Abstract
The α-synuclein protein is associated with several neurodegenarative diseases, including Parkinson’s disease (PD). In humans, only mutated forms of α-synuclein are linked to PD; however, panneural expression of human wild-type (WT) α-synuclein induces Parkinson’s like-symptoms in Drosophila. Here, we report a quantitative proteomic analysis of WT α-synuclein transgenic flies with age-matched controls at the presymptomatic stage utilizing a global isotopic labeling strategy combined with multidimensional liquid chromatographies and tandem mass spectrometry. The analysis includes two biological replicates, in which samples are isotopically labeled in forward and reverse directions. In total, 229 proteins were quantified from assignments of at least two peptide sequences. Of these, 188 (82%) proteins were detected in both forward and reverse labeling measurements. Twelve proteins were found to be differentially expressed in response to the expression of human WT α-synuclein; down-regulations of larval serum protein 2 and fat body protein 1 levels were confirmed by Western blot analysis. Gene Ontology analysis indicates that the dysregulated proteins are primarily associated with cellular metabolism and signaling, suggesting potential contributions of perturbed metabolic and signaling pathways to PD. An increased level of the iron (III)-binding protein, ferritin, typically found in the brains of PD patients, is also observed in presymptomatic WT α-synuclein expressing animals. The observed alterations in both pathology-associated and novel proteins may shed light on the pathological roles of α-synuclein that may lead to the development of diagnostic strategies at the presymptomatic stage.
Keywords: α-synuclein, Drosophila, Parkinson’s disease, proteomics
Introduction
The α-synuclein protein is a member of the synuclein family that consists of α-, β-, and γ- synucleins.1 The primary sequence of α-synuclein consists of 140 amino acid residues, including an amphipathic amino-terminus, a hydrophobic core, and an acidic carboxyl-terminus.2 As a highly abundant protein in the brain, α-synuclein primarily localizes to the presynaptic nerve terminals.3 Although its normal cellular functions remain unknown, recent studies suggest that α-synuclein may be involved in neuronal plasticity,4 lipid transport,2,5 and synaptic membrane biogenesis.6
The association of α-synuclein with neurodegenerative diseases originates from the finding in 1993 that amyloid plaques formed in the brains of Alzheimer’s disease (AD) patients, which consist primarily of amyloid β peptides, also contain a short nonamyloid peptide derived from the precursor protein, α-synuclein.7 In the following years, further evidence of α-synuclein contributions to neurodegeneration emerged. In 1997, a point mutation (A53T) in α-synuclein was reported to cause early onset, familial Parkinson’s disease (PD).8 In the same year, α-synuclein was revealed to be the major component of Lewy bodies (LBs) formed in the substantia nigra of PD patients.9 In 1998, a second PD-linked α-synuclein mutation (A30P) was observed,10 and in 2003, triplication of the α-synuclein gene was determined to contribute to PD.11 In 2004, a third PD linked α-synuclein mutation (E46K)11 was uncovered; several months later, duplication of the α-synuclein locus was found to cause PD.13,14 In addition to PD, α-synuclein inclusions also contribute to dementia with LBs,3,9 multiple system atrophy,15 the Parkinsonism-dementia complex of Guam,16 Hallervorden-Spatz disease,17 and some cases of AD.18,19 The involvement of α-synuclein in such a wide group of neurodegenerative disorders (termed synucleinopathies) indicates that this protein possibly plays a pivotal role in the etiology and pathogenesis of neurodegeneration.
To understand cellular functions of α-synuclein and how this protein contributes to synucleinopathies, especially PD, a variety of α-synuclein-based animal models have been established.20 These include yeast,21 Drosophila,22 mice,23,24 rats,25,26 and primates27 expressing (or overexpressing) either wild-type (WT) or disease-linked mutant human α-synuclein. Among them, Drosophila is an interesting model. This is because the Drosophila organism has a relatively simple central nervous system (CNS) and its genome does not contain an endogenous α-synuclein gene or α-synuclein ortholog; however, transgenic Drosophila expressing both human WT and disease linked α-synuclein mutants (A30P and A53T) develop PD-like symptoms.28 Thus, Drosophila provides a model system with which to study α-synuclein-mediated neurodegeneration associated with PD.
Our group has been studying the Drosophila organism for several years.29–34 We previously reported on proteome analyses of two α-synuclein mutants (A30P and A53T) associated Drosophila PD models.33,34 These studies revealed that as early as day 1 (presymptomatic stage), expression of A30P and A53T α-synuclein in the Drosophila CNS alters the levels of proteins associated with the Actin cytoskeleton, mitochondria, and membrane. Because Drosophila expressing WT α-synuclein develop similar human PD-like symptoms as Drosophila expressing mutant forms of α-synuclein, a proteomic study of the WT α-synuclein Drosophila model is expected to provide insight to molecular pathways involving normal α-synuclein and to provide a more general or comprehensive view of protein alterations that may be associated with PD. Herein, we present a quantitative proteome analysis of a WT α-synuclein Drosophila model at the presymptomatic stage employing a global internal standard technology (GIST) in combination with strong cation exchange (SCX) and reversed-phase liquid chromatographies (RP-LC) coupled to tandem mass spectrometry (MS/MS).
Experimental Section
Drosophila Stocks and Harvesting
This study utilized the following control and PD-generating fly genotypes: elav::Gal4 (Pw+mW.hs=GawBelavC155, Bloomington Stock Center, Indiana University) and UAS- α-synuclein [the P(UAS-Hsap\SNCA.F)5B line was obtained from Mel Feany, Harvard Medical School], respectively. To obtain the elav::Gal4=>UAS::α-synuclein experimental flies, virgin females from the elav::Gal4 line were crossed to males from the UAS-α-synuclein stock. Control and PD-like flies were cultured on standard cornmeal medium, maintained at identical conditions (25 ± 1 °C), and harvested at the same time. To avoid differences that arise from gender, only male flies were used. A population of 250 adult fly heads was collected for each genotype at day 1 posteclosion (within 24 h) for protein extraction. Fly heads were collected and stored as described previously. A new batch of flies was raised for an independent biological replicate measurement.
Protein Sample Preparation
Fly samples were prepared as described previously32 with minor modifications. Briefly, fly head proteins were extracted using a mortar and electric pestle in a 0.2 M phosphate buffer saline solution (pH 7.0) containing 8.0 M urea and 0.1 mM phenylmethylsulfonyl fluoride. After centrifugation (13 000 rpm) for 10 min, the supernatant was collected. A Bradford assay indicated that ~2.5 mg of proteins was obtained from 250 adult fly heads. Three standard proteins (i.e., human hemoglobin, human albumin, and horse heart myoglobin) were each spiked into equal amounts of control and PD-like samples at known concentration ratios of 4:1, 1:1, and 1:3, respectively. Protein mixtures were reduced, alkylated, and tryptically digested as described previously.32 Finally, tryptic peptides were desalted, dried, and stored at −80 °C until future use.
GIST Labeling of Tryptic Peptides
Equal amounts of dried control and PD-like fly tryptic peptides were resuspended in phosphate buffer (pH 7.5) to produce a 1 mg·mL−1 solution. The GIST labeling reaction was processed as described elsewhere.35 Briefly, a 100-fold molar excess of N-acetoxy-d0-succinimide (light) and N-acetoxy-d3-succinimide (heavy) (refer to reference for synthesis) was added to control or PD-like samples (i.e., in one experiment, the control sample was light-labeled and the PD-like sample was heavy-labeled, which was referred to as forward labeling; in a second experiment with an independent biological replicate, the control sample was heavy-labeled and the PD-like sample was light-labeled, which was referred to as reverse labeling). The reagents and protein mixtures were stirred for 5 h at room temperature. At the end of the reaction, the two differentially labeled samples were combined, treated with an excess amount of N-hydroxylamine as previously described,34 desalted, dried, and stored at −80 °C until further analysis.
SCX Chromatography
Offline SCX prefractionation was performed as previously described.34 Briefly, the isotopically labeled tryptic peptides were dissolved in 5.0 mM potassium phosphate buffer solution in 75:25 water:acetonitrile at pH 3.0 (i.e., solvent A) and was injected onto a javelin guard column (10 × 2.1 mm; PolyLC Inc., Southboro, MA) that preceded a polysulfethyl aspartamide column (100 × 2.1 mm, 5 μm, 200 Å; PolyLC Inc., Southboro, MA). Mobile phases consisted of solvent A and B (i.e., solvent A with the addition of 350 mM potassium chloride). Binary gradients with respect to the percentage of solvent B were as follows: 0–5 min, 0%; 5–45 min, 0–40%; 45–90 min, 40–80%; 90–100 min, 80–100%; 100–110 min, 100%; 110–111 min, 100–0%; 111–121 min, 0%. The gradient was delivered at a flow rate of 0.2 mL·min−1 by a Waters 600 multisolvent delivery system (Waters, Milford, MA) and peptides were detected at 214 nm by a Waters 2487 dual λ absorbance detector (Waters, Milford, MA). One minute collections into 96 well plates (Corning Incorporated, Corning, NY) over the 121 min gradient were combined into six fractions as follows: (1) 0–34 min, (2) 34–40 min, (3) 40–44 min, (4) 44–50 min, (5) 50–58 min, and (6) 58–121 min. Pooled fractions were desalted, dried, and stored at −80 °C until further analysis.
