offset by the number of pregnancies at risk of preterm birth each day (denominator). Calculation of the pregnancy risk set also differed by exposure window and is described in detail below.
Ambient Air Quality Data
We obtained ambient air pollution levels from three sources: 1) the U.S. EPA Air Quality System, 2) the Georgia Institute of Technology PM2.5 network,20 and 3) the Aerosol Research and Inhalation Epidemiology Study (ARIES) monitor located near downtown Atlanta.21 The daily air metrics obtained included 1-hour maximum CO, NO2, and SO2, 8-hour maximum O3, and 24-hour average PM10, PM2.5, PM2.5–10, and PM2.5 components. Monitoring instrumentation and methods used are described in the online appendix (eTable 1, http://links.lww.com).
For CO, NO2, SO2, O3, PM10 and PM2.5, we calculated daily population-weighted spatial averages using methods described by Ivy and colleagues (see eAppendix, http://links.lww.com).22 This approach utilized all monitoring data available for each pollutant on a given day within the twenty-county metropolitan Atlanta area and yielded a daily spatial composite metric robust to missing data at individual monitoring sites. There were five CO monitors, six NO2 monitors, five SO2 monitors, five O3 monitors, nine PM10 monitors and eleven PM2.5 monitors used to calculate the daily spatial averages. For the coarse PM measurements (PM2.5–10) and PM2.5 component measurements (PM2.5 sulfate, PM2.5 nitrate, PM2.5 organic carbon, PM2.5 elemental carbon, PM2.5 total carbon, and PM2.5 water-soluble metals), daily measurements from the centrally located ARIES monitor were used. We imputed missing ozone values for six winter months between 1994 and 1996 using results from a statistical model in which temperature and week of year predicted the population-weighted ozone concentrations. Ozone values calculated using this imputation model were highly correlated with the population-weighted spatial average ozone values in winters after 1996 when ozone was monitored (r=0.79 for one-week averages).
Exposure Assignment
To calculate exposures for each study date in the time series, we averaged the daily spatial average pollutant values over the exposure window of interest. For late pregnancy exposure windows, air pollution assigned to each day represents the average pollution levels in the six weeks leading up to the study day, or the one week leading up to the study day. For the 1-month window, for which we analyzed preterm counts by conception date, each study day was assigned the average pollution level in the subsequent 28 days (i.e., pollution during the first month of gestation).
In a complementary approach we created spatial capture areas around each monitor and conducted monitor-specific time-series analyses for the cohort of births with residential geocodes within four miles of each station. This approach allowed for the possibility that ambient measurements close to the maternal residential address better correlate with personal exposures, particularly for primary pollutants which are more spatially heterogeneous (e.g., SO2, CO, NO2). Maternal addresses within four miles of two or more stations were assigned to the closest monitor. We limited the monitor-specific analyses to monitors that recorded daily pollutant concentrations; PM2.5 and PM10 monitors that recorded concentrations only every 3 or 6 days were excluded. In all analyses, exposure was set to missing when more than 15% of days in the averaging window were missing pollutant measurements, with the exception of the imputed spatial average winter ozone values described above.
Analytic Approach
Preterm births were aggregated into daily counts and analyzed using Poisson generalized linear models. Pollutants were examined as continuous variables in single-pollutant models, using scaled variance estimates to account for overdispersion. In the capture-area approach, separate time-series analyses were performed for the population surrounding each monitor, and effect estimates were pooled into a summary risk ratio using inverse-variance weights.
Because ambient air pollution levels exhibit strong seasonal variation, and the incidence of preterm birth may also vary by season,23 we controlled for seasonal trends using parametric cubic splines. We constrained the seasonal spline parameters in the model to be the same across all study years by including a day of year spline (day=1 to 365) with 12 monthly knots. In our descriptive analyses we found that births in April–May (conceptions in July–August) were more likely to be non-Hispanic white, and to married, college-educated mothers.24 Because these sociodemographic factors are related to the risk of preterm birth, we accounted for these seasonal trends explicitly by modeling temporal associations within racial, educational and marital status groups. Thus, each study day had multiple observations representing the counts of preterm birth within racial, educational and marital status strata. Accounting for these subtle trends directly allowed the day-of-year spline to adjust for other seasonal influences on the risk of preterm birth. We also smoothly adjusted for long-term temporal trends in preterm birth using a second cubic spline with knots on June 30th of each year.
First Month of Gestation Exposure Window
To examine whether high pollution levels during the first month of gestation increase the risk of later preterm birth, births were aggregated by conception date (assumed to be 14 days after the LMP date) and each conception date was assigned the average pollution over the subsequent four weeks (i.e., during the first month of gestation). Models took the form:
where Yt,r,k,m represents the number of conceptions on day t within stratum of race r, education k and marital status m who were eventually born preterm. The count was offset by the total number of conceptions on day t within the same racial, educational and marital status strata.
