Abstract
TNT is one of the most commonly used nitro aromatic explosives for landmines of military and terrorist activities. As a result, there is an urgent need for rapid and reliable methods for the detection of trace amount of TNT for screening in airport, analyzing forensic samples and environmental analysis. Driven by the need to detect trace amounts of TNT from environmental samples, this article demonstrates a label-free, highly selective and ultra sensitive para-aminothiophenol (p-ATP) modified gold nanoparticle based dynamic light scattering (DLS) probe for TNT recognition in 100 pico molar (pM) level from ethanol:acetonitile mixture solution. Due to the formation of strong π-donor–acceptor interaction between TNT and p-ATP, para-aminothiophenol attached gold nanoparticles undergo aggregation in the presence of TNT, which changes the DLS intensity tremendously. A detailed mechanism for significant DLS intensity change has been discussed. Our experimental results show that TNT can be detected quickly and accurately without any dye tagging in 100 pM level with excellent discrimination against other nitro compounds.
Keywords: TNT detection, dynamic light scattering, gold nanoparticle, aggregation, plasmonics, selectiviyty
Introduction
Detection of illegally transported explosives materials like 2,4,6-trinitrotoluene has become important for assuring safety at airports and air travel 1–6. TNT is also a major source of hazardous water pollution, which is produced through military preparation of landmines 1–6. As a result, there is an urgent need for rapid and reliable methods of trace detection of TNT, for screening in airport, analyzing forensic samples and environmental analysis. These explosive materials must be detected with rapid response time and preferably without any sample preparation. Although current technologies are quite sensitive, often their selectivity is insufficient for performance in practical applications due to false positive signals 1–6. For growing market needs of the 21st century, future devices must link with selectivity, speed, simplicity and cost effective 6–30. As a result, different sensor concepts for analyzing TNT have been reported in last 10 years 7–18, which include nanotechnology based sensor 7–9,11–13, electrochemistry based sensor 10, fluorescence quenching sensor 15, organic dye based fluorescence resonance energy transfer (FRET)14, conjugated polymer based single and mutiphoton sensor 16–18. But FRET assays identify TNT analyte through a covalently linked label such as a fluorescent or luminescence tag. Necessity of tagging makes it difficult to use them as biosensors for real life. Driven by the need, in this article, we demonstrate that para amino-thiophenol (p-ATP) modified gold nanoparticle based dynamic light scattering (DLS) probe can be used for label-free detection of TNT, with excellent detection limit (100 pico-molar) and selectivity over DNT and other nitrocompounds.
DLS, known as photon correlation spectroscopy (PCS), is a non-invasive well-established technique for measuring the size of particles ranging in size from 0.5 nm to 6 μm 31–35. DLS is an absolute measurement and it is a powerful tool for determining small change in the size of particles 31–35. Noble metal nanostructures attract great interest because of their unique size or shape dependent properties, including large optical field enhancements resulting in the strong scattering and absorption of light 6–30, 36–43. Due to the presence of this surface plasmon resonances, weak scattering effects generally gets significantly enhanced via strong electromagnetic (plasmon) fields at the surfaces of metallic nanostructures 6–30, 36–43. This, together with our ability to make nanomaterials of different sizes and shapes makes them potentially useful assay for possible daily life applications 6–30, 36–43. Recently, we and other groups have shown that this technique coupled with noble metal nanoparticles can be used in ultrasensitive assay for chemical and bilogical detection, where nanoparticles are used as a light-scattering enhancer and DLS as a read-out system 31–35. In this manuscript, for the first time we are demonstrating that p-ATP conjugated gold nanoparticle based DLS assay can be used for selective detection of TNT.
Materials and Experiments
Hydrogen tetrachloroaurate (HAuCl4.3H2O), sodium citrate, p-ATP, 2,6-dinitrotoluene, nitrophenol, acetontrile, ethanol purchased from Sigma-Aldrich and used without further purification. 2,4,6-trnitrotoluene was provided by ERDC, Vicksburg, MS.
Gold Nanoparticle Synthesis
Gold nanoparticles of 13 nm size was synthesized by using HAuCl4, 3H2O and sodium citrate concentration as we reported recently 9,21–24,32. JEM-2100F transmission electron microscope (TEM) and UV-visible absorption spectrum were used to characterize the nanoparticles. The particle concentration was measured by UV-visible spectroscopy using the molar extinction coefficients at the wavelength of the maximum absorption of each gold colloid as reported recently 9,21–24,32.