RP-LC–MS/MS Analysis
RP-LC–MS/MS analysis was performed using an LTQ-FT hybrid linear ion trap Fourier transform ion cyclotron resonance mass spectrometer (Thermo-Electron, San Jose, CA) equipped with a Finnigan Nanospray II electrospray ionization source (ThermoElectron, San Jose, CA) and an UltiMate 3000 system (Dionex Corporation, Sunnyvale, CA). The instrumental configuration has been described elsewhere.34 Briefly, peptides were separated on a 13.2 cm self-pack PicoFrit column (75 μm i.d.; New Objective, Woburn, MA) packed with Magic C18AQ (5 μm, 100 Å; Microm BioResources Inc., Auburn, CA). A sample volume of 6.4 μL was loaded onto a 300 μm i.d. × 5 mm precolumn cartridge (packed with C18 PreMap 100, 5 μm, 100 Å; LC Packings, a Dionex Company, Sunnyvale, CA) at a flow rate of 10 μL·min−1. Binary mobile phases consisting of 96.95:2.95:0.1 water:acetonitrile:formic acid (solvent A) and 99.9:0.1 acetonitrile:formic acid (solvent B) were delivered by an Ultimate micropump (Dionex Corporation, Sunnyvale, CA) at a flow rate of 250 nL·min−1. The same LC gradient was used as previously described.34 Eluted peptides were analyzed using a hybrid LTQ-FT mass spectrometer. The instrument was operated to acquire a full FT-MS scan (m/z range of 300–2000) followed by MS/MS scans of the top three most intense ions in the LTQ in a data-dependent manner. The resolution was set to 100000 (at m/z 400) for the survey FT-MS scan. The data-dependent acquisition uses a 30 s exclusion duration time and a collision energy of 35%.
Data Analysis
Raw MS/MS spectra (.RAW) were processed using the Xcalibur software package Bioworks (version 3.3.1, Thermo Electron, San Jose, CA) to create a peak list (.DTA) with 50 ppm precursor ion tolerance. Individual .DTA files were grouped according to charge states of precursor ions using an in-house written algorithm. Charge-sorted .DTA files were submitted to MASCOT (Matrix Science, Version 2.1) and searched against the National Center for Biotechnology Information nonredundant (NCBInr) Drosophila database (28642 sequence entries) that is correlated with FBgn accession numbers in the FlyBase database for Drosophila protein identification. The same files were also searched against a home-built database containing protein sequences of human albumin, human hemoglobin, and horse heart myoglobin for assignment of standard proteins. The same parameters were utilized for the MASCOT search as previously described.34 Briefly, carbamidomethylation of cysteine residues was used as a fixed modification and acetylation (light or heavy) of lysine residues and the N-termini of peptides were defined as variable modifications. Additional parameters included a maximum of 2 trypsin miscleavages, a precursor tolerance of 15 ppm and a MS/MS tolerance of 0.8 Da. Spectra that led to scores at or above the MASCOT assigned homology score (the homology score defines spectral match at a 95% confidence level) are assigned to specific peptide sequences; peptide sequences matching to multiple FBgn accession numbers were discarded (i.e., only peptides having sequences that are unique to a single protein are considered). To estimate the false positive rate of peptide identifications, one of the most peptide-rich fractions, i.e., SCX fraction 3, was searched against the reverse NCBInr Drosophila database (28642) using the same parameters described above; the false positive rate calculated according to the formula by Gygi and co-workers36 was 2.2%.
Abundance ratios of differentially labeled peptides were computed using peak intensities from extracted ion chromatograms of light- and heavy-labeled peptides with an in-house written algorithm, as previously described by our laboratory.37 For peptides identified in multiple SCX fractions or charge states, peak intensities were summed to obtain abundance ratios. Relative quantification of proteins was obtained by averaging the intensity ratios of multiple derived peptides. Only proteins with a minimum of two peptide sequence identifications from the two independent experiments were considered for quantification. Abundance ratios for proteins reported as differentially expressed in this study were confirmed by manual inspection using the criteria described elsewhere.34
Western Blot Analysis
For Western blot analysis, a new batch of male control and WT α-synuclein flies was raised and harvested to obtain a total of 250 adult fly heads each. Proteins were extracted in 400 μL ice-cold 50 mM Tris buffer (pH 7.5) containing 10 mM NaF, 1 mM Na3VO4, 1 mM phenylmethyl-sulfonyl fluoride, 50 mM dithiothreitol, and 4% CHAPS, as previously described.34 Protein concentrations were determined with a Bradford assay. Equal amounts of proteins were separated by SDS-PAGE with 8% polyacrylamide gels and electroblotted onto polyvinylidene difluoride membranes. Membranes were blocked overnight at 4 °C in Odyssey blocking buffer (LI-COR, Lincoln, NE), then simultaneously incubated with anti-beta-tubulin monoclonal antibody (loading control, 1:5000 dilution; Developmental Studies Hybridoma Bank, Iowa City, IA) and antilarval serum protein 2 monoclonal antibody (1:1000 dilution, kindly provided by Thomas P. Neufeld) or antifat body protein 1 polyclonal antibody (kindly provided by Qisheng Song), or antitroponin-T antibody (1:500, Babraham Bioscience Technologies, Cambridge, UK) for 2 h at room temperature with gentle shaking. Next, infrared dye 700-labeled goat anti-mouse IgG (LI-COR, Lincoln, NE) and infrared dye 800 conjugated affinity purified goat antirat IgG (Rockland Inc., Gilbertsville, PA) or antirabbit IgG (LI-COR, Lincoln, NE) secondary antibodies were added and allowed to incubate for 2 h at room temperature. Immunoblotting bands were detected and quantified utilizing LI-COR Odyssey Infrared imaging system (simultaneous two-color targeted analysis) and software.
Results
Data of Internal Standard Proteins
In the present study, GIST coupled to an LC–MS/MS approach was utilized for protein identification and quantification. Figure 1 shows the schematic representation of the experimental design. Three standard proteins at known concentration ratios were spiked into equal amounts of control and PD-like samples. Human hemoglobin, human albumin, and horse heart myoglobin were chosen as standard proteins due to their unique peptide sequences compared to the Drosophila NCBInr genome database. Spiked concentration ratios for hemoglobin, albumin, and myoglobin were 4:1 (down-regulated), 1:1 (not-regulated), and 1:3 (up-regulated), respectively, in PD-like samples relative to controls. Differentially labeled samples were equally combined, prefractionated by offline SCX, and subjected to RP-LC–LTQ-FT-MS/MS analysis for protein identification and quantification. Two biological replicates isotopically labeled in forward and reverse directions were analyzed. Figure 2 shows an example of mass spectra of differentially labeled peptide pairs of the standard proteins from both forward and reverse labeling measurements. The relative intensities of the example peptide ion mass spectra obtained from the forward labeling experiment for albumin, [VFDEFKPLVEEPQNLIK+2H]2+ (Figure 2a); hemoglobin, [KVLGAFSDGLAHLDNLK+2H]2+ (Figure 2c); and myoglobin, [VEADIAGHGQEVLIR+2H]2+ (Figure 2e) indicate the corresponding peptides as not-regulated, down-regulated, and up-regulated in PD-like samples, respectively. In the reverse labeling experiment, relative intensities of the same corresponding peptides belonging to albumin, hemoglobin, and myoglobin exhibit consistent regulation patterns (Figures 2b, 2d, and 2f, respectively). In the forward labeling experiment, the observed average ratio obtained from multiple peptides for albumin, hemoglobin, and myoglobin was 1.12 ± 0.25, 0.17 ± 0.06, and 3.77 ± 1.01, respectively, as shown in Table 1. The corresponding average ratio obtained from the reverse labeling experiment was 0.94 ± 0.18, 0.37 ± 0.11, and 2.41 ± 1.16, respectively (Table 1).
Figure 1.