Late Gestation Exposure Windows
To investigate the hypothesis that high pollution levels in late pregnancy trigger preterm birth, preterm birth counts were aggregated by birth date rather than at a specific gestational age (e.g., conception). The denominator used to calculate daily rates of preterm birth included all ongoing gestations in utero at risk of preterm birth on a given day. A fetus enters the risk set at 29 weeks’ gestation and exits the risk set either on the date of preterm birth or at 37 completed weeks’ gestation; at 37 weeks’ gestation a fetus is censored from the analysis because it is no longer at risk of the outcome. Exposure assigned to each day in the time series was a lagged moving average of pollution in the previous one week or six weeks. Due to seasonal differences in the gestational age distribution of the risk set,24 we further subdivided each day’s preterm birth counts and risk set by gestational week, calculating a numerator of preterm birth counts within each gestational week between 29 and 36 weeks and a corresponding denominator of ongoing pregnancies in the same gestational week.
The models took the following form:
where Yt,w,r,k,m represents the number of preterm births on day t, in gestational week w, within stratum of infant race r (non-Hispanic black, non-Hispanic white, Hispanic, Asian), maternal education k (<12 years, 12–15 years, 16+ years), and maternal marital status m (married, unmarried). The offset (denominator) is the natural log of the number of ongoing pregnancies on day t, in gestational week w within strata of race (r), education (k) and marital status (m). The pollutant concentration represents either the lagged moving average concentration in the Atlanta area over the previous six weeks, or seven days corresponding to the six weeks or one week before the preterm births on each day of follow-up. The product terms allow for interaction between sociodemographic variables (race, education and marital status) and gestational age because the risk of preterm birth at various gestational weeks differed by these factors.17 Although weekend status is not associated with weekly pollution averages and therefore not a confounder, it was a strong temporal predictor of the outcome, particularly for the subset of induced preterm births; inclusion in the model reduced Poisson overdispersion and, as a consequence, slightly improved precision.
For all exposure windows we conducted sensitivity analyses evaluating the robustness of results to more and less stringent control for long-term and seasonal trends, control for temperature and dew point over the exposure window of interest, and use of a central monitor instead of a population-weighted spatial average to assign exposure. We also conducted stratified analyses by race, marital status and maternal education to observe whether results were consistent across these factors. We conducted all analyses using SAS version 9.1 (SAS Institute Inc., Cary, NC).
RESULTS
Maternal and infant characteristics of the five-county cohort and the cohort of births within four miles of a monitor are displayed in Table 1. Relative to the five-county cohort, the cohort of births within four miles of a monitor had a higher percentage of preterm births (11.7% vs. 10.3%) and were more likely to be black (57% vs. 40%). Mothers were less likely to be married (50% vs. 65%), and had fewer years of education (mean of 12.6 years vs. 13.2 years). On average, there were 12.2 preterm births per day, with 48,843 preterm births (10.3%) over the study period.
Table 1.
Maternal and infant characteristics for births 1 January 1994 – 31 December 2004 in five-county Atlanta and within four miles of a monitoring station included in the capture-area analysis.
| Five-county Atlanta (n=476,489)a No. (%) | Births within 4 miles of a monitor (n=136,858)a No. (%) | |
|---|---|---|
| Preterm birth | 48,843 (10.3) | 15,946 (11.7) |
| Female infant | 233,931 (49.1) | 67,313 (49.2) |
| Maternal age group (years) | ||
| <20 | 49,359 (10) | 19,419 (14) |
| 20–34 | 355,515 (75) | 99,135 (72) |
| 35+ | 71,615 (15) | 18,304 (13) |
| Infant race/ethnicity | ||
| Non-Hispanic white | 199,717 (42) | 33,504 (25) |
| Non-Hispanic black | 190,781 (40) | 78,094 (57) |
| Hispanic | 63,347 (13) | 19,749 (14) |
| Asian | 22,644 (5) | 5,511 (4) |
| Maternal Education (completed years) | ||
| <12 | 92514 (19) | 36794 (27) |
| 12–15 | 223,409 (47) | 63,216 (46) |
| 16+ | 160,566 (34) | 36,848 (27) |
| Married | 307,996 (65) | 68,411 (50) |
| First birth | 208,526 (44) | 60,317 (44) |
| Reported tobacco use | 23,041 (5) | 6,457 (5) |
| Season of birth | ||
| Winter (December–February) | 116,601 (25) | 33,530 (25) |
| Spring (March–May) | 117,642 (26) | 33,446 (24) |
| Summer (June–August) | 121,945 (26) | 34,732 (25) |
| Fall (September–November) | 120,301 (25) | 35,150 (26) |
| Year of birth | ||
| 1994 | 37,899 (8) | 8,757 (6) |
| 1995 | 38,288 (8) | 9,964 (7) |
| 1996 | 38,744 (8) | 10,552 (8) |
| 1997 | 40,463 (9) | 10,724 (8) |
| 1998 | 41,508 (9) | 11,059 (8) |
| 1999 | 43,207 (9) | 13,563 (10) |
| 2000 | 46,375 (10) | 15,217 (11) |
| 2001 | 47,660 (10) | 15,493 (11) |
| 2002 | 47,288 (10) | 14,927 (11) |
| 2003 | 47,421 (10) | 13,744 (10) |
| 2004 | 47,636 (10) | 12,858 (9) |
Excludes plural births, infants with major structural congenital birth defects, and infants who were missing data on race, maternal education and marital status.
