Abstract
Venous thromboembolism (VTE) is a significant cause of morbidity and mortality in patients with cancer. The risk of VTE varies over the natural history of cancer, with the highest risk occurring during hospitalization and following disease recurrence. Patient and disease characteristics are associated with further increased risk of VTE in this setting. Specific factors include cancer type (eg, pancreatic cancer, brain cancer, lymphoma) and the presence of metastatic disease at the time of diagnosis. VTE is a significant predictor of increased mortality during the first year among all types and stages of cancer, with metastatic disease the strongest predictor of mortality. VTE is also associated with early death in ambulatory patients with cancer. These data highlight the need for close monitoring, prompt treatment, and appropriate preventive strategies for VTE in patients with cancer. The American Society of Clinical Oncology and the National Comprehensive Cancer Network have issued guidelines regarding the prophylaxis and treatment of patients with cancer. This review summarizes the impact of VTE on patients with cancer, the effects of VTE on clinical outcomes, the importance of thromboprophylaxis in this population, relevant ongoing clinical trials examining the prevention of VTE, and new pharmacologic treatment options.
Keywords: Venous thromboembolism, VTE, cancer, thromboprophylaxis, anticoagulant, chemotherapy, low–molecular weight heparin, LMWH
INTRODUCTION
Venous thromboembolism (VTE), which includes deep vein thrombosis and pulmonary embolism, is a major complication of cancer and one of the leading causes of death among cancer patients.1,2 Overall, approximately 20% of all VTE cases occur in patients with cancer.3 In addition, VTE affects up to 20% of patients with cancer before death, but has been reported in up to half of cancer patients coming to postmortem examination, highlighting the fact that the true extent of this complication may be underestimated.4,5 Cancer-associated VTE has important clinical and economic consequences, including increased morbidity resulting from hospitalization and anticoagulation use, bleeding complications, increased risk of recurrent VTE, and cancer treatment delays.6 In one analysis, Prandoni and colleagues reported that patients with cancer and VTE were approximately 4 times more likely to develop recurrent thromboembolic complications and twice as likely to develop major bleeding during anticoagulant treatment than those without malignancy.7 The occurrence of VTE in patients with cancer may interfere with planned chemotherapy regimens, worsen patient quality of life,8 and lead to increased consumption of healthcare resources compared with patients without cancer who experience VTE. In a retrospective study of records from 529 cancer patients, the mean hospitalization cost for DVT was $20,065 per episode (2002 dollars)9 compared with a cost of $7712 to $10,804 per episode in a general medical population with VTE.10
VTE is also associated with increased mortality in cancer patients. A retrospective study by Khorana and colleagues found that in-hospital mortality was 2- to 5-fold more common in neutropenic cancer patients hospitalized with thromboembolism compared to those without thromboembolism.11 Similarly, Chew and colleagues determined that the diagnosis of VTE was a significant predictor of increased mortality during the first year among all cancer types examined, with hazard ratios ranging from 1.6 to 4.2 (P <.01).12 The strongest predictor of death in this analysis was metastatic disease at the time of cancer diagnosis, with a hazard ratio ranging from 1.8 to 49.0 (P <.001). In addition, stratified analyses demonstrated that VTE was associated with an increased risk of death for all stages and cancer types with a median overall relative risk of 3.7 (Table 1).12 A prospective study of patients starting new chemotherapy (median follow-up 75 days) found that VTE accounted for 9.2% of deaths.1 In addition, a VTE diagnosis has been associated with an approximately 2-fold increased risk of death within 2 years in patients with breast cancer.13
Table 1.
Effect of VTE on Mortality Risk Within 1 Year of Diagnosis in Patients with Different Cancer Types Stratified by Cancer Stage
| Hazard Ratio by Stage | |||
|---|---|---|---|
| Local | Regional | Remote | |
| Prostate | 5.6* | 4.7* | 2.8† |
| Breast | 6.6* | 2.4† | 1.8‡ |
| Lung | 3.1* | 2.9* | 2.5* |
| Colorectal | 3.2* | 2.2* | 2.0* |
| Melanoma | 14.4* | NA | 2.8† |
| Non-Hodgkin’s lymphoma |
3.2* | 2.0† | 2.3* |
| Uterus | 7.0* | 9.1* | 1.7‡ |
| Bladder | 3.2* | 3.3* | 3.3* |
| Pancreas | 2.3‡ | 3.8* | 2.3* |
| Stomach | 2.4‡ | 1.5‡ | 1.8* |
| Ovary | 11.3† | 4.8‡ | 2.3* |
| Kidney | 3.2‡ | 1.4 | 1.3 |
P <.001;
P <.01;
P <.05.
Reproduced with permission from Archives of Internal Medicine, February 27, 2006, Volume 166, Page 463. Copyright © 2006 American Medical Association. All rights reserved.12
Taken together, these data highlight the need for close monitoring, prompt treatment, and appropriate preventive strategies for VTE in patients with cancer. This review will describe the substantial impact of VTE on patients with cancer, the effects of VTE on clinical outcomes, the importance of thromboprophylaxis in this population, relevant ongoing clinical trial data, and new pharmacologic treatment options for the prevention of VTE.
RISK OF VTE IN PATIENTS WITH CANCER
In addition to the overall increased risk for VTE among patients with cancer, VTE risk is especially high among certain subgroups, such as hospitalized patients, those undergoing active antineoplastic therapy, and those with metastatic disease.14 Cancer patients undergoing major surgery are also at increased risk of VTE.15,16 Other factors that have been associated with increased risk include patient characteristics such as advanced age, ethnicity, and gender; cancer-related factors including cancer type and disease stage; presence of specific biomarkers such as tissue factor and D-dimer; and factors related to systemic treatment such as type of therapeutic agent (Table 2).14
Table 2.
Risk Factors for VTE in Patients With Cancer
| Category | Risk Factor |
|---|---|
| Patient characteristics |
|
| Cancer-related factors |
|
| Biomarkers |
|
| Treatment-related factors |
|
Reprinted from Thrombosis Research, Vol 120 Supplement 2, Alok A. Khorana, Maithili V. Rao, Approaches to risk-stratifying cancer patients for venous thromboembolism, Pages No. S41-S50, Copyright 2007, with permission from Elsevier.14
The presence of metastatic disease is strongly associated with an increased risk for VTE. An analysis of the California Cancer Registry found that the incidence of VTE varied with cancer type, but regardless of cancer type, the incidence was highest among patients initially diagnosed with metastatic-stage disease.12 Among patients with concurrent VTE, 56% had metastatic disease compared with 21% of patients without concurrent VTE (P < .001). Conversely, patients with metastatic disease at the time of cancer diagnosis had a 1.4- to 21.5-fold higher risk of thromboembolism than patients with localized disease for all cancer types analyzed.12
The risk of VTE varies over the natural history of cancer, with the highest risk occurring during hospitalization and following the development of metastatic disease (Figure 1).17 In one study of patients with non-Hodgkin’s lymphoma and VTE, thrombosis was present at diagnosis in 37%, occurred during the first chemotherapy cycle in 22%, and occurred overall within the first 3 cycles in 82%.18 Another study found that the incidence rate of thromboembolism was higher during the first year of follow-up than during the second year for all types and stages of cancer, with the exception of localized pancreatic cancer.12 Similarly, in a study by Alcalay and colleagues of patients with regional stage colon cancer, the 2-year cumulative incidence of VTE was 3.1%, but the incidence rate decreased significantly over time from 5.0% during the first 6 months to 1.4% from 6 months to 1 year. During the second year, the incidence had decreased further to 0.6%.19
FIGURE 1.
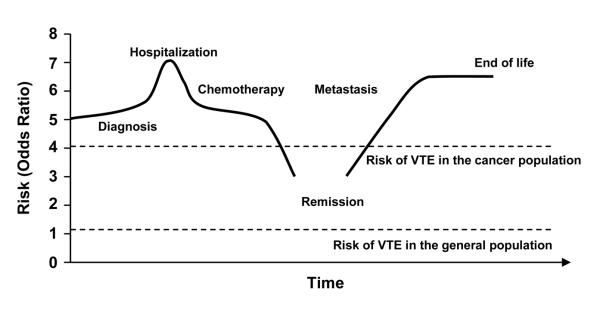
Risk of VTE varies over natural history of cancer. ©2007 Informa Healthcare. Reproduced with permission.17
VTE Risk and Cancer Type
The incidence of VTE may be closely associated with characteristics of tumor biology–not only the extent of metastatic spread, but primarily the rate of growth and spread of the cancer–suggesting that specific cancer types are associated with an increased risk of VTE.
Sites of cancer with the highest rates of VTE include the pancreas (8.1%), kidneys (5.6%), ovaries (5.6%), lungs (5.1%), and stomach (4.9%).20 Among the hematologic malignancies, myeloma (5%), non-Hodgkin’s lymphoma (4.8%), and Hodgkin’s disease (4.6%) had the highest rates of VTE.20 One retrospective record review estimated a cumulative frequency of VTE in patients with diffuse large B-cell lymphoma of 12.8%.18
In an analysis of data from the National Hospital Discharge Survey, the highest incidence of VTE among 19 cancer types included in the analysis occurred in patients with pancreatic cancer (4.3%), whereas the lowest evaluable incidence was in patients with bladder cancer (1.0%).21 In neutropenic cancer patients hospitalized with thromboembolism, Khorana and colleagues reported that the sites of cancer with the highest proportion of patients with VTE were the pancreas (12.1%), brain (9.5%), and endometrium or cervix (9%).11 The risk in hospitalized patients with hematologic disorders was also high; patients with non-Hodgkin’s lymphoma and leukemia accounted for more than one third of all patients with venous events.11 Similarly, a large retrospective cohort study using the discharge database of the University HealthSystem Consortium (N=1,015,598 cancer patients)20 found that 4.1% of patients were diagnosed with VTE. Factors associated with increased risk included black ethnicity and use of chemotherapy.
