Abstract
Background: Non vital bleaching is simple, conservative procedure for esthetic correction of discolored endodontically treated teeth. The aim of this study was to determine and compare the amount of peroxide leakage from four different bleaching agents i.e superoxol, sodium perborate, combination of superoxol & sodium perborate and carbamide peroxide during intracoronal bleaching, as the safe and effective bleaching is the need of the hour.
Materials & Methods: 50 extracted maxillary centrals were selected for the study. Following standardized protocol access, cleaning and shaping by step back technique and obturation was done using guttapercha and AH plus sealer. Access was sealed with Cavit G and outer root surface was coated with wax and nail varnish. The teeth were separated into crown and root and the root portion was placed in plastic tube containing distilled water for 7days.After incubation, 3mm of gutta-percha was removed below CEJ and 2mm glass ionomer cement base was placed. Grouped into five categories based on the bleaching agent placed in pulp chamber as –group1 (control)-distilled water, group 2-sodium perborate with distilled water , group 3- 30% hydrogen peroxide ,group 4-mixture of sodium perborate and 30% hydrogen peroxide and group 5-10% carbamide peroxide gel. Peroxide leakage was measured after 24hrs using ferrothiocyanate method and optical density using spectrophotometer. Statistical analysis of the data was conducted using ANOVA and multiple comparisons within the groups was done using BONFERRONI method (Post-Hoc tests).
Results: The results showed highest peroxide penetration from 30% hydrogen peroxide followed by mixture of sodium perborate with 30% hydrogen peroxide, mixture of sodium perborate with distilled water and least penetration from 10% carbamide peroxide gel. The results were statistically significant.
Conclusion: Radicular peroxide leakage in 10% carbamide peroxide was significantly lower than the other tested bleaching agents making it a very safe alternative for intracoronal bleaching.
How to cite this article: Madhu KS, Hegde S, Mathew S, Lata DA, Bhandi SH, Shruthi N. Comparison of Radicular Peroxide Leakage from four Commonly used Bleaching agents following Intracoronal Bleaching in Endodontically treated teeth - An In Vitro Study. J Int Oral Health 2013; 5(4):49-55.
Key Words: : Intracoronal Bleaching, Hydrogen Peroxide, Sodium Perborate, Carbamide Peroxide
INTRODUCTION
Discoloured teeth especially in the anterior region can result in considerable cosmetic impairment. Intra coronal bleaching is simple, conservative and effective procedure for esthetic restoration of such discolored endodontically treated teeth. Over the years there have been plethoras of bleaching agents used. The most commonly used bleaching agents of this era are Superoxol (30-35% aqueous solutions of Hydrogen peroxide) alone or in combination with Sodium perborate (monohydrate, trihydrate, tetrahydrate). Non vital bleaching techniques used most often are Walking Bleach technique, Thermocatalytic technique or Combination walking bleach technique.
External root resorption is a serious complication of intracoronal bleaching with 30% hydrogen peroxide1 .Bleaching agent placed inside the pulp chamber diffuses through patent dentinal tubules to cervical region of teeth causing root resorption2. Reported incidence of cervical root resorption ranged from 0 to 6.9% in walking bleach technique3.
Sodium perborate moistened with water and Carbamide peroxide (10-37%) gels were such newer bleaching agents introduced over period of time to eliminate the complications but without affecting the bleaching efficacy of traditional agents. Aesthetic outcomes with 37% carbamide peroxide gel in walking bleach technique is acceptable and its use as intracoronal bleaching agent appears to combine efficacy of 35% hydrogen peroxide with safety of sodium perborate.
10% carbamide peroxide gel containing lower hydrogen peroxide concentration also yield whitening results comparable to sodium perborate with water when placed intracoronally4. As the biological threshold of peroxide compounds causing irreversible damage to dental tissues is still unknown, radicular peroxide penetration should be as limited as possible5. The aim of this study was to evaluate and compare radicular peroxide leakage from commonly used bleaching agents so as to assess the safety of the newer generation bleaching agents with that of traditional.
METHODOLOGY
Fifty fresh intact maxillary central incisors without any detectable caries, restoration, fracture or anamolies were collected for this study. All the soft and hard tissue deposits on the teeth were thoroughly cleaned, rinsed and stored in saline. Radicular cementum and cementoenamel junction (CEJ) of the teeth were examined stereomicroscopically( KYOWACZM4) and only teeth with no apparent cementum defects or dentin exposure at CEJ were included in this study.