Descriptive statistics of the five-county pollutant averages for each exposure window are presented in Table 2. The table includes the number of observation days used in each analysis, which differed by the availability of air quality data, and by the time period for which all fetuses at risk could be identified (i.e., without birth data from 2005, the in utero fetuses at risk in late 2004 could not be fully identified). An online appendix provides correlations between the pollutants for each averaging window as well as descriptive statistics of daily pollutant levels, overall and by season (eTables 2–6, http://links.lww.com).
TABLE 2.
Descriptive statistics of pollution levels for each gestational window of exposure using the population-weighted pollutant values (gaseous pollutants, PM10 and PM2.5) and the ARIES station measurements (PM2.5–10 and PM2.5 components).
| POLLUTANT | 4-week averageb | 1-week averagec | 6-week averagec | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. daysa | Mean (SD) | IQR | Range | No. daysa | Mean (SD) | IQR | Range | No. daysa | Mean (SD) | IQR | Range | |
| 1-hour max CO (ppm) | 3806 | 0.9 (0.2) | 0.32 | 0.5–1.7 | 3834 | 0.9 (0.3) | 0.37 | 0.2–2.4 | 3834 | 0.9 (0.2) | 0.30 | 0.5–1.5 |
| 1-hour max NO2 (ppb) | 3780 | 23.5 (4.0) | 5.0 | 12.6–38.5 | 3827 | 23.5 (6.0) | 8.0 | 8.0–46.1 | 3834 | 23.6 (3.5) | 5.0 | 14.7–33.5 |
| 1-hour max SO2 (ppb) | 3742 | 10.5 (3.1) | 4.0 | 3.9–22.7 | 3802 | 10.3 (4.7) | 6.0 | 1.4–30.7 | 3736 | 10.3 (2.6) | 3.0 | 4.2–18.8 |
| 8-hour max O3 (ppb) | 3806 | 44.1 (15.0) | 25 | 18.7–90.1 | 3834 | 44.0 (16.7) | 25 | 10.3–96.9 | 3834 | 44.3 (14.4) | 25 | 20.0–85.0 |
| 24-hour PM10 (μg/m3) | 2916 | 23.9 (6.3) | 8.0 | 10.8–51.8 | 3017 | 23.9 (8.2) | 10 | 7.4–68.7 | 2976 | 23.9 (5.7) | 8.0 | 13.2–43.6 |
| 24-hour PM2.5 (μg/m3) | 1994 | 16.5 (4.0) | 5.0 | 9.8–34.1 | 2111 | 16.4 (5.2) | 6.0 | 6.9–41.9 | 2130 | 16.5 (3.7) | 5.0 | 10.5–30.5 |
| 24-hour PM2.5–10 (μg/m3) | 1734 | 9.1 (2.5) | 2.7 | 4.6–19.2 | 1889 | 9.1 (3.3) | 3.6 | 2.6–25.6 | 1731 | 9.1 (2.2) | 2.5 | 4.9–16.7 |
| 24-hour PM2.5 sulfate (μg/m3) | 1594 | 4.9 (2.2) | 2.8 | 1.9–13.3 | 1782 | 4.8 (2.5) | 3.0 | 1.1–15.6 | 1533 | 4.9 (2.0) | 2.8 | 2.0–11.9 |
| 24-hour PM2.5 nitrate (μg/m3) | 1591 | 1.0 (0.5) | 0.66 | 0.3–2.7 | 1781 | 1.0 (0.6) | 0.75 | 0.2–4.3 | 1531 | 0.9 (0.5) | 0.64 | 0.3–3.6 |
| 24-hour PM2.5 total carbon (μg/m3) | 1951 | 6.0 (1.4) | 1.6 | 3.3–11.4 | 2007 | 5.9 (2.0) | 2.3 | 2.2–15.4 | 2047 | 6.0 (1.2) | 1.7 | 3.7–10.2 |
| 24-hour PM2.5 elemental carbon (μg/m3) | 1951 | 1.6 (0.5) | 0.53 | 0.8–4.3 | 2007 | 1.6 (0.7) | 0.70 | 0.5–7.6 | 2047 | 1.6 (0.5) | 0.55 | 1.0–3.6 |
| 24-hour PM2.5 organic carbon (μg/m3) | 1951 | 4.4 (1.0) | 1.2 | 2.4–8.3 | 2013 | 4.3 (1.4) | 1.7 | 1.5–12.1 | 2047 | 4.4 (0.8) | 1.2 | 2.6–7.4 |
| 24-hour PM2.5 water-soluble metalsd (μg/m3) | 1604 | 0.030 (0.011) | 0.017 | 0.009–0.066 | 1789 | 0.029 (0.015) | 0.020 | 0.005–0.104 | 1540 | 0.030 (0.012) | 0.016 | 0.010–0.079 |
Number of days over time period with nonmissing pollution values
Time period: gases 9/15/1993–2/15/2004 (3806 days), PM10 1/1/1996–2/15/2004 (2968 days), PM2.5 9/1/1998–2/15/2004 (1994 days)
Time period: gases 1/1/94–6/30/2004 (3834 days), PM10 2/1/1996–6/30/04 (3073 days), PM2.5 9/1/1998–6/30/2004 (2130 days)
Water-soluble metal index includes water soluble: Chromium, Copper, Iron, Manganese, Nickel, Vanadium.