VTE Risk and Systemic Cancer Therapy
Many cancer therapies (including surgery, chemotherapy, and hormonal therapy) appear to place patients with cancer at further increased risk for VTE. This appears to also be true of several newer cancer treatments, such as the antiangiogenesis agents thalidomide, lenalidomide, and bevacizumab.4 The use of thalidomide, an immunomodulatory agent with antiangiogenic activity, has been associated with an increased risk of VTE when used concomitantly with chemotherapy or dexamethasone in patients with multiple myeloma.4 In a study presented at the American Society of Hematology 2008 annual meeting, Gray and colleagues characterized the incidence of VTE in 3977 patients with multiple myeloma as well as a variety of solid tumors in a meta-analysis of 17 randomized controlled trials.22 The overall incidence of VTE in the study was 11.7%, and patients treated with thalidomide were at more than a 2-fold increased risk of VTE compared with controls (P <.001). The risk was especially high in patients with multiple myeloma, with approximately 15% of patients experiencing VTE and having a 3-fold increased risk over control patients not receiving thalidomide.22
Lenalidomide, a structural analog of thalidomide, was not associated with an increased risk of VTE in a postmarketing survey of patients with myelodysplastic syndromes. In this survey, the observed risk of VTE was increased in patients treated with lenalidomide and erythropoiesis-stimulating agents (ESAs); there was no increase in VTE risk observed in patients treated with lenalidomide without ESAs.23 According to the ASCO recommendations for VTE prophylaxis and treatment in patients with cancer, patients receiving thalidomide or lenalidomide with chemotherapy or dexamethasone warrant prophylaxis with low–molecular weight heparin (LMWH) or adjusted-dose warfarin (INR ~1.5).4 As more agents with antiangiogenic activity become indicated for the treatment of cancer, it will be important to consider this risk in cancer patients, especially in those already at increased risk from other factors.
Bevacizumab is a monoclonal antibody directed toward vascular endothelial growth factor that has an antiangiogenic effect. Currently, its role in the prevention of VTE is controversial. Bevacizumab has demonstrated a survival benefit in combination with chemotherapy in patients with colorectal cancer and with non-squamous cell lung cancer.24 Scappaticci and colleagues conducted a post-hoc analysis of pooled data from randomized controlled trials evaluating combination treatment with bevacizumab and chemotherapy versus chemotherapy alone in 1745 patients with colorectal, breast, or non–small-cell lung cancer. Compared with chemotherapy alone, bevacizumab was associated with a 2-fold increase in arterial thromboembolic events (P =.031) but was not associated with an increased risk of venous thromboembolic events.24 These data are in contrast to a recent systematic review and meta-analysis by Nalluri et al, which included a total of 7956 patients with a variety of advanced solid tumors from 15 randomized controlled trials. Results indicate that bevacizumab was associated with an increased risk of VTE with a relative risk (RR) of 1.33 (95% CI, 1.13-1.56, P <.001) compared with controls.25
In addition to antineoplastic therapies, certain supportive care measures utilized in cancer treatment appear to increase the risk of VTE. The use of epoetin alfa and darbepoetin alfa for managing anemia in patients undergoing cancer treatment has been associated with thromboembolic complications. Bohlius and colleagues conducted a meta-analysis of 35 studies and reported that treatment with epoetin or darbepoetin increased the risk of thromboembolic events by approximately 67% compared with control patients not receiving these agents (RR = 1.67, 95% CI = 1.35-2.06).26
Additionally, red blood cell transfusions may increase the risk of VTE. One study of patients receiving transfusions reported that 7.2% of patients developed VTE and 5.2% developed arterial thromboembolism compared with 3.7% and 3.0% of patients who did not receive transfusions, respectively. Transfusions were also associated with an increased risk of in-hospital mortality (odds ratio 1.34 [95% CI:1.29-1.38]).27
Clinical Risk Model for Chemotherapy-Associated VTE
Recently, a simple model for predicting chemotherapy-associated VTE was developed and validated to assist in the assessment of VTE risk in ambulatory cancer patients undergoing chemotherapy.28 A total of 2701 patients in the derivation cohort and 1365 patients in the validation cohort were included. Five clinical and laboratory parameters were found to independently predict symptomatic VTE in cancer patients starting a new chemotherapy regimen.28 These parameters were combined into a risk-assessment model that allows classification of patients into 3 groups based on risk factors: 1) site of cancer (very high risk: stomach, pancreas; high risk: lung, lymphoma, gynecologic, bladder, testicular); 2) prechemotherapy platelet count of ≥350 × 109/L; 3) hemoglobin levels <100 g/L or use of red cell growth factors; 4) prechemotherapy leukocyte count >11 × 109/L ; and 5) body mass index ≥35 kg/m2.28 VTE risk score categories using this model have been found to correlate with the development of VTE and with overall survival in patients with cancer undergoing chemotherapy.29
THROMBOPROPHYLAXIS IN CANCER PATIENTS
Key Clinical Trials of Pharmacologic Agents
Pharmacologic prophylactic options for VTE consist of unfractionated heparin (UFH), the class of LMWHs, fondaparinux (an indirect inhibitor of activated factor Xa), and the vitamin K antagonists.4,30 Several novel agents, described below, are also in development. The pharmacologic anticoagulant agents currently being evaluated in cancer patients in phase II or III are provided in Table 3. Results of selected key studies of pharmacologic anticoagulants in cancer patients are discussed below, and an overview of recently published clinical studies is presented in Table 4a–c.
Table 3.
Pharmacologic Anticoagulant Agents Being Evaluated in Phase II or III Clinical Trials in Cancer Patients*
| Agent | Class/ MOA |
Route | Title | NCT Reference |
|---|---|---|---|---|
| Phase III | ||||
| Bemiparin vs placebo | LMWH | SC | CANBESURE Study (Cancer, Bemiparin and Surgery Evaluation) | NCT00219973 |
| Dalteparin vs SOC | LMWH | SC | A Study of Dalteparin Prophylaxis in High-Risk Ambulatory Cancer Patients | NCT00876915 |
| Dalteparin vs SOC | LMWH | SC | Dalteparin in Preventing Blood Clots in Patients With Lung Cancer | NCT00519805 |
| Dalteparin vs placebo | LMWH | SC | Dalteparin Low Molecular Weight Heparin for Primary Prophylaxis of Venous Thromboembolism in Brain Tumour Patients |
NCT00135876 |
| Gemcitabine with or without Dalteparin |
LMWH | SC | Gemcitabine With or Without Dalteparin in Treating Patients With Unresectable or Metastatic Pancreatic Cancer |
NCT00031837 |
| Gemcitabine or Capecitabine With or Without Dalteparin |
LMWH | SC | Gemcitabine With or Without Capecitabine and/or Dalteparin in Treating Patients with Metastatic Pancreatic Cancer |
NCT00662688 |
| Chemotherapy with or without Enoxaparin |
LMWH | SC | Chemotherapy With or Without Enoxaparin in Pancreatic Cancer (PROSPECT) |
NCT00785421 |
| Enoxaparin | LMWH | SC | Enoxaparin Thromboprophylaxis in Cancer Patients With Elevated Tissue Factor Bearing Microparticles |
NCT00908960 |
|
Enoxaparin vs intermittent pneumatic compression |
LMWH | SC | Japanese Efficacy and Safety Study of Enoxaparin in Patients With Curative Abdominal Cancer Surgery |
NCT00723216 |
| Chemotherapy with or without Enoxaparin |
LMWH | SC | Overall Survival of Inoperable Gastric/GastroOesophageal Cancer Subjects on Treating With LMWH + Chemotherapy (CT) vs Standard CT (GASTRANOX) |
NCT00718354 |
|
Fondaparinux with or without inferior vena cava filter |
Indirect factor Xa inhibitor |
SC | Anticoagulation and Inferior Vena Cava Filters in Cancer Patients With a Venous Thromboembolism |
NCT00423683 |
| Semuloparin vs placebo | ULMWH | SC | Evaluation of AVE5026 in the Prevention of Venous Thromboembolism in Cancer Patients Undergoing Chemotherapy (SAVE-ABDO) |
NCT00694382 |
| Tinzaparin | LMWH | SC | Effect of Low Molecular Weight Heparin: Tinzaparin in Lung Tumours (TILT) | NCT00475098 |
|
Tinzaparin vs Warfarin |
LMWH / VKA |
SC | Long-Term innohep® Treatment Versus a Vitamin K Antagonist (Warfarin) for the Treatment of Venous Thromboembolism (VTE) in Cancer |
NCT01130025 |
| Phase II | ||||
| Apixaban vs placebo | Direct factor Xa inhibitor |
Oral | A Phase 2 Pilot Study of Apixaban for the Prevention of Thromboembolic Events in Patients With Advanced (Metastatic) Cancer |
NCT00320255 |
| Combination chemotherapy with wafarin |
VKA | Oral | Combination Chemotherapy Plus Warfarin in Treating Patients With Prostate Cancer |
NCT00014352 |
| Gemcitabine with or without Dalteparin |
LMWH | SC | Gemcitabine With or Without Dalteparin in Treating Patients With Locally Advanced or Metastatic Pancreatic Cancer |
NCT00462852 |
| Dalteparin and Warfarin | LMWH / VKA |
SC / Oral |
The Catheter Study: Central Venous Catheter Sruvival in Cancer Patients Using Low Molecular Weight Heparin (Dalteparin) for the Treatment of Deep Vein Thrombosis |
NCT00216866 |
| Dalteparin | LMWH | SC | Fragmin in Ovarian Cancer: Utility on Survival (FOCUS) | NCT00239980 |
| Dalteparin | LMWH | SC | Treatment of Blood Clots in Children With Cancer | NCT00952380 |
| Enoxaparin | LMWH | SC | Identification and Treatment of Clinically Silent Catheter-Related Deep Vein Thrombosis in Children With Cancer |
NCT00633061 |
| Fondaparinux | Indirect factor Xa inhibitor |
SC | Fondaparinux in Preventing Blood Clots in Patients Undergoing Surgery for Gynecologic Cancer |
NCT00381888 |
| Tinzaparin | LMWH | SC | Tinzaparin for Primary Treatment and Extended Secondary Prophylaxis of Venous Thromboembolism in Patients with Cancer |
NCT00981903 |
| Tinzaparin | LMWH | SC | Tinzaparin in Treating Patients with Metastatic Kidney Cancer That Cannot Be Removed by Surgery |
NCT00293501 |
Search of www.clinicaltrials.gov August 21, 2009; search terms: “venous thromboembolism”, “thromboprophylaxis”, “thrombosis”, “phase II”, “phase III”; conditions = cancer. Completed studies and studies actively recruiting participants are included.