Gold Nanoparticle Surface Modification
For selective detection of TNT, we have modified the gold nanoparticle surface by p-ATP 9:1 volume ratio of freshly prepared AuNPs (10 nM) and amino-thiophenol (10−6 M) was mixed by stirring for 12hrs. Excess amino thiophenol was removed by centrifugation at 8000 rpm for several minutes. By using UV-Vis absorption spectra, we estimated an average of 15–20 amino thiophenol per gold nanoparticle.
Colorimetric Detection of TNT
Colorimetric detection of TNT was carried out using 675μL of 9:1 v/v ratio of 1μM p-ATP anchored GNPs solution (10nM). Then different volumes of TNT stock prepared in 4:1 EtOH/ACN was added to the solution to attain TNT concentrations in the range of 10nM –250μM. The final volume of the solution was adjusted to 750μL. Samples with other nitro explosives were prepared by substituting TNT. Although the color change was instant, photographs were taken after 30 min of TNT addition for full color development. Photographs were taken using Cannon S70 digital camera.
Dynamic Light Scattering Measurement
DLS measurement was performed using Malvern Zetasizer Nano instrument. Conventional DLS becomes ineffective in absorbing media, since the laser beam is absorbed by the sample and can induce an interfering effect. Also, in case of concentrated samples, the artifact of multiple scattering can hamper the measurement. To reduce all the above problem, non-invasive back-scatter (NIBS) technology was used 32. DLS detection of TNT was carried out in the range of 100 fM-250nM using similar procedures, as we described above for colorimetric detection. All diameter reported here is based on DLS intensity average using a non-negative least squares analysis method. For each sample five DLS measurements were conducted with a fixed 10 runs.
Results and Discussion
Our detection is based on the fact that in the presence of TNT, p-ATP conjugated gold nanoparticles undergo aggregation (as shown in Scheme 1) due to the π-donor–acceptor interactions between TNT and p-ATP linked gold nanoparticle 11–13, which enabled the binding of TNT and GNP. One TNT molecule can be involved in π-donor–acceptor interactions with several p-ATP attached GNPs (shown in Scheme 1B). As a result, p-ATP attached GNP undergoes aggregation in the presence of TNT and the size of aggregates should increase as we add higher amount of TNT. In our study, we have used p-ATP as the primary amine for π-donor–acceptor interaction as well as stabilizer for the gold nanoparticles. After the addition of freshly prepared citrate-stabilized gold nanoparticles, the nano-surfaces were modified by p-ATP through the Au-S covalent bond (as shown in Scheme 1). The addition of p-ATP does not change the color and the absorption spectrum of gold nanoparticle remains the same, which indicates that there is no aggregation when 9:1 volume ratio of freshly prepared AuNPs (10nM) and p-ATP (10−6M) was stirred. TEM image of p-ATP modified gold nanoparticle, as shown in Figure 1C, also confirmed it.
Scheme 1.
Schematic representation of p-ATP conjugated gold nanoparticle based TNT detection. A) Shematic representation showing p-ATP modification process. B) Schematic representation showing P-ATP modified gold nanoparticle aggregation in the presence of TNT.
Figure 1.
A) Photograph showing colorimetric image of p-ATP conjugated gold nanoparticle in the presence of A1) without anything, A2) 0.3 mM 2,4 di-nitro toluene (DNT), A3) 0.3 mM nitro-phenol (NP), A4) 0.3 mM picric acid (PA), A5) 150 μM TNT. B) The absorption spectral changes of p-ATP modified gold nanoparticle in the presence of TNT. Absorption spectra remain the same in the presence of DNT, NP or PA. C) TEM image of p-ATP modified gold nanoparticle in the absence of TNT and D) TEM image of p-ATP modified gold nanoparticle in the presence of TNT.
When we added TNT to p-ATP modified gold nanoparticle, it undergoes aggregation (as shown in Figure 1D) and it is due to the strong π-donor–acceptor interaction between TNT and p-ATP as we discussed before. Aggregation in the presence of TNT ions yields both a substantial shift in the plasmon band energy to longer wavelength and a red-to-blue color change (as shown in Figure 1A and 1B). This red shift might be due to two factors. One is the change in the local refractive index on the nanoparticle surface caused by the specific binding of the p-ATP-conjugated oval shape gold nanoparticles with TNT. The other is the interparticle interaction resulting from the assembly of nanoparticles on surface.