Five-County Analysis
Results of the five-county analysis are presented in Table 3. Risk ratio estimates correspond to the relative increase in risk for an interquartile-range (IQR) increase in window-specific pollutant levels (IQRs shown in Table 2). Results were generally consistent with little or no association. There were negative associations between preterm birth rates and SO2 in the first month of gestation and the six-week lagged moving average of PM2.5 sulfate. Over the study period, there was a long-term decreasing trend in pollution levels and slight increase in preterm birth rates. We controlled for these long-term trends using cubic splines with one knot per year; we did not find evidence for residual confounding by these long-terms trends in sensitivity analyses utilizing more and less stringent temporal control. Other sensitivity analyses, including the analysis excluding induced preterm births, did not meaningfully change the results. Stratification by race, maternal education and marital status did not suggest effect modification by these variables. When we examined whether the model residuals were correlated on neighboring days within strata, there was no suggestion of appreciable autocorrelation.
TABLE 3.
Associations between air pollution levels in the three gestational windows of interest and preterm birth for births in five-county Atlanta
| Exposure Window |
|||
|---|---|---|---|
| First month of gestation a RR (95% CI)c | One week lagged moving average b RR (95% CI) c | Six week lagged moving average b RR (95% CI) c | |
| 1-h max CO (ppm) | 1.01 (0.99–1.04) | 1.00 (0.98–1.02) | 0.97 (0.94–1.01) |
| 1-h max NO2 (ppb) | 0.99 (0.98–1.01) | 1.00 (0.98–1.01) | 1.00 (0.98–1.02) |
| 1-h max SO2 (ppb) | 0.97 (0.96–0.99) | 0.99 (0.98–1.01) | 0.99 (0.97–1.01) |
| 8-h max O3 (ppb) | 0.96 (0.92–1.00) | 0.99 (0.96–1.01) | 1.00 (0.95–1.06) |
| 24-h PM10 (μg/m3) | 0.99 (0.97–1.01) | 0.99 (0.97–1.00) | 0.98 (0.95–1.01) |
| 24-h PM2.5 (μg/m3) | 1.00 (0.98–1.03) | 0.98 (0.97–1.00) | 0.99 (0.95–1.02) |
| 24-h PM2.5–10 (μg/m3) | 1.00 (0.97–1.02) | 0.99 (0.97–1.01) | 1.01 (0.98–1.04) |
| 24-h PM2.5 sulfate (μg/m3) | 1.00 (0.97–1.03) | 0.98 (0.96–1.01) | 0.95 (0.90–1.00) |
| 24-h PM2.5 nitrate (μg/m3) | 1.01 (0.97–1.05) | 0.99 (0.96–1.01) | 0.98 (0.93–1.04) |
| 24-h PM2.5 total carbon (μg/m3) | 0.99 (0.97–1.01) | 0.99 (0.97–1.01) | 1.00 (0.97–1.03) |
| 24-h PM2.5 elemental carbon (μg/m3) | 0.99 (0.97–1.02) | 1.00 (0.98–1.02) | 1.00 (0.97–1.04) |
| 24-h PM2.5 organic carbon (μg/m3) | 0.99 (0.97–1.01) | 0.99 (0.97–1.00) | 1.00 (0.97–1.02) |
| 24-h PM2.5 water-soluble metalsd (μg/m3) | 1.01 (0.97–1.05) | 0.98 (0.96–1.01) | 0.96 (0.91–1.03) |
Counts aggregated by conception date, offset by total conceptions, Poisson models control for long term trends, seasonal trends, race/ethnicity, marital status, education.
Counts aggregated by birth date, offset by gestations at risk, pollution corresponds to the one week and six weeks before the preterm births on each day of follow-up, Poisson models control for long term trends, seasonal trends, race/ethnicity, marital status, education, gestational week and interaction between gestational week and maternal characteristics. Extremely preterm births <29 weeks are excluded.
RRs and 95% CIs correspond to an IQR increase in pollutant value for each exposure window reported in Table 2.
Water-soluble metal index includes the following water soluble metals: Chromium, Copper, Iron, Manganese, Nickel, Vanadium.