LMWH = low−molecular-weight heparin; SC = subcutaneous; SOC = standard of care; ULMWH = ultra−low-molecular-weight heparin.
Table 4a.
Recent Studies of Pharmacologic Anticoagulants in Medical Patients With Cancer
| Citation | Patient Population |
Treatments | Primary Outcome |
Result | Significance Level |
Bleeding Rates | Significance Level |
Length of Treatment |
Setting |
|---|---|---|---|---|---|---|---|---|---|
| Hull RD, et al. Ann Intern Med. 201065 |
Acutely ill medical patients |
Enoxaparin 40 mg QD or placebo |
VTE | Enoxaparin 2.5% (45/1818); placebo 4.2% (78/1867) |
P<.042 |
Major bleeding: enoxaparin 0.8%; placebo 0.3% |
P<.05 | 28 d | Prophylaxis |
| De Cicco M, et al. Ann Oncol. 200966 |
Cancer patients with a central vein catheter |
Acenocumarine 1 mg QD or dalteparin 5000 IU QD or no anticoagulant therapy |
Central vein catheter-related thrombosis |
Acenocumarine 21.9% (25/114); dalteparin 40.0% (48/120); no treatment 52.6% (60/114) |
Acenocumarine vs no treatment P<.01; dalteparin vs no treatment P=.05; acenocumarine vs dalteparin P=.01 |
Major bleeding: none observed |
Not reported | Acenocumarine 11 d; dalteparin 8 d |
Prophylaxis |
| Young AM, et al. Lancet. 200967 |
Cancer patients receiving chemotherapy via central venous catheters |
Fixed-dose warfarin 1 mg QD or INR- adjusted warfarin QD or no warfarin |
Catheter- related thrombotic events |
Fixed-dose warfarin 7% (34/471); INR-adjusted warfarin 3% (13/473); no warfarin 6% (24/404) |
Warfarin vs no warfarin P=.98; fixed-dose warfarin vs INRadjusted warfarin P=.002 |
Major bleeding: fixed-dose warfarin 1%; INR-adjusted warfarin 3%; no warfarin <1% |
Warfarin vs no warfarin P=.07; INR-adjusted warfarin vs fixed-dose warfarin P=.09 |
Treatment continued until catheter removal or occurrence of thrombosis |
Prophylaxis |
| Weber C, et al. Support Care Cancer. 200868 |
Terminal cancer |
Nadroparin 2850–3800 IU/kg QD or no treatment |
VTE | Nadroparin 10% (1/10); no treatment 0% (0/10) |
P=1.00 |
Major bleeding: nadroparin 10%; no treatment 0% |
P=1.00 | Treatment continued until death |
Prophylaxis |
| Robins HI, et al. Cancer Chemother Pharmacol. 200869 |
Glioblastoma multiforme |
Dalteparin 5000 IU QD with conventional radiotherapy vs control cohort |
Survival time | Median survival time in dalteparintreated patients 11.9 mo |
P=.47 vs control cohort |
Major bleeding: none reported |
Not reported | ≤24 mo | Prophylaxis |
| Niers TM, et al. J Thromb Haemost. 200770 |
Hematologic malignancy |
Nadroparin 2850 IU QD vs placebo |
Catheter-related thrombosis |
Nadroparin 17% (7/41); placebo 9% (4/46) |
P=.49 |
Major bleeding: none reported |
Not reported | 3 wk | Prophylaxis |
| Meister B, et al. Pediatr Blood Cancer. 200871 |
Acute lymphoblastic leukemia |
Antithrombin alone vs antithrombin + enoxaparin 0.75– 1.2 mg/kg QD |
VTE | Antithrombin alone 12.7% (9/71); antithrombin + enoxaparin 0% |
P=.02 |
Major bleeding: none reported |
Not reported | 1–2 wk during chemotherapy induction and reinduction phases |
Prophylaxis |
| Icli F, et al. J Surg Oncol. 200772 |
Advanced pancreatic cancer |
Combination chemotherapy + nadroparin 2850 IU QD vs combination chemotherapy alone |
Treatment response rate; survival |
Response rate: nadroparin 58.8% (20/34); no nadroparin 12.1% (4/33). Median overall survival time: nadroparin 13.0 mo; no nadroparin 5.5 mo |
Response rate P=.0001; survival time P=.0001 |
Treatment-related bleeding: none reported |
Not reported | Until disease progression |
Prophylaxis |
| Miller KC, et al. Leuk Lymphoma. 200673 |
Patients with multiple myeloma or chronic lymphocytic leukemia treated with thalidomide-based therapies |
Warfarin 1 or 2 mg QD vs. historical studies with similar chemotherapy regimens |
VTE | Warfarin 5.9% (4/68); thalidomide + doxorubicin 27%; thalidomide + epirubicin 26% |
warfarin regimen vs thalidomide + doxorubicin P=.034; warfarin regimen vs thalidomide + epirubicin P=.009 |
Treatment-related bleeding: none reported |
Not reported | 4 mo | Prophylaxis |
| Deitcher SR, et al. Clin Appl Thromb Hemost. 200674 |
Patients with active cancer and acute VTE |
Enoxaparin 1 mg/kg BID X 5 d then 1 mg/kg QD thereafter or enoxaparin 1 mg/kg BID X 5 d then 1.5 mg/kg QD thereafter vs enoxaparin 1 mg/kg BID X 5 d or until INR target achieved then INRadjusted warfarin thereafter |
Recurrent VTE | Enoxaparin 1 mg/kg 3.4% (1/29); enoxaparin 1.5 mg/kg 3.1% (1/32); warfarin 6.7% (2/30) |
Not reported |
Major bleeding: enoxaparin 1 mg/kg 6.5%; enoxaparin 1.5 mg/kg 11.1%; warfarin 2.9% |
Not reported | 180 d | Treatment |
| Ruud E, et al. Acta Paediatr. 200675 |
Children with active cancer and central venous lines |
INR-adjusted warfarin QD vs. no prophylaxis |
Central vein catheter-related VTE |
Warfarin 48% (14/29); no prophylaxis 36% (12/33) |
P=.44 | Bleeding rates not reported |
Not reported | 6 mo | Prophylaxis |
| Ikhlaque N, et al. Am J Hematol. 200676 |
Patients receiving thalidomide therapy |
Low-dose warfarin (1–2 mg/d) or highdose warfarin (adjusted to INR 2–3) vs no prophylaxis |
DVT | Low-dose warfarin 2.7% (1/37); high-dose warfarin 11.1% (2/18); no warfarin 23.7% (18/76) |
P=.01 for any dose of warfarin vs no warfarin |
Clinical bleeding: low-dose warfarin 0%; high-dose warfarin 22.2%; no warfarin 0% |
Not reported | ≤14 mo | Prophylaxis |
| Baz R, et al. Mayo Clin Proc. 200577 |
Multiple myeloma |
Aspirin 81 mg QD initiated at the start of chemotherapy or aspirin 81 mg QD initiated after the start of chemotherapy vs no aspirin |
VTE | Aspirin initiated at start of chemotherapy 19% (11/58); aspirin initiated after start of chemotherapy 15% (4/26); no aspirin 58% (11/19) |
P≤.002 for both aspirin groups vs no aspirin |
Significant bleeding complications: none reported |
Not reported | Median 2 yr | Prophylaxis |
| Karthaus M, et al. Ann Oncol. 200678 |
Cancer patients with central venous catheters |
Dalteparin 5000 IU QD vs placebo |
Catheter-related complications |
Dalteparin 3.7% (11/294); placebo 3.4% (5/145) |
P=.88 |
Any bleeding event: dalteparin 17.5%; placebo 15% |
Not reported | 16 wk | Prophylaxis |
| Verso M, et al. J Clin Oncol. 200579 |
Cancer patients with central venous catheters |
Enoxaparin 40 mg QD vs placebo |
DVT or clinically overt PE |
DVT: enoxaparin 14.1% (22/155 ); placebo 18.0% (28/155 ) |
P=.35 |
Major bleeding: none reported |
Not reported | 6 wk | Prophylaxis |
| Couban S, et al. J Clin Oncol. 200580 |
Cancer patients with central venous catheters |
Warfarin 1 mg QD vs placebo |
Central venous catheter-related thrombosis |
Warfarin 4.6% (6/130); placebo 4.0% (5/125) |
HR 1.20; 95% CI, 0.37–3.94 |
Major bleeding: warfarin 0%; placebo 2% |
P=.07 | Until catheter removal, death, or catheter-related thrombosis |
Prophylaxis |
Table 4c.