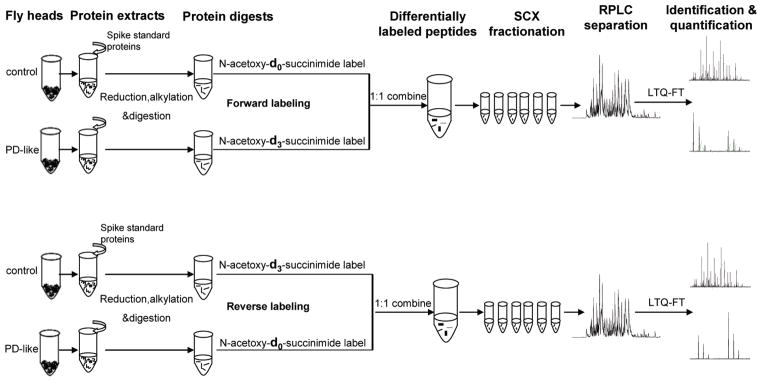
Quantitative proteomic strategy. Standard proteins spiked into control and WT α-synuclein expressing (PD-like) fly samples are human hemoglobin (4:1), human albumin (1:1), and horse heart myoglobin (1:3). Two independent experiments were conducted by isotopically labeling biological replicate samples in forward and reverse directions.
Figure 2.
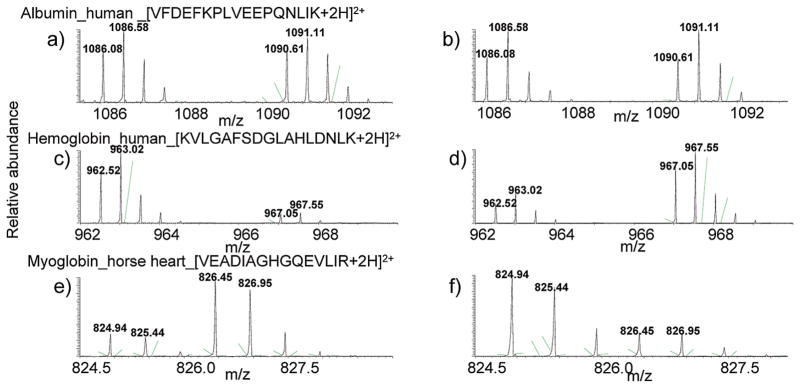
Data for internal standard proteins. (a–f) Example mass spectra for the light and heavy labeled peptide ions [VFDEFKPLVEEPQNLIK+2H]2+ (a and b), [KVLGAFSDGLAHLDNLK+2H]2+ (c and d), and [VEADIAGHGQEVLIR+2H]2+ (e and f) that belong to albumin, hemoglobin, and myoglobin, respectively. (a), (c), and (e) Mass spectra from the forward labeling experiment, and (b), (d), and (f) mass spectra from the reverse labeling experiment.
Table 1.
Quantification of Internal Standard Proteins Spiked into Control and PD-Like Samples
| protein | expected ratio (PD-like/control) | forward labeling observed ratio (PD-like/control) ± SDa | reverse labeling observed ratio (PD-like/control)± SDa |
|---|---|---|---|
| Albumin_human | 1.00 | 1.12 ± 0.25 (5) | 0.94 ± 0.18 (4) |
| Hemoglobin_human | 0.25 | 0.17 ± 0.06 (10) | 0.37 ± 0.11 (8) |
| Myoglobin_horse heart | 3.00 | 3.77 ± 1.01 (7) | 2.41 ± 1.16 (5) |
Numbers in parentheses indicate the total number of peptides used for quantification of proteins. The observed ratio ± SD indicates the average ratio and standard deviation of multiple peptides detected for a protein.
Differential Proteome Analysis of a Drosophila PD Model
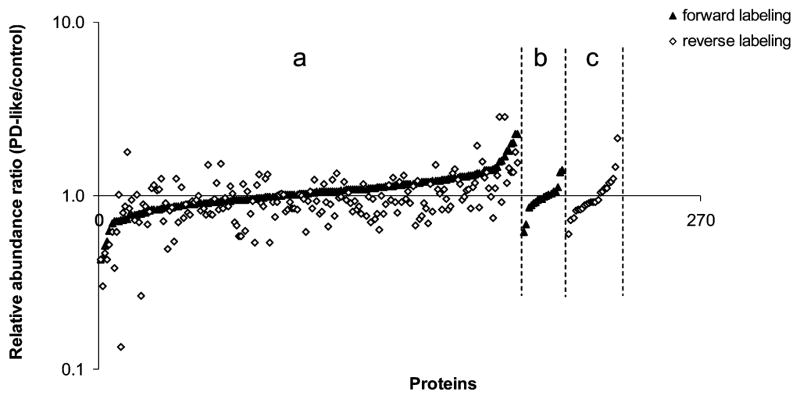
In the present study, a minimum of two peptides from the forward and reverse labeling experiments were required for a protein to be considered for quantification. A total of 229 proteins met this criterion (Supplementary Table SI, Supporting Information), of which 188 (82%) proteins were detected in both forward and reverse labeling experiments (Figure 3a). There are 18 proteins that were uniquely quantified with multiple peptides in the forward labeling experiment (Figure 3b) and 23 proteins that were uniquely detected in the reverse labeling experiment (Figure 3c). Like our quantitative proteomic analysis of the A53T α-synuclein Drosophila PD model as well as many other quantitative proteomic studies,38–40 the majority of proteins identified in the present study did not exhibit changes (i.e., 141/207 proteins from the forward labeling experiment and 127/212 proteins from the reverse labeling experiment show a fold-change between 0.80 and 1.20) in PD-like flies compared to controls. For proteins identified in both forward and reverse labeling experiments, the majority of the proteins exhibited consistent fold changes, i.e., 78% (147/189) of the proteins had relative standard deviations (RSDs) ≤ 25% between the two measurements. Only three proteins displayed an RSD ≥ 50% between the two measurements; presumably, differences are primarily associated with different peptides identified in the two independent measurements, similar to what we observed elsewhere.34 As discussed previously, poor agreement of relative quantities of different peptides from a protein could be arising from several factors, including varying ionization efficiency and posttranslational modifications.40
Figure 3.
Relative quantification of the 229 proteins with a minimum of two peptide sequences from two biological replicates plotted on a logarithm scale. Proteins were classified into three groups: (a) proteins (i.e., 188) identified in both forward and reverse labeling experiments; (b) proteins (i.e., 18) that were uniquely quantified in the forward labeling experiments; and (c) proteins (i.e., 23) that were uniquely identified in the reverse labeling experiment. Each group of proteins was sorted by relative abundance ratios in ascending order.
We applied a 1.5-fold change (in both forward and reverse labeling experiments) as a threshold based on our results of internal standard proteins in the present and previous studies.34 A total of 12 proteins (the α-synuclein protein is not included, because in the present study we are interested in the Drosophila proteome alterations arising from the expression of the human α-synuclein gene) meet the criterion (Table 2), among which 7 proteins were down-regulated, which are encoded by fat body protein 1, phospholipase C at 21C, larval serum protein 2, cuticular protein 64Aa, cuticular protein 97Ea, larval serum protein 1γ, and isocitrate dehydrogenase; the proteins that show up-regulation in PD-like flies are encoded by glycerol 3 phosphate dehydrogenase, ferritin 2 light chain homologue, glutamic acid decarboxylase, ribosomal protein S12, and Muscle LIM protein at 60A.
Table 2.