Capture-Area Analysis
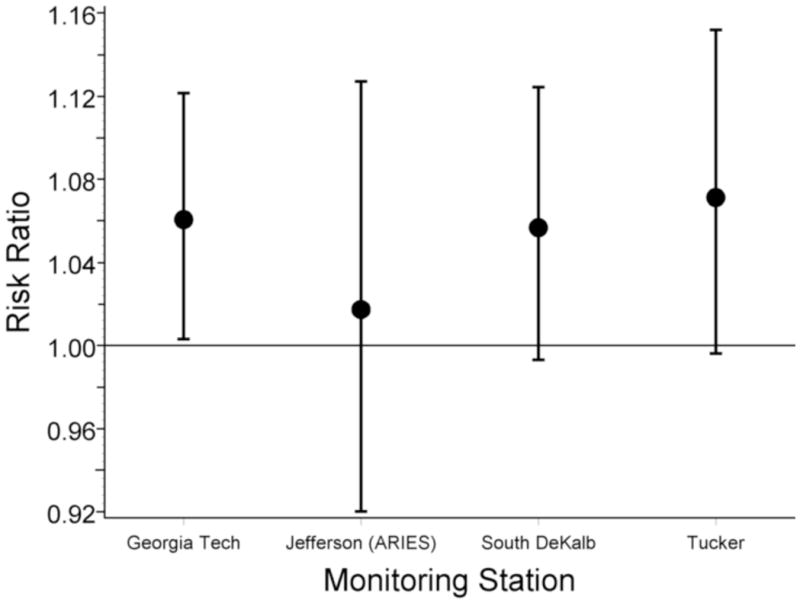
Results for the population of pregnancies within four miles of a monitor are presented in Table 4. Overall effect estimates for each pollutant were obtained using an inverse-variance weighted average of the effect estimates at each monitor, and are scaled to the same IQR values used in the five-county analysis (Table 2). The number of monitor-specific analyses included in the pooled estimate and the number of births captured by the four-mile buffers for each pollutant are also shown in Table 4. Observed effect estimates at each monitor are available in the online appendix (eTable 6). We observed a positive association of preterm birth rates with PM2.5 sulfate in the previous week (RR=1.09 [95% CI = 1.01–1.19]), PM2.5 water-soluble metals in the previous week (1.11 [1.02–1.22]) and NO2 in the previous six weeks (1.06 [1.02–1.09]). Only one monitor (ARIES) provided PM2.5 sulfate and PM2.5 water-soluble metal measurements; the four monitor-specific results pooled in the overall NO2 effect estimate for the six-week window are shown in Figure 2. The wider confidence intervals at the ARIES monitor reflect, in part, the shorter duration of monitoring at that site. Similar associations were observed in the analysis excluding induced preterm births.
TABLE 4.
Associations between air pollution levels in the three gestational windows of interest and preterm birth for births with a maternal residential address within 4 miles of a monitor.
| Pollutant | Exposure Window |
No. monitors (capture areas) | No. births within 4 miles of a monitord | ||
|---|---|---|---|---|---|
| First month of gestationa | One week lagged moving averageb | Six week lagged moving averageb | |||
| RR (95% CI)c | RR (95% CI)c | RR (95% CI)c | |||
| 1-hour max CO (ppm) | 0.99 (0.95–1.02) | 1.01 (0.99–1.03) | 1.01 (0.97–1.06) | 3 | 60,842 |
| 1-hour max NO2 (ppb) | 1.01 (0.99–1.04) | 1.01 (0.98–1.04) | 1.06 (1.02–1.09) | 4 | 68,801 |
| 1-hour max SO2 (ppb) | 1.00 (0.97–1.03) | 0.99 (0.96–1.02) | 0.98 (0.95–1.02) | 3 | 45,974 |
| 8-hour max O3 (ppb) | 0.94 (0.83–1.05) | 1.00 (0.94–1.08) | 1.06 (0.91–1.24) | 3 | 50,994 |
| 24-hour PM10 (μg/m3) | 1.07 (0.99–1.17) | 1.06 (0.99–1.13) | 1.01 (0.90–1.14) | 2 | 27,469 |
| 24-hour PM2.5 (μg/m3) | 0.99 (0.93–1.05) | 1.00 (0.96–1.03) | 1.05 (0.96–1.16) | 6 | 68,643 |
| 24-hour PM2.5–10 (μg/m3) | 1.03 (0.95–1.12) | 1.03 (0.97–1.10) | 1.07 (0.97–1.18) | 1 | 17,086 |
| 24-hour PM2.5 sulfate (μg/m3) | 1.06 (0.94–1.20) | 1.09 (1.01–1.19) | 0.93 (0.77–1.11) | 1 | 17,086 |
| 24-hour PM2.5 nitrate (μg/m3) | 1.03 (0.89–1.20) | 0.98 (0.90–1.08) | 0.86 (0.71–1.04) | 1 | 17,086 |
| 24-hour PM2.5 total carbon (μg/m3) | 1.02 (0.95–1.09) | 1.02 (0.96–1.09) | 0.97 (0.88–1.08) | 1 | 17,086 |
| 24-hour PM2.5 elemental carbon (μg/m3) | 1.01 (0.93–1.10) | 1.04 (0.98–1.10) | 0.97 (0.86–1.08) | 1 | 17,086 |
| 24-hour PM2.5 organic carbon (μg/m3) | 1.02 (0.94–1.10) | 1.01 (0.95–1.08) | 0.98 (0.89–1.07) | 1 | 17,086 |
| 24-hour PM2.5 water-soluble metals (μg/m3)e | 1.07 (0.93–1.24) | 1.11(1.02–1.22) | 0.89 (0.72–1.09) | 1 | 17,086 |
Counts aggregated by conception date, offset by total conceptions, Poisson models control for long term trends, seasonal trends, race/ethnicity, marital status, education
Counts aggregated by birth date, offset by gestations at risk, pollution corresponds to the one week and six weeks before the preterm births on each day of follow-up, Poisson models control for long term trends, seasonal trends, race/ethnicity, marital status, education, gestational week and interaction between gestational week and maternal characteristics. Extremely preterm births <29 weeks are excluded.
RRs and 95% CIs correspond to an IQR increase in pollutant value for each exposure window reported in Table 2 (same IQR values as the five-county analysis).
Exact number of births analyzed differed slightly by exposure window and missing pollutant values.