Following standardized endodontic protocol, access cavities were prepared on the palatal surfaces of all the teeth using Endo Access bur (Dentsply). Working length was established by inserting a #15 k file (MANI) into the root canal until the file tip was just visible at the level of the apical foramen, and the distance between file tip and the rubber stop representing actual tooth length was measured to the nearest tenth of a millimeter. Working length was determined as 1mm short of the actual tooth length.
Root canals were cleaned and shaped using step back technique with endodontic k files (MANI) upto master apical file size # 55. Sodium hypochlorite 2.5% (Multilabs) was used intermittently for irrigating the canal. The canals were coated with AH plus sealer (Dentsply) and obturated with gutta-percha (Dentsply, Maillefer) by cold lateral condensation technique. The access cavities of all the teeth were sealed with temporary filling material- Cavit G (3M,ESPE) .The outer root surfaces including apical foramina and apical third of root were sealed with wax and covered with 2 layers of nail polish.
The teeth were separated into crown and root using modeling wax and suspended into plastic tubes containing 2ml of distilled water such that the entire root including CEJ was immersed in the distilled water (Fig. 2) for 7days at 37o C. After the incubation period, the canals were revisited and intracoronal bleaching procedure was initiated. The 3mm gutta-percha filling was removed from the canal using hot pluggers with labial CEJ as reference point. Remanants of gutta-percha and sealer were removed from access cavity with cotton pellet soaked with distilled water. Glass ionomer cement (GC Gold Label) of 2mm thickness was applied as a barrier on the remaining gutta-percha (Fig.1).
Fig. 2: Mounted teeth with radicular portion including CEJ immersed in distilled water.

Fig. 1: Radiograph of maxillary centrals obturated with gutta-percha & AH plus sealer and 2mm GIC barrier.
Teeth were suspended into plastic tubes containing 2ml of distilled such that the entire root including CEJ was immersed in the distilled water (Fig.2). Teeth
were separated into five groups of 10 teeth each based on type of bleaching agent as follows: Group1 (control) – Distilled water,Group 2 (experimental) – Mixture of sodium perborate trihydrate (LOBA CHEMIE )with distilled water (2g: 1ml), Group3 (experimental) – 30% hydrogen peroxide (20 μl ) (SD Fine Chem),Group 4 (experimental) – Mixture of sodium perborate trihydrate and 30% hydrogen peroxide (2g : 1ml) & Group 5 (experimental) -10% carbamide peroxide gel (Opalescence). The mounted teeth were left at 37oc for 24hrs.
After bleaching procedure, the teeth were removed from plastic tubes and measurement of hydrogen peroxide that may have leached into distilled water from bleaching agent was done using Ferrothiocyanate method. This reaction was colorimetric wherein colorless ferrothiocyanate was oxidized by peroxide to red ferrithiocyanate by the formation of ferrithiocyanate complex. The optical density of the solution was measured using UV Visible spectrophotometer (Perkin Elmer,Lambda 35) at a wavelength of 480nm (at room temperature).
The standard calibration curve was obtained using 30% Hydrogen Peroxide stock solution. Amount of peroxide in the samples were determined by comparing them to standard calibration curve. Statistical analysis of the data was conducted using Anova and multiple comparisons within the groups was done using Bonferroni method (Post-Hoc tests).
RESULTS
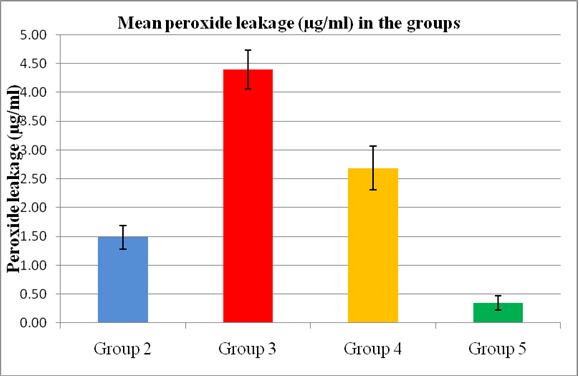
All the bleaching agents used in this study showed peroxide penetration from radicular canal following intracoronal bleaching (Table1, Fig.3).Highest mean peroxide penetration (in μg) was observed from Group 3 (30% hydrogen peroxide) (4.40μg/ml) followed by Group 4 (2.68μg/ml), Group 2 (1.48μg/ml) and least from group 5 (10% carbamide peroxide gel) (0.32μg/ml) (Table1). The difference in mean peroxide leakage between the groups was found to be statistically significant (P<0.001) using ANOVA. Multiple comparisons (Post – Hoc tests) done using Bonferroni test also showed significant difference among the groups. No radicular peroxide penetration was observed in Group 1(control.