Differentially Expressed Proteins in WT α-Synuclein Flies Relative to Controls
| gene name or IDa | peptide sequence identified | RFb | mean ± SDRFc | RRb | mean ± SDRRc |
|---|---|---|---|---|---|
| Fat body protein 1 | R.QVESLIADVLLGR.L | 0.33 | 0.44 ± 0.11 | na | 0.30 ± 0.14 |
| R.LSEIVLHNLR.Q | 0.45 | na | |||
| R.FPQHLLLPR.G | 0.54 | na | |||
| R.DREDTQTLIVPAVQELLPELYLDEEVIQQVR.S | na | 0.20 | |||
| R.TRIEEHELDLSNLVEQQVQGIQQEIVGR.Q | na | 0.40 | |||
| Phospholipase C at 21C | K.AAQQVALSASHEDGGVTR.S | 0.43 | 0.43 | na | 0.43 |
| R.STANGDVATGTGTGSAAGTAGHAPPLQQIR.Q | na | 0.43 | |||
| Larval serum protein 2 | K.TIVSHYWHLMETYPEYHKK.D | 0.43 | 0.54 ± 0.09 | 0.21 | 0.43 ± 0.18 |
| K.NWETFQHVVYWAR.Q | 0.45 | na | |||
| K.TIVSHYWHLMETYPEYHK.K | 0.48 | 0.30 | |||
| K.HDYYFDVHNFK.F | 0.50 | 0.63 | |||
| K.FDVETINVLGNIIQGNADSVDKK.F | 0.52 | na | |||
| R.TYYGVPQWHR.E | 0.58 | 0.47 | |||
| K.VHLEAGVNHIK.R | 0.63 | 0.53 | |||
| K.FDVETINVLGNIIQGNADSVDK.K | 0.63 | na | |||
| K.YDEHGHEIPLEHNYQNFFELEHFK.V | 0.67 | 0.65 | |||
| R.LSHDLGEVPAFNMYVPTESGYASNLR.T | na | 0.24 | |||
| Cuticular protein 64Aa | R.IVEYTADPVHGFNAVVR.R | 0.52 | 0.52 | 0.73 | 0.47 ± 0.37 |
| K.VLAPAPLLHASPLVAK.V | na | 0.20 | |||
| Cuticular protein 97Ea | R.ISRPVYALPPASPAPSSAR.A | na | na | 0.62 | 0.60 ± 0.19 |
| R.TTDVLYSPLQRPARPEPDYSQTQSFGDGPSNVR.I | na | 0.40 | |||
| R.VQYAPAPQPQPQPLPQPQHLPQQHHQPSVGPAPPR.L | na | 0.77 | |||
| Isocitrate dehydrogenase | R.FKDIFEDLYNK.Q | 0.58 | 0.62 ± 0.05 | na | na |
| K.EYEAAGIWYEHR.L | 0.66 | 0.63 ± 0.11 | na | 0.52 | |
| Larval serum protein 1γ | K.FLFEIVHR.I | 0.67 | NA | ||
| K.YDFPLDISEPHNAFPDR.L | 0.59 | 0.52 | |||
| Glycerol 3 phosphate dehydrogenase | R.DLFQANHFR.V | 1.52 | 1.56 ± 0.06 | 1.63 | 2.84 ± 1.69 |
| K.AEGGGIDLISHIITR.H | 1.61 | 2.12 | |||
| K.GLEDKFPLFTAIHK.I | na | 4.77 | |||
| Ferritin 2 light chain homologue | K.RGTLEVDELHSLALALDTEK.Q | 1.52 | 1.69 ± 0.25 | 3.99 | 2.85 ± 1.61 |
| R.DPELAHYFEENFLGK.Q | 1.87 | na | |||
| R.ATHATDAERDPELAHYFEENFLGK.Q | na | 1.71 | |||
| Glutamic acid decarboxylase 1 | K.SCAAVCGLGTDHCIVVPSDEHGK.M | na | na | 2.56 | 2.16 ± 0.58 |
| R.ALPLQQLIEDCATTLK.Y | na | 1.75 | |||
| Ribosomal protein S12 | K.SLIADGLVHGIHQACK.A | 2.21 | 2.26 ± 0.08 | na | 1.79 |
| K.LVTALCNEHQIPLIR.V | 2.32 | 1.79 | |||
| Muscle LIM protein at 60A | K.GYGFGGGAGCLSTDTGAHLNRE.- | 2.05 | 2.28 ± 0.32 | na | 1.56 |
| K.GYGFGGGAGCLSTDTGAHLNR.E | 2.50 | 1.56 |
Gene name or IDs were obtained from the FlyBase database (www.flybase.org).
RF and RR indicate observed intensity ratios (PD-like/control) from the forward and reverse labeling experiment, respectively.
Observed ratio (mean ± SD) indicates the average ratio and standard deviation of multiple peptides detected for a protein. The values without SD denote that the protein was quantified with a single peptide. NA indicates that the protein was not identified.
Western Blot Analysis
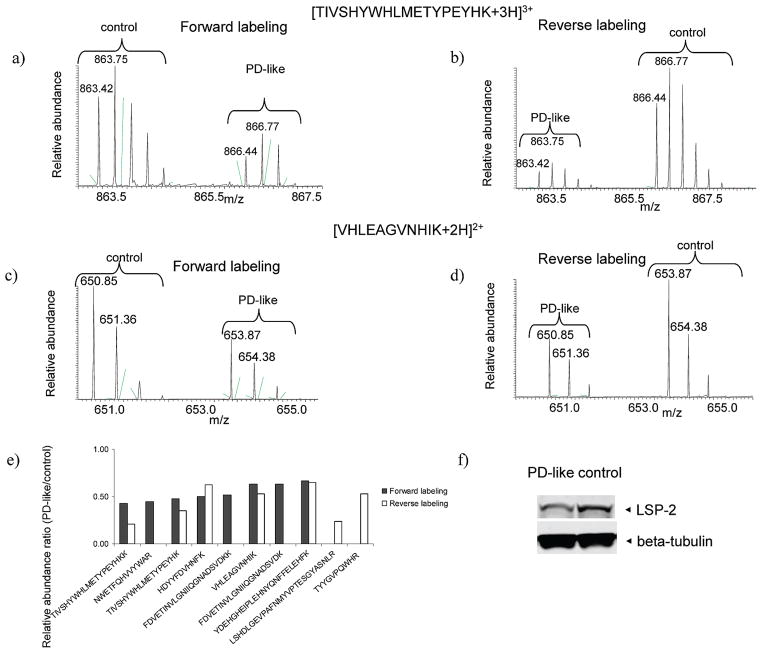
To confirm protein changes observed using the MS-based proteomic approach in the present study, we incorporated Western blot analyses. For example, larval serum protein 2 (LSP-2) was identified with a total of ten peptides from our GIST coupled to LC–MS/MS approach. Example mass spectra from the forward labeling measurement for the peptide ions [TIVSHYWHLMETYPEYHKK+3H]3+ (Figure 4a) and [VHLEAGVNHIK+2H]2+ (Figure 4c) show down-regulation in PD-like flies based on the relative intensities of the peaks. The reverse labeling experiment exhibits consistent down-regulation of the peptides (Figures 4 b and d). The ten peptides (five are in common between the forward and reverse labeling experiments) identified for LSP-2 all display down-regulation in PD-like flies compared to controls (Figure 4e and Table 2). Consistent with the proteomic measurements (i.e., an average of 0.49-fold down-regulation from the forward and reverse labeling experiments), Western blot analysis indicates a 0.58-fold down-regulation of LSP-2 (Figure 4f). Fat body protein 1 (FBP-1), a suggested LSP-2 receptor that is subjected to post-translational cleavage by 20-hydroxy-ecdysone,41 also shows a lower expression level in PD-like flies from the proteomic approach (Table 2). Consistent with previous reports,41,42 Western blot analysis detects three main bands (Figure 5) that correspond to the FBP-1 protein (118 kDa) and two FBP-1 fragments (69 and 50 kDa). Western blot analysis indicates that the native FBP-1 protein levels do not exhibit substantial differences between PD-like flies and controls. However, the two FBP-1 fragments are at lower levels. There is a 0.63- and 0.28-fold down-regulation for the fragments at 69 and 50 kDa, respectively. Overall, there is a 0.34-fold down-regulation for the sum of the three detected bands in PD-like flies, in accordance with our proteomic results (i.e., an average of 0.37-fold down-regulation from the forward and reverse labeling experiments).
Figure 4.
Identification and quantification data for LSP-2. (a, b) Example mass spectra for the light and heavy labeled peptide ion [TIVSHYWHLMETYPEYHKK+3H]3+ identified in the forward and reverse labeling experiment, respectively. (c, d) Example mass spectra for the light and heavy labeled peptide ion [VHLEAGVNHIK+2H]2+ identified in the forward and reverse labeling experiment, respectively. (e) Column representation of identification and quantification of all ten identified peptides. (f) Western blot analysis of LSP-2 with beta-tubulin as the loading control.
Figure 5.