Water-soluble metal index includes the following water soluble metals: Chromium, Copper, Iron, Manganese, Nickel, Vanadium.
Figure 2.
Monitor-specific adjusted risk ratios (circles) and 95% CIs (vertical bars) for preterm birth associated with a 5 ppb increase in NO2 levels in the preceding six weeks. Adjusted for long term trends, seasonal trends, race/ethnicity, marital status, education, gestational week and interaction between gestational week and maternal characteristics. Monitoring time periods: Georgia Tech 1/94–12/04, Jefferson St. 8/98–12/04, South DeKalb 1/94–12/04, Tucker 4/95–12/04.
DISCUSSION
We investigated the relationship between 13 ambient air pollutants during three gestational windows and the incidence of preterm birth. Most of the 78 relationships examined were consistent with little or no association. However, three air pollutants were associated with higher risk in the capture-area approach: NO2 in the six weeks before birth, PM2.5 sulfate in week before birth and PM2.5 water-soluble metals in the week before birth. In contrast, two pollutants were associated with lower risk in the larger five-county analysis: PM2.5 sulfate in the six weeks before birth and SO2 in the first month of gestation. (It should be noted that point-source plume touchdowns lead to considerable spatial heterogeneity of SO2 concentrations in Atlanta.)25
Most previous studies have used spatio-temporal contrasts of exposure, comparing pregnant women across both space and time. Residual confounding by spatially-varying individual risk factors such as socioeconomic status (which can be difficult to quantify and adequately control) is a concern.26 To reduce the plausibility of confounding by individual-level risk factors, we conducted a temporal analysis in which comparisons were made across days within the same location. Furthermore, we were able to incorporate finer spatial resolution of ambient air pollution concentrations using population capture areas around each monitor, while still maintaining the purely temporal nature of the analysis.
It is possible that the finer spatial scale of exposure assignment provided by the capture-area approach better approximated exposures for mothers living near the monitor, particularly for primary pollutants such as NO2.25 Although this approach is intuitively appealing, it is unclear whether the closest monitor better approximates personal exposures when compared with a citywide metric.27,28 Pregnant women may spend a large portion of their day away from their residence, and with a recent study showing that 22% of women in Atlanta change residences during pregnancy,29 exposure assignment based on the residence at time of birth is problematic for assessment of early gestational windows. For spatially homogenous pollutants such as O3 and PM2.5 sulfate, which have strong spatial correlations, the five-county population-weighted average may provide a better measure of population average exposure while permitting analysis of the entire birth cohort.
Differences in results from the two approaches may also reflect differences in population susceptibility. Mothers residing near a monitor were more likely to be black, less educated, unmarried and were at an overall higher risk of preterm birth. Increased sensitivity to the adverse effects of air pollution in lower socioeconomic (SES) groups could be explained by a lack of access to health care, nutritional deficiencies,30 or concurrent exposure to other occupational and environmental hazards. Effect modification by SES-related characteristics could also act indirectly through better exposure assessment in individuals who have less access to air conditioning, live in older inner-city housing with greater pollutant infiltration,31 have occupations or activity patterns that involve more time spent outdoors, or are less likely to change residences in response to a growing family. However, when we stratified the five-county population by available SES-related variables, such as race, maternal education and marital status, results did not suggest effect modification by these variables.
One positive association observed in the capture area but not in the five-county approach was for PM2.5 sulfate, perhaps the most spatially homogeneous pollutant we analyzed.25 Although PM2.5 sulfate was measured at only one monitor (ARIES), total PM2.5 is strongly correlated with PM2.5 sulfate (r = 0.84) and was monitored at six stations. Although the risk ratio for PM2.5 at the ARIES monitor was also elevated at 1.06 (95% CI = 0.98–1.14), the pooled estimate for one-week lagged PM2.5 was 1.00 (0.96–1.03). This suggests that the PM2.5 sulfate association may not have been consistent across monitoring stations or subpopulations living near the monitors. In contrast, PM2.5 water-soluble metals are more spatially heterogeneous, and it is possible that the capture-area approach provided a better exposure estimate compared with the five-county approach. However, in light of the number of associations examined, and the fact that these PM2.5 constituents have not been previously assessed in relation to preterm birth, these associations warrant cautious interpretation and assessment in other populations.
The positive association between NO2 in late pregnancy and preterm birth is perhaps more compelling. Effect estimates were similar across the four individual NO2 monitors, and the spatial heterogeneity of this primary pollutant could explain why an association was not observed in the five-county analysis. Previous studies investigating NO2 in late pregnancy have yielded mixed results. Associations between preterm birth and ambient levels of NO2 in late pregnancy have been observed in the Czech Republic5 and Korea,8 but not in Los Angeles,11 Sydney,15 or Brisbane.6 In Vancouver, the association between NO2 in the last four weeks of gestation and preterm birth was suggestive (RR= 1.08 [95% CI = 0.99–1.17 per 10 ppb]).10 As in any study of ambient air pollution, the specific pollutants examined may serve as surrogates for other unmeasured (or less well measured) pollutants. Several studies have observed associations between preterm birth and traffic sources or traffic-related pollutants other than NO2.7,8,10,11,13,16 In Atlanta, using a spatial resolution of four miles around each monitor, NO2 may act as a surrogate for other pollutants emitted from internal combustion engines. In contrast to previous reports, we did not observe associations of preterm birth with CO, PM2.5, PM10, SO2 or O3.