To understand whether our assay is highly selective, we have also performed colorimetric response upon addition of 2,4 -dinitrotoluene (DNT), 2,4,6-trinitrophenol (TNP) or picric acid (PA) and nitrophenol. Figure 1A shows the colorimetric response of our probe in the presence of various nitro-compounds. Figure 1B demonstrates the absorption spectra response in the presence of various organic nitro compounds. Our absorption spectra measurement (as shown in Figure 1B) clearly shows that there are no visible bands between 600 to 800 nm in the presence of 500 μM DNT, PA or NP, whereas we observed a very strong absorption bands with peaks at 650 nm in presence of 200 μM TNT, which is due to the formation of p-ATP conjugated nano aggregates via strong π-donor–acceptor interaction. This may be due to the fact that the reduction potential for TNT is much lower (−565 mV) that DNT (−674 mV) or TNP (−764 mV) 44.
Our experimental absorption and colorimetric study clearly demonstrate that p-ATP modified gold nanoparticle does not undergo aggregation in the presence of DNT and other nitro compounds, which allows us to selectively detect TNT. In case of DNT, due to the lack of –NO2 group in the fourth position of the benzene ring, partial negative charge may not be distributed throughout the DNT molecular ring. Due to the lack of enough anionic charge, DNT may not form strong π-donor–acceptor interaction like TNT and as a result, the aggregation of p-ATP modified gold nanoparticles is prevented. The same phenomenon is true for nitro phenol. So our result clearly shows excellent selectivity of our probe over DNT, PA and nitro phenol.
To evaluate the sensitivity of our p-ATP attached gold nanoparticle based colorimetric assay, different concentrations of TNT from one stock solution were evaluated. As shown in Figure 2A, our colorimetric assay is highly sensitive to the concentration of TNT. Figure 2B shows how the absorption spectra change with the concentration of TNT. Our experimental results (as shown in Figure 2A) clearly demonstrate that the sensitivity of our colorimetric assay is as low as 50 μM TNT. We have also performed TEM experiment to understand how the cluster size changes with the concentration of TNT. As shown in Figure 2C, our TEM data clearly shows that as TNT concentration increases from 5 nm to 500 μM, the nano-aggregate size increases tremendously. Our experimental results also demonstrate that colorimetric response is only visible when the aggregate sizes are quite big.
Figure 2.
A) Photograph showing colorimetric change upon the addition of different concentrations of TNT on ATP modified gold nanoparticle: A1) 1 nM TNT, A2) 100 nM TNT, A3) 500 nM TNT, A4) 1 μM TNT, A5) 50 μM TNT, A6) 100 μM TNT, A7) presence of 250 μM TNT. Our data clearly demonstrate that the sensitivity of colorimetric assay is around 50 μM. B) The plot demonstrates the absorption spectral changes of ATP modified gold nanoparticle in the presence of different concentrations of TNT. C) TEM images showing how gold nanoparticle aggregate sizes vary with the concentration of TNT. C1) 5 nM TNT, C2) 100 nM TNT, C3) 50 μM TNT, C4) 500 μM TNT.