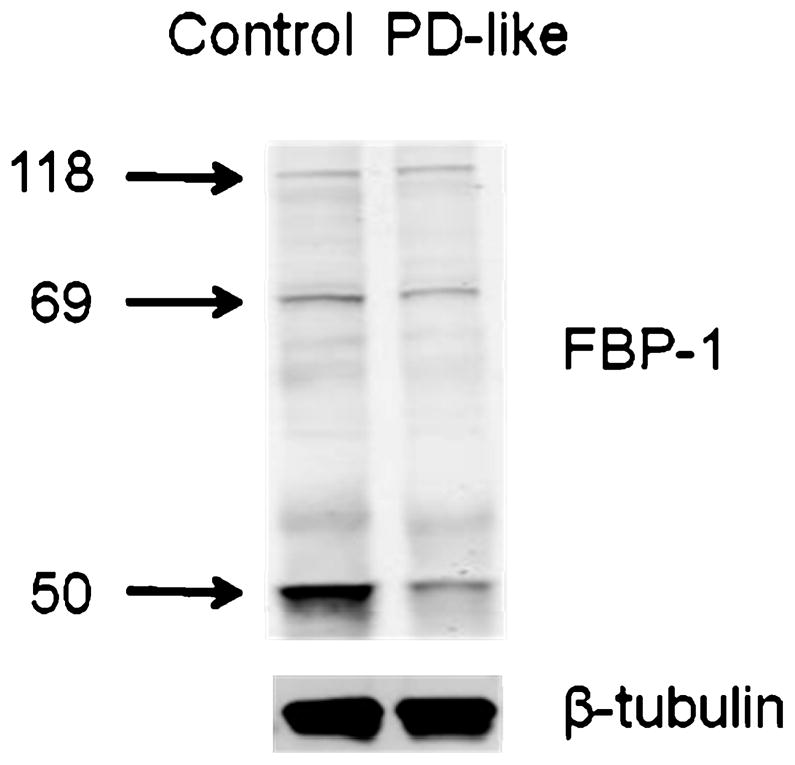
Western blot analysis of FBP-1 using polyclonal antibodies (top) with beta-tubulin (bottom) as the loading control. Three bands corresponding to the FBP-1 protein (118 kDa) and two FBP-1 fragments (69 and 50 kDa) were detected.
Discussion
Overview
The present study describes a quantitative proteome analysis of a WT α-synuclein Drosophila model of PD utilizing a well-characterized GIST coupled to LC–MS/MS approach. Similar to our previous study,34 we incorporated multiple standard proteins at an early stage of sample preparation and analyzed two independent biological replicates labeled in forward and reverse directions to assess experimental variability associated with sample handling and preparation. The data from standard proteins obtained in this study and in our previous study indicate that the applied strategy accurately predicts relative protein abundance in PD-like flies compared to controls [we note that statistic evaluations (e.g., P-values) are desirable to assess the significance of protein alterations besides the amplitude of changes (currently employed) and plan to carry out more than two biological replicates to draw statistic conclusions in the future analyses].
A total of 229 proteins were quantified with a minimum of two peptides from the forward and reverse labeling experiments. Quantitative results from the two independent measurements are in overall agreement (i.e., 78% proteins exhibit a RSD ≤ 25%). A total of 12 proteins were dysregulated in WT α-synuclein Drosophila. Down-regulations of LSP-2 and FBP-1 expression levels were confirmed by Western blot analysis. Gene ontology analysis43 indicates that these dysregulated proteins are primarily associated with cellular metabolism and signaling (Figure 6). It is instructive to provide a brief discussion of the relevance of individual proteins.
Figure 6.
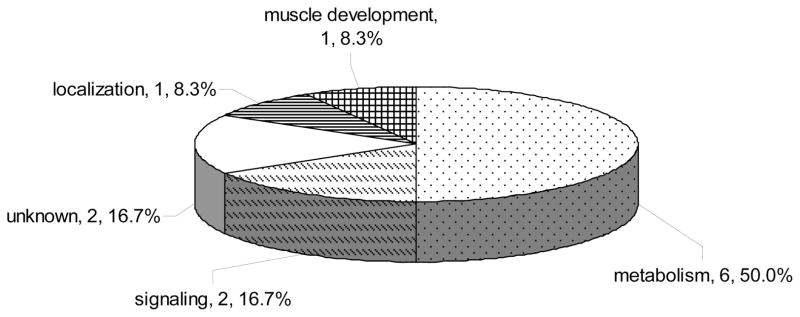
Pie chart representation of biological process for the 12 differentially expressed proteins in WT α-synuclein expressing flies. A protein was grouped into a single well-known category if it was associated with several biological processes.
Cell Metabolism
Cellular metabolism associated proteins include LSP-2, LSP-1γ, isocitrate dehydrogenase (ICDH), ribosomal protein S12, glycerol 3 phosphate dehydrogenase (GPDH), and ferritin 2 light chain homologue (Fer2LCH). LSP-2 and LSP-1γ (LSP-1γ is one of the three subunits of LSP-1; the other two subunits, LSP-1α and LSP-1β, were not detected in the present study) are major hemolymph proteins in Drosophila that serve as energy and amino acid pools for the production of adult cuticle structures.41,44 LSP-2 is observed throughout the Drosophila adult life and is mostly expressed in the adipose tissue of the head after eclosion but at a significantly lower level than in the fat body cells of third instar larvae.44,45 LSP-1γ is also synthesized during the larval development and the amount of LSP-1γ appears to decline in late larval life, at a faster rate than LSP-2 but a slower rate than LSP-1α and LSP-1β.45 Both LSP-2 and LSP-1γ were down-regulated in PD-like animals (Table 1). It is interesting to mention that FBP-1 (the receptor of LSPs) and two cuticular proteins including cuticular protein 64Aa and cuticular protein 97Ea (their production appears to depend on accumulation of LSPs),41,44 were also down-regulated in WT α-synuclein flies (Table 1). Notably, FBP-1, which was previously reported as exclusively expressed in the late third instar fat body tissues,41,46 was identified by our laboratory in 1-day-old fly heads (A30P, A53T, WT, and controls)32–34 and was also down-regulated in A30P and A53T α-synuclein Drosophila models relative to controls.32,34 It has been demonstrated that LSP-2, LSP-1γ, and FBP-1 are development- and ecdysteroid-regulated;41,47 thus, down-regulation of these proteins in WT α-synuclein flies suggests that expression of human WT α-synuclein protein may interfere with normal cell development.
ICDH is an enzyme that catalyzes the oxidative decarboxylation of isocitrate to produce alpha-ketoglutarate in the citric acid cycle.48 In Drosophila, this reaction produces 20% of the NADPH for lipogenesis during development.49 Down-regulation of ICDH in WT α-synuclein flies suggests that the metabolic pathway to generate energy in the citric acid cycle was likely perturbed due to the introduction of the foreign α-synuclein gene to the Drosophila CNS. Possible disturbance of the Drosophila cellular metabolism may also be seen from the up-regulation of the ribosomal protein S12, a structural constituent of the ribosome, which serves as the cellular machinery that synthesizes proteins. Upregulation of ribosomal proteins was also observed in A53T α-synuclein flies; dysregulation of ribosomal proteins may interfere with the interaction of other proteins.34
Drosophila GPDH has two well-characterized metabolic functions, including bridging glycolysis and triglyceride metabolism and serving in the glycerol-3-phosphate shuttle to regenerate NAD+ from NADH in mitochondria.50 The glycerol-3-phosphate shuttle contributes to oxidative phosphorylation by providing NAD+. Thus, an increased level of GPDH in α-synuclein expressing flies raises the possibility that cells undergo increased oxidative phosphorylation, which would generate more ROS that damage cells. The hypothesis of increased oxidative stress due to the expression of the α-synuclein gene appears to be supported by the increased level of Fer2LCH in PD-like flies (Table 2). Fer2LCH is one of the two subunits of Drosophila secreted ferritin and is expressed at all stages of fly life.51 Fer2LCH plays a role in iron transport in Drosophila and ferritin synthesis directly correlates with cellular iron concentration.52 Because iron can facilitate the production of various ROS through Fenton chemistry, it seems reasonable to speculate that an elevated level of Fer2LCH may be associated with increased cellular oxidative stress. Interestingly, an increased level of ferritin has also been found in the substantia nigra of postmortem PD patients53,54 and oxidative stress has been widely accepted as one of the hallmarks of PD pathology.54 Taken together, our data suggest that expression of human WT α-synuclein may induce elevated cellular oxidative stress and potentially disturb cellular metabolism. These factors may contribute to the later development of PD-like symptoms in flies.
Cell Signaling
Glutamic acid decarboxylase 1 (GAD1) and phospholipase C at 21C are associated with cell signaling pathways. Drosophila GAD1 is expressed exclusively in the nervous system;55 it catalyzes the synthesis of the neurotransmitter, γ-aminobutyric acid (GABA) from glutamate56 and its production is mediated downstream of GAD1.57 Drosophila GAD1 is also required in presynaptic motor neurons for proper postsynaptic glutamate receptor levels.56 The presynaptic GAD1 expression level directly correlates with postsynaptic glutamate receptor levels; overexpression of Drosophila GAD1 leads to the increase of glutamate receptor levels in the postsynaptic muscle.56 The increased level of GAD1 in WT α-synuclein flies likely reflects cellular self-regulation mechanism due to the following factors: (i) Drosophila lack an endogenous α-synuclein gene;28 (ii) α-synuclein is predominantly localized to presynaptic terminals58 and may play a role in synaptic plasticity and vesicular transport;1,3 (iii) α-synuclein inhibits tyrosine hydroxylase activity and overexpression of α-synuclein reduces dopamine synthesis;59 and, (iv) neurochemical studies indicate that neurotransmitters, such as dopamine, GABA, and glutamate, functionally interact in the brain, and the dopamine-GABA interaction might be reciprocal.60,61 Presumably, the expression of the human α-synuclein gene in the Drosophila CNS may inhibit the release of dopamine,59 which may induce elevated expression of GAD1 to produce more GABA and/or to increase postsynaptic glutamate receptor levels to compensate for perturbed dopamine neurotransmitters. Nevertheless, further studies are required to investigate the signaling pathways involving these neurotransmitters and receptors.