One possible explanation for some of the null results could be an underestimation of effects due to the use of ambient measurements instead of personal exposures. By using a population-weighted spatial average in the five-county analysis and conducting capture area analyses at a finer spatial scale, we attempted to improve longitudinal correlations between ambient measures and average population exposures. Nonetheless, bias toward the null may have obscured true effects. In addition to exposure measurement error, gestational age is known to be measured with error on birth records.32 The degree and direction of misclassification, however, is likely to be independent of short-term changes in air pollution, and the reduction of power resulting from outcome misclassification was mitigated by our large sample size. Finally, as in previous studies, we could identify only conceptions resulting in a live birth; associations between early pregnancy air pollution exposure and preterm birth would be underestimated if air pollution increased the risk of fetal loss in addition to preterm birth.
In summary, we observed some evidence of an effect for NO2, PM2.5 sulfate and PM2.5 water-soluble metals during late pregnancy on the risk of preterm birth. However, these findings should be interpreted with caution in light of the multiple pollutants and gestational windows investigated, and the lack of strong a priori evidence for an effect of these pollutants. Nonetheless, because small increases in risk associated with a ubiquitous exposure could have large public health impacts, the relationship between air pollution and preterm birth merits further investigation.
Supplementary Material
Acknowledgments
Funding: Supported by the STAR Fellowship Program of the United States Environmental Protection Agency, and grant number R01-ES-012967-02S2A1 from the National Institute of Environmental Health Sciences, NIH.
We are grateful to the Georgia Division of Public Health, Office of Health Information and Policy for providing the vital record data.
Footnotes
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the United States Environmental Protection Agency.
References
- 1.Martin JA, Hamilton BE, Sutton PD, Ventura S, Menacker F, Kirmeyer SMLM. National vital statistics reports. 6. Vol. 56. Hyattsville, MD: National Center for Health Statistics; 2007. Births: Final data for 2005. [PubMed] [Google Scholar]
- 2.Glinianaia SV, Rankin J, Bell R, Pless-Mulloli T, Howel D. Particulate air pollution and fetal health: a systematic review of the epidemiologic evidence. Epidemiology. 2004;15(1):36–45. doi: 10.1097/01.ede.0000101023.41844.ac. [DOI] [PubMed] [Google Scholar]
- 3.Lacasana M, Esplugues A, Ballester F. Exposure to ambient air pollution and prenatal and early childhood health effects. Eur J Epidemiol. 2005;20(2):183–199. doi: 10.1007/s10654-004-3005-9. [DOI] [PubMed] [Google Scholar]
- 4.Sram RJ, Binkova B, Dejmek J, Bobak M. Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect. 2005;113(4):375–82. doi: 10.1289/ehp.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bobak M. Outdoor air pollution, low birth weight, and prematurity. Environ Health Perspect. 2000;108(2):173–176. doi: 10.1289/ehp.00108173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen C, Neller A, Williams G, Simpson R. Maternal exposure to low levels of ambient air pollution and preterm birth in Brisbane, Australia. BJOG. 2006;113(8):935–941. doi: 10.1111/j.1471-0528.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- 7.Huynh M, Woodruff TJ, Parker JD, Schoendorf KC. Relationships between air pollution and preterm birth in California. Paediatr Perinat Epidemiol. 2006;20(6):454–461. doi: 10.1111/j.1365-3016.2006.00759.x. [DOI] [PubMed] [Google Scholar]
- 8.Leem JH, Kaplan BM, Shim YK, Pohl HR, Gotway CA, Bullard SM, Rogers JF, Smith MM, Tylenda CA. Exposures to air pollutants during pregnancy and preterm delivery. Environ Health Perspect. 2006;114(6):905–910. doi: 10.1289/ehp.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maroziene L, Grazuleviciene R. Maternal exposure to low-level air pollution and pregnancy outcomes: a population-based study. Environ Health. 2002;1(1):6. doi: 10.1186/1476-069X-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S, Krewski D, Shi Y, Chen Y, Burnett RT. Association between gaseous ambient air pollutants and adverse pregnancy outcomes in Vancouver, Canada. Environ Health Perspect. 2003;111(14):1773–1778. doi: 10.1289/ehp.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritz B, Yu F, Chapa G, Fruin S. Effect of air pollution on preterm birth among children born in Southern California between 1989 and 1993. Epidemiology. 2000;11(5):502–511. doi: 10.1097/00001648-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Sagiv SK, Mendola P, Loomis D, Herring AH, Neas LM, Savitz DA, Poole C. A time-series analysis of air pollution and preterm birth in Pennsylvania, 1997–2001. Environ Health Perspect. 2005;113(5):602–606. doi: 10.1289/ehp.7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilhelm M, Ritz B. Local variations in CO and particulate air pollution and adverse birth outcomes in Los Angeles County, California, USA. Environ Health Perspect. 2005;113(9):1212–1221. doi: 10.1289/ehp.7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X, Ding H, Wang X. Acute effects of total suspended particles and sulfur dioxides on preterm delivery: a community-based cohort study. Arch Environ Health. 1995;50(6):407–415. doi: 10.1080/00039896.1995.9935976. [DOI] [PubMed] [Google Scholar]