Table 1: Mean Radicular peroxide leakage after 24hrs at 37oC
| Group | Mean | Std dev | Min | Max | F | P-Value |
| Group 2 | 1.483839 | 0.199576 | 1.164360 | 1.711216 | 388.217 | <0.001 |
| Group 3 | 4.400495 | 0.334762 | 3.964056 | 5.078701 | ||
| Group 4 | 2.686662 | 0.377785 | 2.226674 | 2.226674 | ||
| Group 5 | 0.342505 | 0.125802 | 0.177925 | 0.494526 |
Fig. 3: Bar graph comparing mean peroxide leakage from four groups of bleaching agents.
DISCUSSION
Superoxol (30% hydrogen peroxide) showed the maximum peroxide leakage among all the bleaching agents tested in the study. This was followed by the mixture of sodium perborate with 30% hydrogen
peroxide and then sodium perborate with distilled water. Least leakage was observed in 10 % carbamide peroxide gel (Table1, Fig.3).
Discoloration of the teeth could be due to incorporation of breakdown products from pulpal hemorrhage, incompletely removed pulpal remnants, pulp necrosis or from root canal filling materials and sealers containing eugenol or silver salts6-7. This condition presents an important esthetic concern for the patient. Walking bleach technique is the intra coronal bleaching procedure used widely because of its efficiency, simplicity and economics as compared to the prosthetic treatment.
In this study maxillary centrals were endodontically treated and the canals were obturated using gutta-percha and AH plus sealer. It has been reported that AH plus sealer showed excellent sealing ability by close adaptation to dentin when compared to other root canal sealers8-9. 2mm thick glass ionomer cement was applied over the canal filling, because it prevented leakage of bleaching agents ,ensured effective bleaching ,served as base for final restoration and resulted in more acceptable aesthetic results particularly in cervical region10-11.
Previous studies of peroxide penetration have utilized different time periods12-13. This study examined penetration levels after 24hrs. Radicular penetration of peroxide was evaluated by colorimetric Ferrothiocya -nate method as it was simple, precise and has been used by number of researchers11-12, 14-15.
In this study, highest peroxide penetration was observed in Group 3 i.e 30% hydrogen peroxide (Table1). The above finding was consistent with the earlier study conducted ,which observed that use of 35% hydrogen peroxide resulted in greater amounts of radicular peroxide when compared with 35% bleaching gel16.
Second highest penetration was seen in Group 4 i.e mixture of sodium perborate and 30% hydrogen peroxide (Table1). Similar study conducted with this combination also showed higher peroxide leakage17.
Group 2 i.e combination of sodium perborate and water also showed peroxide penetration (Table1), but lesser than the other two bleaching agents (Fig.3). Invitro studies have shown that applications of sodium perborate mixed with water were equally effective as applying sodium perborate and 30% hydrogen peroxide solution and also showed relatively less risk of external cervical resorption18-19. Studies have shown that all three forms of sodium perborate (mono hydrate, tri hydrate and tetra hydrate) yields similar results at the end of experimental period19.
Least amount of peroxide penetration was observed with Group 5 i.e 10% carbamide peroxide (Table1). The 10% carbamide peroxide used previously for internal bleaching of non vital teeth had shown similar performance to sodium perborate for internal bleaching4,20 . Studies have shown that carbamide peroxide releases hydrogen peroxide at slower rate and at lower concentration (3.6% H2O2) than superoxol and gives satisfying esthetic results16-17.
Incidence of invasive cervical resorption in association with bleaching procedures was reported to be 6.9%3. There are various hypothesis put forward to show the detrimental effects of bleaching agents. Bleaching agents cause superficial structural changes to dentin and acidic pH probably produces acid etch effect on dentine, opening up the smear layer covered cut surface of dentinal tubules increasing permeability21. It was hypothesized that bleaching agents penetrating patent dentinal tubules may cause dentin denaturation at CEJ initiating foreign body reaction1-2. Histopathol-ogical findings of the study revealed that external cervical root resorption could occur within few months of intra coronal bleaching22. Cervical resorption reported usually as asymptomatic and detected only through routine radiographs14.