Phospholipase C at 21C is one of the Drosophila phosphatidylinositol-specific phospholipases C (PI-PLCs) and it is also expressed in the Drosophila CNS.62 The PI-PLCs play a central role in transmembrane signaling pathways by catalyzing phospholipids to produce two second messenger molecules, including inositol trisphosphate and diacylglycerol.62 Morris and coworkers63 have reported that α-synuclein inhibits phospholipase D2, one member of the other major classes of phospholipases, which catalyzes the hydrolysis of phosphatidylcholine to phosphatidic acid. Phosphatidic acid can undergo further metabolism to yield the same secondary messenger directly from hydrolysis of phosphatidylinositol 4,5-bisphosphate with the catalysis of PI-PLCs-diacylglycerol, which is involved in a variety of cellular processes, such as secretion, contraction, cell growth and differentiation, and synaptic transmission.64 Collectively, these data suggest that α-synuclein may play a role in phospholipid metabolism and/or associated phospholipid signaling cascades, including signal transduction and signal-induced cytoskeletal reorganization. This hypothesis appears to be supported by the previously suggested lipid binding role of α-synuclein due to its specific primary structural features, i.e., an amphipathic amino-terminus and a fairly acidic carboxyl-terminus.2 One possible association with the down-regulation of the signaling protein, phospholipase C at 21C, is the up-regulation of the cytoskeletal protein, muscle LIM protein at 60A (Mlp60A). Mlp60A is associated with the Actin cytoskeleton and plays a role in myogenic differentiation.65,66 Arber et al.66 found that muscle LIM protein was strongly up regulated in denervated or paralyzed skeletal muscle in adult rats; substantially high levels of muscle LIM protein appear to be toxic or interfere with cell differentiation. Notably, Mlp60A was also up-regulated in A53T α-synuclein flies compared to controls.34 Thus, dysregulation of cellular signaling proteins and Actin cytoskeletal Mlp60A may be associated with α-synuclein-mediated PD.
Comparison of Drosophila proteome responses to WT and mutant forms of α-synuclein
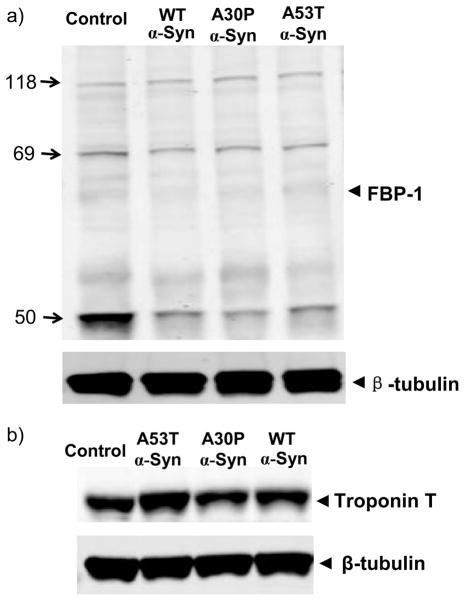
We previously reported on proteome analyses of A30P and A53T α-synuclein Drosophila PD models.33,34 A comparison of the Drosophila proteome responses to WT and mutant forms of α-synuclein indicates that there is low overlap among these differentially expressed proteins. Specifically, there are five proteins (encoded by retinal degeneration A, Odorant-binding protein 99b, Fbp-1, chaoptic, and Rtnl1) in common between the A30P and the A53T α-synuclein flies; two proteins (encoded by Fbp-1 and Lsp-2) are in common between the A30P and WT α-synuclein flies; four proteins (encoded by Fbp-1, Muscle LIM protein at 60A, Glycerol 3 phosphate dehydrogenase, and cuticular protein 64Aa) overlap between the A53T and WT α-synuclein animals; and only one protein (i.e., FBP-1) is in common in all three forms of α-synuclein animals. There may be several reasons associated with the low overlap. For example, the three α-synuclein variants have distinct structures and properties: (i) the A30P mutation disrupts an α-helical structure in the N-terminal region found in WT α-synuclein, whereas the A53T mutation leaves this region unaffected;67 (ii) in vitro studies indicate that both mutations accelerate the α-synuclein oligomerization, whereas the rate of mature fibril formation is increased by the A53T mutation and decreased by the A30P mutation;67,68 (iii) the A30P mutation has poor membrane-binding capacity in comparison with A53T and WT α-synucleins.21,69 These property differences raise the possibility that the Drosophila proteome responses differentially to the expression of the WT, A30P, and A53T α-synuclein. Western blot analyses (Figure 7) validated the distinct expression patterns of FBP-1 (down-regulated in all three α-synuclein forms, Figure 7a) and troponin T (exclusively up-regulated in A53T α-synuclein, Figure 7b), demonstrating confidence of our proteomic measurements. In addition, the relative low proteome coverage obtained from the GIST coupled to LC–MS/MS approach (possible reasons were discussed in detail in reference 34) may play a role in the observed low overlap; many low abundant proteins that were not identified in these studies may commonly disturbed.
Figure 7.
Western blot analyses of FBP-1 (a) and Troponin-T (b) for the three (WT, A30P, and A53T) α-synuclein Drosophila PD models using anti-FBP-1 polyclonal antibodies and anti-Troponin-T antibody with beta-tubulin (bottom) as the loading control.
Conclusions
In the present work, we performed a quantitative proteomic analysis of a WT α-synuclein Drosophila PD model with age-matched controls at the presymptomatic stage utilizing global isotopic labeling strategy combined with multidimensional liquid chromatographies and tandem mass spectrometry. Data of multiple internal standard proteins in both forward and reverse labeling experiments and Western blot analyses provide evidence of the validity of the proteome measurements. Our proteomic analyses revealed that Drosophila proteins primarily associated with metabolism and cellular signaling were perturbed in response to the expression of the human WT α-synuclein gene. Because WT α-synuclein expressing animals develop human PD-like symptoms, proteome changes arising from the expression of the WT α-synuclein gene may provide clues to understanding α-synuclein-mediated neurotoxicity in PD. An increased level of the iron (III)-binding protein, ferritin, typically found in the postmortem brains of PD patients, is also observed in presymptomatic WT α-synuclein expressing animals. The observed alterations in both pathology-associated and novel proteins may shed light on molecular events involving α-synuclein, which may lead to the development of intervening strategies at the presymptomatic stage of PD. Finally, according to our knowledge, none of the 12 differentially expressed proteins has known definitive link to the neural pathology in PD [it appears that there are only a few proteins (e.g., α-synuclein and Parkin) that have demonstrated associations with the PD neuronal pathology]. Thus, further studies are warranted to exam whether the altered proteins are involved in the neuronal pathology and etiology in PD.
Supplementary Material
Acknowledgments
We gratefully acknowledge financial support from the National Institute of Heath (NIH #RO1-AG-024547-3); additional partial support is provided from the Analytical Node of the Indiana University METACyt Initiative (funded by the Lilly Endowment). We also acknowledge the following invaluable contributions: Dr. Thomas P. Neufeld (University of Minnesota) for providing the larval serum protein 2 antibody; Dr. Qisheng Song (University of Missouri, Columbia) for providing the fat body protein 1 antibody; Dr. Kenneth P. Nephew and Xinghua Long (Department of Medical Sciences, Indiana University, Bloomington, IN) for providing assistance with Western blot analysis. The anti beta-tubulin antibody developed by Michael Klymkowsky was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA.