Recent Studies of Pharmacologic Anticoagulants in Ambulatory Cancer Patients
| Citation | Patient Population |
Treatments | Primary Outcome |
Result | Significance Level |
Bleeding Rates |
Significance Level |
Length of Treatment |
Setting |
|---|---|---|---|---|---|---|---|---|---|
| Cini M, et al. Eur J Haematol. 201085 |
Multiple myeloma |
Thalidomide- dexamethasone or thalidomide- dexamethasone + warfarin |
VTE | No prophylaxis 26.3% (5/19); warfarin 10.6% (26/246) |
P=.095 |
Major bleeding: none recorded |
Not specified | 120 d | Prophylaxis |
| Agnelli G, et al. Lancet Oncol. 200943 |
Lung, GI, pancreatic, breast, ovarian, or head and neck cancer |
Nadroparin 3800 IU QD or placebo |
Composite of symptomatic venous or arterial thromboembolic events |
Nadroparin 2.0% (5/769); placebo 3.9% (5/381) |
P=.02 | Major bleeding: nadroparin 0.7%; placebo 0% |
P=.18 | ≤4 mo | Prophylaxis |
| Lee AY, et al. J Clin Oncol. 200586 |
Patients with solid tumors and VTE |
Dalteparin 200 U/kg QD X 1 mo then 150 U/kg QD X 5 mo or dalteparin 200 U/kg X 7 d then INR-adjusted coumarin derivative X 6 mo |
All-cause mortality at 12 mo |
Dalteparin 20% (15/75); warfarin 36% (26/75) in patients with no metastases |
P=.03 | Not specified | Not specified | 6 mo | Prophylaxis |
| Hull RD, et al. Am J Med. 200687 |
Patients with cancer and VTE |
Tinzaparin 175 U/kg QD vs usual care (UFH + warfarin) |
Recurrent VTE or death at 3 mo |
Recurrent VTE: tinzaparin 6% (6/100); usual care 10% (10/100) death: tinzaparin 20% (20/100); usual care 19% (19/100) |
Recurrent VTE P=NS; death P=NS |
Major bleeding: tinzaparin 7%; usual care 7% |
P=NS | 3 mo | Treatment |
| Romera A, et al. Eur J Vasc Endovasc Surg. 200988 |
Patients with VTE including 28.6% (69/241) with cancer |
Tinzaparin 175 IU/kg QD or INR-adjusted acenocoumarol |
Recurrent VTE at 6 mo and 1 yr |
Cancer population: 6 mo: tinzaparin 5.5% (2/36); warfarin 9.1% (3/33). 1 yr: tinzaparin 5.5% (2/36); warfarin 21.2% (7/33) |
6 mo P=.58; 1 yr P=.06 |
Major bleeding in total population: tinzaparin 0.8%; warfarin 2.5% |
P=.6 | 6 mo | Treatment |
Key Studies in Surgical Cancer Patients
LMWHs, including enoxaparin, dalteparin, and tinzaparin, are available in the US for use in thromboprophylaxis.31 ENOXACAN I and II were large randomized trials evaluating enoxaparin thromboprophylaxis in cancer patients. In ENOXACAN I, enoxaparin was compared directly with UFH for its ability to prevent deep vein thrombosis (DVT) in 631 patients undergoing elective cancer surgery.32 Overall, 16.5% of patients developed thromboembolic complications, with no statistically significant difference between the 2 groups. There were also no significant differences in bleeding events, other complications, and mortality. The ENOXACAN II study evaluated the duration of prophylaxis for VTE with enoxaparin in cancer patients following surgery for cancer. Enoxaparin was given for approximately 1 week (6-10 days), and patients were thereafter randomized to receive enoxaparin or placebo for an additional 21 days, for total treatment duration of about 1 month. Patients receiving enoxaparin for 1 month had a significantly reduced incidence of thrombosis compared with enoxaparin given for 1 week followed by placebo.33 The rates of VTE were 12.0% in the placebo group and 4.8% in the enoxaparin group, corresponding to a reduction in risk of 60% (P =.02). There were no significant differences in the rates of bleeding or other complications during the study.33
Key Studies in Hospitalized Cancer Patients
Thromboprophylaxis has been shown to decrease DVT specifically in high-risk hospitalized patients. Key trials include a study comparing enoxaparin with placebo for the prevention of VTE in acutely ill medical patients (MEDENOX).34 In that study, prophylactic treatment with 40 mg per day of subcutaneous enoxaparin safely reduced the risk of VTE in patients with acute medical illnesses including cancer, with no difference in the rates of adverse events between the active comparator and placebo. Similarly, dalteparin 5000 IU once daily was shown in the PREVENT trial to reduce the risk of VTE in acutely ill medical patients, with a low overall incidence of major bleeding.35 Comparable results to LMWH have been reported with fondaparinux in the ARTEMIS trial, in which fondaparinux (2.5 mg subcutaneously for 6-14 days) was found to be effective in preventing symptomatic and asymptomatic VTE in older acute medical patients.36 VTE was detected in 5.6% (18/321) of patients treated with fondaparinux and 10.5% (34/323) of patients given placebo, a relative risk reduction of 46.7% (95% confidence interval [CI] 7.7% to 69.3%). Symptomatic VTE occurred in 5 patients in the placebo group and none in the fondaparinux group (P =.029). The frequency of major bleeding was similar for both fondaparinux and placebo, with major bleeding occurring in 1 patient (0.2%) in each group.36
Key Studies in Ambulatory Cancer Patients
Several randomized controlled trials of thromboprophylaxis in ambulatory cancer patients have been reported.37 In the PROSPECT-CONKO 004 study (a prospective, randomized trial in patients with pancreatic cancer undergoing chemotherapy and also receiving enoxaparin) compared concomitant treatment with enoxaparin to no anticoagulation in 312 patients. Within the first 12 weeks, enoxaparin at 1 mg/kg/day was associated with a relative risk reduction in the incidence of clinically relevant VTE of 65% (from 14.5% to 5%). Preliminary data show no differences between the observational and enoxaparin groups for the secondary endpoints of time to progression (19 vs 22 weeks, respectively) and overall survival (29 vs 31 weeks, respectively). Additionally, there was no increased risk of bleeding events with the use of enoxaparin in this setting (observational 9.9% and enoxaparin 6.3%; P=NS).38
In the FAMOUS trial, dalteparin 5000 IU daily was not found to have a significant impact on the risk of VTE compared with placebo in patients with advanced cancer.39 Dalteparin has also been compared with placebo in 186 patients with newly diagnosed malignant glioma (PRODIGE).40 Patients received dalteparin subcutaneously once daily for 6 months, starting within the first month of surgery. Twenty one patients developed VTE during the first 6 months: 9 patients receiving dalteparin and 12 receiving placebo (11% and 17%, respectively; HR=0.7, 95% CI: 0.37-1.5, P =.3). Over 12 months there were 5 (5.1%) major bleeding events with dalteparin (all intracranial), and 1 (1.2%) with placebo (HR=4.0, 95% CI: 0.5-34, P =.2). Survival was comparable between treatment arms. A randomized controlled trial of dalteparin prophylaxis in solid tumor patients by Sideras and colleagues found no survival benefit in 141 patients with advanced cancer treated with daily injections of 5000 U of dalteparin compared with placebo.41 A randomized controlled clinical trial of dalteparin in patients with advanced pancreatic cancer (UK FRAGEM study) reported a significant reduction in the risk of VTE (RR=0.38, 95% CI: 0.17-0.84, P <.02).42 Although dalteparin was administered at weight-adjusted doses of 200 IU/kg/day for 4 weeks followed by 150 IU/kg/day for 8 additional weeks, no increase in major bleeding was observed.
