Abstract
Background: Aim of the study was to investigate the relationship of melanin pigment and inflammatory process within gingival tissues based on clinical and genetic analysis by differential display technique and DNA sequencing.
Materials and Methods: Seventy gingival biopsy specimens were taken from individuals with melanin pigmentation as well as healthy and inflamed gingiva. Specimens were examined by differential display technique using six different arbitrary primers. Cloning, sequencing and sequence analysis for six different genes were performed.
Results: Gingival specimens with hyperpigmentation (clinical melanin score = 3) showed presence of both, down- and up-regulatory genes when compared with the gingival specimen with clinical melanin score 0. These genes may have a role in curtailing the progress of gingival inflammation associated with melanin hyperpigmentation.
Conclusion: Melanin hyper pigmentation may possess a defensive role against progress of gingival inflammation
How to cite this article:Eid HA, Syed S, Soliman AN. The Role of Gingival Melanin Pigmentation in Inflammation of Gingiva, Based on Genetic Analysis. J Int Oral Health 2013; 5(4):1-7.
Key Words: : Gingiva, Melanin hyper pigmentation, Differential display technique
Introduction:
Oral pigmented lesions can have various etiologies including drugs, heavy metals, endocrine disturbance, inflammation, and genetics.1-3 Smoking also stimulates melanin production leading to exceedingly evident intra oral pigmentation.3-4 Gingiva is the most commonly pigmented intra oral site, in addition to being the most readily seen during inspection. The most common reason for gingival pigmentation is melanin, though other pigments, such as, oxyhemoglobin, reduced hemoglobin and carotene, which contribute to the normal color of the integument, are also found in the masticatory mucosa.2
Melanin is a light-absorbent polymeric pigment found widely dispersed in nature. It is one of the fundamental pigments that give color to mammalian tissues.3 It is found in the oral cavity as early as 3 hours after birth. It is a non–hemoglobin-derived pigment formed by melanocytes that are located in the basal and suprabasal layers of gingival epithelium.5 Melanophores and melanophages are also cells present in epithelium and connective that have phagocytosed melanin granules and impart colour.6 Depending on the amount
and area of distribution of melanin the effect of pigmentation varies from light to dark brown or even black.2 The intensity of pigmentation largely depends on the activity of melanocytes rather than their number.7
Gingival hyper-pigmentation also known as racial gingival pigmentation is accepted to be a genetic trait. It is now well-established that pigmentation normally occurs in the oral mucous membrane of many ethnic groups.This normally occurring hyperpigmentation is benign in most cases and does not pose any medical concern.7-8
It has been hypothesized that oral mucosal melanin can act as a defense barrier by scavenging antioxidants and preventing oxidative stress.4,9 This action is carried out by binding toxins such as free radicals and polycyclic compounds to melanin. Melanin is known to scavenge free radicals associated with superoxide anion, which are generated as a result of 'respiratory burst' of phagocytosis. These free radicals play potential role in matrix destruction in inflamed periodontium. The oxygen derived from the free radicals can depolarize photoglycans and hyaluronan, active neutrophil collagenase which in turn initiates matrix degradation.9 The aim of this work was to correlate the presence of melanin hyper-pigmentation and the progress of gingival inflammatory process based on clinical and genetic analysis by differential display technique and DNA sequencing.
Materials and Methods:
Study inclusion criteria
This study was carried out concurrently with a parallel study on the histopathological aspects of melanin pigmented gingiva.10 Seventy patients were included that were indicated for gingivectomy (crown lengthening) for operative and/or fixed crown and bridge procedure. All of them were free of any systemic disease and were not undergoing any kind of drug therapy. Each patient received thorough scaling and root planning four weeks prior to gingivectomy to eliminate any gingival inflammation related to local factors (plaque and calculus).
After scaling and root planning, gingival bleeding scores were calculated weekly for the next four weeks and the average bleeding score was recorded.11 The presence of bleeding within 10 seconds indicated a positive score, which was expressed as a percentage of the total number of gingival margins examined. Plaque indices were scored12 on the basis of plaque at the gingival area of the teeth. Gingival bleeding, if any, was determined by gentle probing of the gingival crevices on the distofacial, facial, mesiofacial and the entire lingual margin with a William's graduated periodontal probe (Nordent, USA). Gingival Index13 was recorded (4 gingival scoring units per tooth) and subjects with mild to moderate degree of gingivitis were included. The same investigator carried out all clinical measurements for standardization. At the end of the fourth week gingivectomy procedure was done to obtain the gingival biopsy specimens. Specimens including free and part of the attached gingiva were secured from the vestibular surfaces of the maxillary or mandibular teeth of 70 patients recruited for this study. Gingival biopsies were immediately transferred to the molecular biotechnology lab and prepared for genetic analysis using differential display technique and DNA sequencing.