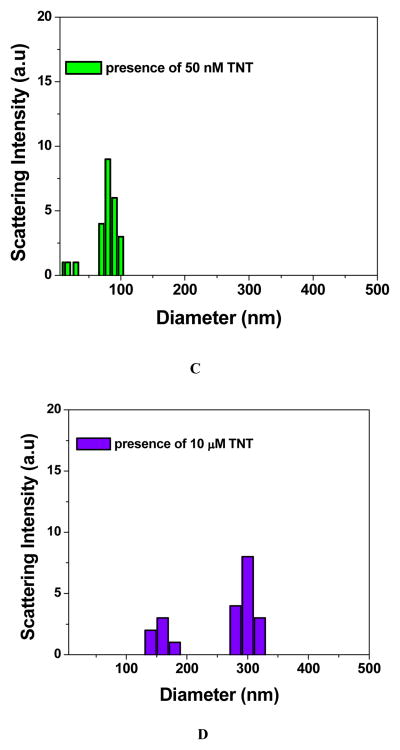
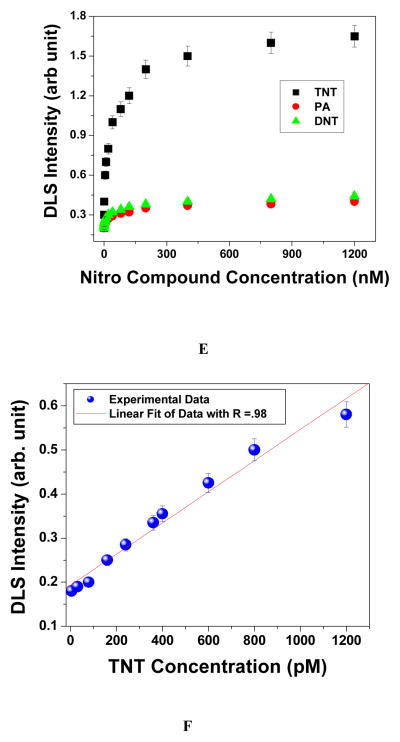
To improve the assay sensitivity towards TNT detection, we have employed p-ATP attached gold nanoparticle based DLS technique. DLS is a powerful method to determine the small change in the size of the particles. As shown in Figure 3B–3D and 2C, when different concentrations of TNT was added to p-ATP modified gold nanoparticle, at a lower concentration of TNT, only a smaller aggregate is formed and as a result, colorimetric assay is not able to respond. Since DLS has capability to separate dimer from the monomer 31–35, p-ATP conjugated gold nanoparticle based DLS assay should be able to show the response at very low concentration of TNT, when only dimers, trimers and slightly bigger aggregates are formed. Figure 3 clearly shows that DLS technique is highly sensitive to the concentration of TNT. Our experimental results clearly demonstrate (as shown in Figure 3E and 3F) that the sensitivity of our DLS assay for TNT detection is as low as 100 pM. Our result shows that DLS assay is about 5 orders of magnitude more sensitive that the usual colorimetric technique. Our experimental results also show that as we increase the concentration of TNT above 300 nM, the DLS intensity remains almost unchanged. It may be due to the fact that as we increase the concentration of TNT, aggregate or cluster size increases. And after certain concentration of TNT, when size becomes > 1 μm, it is close to the saturation point of DLS instrument and as a result, DLS signal remains unchanged.
Figure 3.
Size distributions of p-ATP attached gold nanoparticles measured by DLS, A) in the absence of TNT, B) in the presence of 500 pM TNT, c) in the presence of 15 nM TNT, D) in the presence of 300 nM TNT. E) Plot demonstrating how DLS intensity varies with TNT/PA/DNT concentration from 1 pM to 1200 nM; F) Plot demonstrating how DLS intensity varies with TNT concentration from 1 pM to 1200 pM.
To understand whether our DLS assay is highly selective, we have also performed DLS experiment with DNT, PA and NP. Figure 3A shows the p-ATP attached gold nanoparticle based DLS response in the presence of different concentrations of various nitro-compounds. Our results clearly show that our DLS assay is highly selective for TNT and it can easily separate it from other nitro compounds. We have also tested how stable is our p-ATP attached gold nanoparticle in the presence of trace acid or base contaminants which change the pH of the solution. We found that p-ATP attached gold nanoparticle is highly stable between pH 5.5 to 8.5, which indicates that presence of trace acid or base contaminants will not perturb our assay activity when pH of the solution in the range of 5.5 to 8.5.
Conclusion
In conclusion, in this article, we have demonstrated for the first time p-ATP conjugated gold nanoparticle based highly selective and ultra sensitive DLS probe for the TNT recognition in 100 pM level in aqueous solution. We have shown that due to the formation of strong π-donor–acceptor interaction between TNT and p-ATP P, para amino-thiophenol attached gold nanoparticles undergo aggregation in the presence of TNT, resulting in a tremendous DLS intensity change. Our experimental results show that TNT can be detected quickly and accurately without any dye tagging in 100 pM level with excellent discrimination against other nitro aromatic compounds. Our experiment also demonstrated that the sensitivity of our DLS assay to detect TNT level in water is about 5 orders of magnitude higher than the colorimetric technique. Our experimental results reported here open up a new possibility for rapid, easy and reliable diagnosis of TNT from environmental sample by measuring the DLS intensity. Given the simplicity, speed, and sensitivity of this approach, the described methodology could easily be extended to a high throughput format and become a new method of choice in all applications that require an assay for explosive detection. It is probably possible to improve the our DLS assay sensitivity by several orders of magnitudes by choosing proper materials and as a result, we still need a much greater understanding on how to control surface architecture in order to stabilize and maximize the DLS response.