The LMWH nadroparin was found to reduce the incidence of thromboembolic events in ambulatory cancer patients receiving chemotherapy.43 The PROTECHT trial was a randomized, double-blind, placebo-controlled study designed to evaluate the efficacy of nadroparin versus placebo for prophylaxis of thromboembolic events in 1166 patients receiving chemotherapy for advanced cancer. Patients had metastatic or locally advanced lung, breast, gastrointestinal, ovary, or head and neck cancer with an Eastern Cooperative Oncology Group (ECOG) performance status ≤2. Of the 769 patients treated with nadroparin, 2.0% had a thromboembolic event compared with 3.9% of patients receiving placebo (P =.02), although the difference for VTE did not reach statistical significance. The incidence of minor bleeding in the nadroparin group (<8%) was comparable to that of the placebo group.
Certoparin, another LMWH, has been evaluated in 2 double-blind, placebo-controlled studies (TOPIC-1 and TOPIC-2) that randomized patients with advanced breast cancer (N=353) or non–small-cell lung cancer (N=547) to certoparin 3000 U daily or placebo for prevention of chemotherapy-associated VTE.44 The overall rate of symptomatic and asymptomatic thrombosis in breast cancer patients was 4% for certoparin and 3.9% for placebo. Rates of major bleeding complications over 6 months of therapy were 1.7% for certoparin and 0% for placebo. Rates of thrombosis were higher in patients with lung cancer compared with rates in breast cancer patients, and showed a trend toward a reduction in thrombosis with certoparin (4.5% vs 8.3% for placebo, P =.07). Certoparin was especially effective in stage IV disease (3.5% vs 10.1% placebo; P =.03).
Taken together, the findings of these trials of LMWHs in the ambulatory cancer care setting suggest that these agents have the most benefit in patients at high risk of VTE, such as those with pancreatic cancer. A systematic review and meta-analysis of 8 randomized controlled trials enrolling ambulatory cancer patients has indicated a favorable benefit-to-risk ratio for the use of thromboprophylaxis in patients with advanced pancreatic cancer.37
Impact of Anticoagulants on Cancer Patient Survival
Anticoagulants have been postulated to improve survival in cancer patients.45 In a systematic review identifying 11 randomized, controlled trials, anticoagulants (particularly LMWH) showed significantly improved survival at 1 year in cancer patients without VTE while increasing the risk for bleeding complications.46 Improved survival with anticoagulation may be dependent on tumor type and disease stage. The meta-analysis of randomized controlled trials of prophylactic LMWHs in ambulatory cancer patients found no evidence of a survival benefit with the use of these agents in this setting.37 Given the limitations of available data, the use of anticoagulants as antineoplastic therapy cannot be recommended until additional randomized controlled trials have been conducted. Several trials are ongoing to test the effects of LMWH on survival in patients with cancer.47
Current ASCO and NCCN Guideline Recommendations
The American Society of Clinical Oncology (ASCO)4 and the National Comprehensive Cancer Network (NCCN),30 among other professional organizations, have developed guidelines for VTE prophylaxis and treatment in patients with cancer. As summarized in these guidelines, the primary goal of thromboprophylaxis in patients with cancer is to prevent VTE, including pulmonary embolism and early death from these complications. Both guidelines support the use of pharmacologic VTE prophylaxis in hospitalized cancer patients unless contraindications to prophylactic anticoagulation are present. It must be acknowledged, however, that these recommendations are based on studies of seriously ill medical patients only a small subgroup of which were actually cancer patients. While guideline panels and most clinicians have found it reasonable to extrapolate the results of these studies to the cancer population, more direct evidence on the risk of VTE in hospitalized cancer patients is needed.
In addition to cancer patients hospitalized for medical care, prophylaxis should include cancer patients undergoing major surgery and those with cancer and established VTE to prevent recurrence of thromboembolic events. According to the ASCO guidelines, low-dose UFH or LMWH is the recommended prophylaxis in patients undergoing laparotomy, laparoscopy, or thoracotomy.4 Prophylaxis should be initiated before surgery or as early as possible in the postoperative period, and be continued for at least 7 to 10 days after surgery. Prophylaxis may be prolonged for up to 4 weeks in obese patients, patients undergoing major abdominal or pelvic surgery for cancer, and patients with a history of VTE. Mechanical methods of VTE prophylaxis may be used with pharmacologic anticoagulation but should not be used alone except in patients with active bleeding, for whom the medications are contraindicated.4 Treatment with LMWH is preferred in cancer patients with established VTE for the initial 5 to 10 days of treatment and should be given up to 6 months or longer to prevent VTE recurrence. Vitamin K antagonists with a targeted international normalized ratio (INR) of 2-3 are acceptable for extended secondary prophylaxis when LMWH is not available. Indefinite anticoagulant therapy should be considered for patients with active cancer.
Routine thromboprophylaxis is currently not recommended in ambulatory patients with cancer who are receiving systemic chemotherapy due to the lower risk of VTE in this setting along with an increased risk of major bleeding in these patients.4 However, ASCO guidelines recommend anticoagulation for VTE prophylaxis specifically in patients receiving thalidomide or lenalidomide adjunctively with chemotherapy or dexamethasone due to the high risk of thrombosis associated with these treatment regimens.4 The evaluation of various biomarkers to enhance clinical prediction tools for the identification of cancer patients at increased VTE risk who may benefit from thromboprophylaxis is an area of active investigation.48-50 Current studies of VTE prophylaxis in ambulatory cancer patients at high risk for VTE based on cancer type, eg, pancreatic cancer or risk model evaluation, may lead to future recommendations for prophylaxis in such settings.
The NCCN guidelines recommend LMWHs, fondaparinux, or UFHs for acute treatment of VTE while the diagnosis and risk are being assessed, with LMWHs preferred in patients who are expected to receive chronic anticoagulation therapy; warfarin can be used in patients requiring chronic anticoagulation but should be started in a 5- to 7-day transition period with the LMWH, fondaparinux, or UFHs and be monitored to INR.30 The guidelines state that LMWHs such as enoxaparin, dalteparin, and tinzaparin are commonly considered therapeutically equivalent, but each has distinct pharmacokinetics and few clinical studies have directly compared the clinical effects of these agents.30 LMWH heparin as monotherapy (without warfarin) is recommended for treatment of proximal DVT or PE, and prevention of recurrent VTE in patients with advanced or metastatic cancer.30 Indefinite anticoagulation should be considered if cancer is active or important risk factors are persistent.30
There are few data on the impact of thrombosis on quality of life in cancer patients. Likewise, the impact of VTE on the delivery of optimal cancer treatment has received little attention. The prospective international Perceive Registry is designed to study the extent to which VTE complicates the course of common solid tumor malignancies and subsequent clinical outcomes.51 In addition, a prospective, randomized clinical trial will compare the safety and efficacy of LMWH prophylaxis (dalteparin) versus no treatment in reducing VTE in high-risk ambulatory cancer patients initiating chemotherapy.52
Representatives of the major international guidelines panels have recently issued a call to action for improved treatment and prevention strategies as well as a sustained research effort to further our understanding of the relationship between cancer and thrombosis in order to reduce the burden of VTE and its consequences on patients with cancer.53
New Pharmacologic Options for the Treatment and Prevention of VTE
Evaluation of new anticoagulants is important in order to enhance treatment options available for patients with cancer. New agents for the pharmacologic treatment and prevention of VTE include the parenteral agents bemiparin and semuloparin as well as the oral agents rivaroxaban and apixaban. It is anticipated that oral agents may provide greater convenience of administration, while parenteral agents continue to be more suitable in the hospital setting for patients undergoing active cancer treatment, as well as for some patients with advanced malignancy.