Melanin pigmentation score
Since the identification of induced and suppressed genes was the objective of this study, differential display technique was used to characterize the genetic variation (at RNA level) between gingival tissues with clinical melanin pigmentation sore 0&3.
Clinical assessment was carried out according to Dummets criteria14-15. Dummetts oral pigmentation score was recorded for each patient to assess the degree of gingival melanin pigmentation. A score of '0' was recorded for pink gingival tissue with no clinical pigmentation and a score of '3' for deep brown or blue/black tissue for heavy clinical pigmentation.
Study groups:
Test group 1 - 20 individuals with no clinical pigmentation (score 0), with gingival bleeding.
Test group 2 - 20 individuals with heavy clinical pigmentation (score 3), with gingival bleeding.
Negative control (Group 3) - 15 individuals with no clinical pigmentation (score 0), with no gingival bleeding (score 0).
Positive control (Group 4) - 15 individuals with heavy clinical pigmentation (score 3), with no gingival bleeding (score 0).
Differential display analysis
RNA isolation protocol Total RNA was extracted from gingival tissue using RNeasy Isolation kit (QIAgene, Germany) according to the manufacturer's instructions.
Reverse transcription of RNA Reverse transcription reaction was performed using oligo (dT) primer. Each 25 μl reaction mixture contained 2.5 μl (5x) buffer with MgCl2, 2.5 μl (2.5 mM) dNTPs, 1 μl (10 pmol) primer, 2.5 μl RNA (2mg/ml) and 0.5 unit reverse transcriptase enzyme (Promega, USA). PCR amplification was performed in a thermal cycler programmed at 95 °C for 5 minutes, 42 °C for 1 hour, 72 °C for 10 minutes (enzyme inactivation) and the product was stored at 4 °C until use.
-
Differential display PCR
Six primers were used in the differential display analysis (Table 1). The reaction mixture for differential display PCR was carried out in total volume 25 μl containing 2.5 μl 10x buffer with Mg Cl2, 2μl 2.5 mMdNTPS, 1μl 10 pmol, 1.5 μlcDNA and 0.2 μl (5 units/μl) TaqDNA polymerase. PCR amplification was performed in a thermal cycler (Eppendorf, Germany) programmed for one cycle at 95 °C for 5minutes, then 34 cycles as follows: 30 seconds at 95°C for denaturation, one minute at 30-32°C for annealing and one minute at 72 °C for elongation. Reaction was then incubated at 72 °C for 10 minutes for final extension.
Two μl of loading dye was added prior to loading of 10 μl per gel slot. Electrophoresis was performed at 80 Volt with 0.5 x TBE as running buffer in 1.5 % agarose/0.5 x TBE gels and then the gel was stained in 0.5 μg/ cm3 (w/v) ethidiumbromide solution and destined in deionized water. Finally the gel was visualized and photographed using a gel documentation system.
-
Sequencing of suspected upregulated gene
The upregulated band was excised and purified using Agarose DNA purified gel (Qiagene, Germany) following the manufactures procedures. DNA sequence was performed by Macrogene Company (Seoul, Korea). The sequence was submitted to NCBI GenBank database and DNA sequences were aligned in CLUSTAL W (1.8) program (http//www2.ebi.ac.uk/clustalw). The obtained sequence was translated in vitro, the suspected defense genes translation product (amino acid sequences) submitted to the EMBL blastx database Genbank.
Table 1: Primers used in this study for differential display.
| P1 | GAGAGCCAAC |
| P2 | ATGCCCCTGT |
| P3 | AGCCACCGAA |
| P4 | CCTTGACGCA |
| P5 | TTCGACCCAG |
| P6 | AAAGCTGCGG |
Results
Differentially displayed cDNAs of gingival cells with clinical melanin pigmentation score of 0 and 3 using 6 primers (Table 1) corresponding to well know tumor marker genes is show in figures 1, 2 and 3. Many up and down regulated genes were observed with all the primers used in this study.