The penetration of bleaching agents is affected by various factors including dental tissue characteristics, pH, concentration, active ingredients, contact time of bleaching agents and application of heat during bleaching process5, 11, 15, 23-24. Other factors include ceme-ntoenamel junction morphology, patient age and cementum defects15.
It was argued that greater hydrogen peroxide concentration of bleaching agents resulted in higher levels of peroxide penetration23. This study also found correlation between concentration and peroxide penetration, with lower peroxide penetration levels found in bleaching agents with lesser hydrogen peroxide percentage (Table1, Fig.3).
The teeth used in this study were not stained prior to experiment. In stained teeth however, the conversion of hydrogen peroxide to active oxygen may lead to oxidation of for example blood products within dentinal tubules with the resultant reduction in leakage of hydrogen peroxide during bleaching process5. In vivo studies have shown relatively lesser percent of damage following bleaching when compared to in vitro study. Atleast two forces that was thought to work against diffusive flux of molecules of bleaching agent towards pulp: positive pulpal pressure and osmotic pressure of gels24-25.
CONCLUSION
Within the limitations of this study, it can be concluded that all bleaching agents used in this study showed peroxide penetration to the extraradicular region of the teeth. One of the major concerns using walking bleach on discolored pulpless tooth was to find a compromise between esthetic results, risk of initiating external root resorption and time necessary to obtain best esthetic result. Although bleaching efficacy of these agents were not evaluated , considering the low rate and low levels of extraradicular diffusion found in this study and successful whitening effects reported in the literature4, 10% carbamide peroxide gel may prove to be the intra coronal bleaching agent of choice. Further studies should be conducted to focus on the complete elimination of peroxide penetration into extra radicular region of tooth as the biological threshold for peroxide compounds is still unknown.
Footnotes
Source of Support: Nil
Conflict of Interest: None Declared
REFERENCES
- 1.GW Harrington, E Natkin. External resorption associated with bleaching of pulpless teeth. J Endod. 1979;5(11):344–348. doi: 10.1016/S0099-2399(79)80091-6. [DOI] [PubMed] [Google Scholar]
- 2.M Cvek, AM Lindvall. External root resorption following bleaching of pulpless teeth with oxygen peroxide. Endod Dental Traumatol. 1985;1(2):56–60. doi: 10.1111/j.1600-9657.1985.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 3.S Friedman, I Rotstein, H Libfeld, A Stabholz, I Heling. Incidence of external root resorption and esthetic results in 58 bleached pulpless teeth. Endod Dent Traumatol. 1988;4(1):23–26. doi: 10.1111/j.1600-9657.1988.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 4.GA Perrine, RB Reichl, MK Baisden, SO Hondrum. Comparison of 10% carbamide peroxide and sodium perborate for intracoronal bleaching. Gen Dent. 2000;48(3):264–270. [PubMed] [Google Scholar]
- 5.R Weiger, A Kuhn, C Lost. Radicular penetration of hydrogen peroxide during intracoronal bleaching with various forms of sodium perborate. Int Endod J. 1994;27(6):313–317. doi: 10.1111/j.1365-2591.1994.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 6.A Watts, M Addy. Tooth discoloration and staining: a review of literature. Br Dent J. 2001;190(6):309–316. doi: 10.1038/sj.bdj.4800959. [DOI] [PubMed] [Google Scholar]
- 7.L Boksman, RE Jordan, DH Skinner. Non vital bleaching –internal and external. Aust Dent J. 1983;28(3):149–152. doi: 10.1111/j.1834-7819.1983.tb05270.x. [DOI] [PubMed] [Google Scholar]
- 8.O Zmener, C Spielberg, F Lamberghini, M Rucci. Sealing properties of new epoxy resin based root canal sealer. Int Endod J. 1997;30(5):332–334. doi: 10.1046/j.1365-2591.1997.00086.x. [DOI] [PubMed] [Google Scholar]