Bemiparin
Bemiparin, a LMWH with antifactor Xa/antifactor IIa activity,54 has been studied for the prevention of VTE with prolonged use in cancer patients undergoing abdominal or pelvic surgery.55 In the CANBESURE study, Kakkar and colleagues randomized 703 cancer surgery patients to receive once-daily subcutaneous injections of bemiparin 3500 IU (with the first dose 6 hours after surgery) for approximately 1 week. Patients were then randomized to receive bemiparin or placebo for an additional 3 weeks. Major VTE (composite of proximal DVT, nonfatal PE, and VTE-related deaths) occurred in 0.4% of patients in the bemiparin group compared with 3.3% in the placebo group (relative risk ratio 87.9%; 95% CI: 98.5%, 4.0%; P=.016). Bemiparin was found to significantly reduce the rate of major VTE without significantly increasing the risk of hemorrhagic complications compared with 1 week of bemiparin prophylaxis and subsequent placebo.55
Semuloparin
Semuloparin, another parenteral agent, is a subcutaneous ultra-LMWH that acts as a factor Xa inhibitor with residual anti-IIa activity.56,57 Semuloparin is being studied for VTE prevention in patients with cancer and also in patients undergoing major abdominal or orthopedic surgery.58 The dose response of semuloparin was recently examined in patients undergoing total knee replacement surgery (TREK study).56 There was a significant dose response across the 5 semuloparin doses tested, with the incidence of VTE ranging from 5.3% (60 mg/day) to 44.1% (5 mg/day) for semuloparin. The 3 highest doses of semuloparin (20, 40, and 60 mg) were significantly more effective at reducing confirmed VTE compared with 40 mg/day enoxaparin (used as calibrator), reducing the risk of VTE by 58%, 61%, and 85%, respectively. Six patients in the semuloparin groups (4 in the 60-mg group, 1 in the 40-mg group, and 1 in the 20-mg group) experienced major bleeding compared with none in the enoxaparin calibrator group. The 20-mg dose was selected for further investigation and is being studied in several ongoing phase III trials. Two of these trials are studying semuloparin use in cancer patients. The SAVE-ONCO trial is evaluating semuloparin for the prevention of VTE in cancer patients undergoing chemotherapy (NCT00694382).59 The SAVE-ABDO trial (NCT00679588) is evaluating semuloparin compared with enoxaparin for the prevention of VTE in patients undergoing major surgery of the abdomen and/or pelvis, and includes patients undergoing cancer surgery.60
Rivaroxaban
Rivaroxaban is an oral direct inhibitor of factor Xa61 and is being studied for the prevention of deep vein thrombosis and pulmonary embolism in patients undergoing hip or knee replacement (RECORD 1-4).62 The MAGELLAN trial will evaluate whether extended therapy with oral rivaroxaban can prevent blood clots in the leg and lung that can occur in patients hospitalized for acute illness (including active cancer patients); results will be compared with a standard regimen of enoxaparin.63
Apixaban
Apixaban is another oral direct inhibitor of factor Xa. As demonstrated in an interim analysis of a phase II study, apixaban was found to be well tolerated in patients with metastatic cancer. Incidence of major bleeding and thrombosis among 125 patients were very low (major bleeding: 2 patients receiving apixaban 20 mg and 1 patient receiving placebo; thrombosis: all 3 cases in placebo group).64
CONCLUSIONS
VTE is a common complication of cancer and cancer treatment and is associated with considerable morbidity and mortality. Hospitalized medical and surgical patients with cancer are at increased risk for VTE and should be considered for pharmacologic prophylaxis if no contraindication to anticoagulation is present. Patients with cancer treated for documented VTE should be considered for continued anticoagulation, preferably with LMWH, for up to 6 months or longer in the presence of active malignancy. Routine thromboprophylaxis in ambulatory patients with cancer is not currently recommended. Nevertheless, many ambulatory cancer patients are also at an increased risk for thrombosis. Although results from randomized controlled trials are still needed, thromboprophylaxis may be considered in selective high-risk patients such as those with multiple myeloma receiving thalidomide or lenalidomide plus chemotherapy. Consideration of prophylactic anticoagulation in patients with cancer must always balance the risk of VTE with the increased risk of bleeding. Improved methods for the identification of ambulatory patients with cancer at increased risk for VTE, including assessing clinical risk factors and utilizing biomarkers, are under investigation and should enable safe, effective, and targeted thromboprophylaxis.
Table 4b.
Recent Studies of Pharmacologic Anticoagulants in Surgical Patients With Cancer
| Citation | Patient Population |
Treatments | Primary Outcome |
Result | Significance Level |
Bleeding Rates |
Significance Level |
Length of Treatment |
Setting |
|---|---|---|---|---|---|---|---|---|---|
| Einstein MH, et al. Obstet Gynecol. 200881 |
Gynecologic cancer surgery |
Dual prophylaxis with sequential compression devices alone or compression devices + UFH 5000 U Q12h or Q8h |
VTE | Dual prophylaxis with prolonged prophylaxis in high-risk patients resulted in a significant reduction in VTE rate from 6.5% (19/294) in 2005 to 1.9% (6/311) in 2006 |
OR 0.33; 95% CI 0.12–0.88 |
Median blood loss: 2005: 250 mL; 2006: 200 mL |
P=.22 | Until hospital discharge, extended to 2 wk post-hospital discharge in high-risk patients |
Prophylaxis |
| Shukla PJ, et al. Indian J Gastroenterol. 200882 |
Colorectal cancer surgery |
Dalteparin 2500 IU QD X 6 d or no prophylaxis |
DVT | No DVT occurred in either group |
Not reported | Not specified | Not reported | 6 d | Prophylaxis |
| Simonneau G, et al. J Thromb Haemost. 200683 |
Colorectal cancer surgery |
Nadroparin 2850 IU QD vs enoxaparin 40 mg QD |
VTE | Nadroparin 15.9% (74/464); enoxaparin 12.6% (61/486) |
P=NS |
Major bleeding: nadroparin 7.3%; enoxaparin 11.5% |
P=.012 | 7–11 d | Prophylaxis |
| Perry SL, et al. J Neurooncol. 200984 |
Patients with brain tumors |
Tinzaparin 4500 IU QD |
Safety outcomes |
CNS hemorrhage 5% (2/40) |
Not reported |
CNS hemorrhage: grade 1: 2.5%; grade 2: 2.5% |
Not reported | 12 mo | Prophylaxis |
Disclosures and Acknowledgements
Dr. Lyman is supported by grants from the National Heart, Lung and Blood Institute (1R01HL095109-01) and the National Cancer Institute (RC2CA148041-01). The opinions expressed in this article are those of the author. The author received no honoraria or other form of financial support related to the development of this manuscript. Editorial support for this article was provided by Peloton Advantage LLC and funded by sanofi aventis U.S. However, the author had complete independence in defining content and in all editorial decisions with respect to this article.
Footnotes
Financial disclosures: The author has nothing to disclose.
References
- 1.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632–634. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 2.Lyman GH, Khorana AA. Cancer, clots and consensus: new understanding of an old problem. J Clin Oncol. 2009;27:4821–4826. doi: 10.1200/JCO.2009.22.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee AY. Management of thrombosis in cancer: primary prevention and secondary prophylaxis. Br J Haematol. 2005;128:291–302. doi: 10.1111/j.1365-2141.2004.05292.x. [DOI] [PubMed] [Google Scholar]
- 4.Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25:5490–5505. doi: 10.1200/JCO.2007.14.1283. [DOI] [PubMed] [Google Scholar]
- 5.Gao S, Escalante C. Venous thromboembolism and malignancy. Expert Rev Anticancer Ther. 2004;4:303–320. doi: 10.1586/14737140.4.2.303. [DOI] [PubMed] [Google Scholar]
- 6.Khorana AA. Cancer and thrombosis: implications of published guidelines for clinical practice. Ann Oncol. 2009;20:1619–1630. doi: 10.1093/annonc/mdp068. [DOI] [PubMed] [Google Scholar]
- 7.Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 8.Lee AY. Cancer and venous thromboembolism: prevention, treatment and survival. J Thromb Thrombolysis. 2008;25:33–36. doi: 10.1007/s11239-007-0102-0. [DOI] [PubMed] [Google Scholar]
- 9.Elting LS, Escalante CP, Cooksley C, et al. Outcomes and cost of deep venous thrombosis among patients with cancer. Arch Intern Med. 2004;164:1653–1661. doi: 10.1001/archinte.164.15.1653. [DOI] [PubMed] [Google Scholar]
- 10.Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy. 2009;29:943–953. doi: 10.1592/phco.29.8.943. [DOI] [PubMed] [Google Scholar]
- 11.Khorana AA, Francis CW, Culakova E, Fisher RI, Kuderer NM, Lyman GH. Thromboembolism in hospitalized neutropenic cancer patients. J Clin Oncol. 2006;24:484–490. doi: 10.1200/JCO.2005.03.8877. [DOI] [PubMed] [Google Scholar]