Fig. 1: Differential display-PCR using primer P1 and P2 (M; DNA marker 3Kbp ladder, lane 1; gingival normal cells, lanes 2-5 different stages of gingival clinical melanin pigmentation score 0&3).
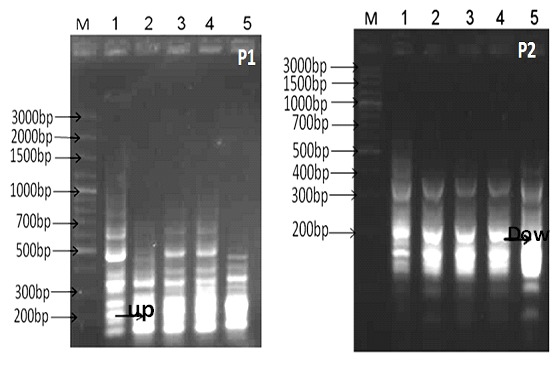
Fig. 2: Differential display-PCR using primer P1 and P2 (M; DNA marker 3Kbp ladder, lane 1; gingival normal cells, lanes 2-5 different stages of gingival clinical melanin pigmentation score 0&3).
With primer P1 about 40 band patterns were obtained, the molecular weights ranged from 600-100 bp. One of the up-regulated gene was isolated, cloned and sequenced. The sequence analysis revealed that the obtained gene was serine/threonine-proteinkinase LMTK2. In case of primer P2, about 55 genes were obtained. All the amplified genes were monomorphic genes except one down regulation gene in sample 5. This gene was isolated from the gel, purified and sequenced. The sequence analysis lead to follicle-stimulatinghormone beta gene.
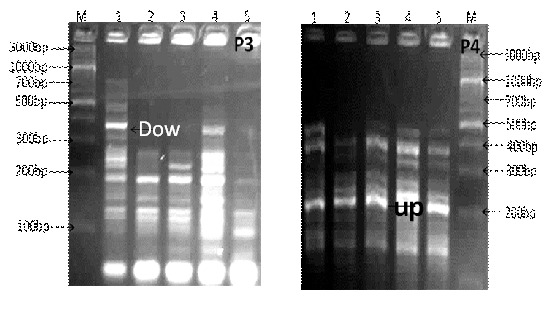
About 50 different genes were observed with primer P3. There were many up and down regulated genes among the amplified ones. The molecular weights of the obtained genes ranged from 500-30bp. One of the down regulated gene was selected randomly and isolated from the control lane, purified and sequenced. The sequence analysis resulted in gene Ankyrin repeat domain-containing protein 31. With primer P4 about 41 genes were obtained. Among these genes one upregulated gene that was isolated from sample 4. This gene was purified and subjected to DNA sequence. Sequence analysis resulted in Serine/threonine-proteinkinaseLMTK2 gene. This gene was similar to the one obtained with primer P1.
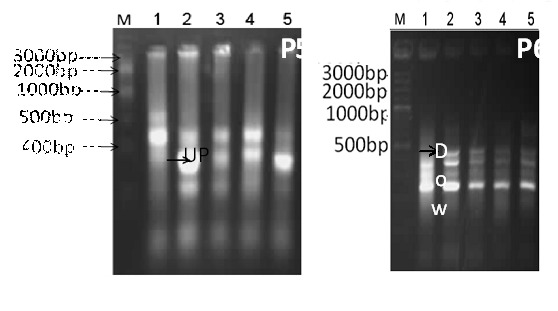
Differential display-PCR with primer P5 was successful in differentiating the examined samples. About 21 genes were observed and most of them were down regulated. One upregulated gene was isolated from sample 2 and sequenced. The sequence results revealed the tested gene to be Phenylalanyl-tRNA synthetase. On the other hand, primer P6 produced unique band patterns except with one gene which was down regulated. The amplified genes molecular weights ranged from 500-200bp. The isolated gene was found to be growth/differentiation factor 3 after subjecting it to DNA sequencing.
Fig. 3: Differential display-PCR using primer P3 and P4 (M; DNA marker 3Kbp ladder, lane 1; gingival tissues without melanin hyper-pigmentation, lanes 2-5 different stages of gingival tissues with clinical melanin pigmentation score 0 &3).