- 9.E Balguerie, MG Gurgel, F Diemer, P Calas. Root canal sealers:scanning electron microscope study of tubular penetration. European Cells and Materials. 2007;13:29. [Google Scholar]
- 10.T Lambrianidis, A Kapalas, M Mazinis. Effect of calcium hydroxide as supplementary barrier in radicular penetration of hydrogen peroxide during intracoronal bleaching in vitro. Int Endod J. 2002;35(12):985–990. doi: 10.1046/j.1365-2591.2002.00580.x. [DOI] [PubMed] [Google Scholar]
- 11.I Rotstein, D Zysking, I Lewinstein, N Bamberger. Effect of different protective base materials on hydrogen peroxide leakage during intracoronal bleaching in vitro. J Endod. 1992;18(3):114–117. doi: 10.1016/S0099-2399(06)81310-5. [DOI] [PubMed] [Google Scholar]
- 12.E Koulaouzidou, T Lambrianidis, P Beltes, K Lyroudia, C Papadopoulos. Role of cementoenamel junction on the radicular penetration of 30% hydrogen peroxide during intracoronal bleaching in vitro. Endod Dent Traumatol. 1996;12(3):146–150. doi: 10.1111/j.1600-9657.1996.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 13.JJ Smith, CJ Cunningham, S Montgomery. Cervical canal leakage after internal bleaching procedures. J Endod. 1992;18(10):476–481. doi: 10.1016/s0099-2399(06)81346-4. [DOI] [PubMed] [Google Scholar]
- 14.I Rotstein. In vitro determination and quanti-fication of 30% hydrogen peroxide penetration through dentin and cementum during bleaching. Oral Surg Oral Med Oral Path. 1991;72(5):602–606. doi: 10.1016/0030-4220(91)90500-c. [DOI] [PubMed] [Google Scholar]
- 15.I Rotstein, Y Torek, R Misgav. Effect of cementum defects on radicular penetration of 30% H2O2 during intracoronal bleaching. J Endod. 1991;39:17(5):230–2333. doi: 10.1016/S0099-2399(06)81927-8. [DOI] [PubMed] [Google Scholar]
- 16.GP Lee, MY Lee, SOY Lum, RSC Poh, KC Lim. Extraradicular diffusion of hydrogen peroxide and pH changes associated with intracoronal bleaching of discoloured teeth using different bleaching agents. Int Endod J. 2004;37(7):500–506. doi: 10.1111/j.1365-2591.2004.00838.x. [DOI] [PubMed] [Google Scholar]
- 17.O Gokay, F Ziraman, AC Asal, OM Saka. Radicular peroxide penetration from carbamide peroxide gels during intracoronal bleaching. Int Endod J. 2008;41(7):556–560. doi: 10.1111/j.1365-2591.2008.01384.x. [DOI] [PubMed] [Google Scholar]
- 18.S Friedman. In vitro efficacy of sodium perborate preparations used for intracoronal bleaching of discolored nonvital teeth. Endod Dent Traumatol. 1991;7(4):177–180. doi: 10.1111/j.1600-9657.1991.tb00204.x. [DOI] [PubMed] [Google Scholar]
- 19.H Ari, M Ungor. In vitro comparison of different types of sodium perborate used for intra coronal bleaching of discolored teeth. Int Endod J. 2002;35(5):433–436. doi: 10.1046/j.1365-2591.2002.00497.x. [DOI] [PubMed] [Google Scholar]
- 20.M Bizhang, A Heiden, U Blunk, S Zimmer, R Seemann, F Roulet. Intracoronal Bleaching of discolored non vital teeth. J Dent Res. 2001;80:710–713. [Google Scholar]
- 21.I Rotstein, E Danker, A Goldman, I Heling. Histochemical analysis of dental hard tissues following bleaching. J Endod. 1996;22(1):23–26. doi: 10.1016/S0099-2399(96)80231-7. [DOI] [PubMed] [Google Scholar]
- 22.D Heller, J Skriber, LM Lin. Effect of intracoronal bleaching on external cervical root resorption. J Endod. 1992;18(4):145–148. doi: 10.1016/S0099-2399(06)81407-X. [DOI] [PubMed] [Google Scholar]
- 23.JS Cooper, TJ Bokmeyer, WH Bowles. Penetration of the pulp chamber by carbamide peroxide bleaching agents. J Endod. 1992;18(7):315–317. doi: 10.1016/S0099-2399(06)80479-6. [DOI] [PubMed] [Google Scholar]
- 24.CT Hanks, JC Fat, JC Wataha, JF Corcoran. Cytotoxicity and dentin permeability of carbamide peroxide and hydrogen peroxide vital bleaching materials, invitro. J Dent Res. 1993;72(5):931–938. doi: 10.1177/00220345930720051501. [DOI] [PubMed] [Google Scholar]
- 25.W Thitinanthapan, P Stamanonont, N Vongsavan. In vitro penetration of the pulp chamber by three brands of carbamide peroxide. J Esth Rest Dent. 1999;11(5):259–264. doi: 10.1111/j.1708-8240.1999.tb00407.x. [DOI] [PubMed] [Google Scholar]