- 12.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166:458–464. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 13.Chew HK, Wun T, Harvey DJ, Zhou H, White RH. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol. 2007;25:70–76. doi: 10.1200/JCO.2006.07.4393. [DOI] [PubMed] [Google Scholar]
- 14.Khorana AA, Rao MV. Approaches to risk-stratifying cancer patients for venous thromboembolism. Thromb Res. 2007;120(Suppl 2):S41–S50. doi: 10.1016/S0049-3848(07)70129-9. [DOI] [PubMed] [Google Scholar]
- 15.Behranwala KA, Williamson RC. Cancer-associated venous thrombosis in the surgical setting. Ann Surg. 2009;249:366–375. doi: 10.1097/SLA.0b013e318195c50c. [DOI] [PubMed] [Google Scholar]
- 16.Osborne NH, Wakefield TW, Henke PK. Venous thromboembolism in cancer patients undergoing major surgery. Ann Surg Oncol. 2008;15:3567–3578. doi: 10.1245/s10434-008-0151-4. [DOI] [PubMed] [Google Scholar]
- 17.Rao MV, Francis CW, Khorana AA. Who’s at risk for thrombosis? Approaches to risk stratifying cancer patients. In: Khorana AA, Francis CW, editors. Cancer-Associated Thrombosis: New Findings in Translational Science, Prevention, and Treatment. Informa Healthcare USA, Inc; New York, NY: 2007. pp. 169–192. [Google Scholar]
- 18.Komrokji RS, Uppal NP, Khorana AA, et al. Venous thromboembolism in patients with diffuse large B-cell lymphoma. Leuk Lymphoma. 2006;47:1029–1033. doi: 10.1080/10428190600560991. [DOI] [PubMed] [Google Scholar]
- 19.Alcalay A, Wun T, Khatri V, et al. Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol. 2006;24:1112–1118. doi: 10.1200/JCO.2005.04.2150. [DOI] [PubMed] [Google Scholar]
- 20.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110:2339–2346. doi: 10.1002/cncr.23062. [DOI] [PubMed] [Google Scholar]
- 21.Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olson RE. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med. 2006;119:60–68. doi: 10.1016/j.amjmed.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 22.Gray KN, Chu D, Wu S, Lin RZ. Risk of venous thromboembolism with thalidomide in cancer patients: a systematic review and meta-analysis of randomized controlled trials [abstract 3820] Blood. 2008;112 Abstract 3820. [Google Scholar]
- 23.Yang X, Brandenburg NA, Freeman J, et al. Venous thromboembolism in myelodysplastic syndrome patients receiving lenalidomide: results from postmarketing surveillance and data mining techniques. Clin Drug Investig. 2009;29:161–171. doi: 10.2165/00044011-200929030-00003. [DOI] [PubMed] [Google Scholar]
- 24.Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–1239. doi: 10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- 25.Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. 2008;300:2277–2285. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 26.Bohlius J, Wilson J, Seidenfeld J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst. 2006;98:708–714. doi: 10.1093/jnci/djj189. [DOI] [PubMed] [Google Scholar]
- 27.Khorana AA, Francis CW, Blumberg N, Culakova E, Refaai MA, Lyman GH. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168:2377–2381. doi: 10.1001/archinte.168.21.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuderer NM, Khorana AA, Francis CW, et al. Venous thromboembolism risk model predicts early progression and overall mortality in cancer patients receiving chemotherapy [abstract 172] Blood. 2008;112 Abstract 172. [Google Scholar]
- 30.Streiff MB, Baird MF, Bennett CL, et al. [Accessed August 23, 2010];Venous thromboembolic disease. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 31.Lyman GH. Thromboprophylaxis with low-molecular-weight heparin in medical patients with cancer. Cancer. 2009;115:5637–5650. doi: 10.1002/cncr.24665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: a double-blind randomized multicentre trial with venographic assessment. ENOXACAN Study Group. Br J Surg. 1997;84:1099–1103. [PubMed] [Google Scholar]
- 33.Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346:975–980. doi: 10.1056/NEJMoa012385. [DOI] [PubMed] [Google Scholar]
- 34.Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341:793–800. doi: 10.1056/NEJM199909093411103. [DOI] [PubMed] [Google Scholar]
- 35.Leizorovicz A, Cohen AT, Turpie AG, Olsson CG, Vaitkus PT, Goldhaber SZ. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110:874–879. doi: 10.1161/01.CIR.0000138928.83266.24. [DOI] [PubMed] [Google Scholar]
- 36.Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332:325–329. doi: 10.1136/bmj.38733.466748.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuderer NM. Low-molecular-weight heparin for venous thromboprophylaxis in ambulatory cancer patients: A systematic review meta-analysis of randomized controlled trials [abstract]. Presented at the 51st annual meeting of the American Society of Hematology; New Orleans, LA. December 5-8, 2009. [Google Scholar]
- 38.Riess H, Pelzer U, Deutschinoff G, et al. A prospective, randomized trial of chemotherapy with or without the low molecular weight heparin (LMWH) enoxaparin in patients (pts) with advanced pancreatic cancer (APC): results of the CONKO 004 trial [abstract LBA4506]. Presented at: 2009 Annual Meeting of the American Society of Clinical Oncology; Orlando, FL. May 29-June 2, 2009. [Google Scholar]
- 39.Kakkar AK, Levine MN, Kadziola Z, et al. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS) J Clin Oncol. 2004;22:1944–1948. doi: 10.1200/JCO.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Perry JR, Rogers L, Laperriere N, et al. PRODIGE: a phase III randomized placebo-controlled trial of thromboprophylaxis using dalteparin low molecular weight heparin (LMWH) in patients with newly diagnosed malignant glioma [abstract 2011] J Clin Oncol. 2007;25(Suppl 18):2011. [Google Scholar]
- 41.Sideras K, Schaefer PL, Okuno SH, et al. Low-molecular-weight heparin in patients with advanced cancer: a phase 3 clinical trial. Mayo Clin Proc. 2006;81:758–767. doi: 10.4065/81.6.758. [DOI] [PubMed] [Google Scholar]
- 42.Maraveyas A, Waters J, Roy R, et al. Gemcitabine with or without prophylactic weight-adjusted dalteparin in patients with advanced or metastatic pancreatic cancer (APC): a multicentre, randomised phase IIB trial (the UK FRAGEM study) [abstract O-6503]. Presented at the Joint ECCO 15–34th ESMO Multidisciplinary Congress; Berlin, Germany. September 20-24, 2009. [Google Scholar]
- 43.Agnelli G, Gussoni G, Bianchini C, et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009;10:943–949. doi: 10.1016/S1470-2045(09)70232-3. [DOI] [PubMed] [Google Scholar]
- 44.Haas SK, Kakkar AK, Kemkes-Matthes B, et al. Prevention of venous thromboembolism with low-molecular-weight heparin in patients with metastatic breast or lung cancer - results of the TOPIC studies [abstract 1707]. Presented at the XX Congress International Society on Thrombosis and Haemostasis; Sydney, Australia. August 6-12, 2005. [Google Scholar]
- 45.Cunningham MS, Preston RJ, O’Donnell JS. Does antithrombotic therapy improve survival in cancer patients? Blood Rev. 2009;23:129–135. doi: 10.1016/j.blre.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Kuderer NM, Khorana AA, Lyman GH, Francis CW. A meta-analysis and systematic review of the efficacy and safety of anticoagulants as cancer treatment: impact on survival and bleeding complications. Cancer. 2007;110:1149–1161. doi: 10.1002/cncr.22892. [DOI] [PubMed] [Google Scholar]
- 47.Kuderer NM, Ortel TL, Francis CW. Impact of venous thromboembolism and anticoagulation on cancer and cancer survival. J Clin Oncol. 2009;27:4902–4911. doi: 10.1200/JCO.2009.22.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Connolly GC, Kuderer NM, Culakova E, Francis CW, Lyman GH, Khorana AA. Leukocytosis, thrombosis and mortality in cancer [abstract OC-TU-018]. Presented at XXII Congress International Society on Thrombosis and Haemostasis; Boston, MA. July 11-16, 2009. [Google Scholar]
- 49.Sud R, Khorana AA. Cancer-associated thrombosis: risk factors, candidate biomarkers and a risk model. Thromb Res. 2009;123(Suppl 4):S18–S21. doi: 10.1016/S0049-3848(09)70137-9. [DOI] [PubMed] [Google Scholar]
- 50.Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27:4839–4847. doi: 10.1200/JCO.2009.22.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petralia GA, Rickard M, Baginski M, et al. Venous thromboembolism and cancer: The PERCEIVE Registry [abstract P-T-489]. Presented at the XXI Congress International Society on Thrombosis and Haemostasis; Geneva, Switzerland. July 6-2, 2007. [Google Scholar]
- 52.Clinicaltrials.gov website [Accessed December 22, 2009];Clinicaltrials.gov Identifier: NCT00876915. A Study of Dalteparin Prophylaxis in High-Risk Ambulatory Cancer Patients. Available at: http://clinicaltrials.gov/ct2/results?term=NCT00876915.