Discussion
Gingivitis is the most prevalent form of periodontal disease and in most cases requires maintaining meticulous dental hygiene along with periodontal therapy for its treatment.9 Gingiva accounts for 60% of oral melanin pigmentation. The distribution of oral melanin pigmentation in the hard palate is 61%, in the mucous membrane 22%, and in the tongue 15%8. Also the number of melanophores and melanin granules in the epithelium and the inflammatory cells of the subepithelial connective tissue gradually decrease from the free gingival groove area to the free gingival crest and mucogingival junction.5,8 Considering the distribution of oral melanin in gingiva, the gingival pigmentation provides a suitable platform to study the possible defensive role of melanins in the oral environment. However, the defensive role of melanin pigmented gingiva in the presence of plaque induced inflammation is not fully understood.16
In this study primers P1 and P2 resulted in the isolation of upregulated gene serine/threonine-proteinkinase(STK). Enomoto et al17 have demonstrated the role of activated STK 38 in prevention of cell death. On the other hand knockdown of STK 38 has enhanced the H2O2-induced JNK phosphorylation and cell death. This can be a probable, if not definitive, indication of the relation of gingival melanin and reduced gingival inflammation demonstrated in this study. Primer P2 resulted in a down regulation of follicle stimulating hormone (FSH) beta gene. The administration of FSH in experimental mice leads to the initiation of ovulatory process. Mammalian ovulation is known to be a highly regulated, inflammation-like process.18 Therefore, the down regulation of FSH gene may play a role in curtailing the inflammation process in the gingival tissue.
Ankyrin repeat domains are one of the most common protein domains historically associated with eukaryotic organisms. Proteins containing these repeats are referred to as Anks and mediate many cellular processes including cell cycle progression, transcription and cytoskeletal organization.16,19Anks have been implicated in the formation and progression of tumors.20 These proteins are possibly involved in relaying signals from the cytoskeleton/sarcomere to the nucleus.21-22Ankyrin repeat domain-containing protein have been used as DNA marker for gingival inflammationand melanoma progression.The down regulation of this gene observed with primer 3,might point towards melanins' role in resisting the development of gingival inflammation.
The up regulation of phenylalanyl-tRNA synthetase demonstrated with primer 5 in the presence of melanin indicate an unexplained relation with gingival inflammation. This can be the focus of a separate study.Epidermal growth factor which is secreted from salivary glands has a potential role in oral wound healing23. As shown by the results obtained in this study,down regulation was also observed for growth factor genes with primer P6. It can be hypothesized that upregulation of growth factor gene in association with gingival melanin can alleviate gingival inflammation.
Conclusion
Gingival pigmentation provides an appropriate platform to investigate the likely defense-role of melanin in oral environment. A kind of epigenetics can be assumed to be controlling the inflammatory process of gingival cells. The induced and suppressed genes isolated in this study draw attention to the plausible promising role of melanin in presence of gingivitis. This role can well be guided by the five isolated genes and many more. However, the understanding of its exact mechanism needs further investigation.
Acknowledgement
Sincere appreciation forAssociate Professor Dr. Elsayed Hafez, of Molecular Biotechnology, City of Science & Technology, New Borg Alarab Alexandria, Egypt, for his remarkable effort in providing the genetic analysis work required for this study.