Footnotes
Supporting Information Available: Detailed information of the identification and relative quantification of the 229 proteins from the forward and reverse labeling experiments. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Clayton DF, George JM. The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998;21(6):249–254. doi: 10.1016/s0166-2236(97)01213-7. [DOI] [PubMed] [Google Scholar]
- 2.Jo EJ, McLaurin J, Yip CM, St George-Hyslop P, Fraser PE. alpha-synuclein membrane interactions and lipid specificity. J Biol Chem. 2000;275(44):34328–34334. doi: 10.1074/jbc.M004345200. [DOI] [PubMed] [Google Scholar]
- 3.Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2(7):492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- 4.Clayton DF, George JM. Synucleins in synaptic plasticity and neurodegenerative disorders. J Neurosci Res. 1999;58(1):120–129. [PubMed] [Google Scholar]
- 5.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273(16):9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 6.Murphy DD, Rueter SM, Trojanowski JQ, Lee VMY. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20(9):3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DAC, Kondo J, Ihara Y, Saitoh T. Molecular-Cloning of Cdna-Encoding an Unrecognized Component of Amyloid in Alzheimer-Disease. Proc Natl Acad Sci USA. 1993;90(23):11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 9.Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M. alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 10.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18(2):106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 11.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R. alpha-synuclein locus triplication causes Parkinson’s disease. Science. 2003;302(5646):841–841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 12.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55(2):164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 13.Ibanez P, Bonnet AM, Debarges B, Lohmann E, Tison F, Pollak P, Agid Y, Durr A, Brice A. Alpha-synuclein gene duplication is responsible for familial Parkinson’s disease. Neurology. 2004;62(7):A25–A25. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- 14.Ibanez P, Bonnet AM, Debarges B, Lohmann E, Tison F, Pollak P, Agid Y, Durr A, Brice A, Genetic FPsD. Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet. 2004;364(9440):1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- 15.Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T, Trojanowski JQ, Lee VMY. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44(3):415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- 16.Yamazaki M, Arai Y, Baba M, Iwatsubo T, Mori O, Katayama Y, Oyanagi K. alpha-synuclein inclusions in amygdala in the brains of patients with the Parkinsonism-dementia complex of Guam. J Neuropath Exp Neur. 2000;59(7):585–591. doi: 10.1093/jnen/59.7.585. [DOI] [PubMed] [Google Scholar]
- 17.Wakabayashi K, Yoshimoto M, Fukushima T, Koide R, Horikawa Y, Morita T, Takahashi H. Widespread occurrence of alpha-synuclein/NACP-immunoreactive neuronal inclusions in juvenile and adult-onset Hallervorden-Spatz disease with Lewy bodies. Neuropath Appl Neuro. 1999;25(5):363–368. doi: 10.1046/j.1365-2990.1999.00193.x. [DOI] [PubMed] [Google Scholar]
- 18.Lippa CF, Schmidt ML, Lee VMY, Trojanowski JQ. Antibodies to alpha-synuclein detect Lewy bodies in many Down’s syndrome brains with Alzheimer’s disease. Ann Neurol. 1999;45(3):353–357. doi: 10.1002/1531-8249(199903)45:3<353::aid-ana11>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Lippa CF, Fujiwara H, Mann DMA, Giasson B, Baba M, Schmidt ML, Nee LE, O’Connell B, Pollen DA, George-Hyslop PS. Lewy bodies contain altered alpha-synuclein in brains of many familiar Alzheimer’s disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol. 1998;153(5):1365–1370. doi: 10.1016/s0002-9440(10)65722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maries E, Dass B, Collier TJ, Kordower JH, Steece-Collier K. The role of alpha-synuclein in Parkinson’s disease: Insights from animal models. Nat Rev Neurosci. 2003;4(9):727–738. doi: 10.1038/nrn1199. [DOI] [PubMed] [Google Scholar]
- 21.Outeiro TF, Lindquist S. Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science. 2003;302(5651):1772–1775. doi: 10.1126/science.1090439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feany MB, Bender WW. A Drosophila model of Parkinson’s disease. Nature. 2000;404(6776):394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 23.Klein RL, King MA, Hamby ME, Meyer EM. Dopaminergic cell loss induced by human A30P alpha-synuclein gene transfer to the rat substantia nigra. Hum Gene Ther. 2002;13(5):605–612. doi: 10.1089/10430340252837206. [DOI] [PubMed] [Google Scholar]
- 24.Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VMY. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34(4):521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 25.Lo Bianco C, Ridet JL, Schneider BL, Deglon N, Aebischer P. alpha-Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson’s disease. Proc Natl Acad Sci USA. 2002;99(16):10813–10818. doi: 10.1073/pnas.152339799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada M, Iwatsubo T, Mizuno Y, Mochizuki H. Overexpression of alpha-synuclein in rat substantia nigra results in loss of dopaminergic neurons, phosphorylation of alpha-synuclein and activation of caspase-9: resemblance to pathogenetic changes in Parkinson’s disease. J Neurochem. 2004;91(2):451–461. doi: 10.1111/j.1471-4159.2004.02728.x. [DOI] [PubMed] [Google Scholar]
- 27.Kirik D, Annett LE, Burger C, Muzyczka N, Mandel RJ, Bjorklund A. Nigrostriatal alpha-synucleinopathy induced by viral vector-mediated overexpression of human alpha-synuclein: A new primate model of Parkinson’s disease. Proc Natl Acad Sci USA. 2003;100(5):2884–2889. doi: 10.1073/pnas.0536383100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF. The genome sequence of Drosophila melanogaster. Science. 2000;287(5461):2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- 29.Taraszka JA, Gao XF, Valentine SJ, Sowell RA, Koeniger SL, Miller DF, Kaufman TC, Clemmer DE. Proteome profiling for assessing diversity: Analysis of individual heads of Drosophila melanogaster using LC-ion mobility-MS. J Proteome Res. 2005;4(4):1238–1247. doi: 10.1021/pr050037o. [DOI] [PubMed] [Google Scholar]
- 30.Taraszka JA, Kurulugama R, Sowell RA, Valentine SJ, Koeniger SL, Arnold RJ, Miller DF, Kaufman TC, Clemmer DE. Mapping the proteome of Drosophila melanogaster: Analysis of embryos and adult heads by LC–IMS-MS methods. J Proteome Res. 2005;4(4):1223–1237. doi: 10.1021/pr050038g. [DOI] [PubMed] [Google Scholar]
- 31.Sowell RA, Hersberger KE, Kaufman TC, Clemmer DE. Examing the proteome of Drosophila across organism lifespan. J Proteome Res. 2007;6(9):3637–3647. doi: 10.1021/pr070224h. [DOI] [PubMed] [Google Scholar]
- 32.Xun ZY, Sowell RA, Kaufman TC, Clemmer DE. Protein expression in a Drosophila model of Parkinson’s disease. J Proteome Res. 2007;6(1):348–357. doi: 10.1021/pr060488o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xun ZY, Sowell RA, Kaufman TC, Clemmer DE. Lifetime proteomic profiling of an A30P α-synuclein Drosophila model of Parkinson’s disease. J Proteome Res. 2007;6(9):3729–3738. doi: 10.1021/pr0700504. [DOI] [PubMed] [Google Scholar]
- 34.Xun ZY, Sowell RA, Kaufman TC, Clemmer DE. Quantitative Proteomics of a Presymptomatic A53T α-synuclein Drosophila Model of Parkinson’s Disease. Mol Cell Proteomics. 2008;7:1191–1203. doi: 10.1074/mcp.M700467-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chakraborty A, Regnier FE. Global internal standard technology for comparative proteomics. J Chromatogr A. 2002;949(1–2):173–184. doi: 10.1016/s0021-9673(02)00047-x. [DOI] [PubMed] [Google Scholar]
- 36.Peng JM, Elias JE, Thoreen CC, Licklider LJ, Gygi SP. Evaluation of multidimensional chromatography coupled with tandem mass spectrometry (LC/LC–MS/MS) for large-scale protein analysis: The yeast proteome. J Proteome Res. 2003;2(1):43–50. doi: 10.1021/pr025556v. [DOI] [PubMed] [Google Scholar]