- 53.Khorana AA, Streiff MB, Farge D, et al. Venous thromboembolism prophylaxis and treatment in cancer: a consensus statement of major guidelines panels and call to action. J Clin Oncol. 2009;27:4919–4926. doi: 10.1200/JCO.2009.22.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinez-Gonzalez J, Vila L, Rodriguez C. Bemiparin: second-generation, low-molecular-weight heparin for treatment and prophylaxis of venous thromboembolism. Expert Rev Cardiovasc Ther. 2008;6:793–802. doi: 10.1586/14779072.6.6.793. [DOI] [PubMed] [Google Scholar]
- 55.Kakkar VV, Balibrea J, Martinez-Gonzalez J, Prandoni P. Late breaking clinical trial: a randomized double blind trial to evaluate the efficacy and safety of prolonging the thromboprophylaxis with bemiparin in patients undergoing cancer abdominal or pelvic surgery (The CANBESURE Study) [abstract LB-MO-002]. Presented at the XXII Congress International Society on Thrombosis and Haemostasis; Boston, MA. July 11-16, 2009. [Google Scholar]
- 56.Lassen MR, Dahl OE, Mismetti P, Destree D, Turpie AG. AVE5026, a new hemisynthetic ultra-low-molecular-weight heparin for the prevention of venous thromboembolism in patients after total knee replacement surgery—TREK: a dose-ranging study. J Thromb Haemost. 2009;7:566–572. doi: 10.1111/j.1538-7836.2009.03301.x. [DOI] [PubMed] [Google Scholar]
- 57.Viskov C, Just M, Laux V, Mourier P, Lorenz M. Description of the chemical and pharmacological characteristics of a new hemisynthetic ultra-low-molecular-weight heparin, AVE5026. J Thromb Haemost. 2009;7:1143–1151. doi: 10.1111/j.1538-7836.2009.03447.x. [DOI] [PubMed] [Google Scholar]
- 58.National Institute for Health Research . AVE-5026 for the prevention of venous thromboembolism in patients at risk. National Horizon Scanning Centre; [Accessed July 14, 2009]. Available at: www.pcpoh.bham.ac.uk/publichealth/horizon/outputs/documents/2008/may-august/AVE-5026_.pdf. [Google Scholar]
- 59.Evaluation of AVE5026 in the prevention of venous thromboembolism in cancer patients undergoing chemotherapy (SAVE-ONCO) US National Institutes of Health; [Accessed July 21, 2009]. Clinicaltrials.gov identifier: NCT00694382. Available at: http://clinicaltrials.gov/ct2/show/NCT00694382?term=ave+5026&rank=7. [Google Scholar]
- 60.Evaluation of AVE5026 as compared to enoxaparin for the prevention of venous thromboembolism in patients undergoing major abdominal surgery (SAVE-ABDO) US National Institutes of Health; [Accessed January 27, 2010]. Clinicaltrials.gov Identifier: NCT00679588. Available at: http://clinicaltrials.gov/ct2/show/NCT00679588?term=save+abdo&rank=1. [Google Scholar]
- 61.Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–2775. doi: 10.1056/NEJMoa0800374. [DOI] [PubMed] [Google Scholar]
- 62.Ageno W. Rivaroxaban for the prevention of venous thromboembolism following major orthopedic surgery: the RECORD trials. Expert Rev Cardiovasc Ther. 2009;7:569–576. doi: 10.1586/erc.09.37. [DOI] [PubMed] [Google Scholar]
- 63.Venous thromboembolic event (VTE) prophylaxis in medically ill patients. US National Institutes of Health; [Accessed July 22, 2009]. Clinicaltrials.gov identifier: NCT00571649. Available at: http://clinicaltrials.gov/ct2/show/NCT00571649?term=magellan+rivaroxaban&rank=1. [Google Scholar]
- 64.Liebman H, Levine MN, Deitchman D, et al. Apixaban in patients with metastatic cancer: a randomized phase II feasibility study [abstract PP-WE-489]. Presented at the XXII Congress International Society on Thrombosis and Haemostasis; Boston, MA. July 11-16, 2009. [Google Scholar]
- 65.Hull RD, Schellong SM, Tapson VF, et al. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8–18. doi: 10.7326/0003-4819-153-1-201007060-00004. [DOI] [PubMed] [Google Scholar]
- 66.De Cicco M, Matovic M, Balestreri L, et al. Early and short-term acenocumarine or dalteparin for the prevention of central vein catheter-related thrombosis in cancer patients: a randomized controlled study based on serial venographies. Ann Oncol. 2009;20:1936–1942. doi: 10.1093/annonc/mdp235. [DOI] [PubMed] [Google Scholar]
- 67.Young AM, Billingham LJ, Begum G, et al. Warfarin thromboprophylaxis in cancer patients with central venous catheters (WARP): an open-label randomised trial. Lancet. 2009;373:567–574. doi: 10.1016/S0140-6736(09)60205-1. [DOI] [PubMed] [Google Scholar]
- 68.Weber C, Merminod T, Herrmann FR, Zulian GB. Prophylactic anti-coagulation in cancer palliative care: a prospective randomised study. Support Care Cancer. 2008;16:847–852. doi: 10.1007/s00520-007-0339-3. [DOI] [PubMed] [Google Scholar]
- 69.Robins HI, O’Neill A, Gilbert M, et al. Effect of dalteparin and radiation on survival and thromboembolic events in glioblastoma multiforme: a phase II ECOG trial. Cancer Chemother Pharmacol. 2008;62:227–233. doi: 10.1007/s00280-007-0596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niers TM, Di NM, Klerk CP, Baarslag HJ, Buller HR, Biemond BJ. Prevention of catheter-related venous thrombosis with nadroparin in patients receiving chemotherapy for hematologic malignancies: a randomized, placebo-controlled study. J Thromb Haemost. 2007;5:1878–1882. doi: 10.1111/j.1538-7836.2007.02660.x. [DOI] [PubMed] [Google Scholar]
- 71.Meister B, Kropshofer G, Klein-Franke A, Strasak AM, Hager J, Streif W. Comparison of low-molecular-weight heparin and antithrombin versus antithrombin alone for the prevention of symptomatic venous thromboembolism in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008;50:298–303. doi: 10.1002/pbc.21222. [DOI] [PubMed] [Google Scholar]
- 72.Icli F, Akbulut H, Utkan G, et al. Low molecular weight heparin (LMWH) increases the efficacy of cisplatinum plus gemcitabine combination in advanced pancreatic cancer. J Surg Oncol. 2007;95:507–512. doi: 10.1002/jso.20728. [DOI] [PubMed] [Google Scholar]
- 73.Miller KC, Padmanabhan S, Dimicelli L, et al. Prospective evaluation of low-dose warfarin for prevention of thalidomide associated venous thromboembolism. Leuk Lymphoma. 2006;47:2339–2343. doi: 10.1080/10428190600799631. [DOI] [PubMed] [Google Scholar]
- 74.Deitcher SR, Kessler CM, Merli G, Rigas JR, Lyons RM, Fareed J. Secondary prevention of venous thromboembolic events in patients with active cancer: enoxaparin alone versus initial enoxaparin followed by warfarin for a 180-day period. Clin Appl Thromb Hemost. 2006;12:389–396. doi: 10.1177/1076029606293692. [DOI] [PubMed] [Google Scholar]
- 75.Ruud E, Holmstrom H, De Lange C, Hogstad EM, Wesenberg F. Low-dose warfarin for the prevention of central line-associated thromboses in children with malignancies—a randomized, controlled study. Acta Paediatr. 2006;95:1053–1059. doi: 10.1080/08035250600729092. [DOI] [PubMed] [Google Scholar]
- 76.Ikhlaque N, Seshadri V, Kathula S, Baumann MA. Efficacy of prophylactic warfarin for prevention of thalidomide-related deep venous thrombosis. Am J Hematol. 2006;81:420–422. doi: 10.1002/ajh.20625. [DOI] [PubMed] [Google Scholar]
- 77.Baz R, Li L, Kottke-Marchant K, et al. The role of aspirin in the prevention of thrombotic complications of thalidomide and anthracycline-based chemotherapy for multiple myeloma. Mayo Clin Proc. 2005;80:1568–1574. doi: 10.4065/80.12.1568. [DOI] [PubMed] [Google Scholar]
- 78.Karthaus M, Kretzschmar A, Kroning H, et al. Dalteparin for prevention of catheter-related complications in cancer patients with central venous catheters: final results of a double-blind, placebo-controlled phase III trial. Ann Oncol. 2006;17:289–296. doi: 10.1093/annonc/mdj059. [DOI] [PubMed] [Google Scholar]
- 79.Verso M, Agnelli G, Bertoglio S, et al. Enoxaparin for the prevention of venous thromboembolism associated with central vein catheter: a double-blind, placebo-controlled, randomized study in cancer patients. J Clin Oncol. 2005;23:4057–4062. doi: 10.1200/JCO.2005.06.084. [DOI] [PubMed] [Google Scholar]
- 80.Couban S, Goodyear M, Burnell M, et al. Randomized placebo-controlled study of low-dose warfarin for the prevention of central venous catheter-associated thrombosis in patients with cancer. J Clin Oncol. 2005;23:4063–4069. doi: 10.1200/JCO.2005.10.192. [DOI] [PubMed] [Google Scholar]
- 81.Einstein MH, Kushner DM, Connor JP, et al. A protocol of dual prophylaxis for venous thromboembolism prevention in gynecologic cancer patients. Obstet Gynecol. 2008;112:1091–1097. doi: 10.1097/AOG.0b013e31818b1486. [DOI] [PubMed] [Google Scholar]
- 82.Shukla PJ, Siddachari R, Ahire S, et al. Postoperative deep vein thrombosis in patients with colorectal cancer. Indian J Gastroenterol. 2008;27:71–73. [PubMed] [Google Scholar]
- 83.Simonneau G, Laporte S, Mismetti P, et al. A randomized study comparing the efficacy and safety of nadroparin 2850 IU (0.3 mL) vs. enoxaparin 4000 IU (40 mg) in the prevention of venous thromboembolism after colorectal surgery for cancer. J Thromb Haemost. 2006;4:1693–1700. doi: 10.1111/j.1538-7836.2006.02083.x. [DOI] [PubMed] [Google Scholar]
- 84.Perry SL, Bohlin C, Reardon DA, et al. Tinzaparin prophylaxis against venous thromboembolic complications in brain tumor patients. J Neurooncol. 2009;95:129–134. doi: 10.1007/s11060-009-9911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cini M, Zamagni E, Valdre L, et al. Thalidomide-dexamethasone as up-front therapy for patients with newly diagnosed multiple myeloma: thrombophilic alterations, thrombotic complications, and thromboprophylaxis with low-dose warfarin. Eur J Haematol. 2010;84:484–492. doi: 10.1111/j.1600-0609.2010.01434.x. [DOI] [PubMed] [Google Scholar]
- 86.Lee AY, Rickles FR, Julian JA, et al. Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. J Clin Oncol. 2005;23:2123–2129. doi: 10.1200/JCO.2005.03.133. [DOI] [PubMed] [Google Scholar]
- 87.Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119:1062–1072. doi: 10.1016/j.amjmed.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 88.Romera A, Cairols MA, Vila-Coll R, et al. A randomised open-label trial comparing long-term sub-cutaneous low-molecular-weight heparin compared with oral-anticoagulant therapy in the treatment of deep venous thrombosis. Eur J Vasc Endovasc Surg. 2009;37:349–356. doi: 10.1016/j.ejvs.2008.11.030. [DOI] [PubMed] [Google Scholar]