Acknowledgments
Dr. Ray thanks DOD grant # W 912HZ-06-C-0057 and NSF-PREM grant # DMR-0611539, for their generous funding. We also thank reviewers whose valuable suggestions improved the quality of the manuscript.
References
- 1.Lubin AA, Plaxco KW. Acc Chem Res. 2010;43:496–505. doi: 10.1021/ar900165x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith KD, McCord BR, MacCrehan WA, Mount K, Rowe WF. J Forensic Sci. 1999;44:789–794. [Google Scholar]
- 3.Thomas SW, III, Joly GD, Swager TM. Chem Rev. 2007;107:1339–1386. doi: 10.1021/cr0501339. [DOI] [PubMed] [Google Scholar]
- 4.Dillewijn PV, Couselo JL, Corredoira E, Delgado A, Wittich RM, Ballester A, Ramos JL. Environ Sci Technol. 2008;42:7405–7410. doi: 10.1021/es801231w. [DOI] [PubMed] [Google Scholar]
- 5.Brettell TA, Butler JM, Almirall JR. Anal Chem. 2007;79:4365–4384. doi: 10.1021/ac070871s. [DOI] [PubMed] [Google Scholar]
- 6.McQuade DT, Pullen AE, Swager TM. Chem Rev. 2000;100:2537–2574. doi: 10.1021/cr9801014. [DOI] [PubMed] [Google Scholar]
- 7.Riskin M, Tel-Vered R, Lioubashevski O, Willner IJ. Am Chem Soc. 2009;131:7368–7378. doi: 10.1021/ja9001212. [DOI] [PubMed] [Google Scholar]
- 8.Aguilar DA, Forzani ES, Leright M, Tsow F, Cagan A, Iglesias RA, Nagahara LA, Amlani J, Tsui R, Tao NJ. Nano Lett. 2010;10:380–384. doi: 10.1021/nl902382s. [DOI] [PubMed] [Google Scholar]
- 9.Dasary SSR, Singh AK, Senapati D, Yu H, Ray PC. J Am Chem Soc. 2009;131:13806–13812. doi: 10.1021/ja905134d. [DOI] [PubMed] [Google Scholar]
- 10.Alizadeh T, Zare M, Ganzali MR, Norouzi P, Tavana B. Biosensor and Bioelectronics. 2010;25:1166–1172. doi: 10.1016/j.bios.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Riskin M, Tel-Vered R, Lioubashevski O, Willner IJ. Am Chem Soc. 2009;131:7368–7378. doi: 10.1021/ja9001212. [DOI] [PubMed] [Google Scholar]
- 12.Riskin M, Tel-Vered R, Bourenko T, Granot E, Willner I. J Am Chem Soc. 2008;130:9726–9733. doi: 10.1021/ja711278c. [DOI] [PubMed] [Google Scholar]
- 13.Forzani ER, Lu D, Leright MJ, Aguilar DA, Tsow F, Iglesias RA, Zhang Q, Lu J, Li J, Tao N. J Am Chem Soc. 2009;131:1390–1391. doi: 10.1021/ja809104h. [DOI] [PubMed] [Google Scholar]
- 14.Andrew TL, Swager TMJ. Am Chem Soc. 2007;129:7254–7255. doi: 10.1021/ja071911c. [DOI] [PubMed] [Google Scholar]
- 15.Narayanan A, Varnavski OP, Swager TM, Goodson T., III J Phys Chem C. 2008;112:881–884. [Google Scholar]
- 16.Freeman R, Willner I. Nano Lett. 2009;9:322–326. doi: 10.1021/nl8030532. [DOI] [PubMed] [Google Scholar]
- 17.Cerruti M, Jaworski J, Raorane D, Zueger C, Varadarajan J, Carraro C, Lee SK, Maboudian R, Majumdar A. Anal Chem. 2009;81:4192–4199. doi: 10.1021/ac8019174. [DOI] [PubMed] [Google Scholar]
- 18.Gao D, Wang Z, Liu B, Ni L, Wu M, Zhang Z. Anal Chem. 2008;80:8545–8553. doi: 10.1021/ac8014356. [DOI] [PubMed] [Google Scholar]