- 37.Liu XY, Miller BR, Rebec GW, Clemmer DE. Protein Expression in the Striatum and Cortex Regions of the Brain for a Mouse Model of Huntington’s Disease. J Proteome Res. 2007;6(8):3134–4142. doi: 10.1021/pr070092s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiang MC, Juo CG, Chang HH, Chen HM, Yi EC, Chern Y. Systematic uncovering of multiple pathways underlying the pathology of Huntington disease by an acid-cleavable isotope-coded affinity tag approach. Mol Cell Proteomics. 2007;6(5):781–797. doi: 10.1074/mcp.M600356-MCP200. [DOI] [PubMed] [Google Scholar]
- 39.McClatchy DB, Liao LJ, Park SK, Venable JD, Yates JR. Quantification of the synaptosomal proteome of the rat cerebellum during post-natal development. Genome Res. 2007;17(9):1378–1388. doi: 10.1101/gr.6375007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qian WJ, Monroe ME, Liu T, Jacobs JM, Anderson GA, Shen YF, Moore RJ, Anderson DJ, Zhang R, Calvano SE. Quantitative proteome analysis of human plasma following in vivo lipopolysaccharide administration using O-16/O-18 labeling and the accurate mass and time tag approach. Mol Cell Proteomics. 2005;4(5):700–709. doi: 10.1074/mcp.M500045-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burmester T, Antoniewski C, Lepesant JA. Ecdysone-regulation of synthesis and processing of Fat Body Protein 1, the larval serum protein receptor of Drosophila melanogaster. Eur J Biochem. 1999;262(1):49–55. doi: 10.1046/j.1432-1327.1999.00315.x. [DOI] [PubMed] [Google Scholar]
- 42.Sun Y, An S, Henrich VC, Sun X, Song Q. Proteomic identification of PKC-mediated expression of 20E-induced protein in Drosophila melanogaster. J Proteome Res. 2007;6(11):4478–4488. doi: 10.1021/pr0705183. [DOI] [PubMed] [Google Scholar]
- 43.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT. Gene Ontology: tool for the unification of biology. Nat Genet. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benes H, Edmondson RG, Fink P, Kejzlarovalepesant J, Lepesant JA, Miles JP, Spivey DW. Adult Expression of the Drosophila Lsp-2 Gene. Dev Biol. 1990;142(1):138–146. doi: 10.1016/0012-1606(90)90157-e. [DOI] [PubMed] [Google Scholar]
- 45.Powell D, Sato JD, Brock HW, Roberts DB. Regulation of Synthesis of the Larval Serum-Proteins of Drosophila-Melanogaster. Dev Biol. 1984;102(1):206–215. doi: 10.1016/0012-1606(84)90185-4. [DOI] [PubMed] [Google Scholar]
- 46.Antoniewski C, Laval M, Dahan A, Lepesant JA. The Ecdysone Response Enhancer of the Fbp1 Gene of Drosophila-Melanogaster Is a Direct Target for the Ecr/Usp Nuclear Receptor. Mol Cell Biol. 1994;14(7):4465–4474. doi: 10.1128/mcb.14.7.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lepesant JA, Maschat F, Kejzlarovalepesant J, Benes H, Yanicostas C. Developmental and Ecdysteroid Regulation of Gene-Expression in the Larval Fat-Body of Drosophila-Melanogaster. Arch Insect Biochem. 1986:133–141. [Google Scholar]
- 48.Kuhn DT, Cunningham GN. Isocitrate Dehydrogenase in Drosophila-Melanogaster Imaginal Disks - Pattern Development and Alteration by Homeotic Mutant-Genes. Dev Genet. 1986;7(1):21–34. doi: 10.1002/dvg.1020070103. [DOI] [PubMed] [Google Scholar]
- 49.Geer BW, Krochko D, Oliver MJ, Walker VK, Williamson JH. Comparative-Study of the Nadp-Malic Enzymes from Drosophila and Chick Liver. Comp Biochem Physiol B: Biochem Mol Biol. 1980;65(1):25–34. [Google Scholar]
- 50.Merritt TJS, Sezgin E, Zhu CT, Eanes WF. Triglyceride pools, flight and activity variation at the Gpdh locus in Drosophila melanogaster. Genetics. 2006;172(1):293–304. doi: 10.1534/genetics.105.047035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Georgieva T, Dunkov BC, Dimov S, Ralchev K, Law JH. Drosophila melanogaster ferritin: cDNA encoding a light chain homologue, temporal and tissue specific expression of both subunit types. Insect Biochem Mol Biol. 2002;32(3):295–302. doi: 10.1016/s0965-1748(01)00090-x. [DOI] [PubMed] [Google Scholar]
- 52.Casey JL, Hentze MW, Koeller DM, Caughman SW, Rouault TA, Klausner RD, Harford JB. Iron-Responsive Elements - Regulatory Rna Sequences That Control Messenger-Rna Levels and Translation. Science. 1988;240(4854):924–928. doi: 10.1126/science.2452485. [DOI] [PubMed] [Google Scholar]
- 53.Riederer P, Sofic E, Rausch WD, Schmidt B, Reynolds GP, Jellinger K, Youdim MBH. Transition-Metals, Ferritin, Glutathione, and Ascorbic-Acid in Parkinsonian Brains. J Neurochem. 1989;52(2):515–520. doi: 10.1111/j.1471-4159.1989.tb09150.x. [DOI] [PubMed] [Google Scholar]
- 54.Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- 55.Jackson FR, Newby LM, Kulkarni SJ. Drosophila Gabaergic Systems - Sequence and Expression of Glutamic-Acid Decarboxylase. J Neurochem. 1990;54(3):1068–1078. doi: 10.1111/j.1471-4159.1990.tb02359.x. [DOI] [PubMed] [Google Scholar]
- 56.Featherstone DE, Rushton EM, Hilderbrand-Chae M, Phillips AM, Jackson FR, Broadie K. Presynaptic glutamic acid decarboxylase is required for induction of the postsynaptic receptor field at a glutamatergic synapse. Neuron. 2000;27(1):71–84. doi: 10.1016/s0896-6273(00)00010-6. [DOI] [PubMed] [Google Scholar]
- 57.Kuppers B, Sanchez-Soriano N, Letzkus J, Technau GM, Prokop A. In developing Drosophila neurones the production of gamma-amino butyric acid is tightly regulated downstream of glutamate decarboxylase translation and can be influenced by calcium. J Neurochem. 2003;84(5):939–951. doi: 10.1046/j.1471-4159.2003.01554.x. [DOI] [PubMed] [Google Scholar]
- 58.Shibayamaimazu T, Okahashi I, Omata K, Nakajo S, Ochiai H, Nakai Y, Hama T, Nakamura Y, Nakaya K. Cell and Tissue Distribution and Developmental-Change of Neuron-Specific 14 Kda Protein (Phosphoneuroprotein-14) Brain Res. 1993;622(1–2):17–25. doi: 10.1016/0006-8993(93)90796-p. [DOI] [PubMed] [Google Scholar]
- 59.Perez RG, Waymire JC, Lin E, Liu JJ, Guo FL, Zigmond MJ. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002;22(8):3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harsing LG, Zigmond MJ. Influence of dopamine on GABA release in striatum: Evidence for D-1-D-2 interactions and non-synaptic influences. Neuroscience. 1997;77(2):419–429. doi: 10.1016/s0306-4522(96)00475-7. [DOI] [PubMed] [Google Scholar]
- 61.Segovia G, Mora F. Dopamine and GABA increases produced by activation of glutamate receptors in the nucleus accumbens are decreased during aging. Neurobiol Aging. 2005;26(1):91–101. doi: 10.1016/j.neurobiolaging.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 62.Shortridge RD, Yoon J, Lending CR, Bloomquist BT, Perdew MH, Pak WL. A Drosophila Phospholipase-C Gene That Is Expressed in the Central-Nervous-System. J Biol Chem. 1991;266(19):12474–12480. [PubMed] [Google Scholar]
- 63.Jenco JM, Rawlingson A, Daniels B, Morris AJ. Regulation of phospholipase D2: Selective inhibition of mammalian phospholipase D isoenzymes by alpha- and beta-synucleins. Biochemistry. 1998;37(14):4901–4909. doi: 10.1021/bi972776r. [DOI] [PubMed] [Google Scholar]
- 64.Moolenaar WH. Lysophosphatidic Acid, a Multifunctional Phospholipid Messenger. J Biol Chem. 1995;270(22):12949–12952. doi: 10.1074/jbc.270.22.12949. [DOI] [PubMed] [Google Scholar]
- 65.Stronach BE, Siegrist SE, Beckerle MC. Two muscle-specific LIM proteins in Drosophila. J Cell Biol. 1996;134(5):1179–1195. doi: 10.1083/jcb.134.5.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arber S, Halder G, Caroni P. Muscle Lim Protein, a Novel Essential Regulator of Myogenesis, Promotes Myogenic Differentiation. Cell. 1994;79(2):221–231. doi: 10.1016/0092-8674(94)90192-9. [DOI] [PubMed] [Google Scholar]
- 67.Bussell R, Eliezer D. Residual Structure and Dynamics in Parkinson’s Disease-associated Mutants of α-Synuclein. J Biol Chem. 2001;276(49):45996–46003. doi: 10.1074/jbc.M106777200. [DOI] [PubMed] [Google Scholar]
- 68.Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D, Kaufman SA, Martin F, Sitney K, Denis P. Both Familial Parkinson’s Disease Mutations Accelerate a-Synuclein Aggregation. J Biol Chem. 1999;274(14):9843–9846. doi: 10.1074/jbc.274.14.9843. [DOI] [PubMed] [Google Scholar]
- 69.Jo E, Fuller N, Rand RP, St George-Hyslop P, Fraser PE. Defective membrane interactions of familial Parkinson’s disease mutant A30P alpha-synuclein. J Mol Biol. 2002;315(4):799–807. doi: 10.1006/jmbi.2001.5269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.