- 15.Jalaludin B, Mannes T, Morgan G, Lincoln D, Sheppeard V, Corbett S. Impact of ambient air pollution on gestational age is modified by season in Sydney, Australia. Environ Health. 2007;6:16. doi: 10.1186/1476-069X-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilhelm M, Ritz B. Residential proximity to traffic and adverse birth outcomes in Los Angeles county, California, 1994–1996. Environ Health Perspect. 2003;111(2):207–216. doi: 10.1289/ehp.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Institute of Medicine of the National Academies. Preterm Birth Causes, Consequences and Prevention. Washington D.C: National Academies Press; 2007. [PubMed] [Google Scholar]
- 18.Klebanoff MA, Shiono PH. Top down, bottom up and inside out: reflections on preterm birth. Paediatr Perinat Epidemiol. 1995;9(2):125–129. doi: 10.1111/j.1365-3016.1995.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 19.Savitz DA, Dole N, Herring AH, Kaczor D, Murphy J, Siega-Riz AM, Thorp JM, Jr, MacDonald TL. Should spontaneous and medically indicated preterm births be separated for studying aetiology? Paediatr Perinat Epidemiol. 2005;19(2):97–105. doi: 10.1111/j.1365-3016.2005.00637.x. [DOI] [PubMed] [Google Scholar]
- 20.Butler AJ, Andrew MS, Russell AG. Daily sampling of PM2.5 in Atlanta: results of the first year of the assessment of spatial aerosol composition in Atlanta study. J Geophys Res. 2003;108(D1) [Google Scholar]
- 21.Van Loy M, Bahadori T, Wyzga R, Hartsell B, Edgerton E. The Aerosol Research and Inhalation Epidemiology Study (ARIES): PM2.5 mass and aerosol component concentrations and sampler intercomparisons. J Air Waste Manag Assoc. 2000;50(8):1446–1458. doi: 10.1080/10473289.2000.10464187. [DOI] [PubMed] [Google Scholar]
- 22.Ivy D, Mulholland J, Russell A. Development of Ambient Air Quality Population- Weighted Metrics for Use in Time-Series Health Studies. J Air Waste Manag Assoc. 2008;58(5):711–720. doi: 10.3155/1047-3289.58.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SJ, Steer PJ, Filippi V. Seasonal patterns and preterm birth: a systematic review of the literature and an analysis in a London-based cohort. BJOG. 2006;113(11):1280–1288. doi: 10.1111/j.1471-0528.2006.01055.x. [DOI] [PubMed] [Google Scholar]
- 24.Darrow LA, Strickland MJ, Klein M, et al. Seasonality of birth and implications for temporal studies of preterm birth. Epidemiology. 2009;20:XXX–XXX. doi: 10.1097/EDE.0b013e3181a66e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wade K, Mulholland J, Marmur A, Russell A, Hartsell B, Edgerton E, Klein M, Waller L, Peel J, Tolbert P. Instrument error and spatial variability of ambient air pollution in Atlanta, Georgia. J Air Waste Manag Assoc. 2006 Jun;56:876–888. doi: 10.1080/10473289.2006.10464499. [DOI] [PubMed] [Google Scholar]
- 26.Hertz-Picciotto I. Environmental Epidemiology. In: Rothman K, Greenland S, editors. Modern Epidemiology. 2. Philadelphia: Lippincott Williams and Wilkins; 1998. pp. 555–583. [Google Scholar]
- 27.Ebelt ST, Petkau AJ, Vedal S, Fisher TV, Brauer M. Exposure of chronic obstructive pulmonary disease patients to particulate matter: relationships between personal and ambient air concentrations. J Air Waste Manag Assoc. 2000;50(7):1081–1094. doi: 10.1080/10473289.2000.10464166. [DOI] [PubMed] [Google Scholar]
- 28.Kim D, Sass-Kortsak A, Purdham JT, Dales RE, Brook JR. Associations between personal exposures and fixed-site ambient measurements of fine particulate matter, nitrogen dioxide, and carbon monoxide in Toronto, Canada. J Expo Sci Environ Epidemiol. 2006;16(2):172–183. doi: 10.1038/sj.jea.7500446. [DOI] [PubMed] [Google Scholar]
- 29.Miller A, Siffel C, Correa A. Residential mobility during pregnancy in Atlanta (abstract) Am J Epidemiol. 2007;165:S149. [Google Scholar]
- 30.Kannan S, Misra DP, Dvonch JT, Krishnakumar A. Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ Health Perspect. 2006;114(11):1636–1642. doi: 10.1289/ehp.9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan WR, Price PN, Sohn MD, Gadgil AJ. Analysis of US Residential Air Leakage Database. Berkeley: Indoor Environment Department, Lawrence Berkeley National Laboratory; 2003. [Google Scholar]
- 32.Wier ML, Pearl M, Kharrazi M. Gestational age estimation on United States livebirth certificates: a historical overview. Paediatr Perinat Epidemiol. 2007;21 (Suppl 2):4–12. doi: 10.1111/j.1365-3016.2007.00856.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