Acknowledgments
Sincere appreciation forAssociate Professor Dr. Elsayed Hafez, of Molecular Biotechnology, City of Science & Technology, New Borg Alarab Alexandria, Egypt, for his remarkable effort in providing the genetic analysis work required for this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None Declared
References
- 1.A Kauzman, M Pavone, N Blanas, G Bradley. Pigmented lesions of the oral cavity: review, differential diagnosis, and case presentations. J Can Dent Assoc. 2004;70(10):682–683. [PubMed] [Google Scholar]
- 2.GW Mirowski, JS Waibel. Pigmented lesions of the oral cavity. DermatolTher. 2002;15:218–228. [Google Scholar]
- 3.M Meleti, P Vescovi, WJ Mooi, I van der Waal. Pigmented lesions of the oral mucosa and perioral tissues: a flow-chart for the diagnosis and some recommendations for the management. Oral Surg Oral Med Oral Pathol Oral RadiolEndod. 2008;105(5):606–616. doi: 10.1016/j.tripleo.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 4.CA Hedin, T Axell. Oral melanin pigmentation in 467 Thai and Malaysian people with special emphasis on smoker's melanosis. J Oral Pathol Med. 1991;20(1):8–12. doi: 10.1111/j.1600-0714.1991.tb00879.x. [DOI] [PubMed] [Google Scholar]
- 5.E Hubert, Schroeder Melanin containing organelles in cells of the human gingiva I. Epithelial Melanocytes. J Periodontol Res. 1969;4(1):1–18. doi: 10.1111/j.1600-0765.1969.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 6.T Sorsa, H Saari, YT Kontinen. Non-proteolytic activity of latent human neutrophil collagenase and its role in matrix destruction in periodontal disease. Int J Tissue Reac. 1989;9(4):153. [PubMed] [Google Scholar]
- 7.H Tal, D Oegiesser, M Tal. Gingival depigmentation by erbium:YAG laser: clinical observations and patient responses. J Periodontol. 2003;74(11):1660–1667. doi: 10.1902/jop.2003.74.11.1660. [DOI] [PubMed] [Google Scholar]
- 8.PM Bartold. The effect of oxygen derived free radicals on radicals on gingival proteogycans and hyaluronic acid. J Peridontol Res. 1984;19(4):390. doi: 10.1111/j.1600-0765.1984.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 9.RC Page. Gingivitis. J Clin Periodontol. 1986;13(5):345–349. doi: 10.1111/j.1600-051x.1986.tb01471.x. [DOI] [PubMed] [Google Scholar]
- 10.HA Eid. Impact of melanin pigmentation on gingival inflammation: Histopathological and biochemical analysis. Egy Dent J. 2012;58 (2):1289–1294. [Google Scholar]
- 11.J Ainamo, I Bay. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25(4):229–235. [PubMed] [Google Scholar]
- 12.J Silness, H Loe. Periodontal disease in pregnancy. Correlation between oral hygiene and periodontal condition. Acta Odont Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 13.H Loe, J Silness. Periodontal disease in pregnancy. Acta Odont Scand. 1963;21:532–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 14.CO Dummett. First symposium on oral pigmentation. J Periodontol. 1960;31:345. [Google Scholar]
- 15.CO Dummett, JS Sakumara, G Barens. The relationship of patient skin complexion to oral mucosa pigmentation and tooth colour. J Prosth Dent. 1980;(4):392–6. doi: 10.1016/0022-3913(80)90207-3. [DOI] [PubMed] [Google Scholar]
- 16.AW Barrett, C Scully. Human oral mucosal melanocytes: a review. J Oral Pathol Med. 1994;23(3):97–103. doi: 10.1111/j.1600-0714.1994.tb01095.x. [DOI] [PubMed] [Google Scholar]
- 17.K Enomoto, K Sakurai. A Case of Breast Cancer Postoperative Metastases to the Liver Obtained cCR. GanTo Kagaku Ryoho. 2011;38(12):2075–2077. [PubMed] [Google Scholar]
- 18.JS Richards, DL Russell, S Ochsner, L Espey. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu. Rev. Physiol. 2002;64:69–92. doi: 10.1146/annurev.physiol.64.081501.131029. [DOI] [PubMed] [Google Scholar]
- 19.LK Mosavi, TJ Cammett, DC Desrosiers, ZY Peng. The ankyrin repeat as molecular architecture for protein recognition. Protein Sci. 2004;13(6):1435–1448. doi: 10.1110/ps.03554604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.J Li, A Mahajan, MD Tsai. Ankyrin repeat: a unique motif mediating protein-protein interactions. Biochemistry. 2006;45(51):15168–15178. doi: 10.1021/bi062188q. [DOI] [PubMed] [Google Scholar]
- 21.S Kojic, E Medeot, E Guccione, H Krmac, I Zara, V Martinelli, G Valle, G Faulkner. The Ankrd2 protein, a link between the sarcomere and the nucleus in skeletal muscle. J Mol Biol. 2004;339(2):313–325. doi: 10.1016/j.jmb.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 22.MK Miller, ML Bang, CC Witt, D Labeit, C Trombitas, K Watanabe, H Granzier, AS McElhinny, CC Gregorio, S Labeit. The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and DARP as a family of titin filament-based stress response molecules. J Mol Biol. 2003;333(5):951–964. doi: 10.1016/j.jmb.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 23.JL Herrera, MF 2nd Lyons, LF Johnson. Saliva: its role in health and disease. J Clin Gastroenterol. 1988;10(5):569–578. doi: 10.1097/00004836-198810000-00019. [DOI] [PubMed] [Google Scholar]