- 19.Wang S, Singh AK, Senapati D, Neely A, Yu H, Ray PC. Chem A-Eur J. 2010;16:5600–5606. doi: 10.1002/chem.201000176. [DOI] [PubMed] [Google Scholar]
- 20.Stewart ME, Anderton CR, Thompson LB, Maria J, Gray SK, Rogers JA, Nuzzo RG. Nanostructured Plasmonic Sensors. Chem Rev. 2008;108:494–521. doi: 10.1021/cr068126n. [DOI] [PubMed] [Google Scholar]
- 21.Ray PC. Chem Rev. 2010 doi: 10.1021/cr900335q. (ASAP Article) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffin J, Singh AK, Senapati D, Rhodes P, Mitchell K, Robinson B, Yu E, Ray PC. Chem Eur J. 2009;15:342–351. doi: 10.1002/chem.200801812. [DOI] [PubMed] [Google Scholar]
- 23.Darbha GK, Singh AK, Rai US, Yu E, Yu H, Ray PC. J Am Chem Soc. 2008;130:8038. doi: 10.1021/ja801412b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray PC. Angew Chem Int Ed. 2006;45:1151–1154. doi: 10.1002/anie.200503114. [DOI] [PubMed] [Google Scholar]
- 25.Laurence TA, Braun G, Talley C, Schwartzberg A, Moskovits M, Reich N, Huser T. J Am Chem Soc. 2009;131:162–169. doi: 10.1021/ja806236k. [DOI] [PubMed] [Google Scholar]
- 26.Graham D, Thimpson DG, Smith WE, Faulds K. Nat Nanotechnol. 2008;3:548–551. doi: 10.1038/nnano.2008.189. [DOI] [PubMed] [Google Scholar]
- 27.Griffin J, Singh AK, Senapati D, Lee E, Gaylor K, Jones-Boone J, Ray PC. Small. 2009;5:839–845. doi: 10.1002/smll.200801334. [DOI] [PubMed] [Google Scholar]
- 28.Donath E. Nat Nanotech. 2009;4:215–216. doi: 10.1038/nnano.2009.64. [DOI] [PubMed] [Google Scholar]
- 29.Singh AK, Senapati D, Wang S, Griffin J, Neely A, Candice P, Naylor KM, Varisli B, Kalluri JR, Ray PC. ACS Nano. 2009;3:1906–1912. doi: 10.1021/nn9005494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darbha GK, Singh AK, Rai US, Yu E, Yu H, Ray PC. J Am Chem Soc. 2008;130:8038. doi: 10.1021/ja801412b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian X, Zhou X, Nie S. J Am Chem Soc. 2008;130:14934–14935. doi: 10.1021/ja8062502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalluri JR, Arbneshi T, Khan SF, Neely A, Candice P, Varisli B, Washington M, McAfee S, Robinson B, Banerjee S, Singh AK, Senapati D, Ray PC. Angew, Chem, Int Ed. 2009;48:9668–9671. doi: 10.1002/anie.200903958. [DOI] [PubMed] [Google Scholar]
- 33.Du BA, Li ZP, Liu CH. Angew, Chem, Int Ed. 2008;47:8022–8025. doi: 10.1002/anie.200603331. [DOI] [PubMed] [Google Scholar]
- 34.Ipe BI, Shukla A, Liu H, Zou B, Rehage H, Niemeyer CM. Chem Phys Chem. 2006;7:1112. doi: 10.1002/cphc.200500660. [DOI] [PubMed] [Google Scholar]
- 35.Jans H, Liu X, Austin L, Maes G, Huo Q. Anal Chem. 2009;81:9425–9432. doi: 10.1021/ac901822w. [DOI] [PubMed] [Google Scholar]
- 36.Camden JP, Dieringer JA, Wang Y, Masaiello DJ, Marks LD, Schatz GC, Van Duyne RP. J Am Chem Soc. 2008;130:12616–12617. doi: 10.1021/ja8051427. [DOI] [PubMed] [Google Scholar]
- 37.Mallouk TE, Yang P. J Am Chem Soc. 2009;131:7937–7939. doi: 10.1021/ja9038104. [DOI] [PubMed] [Google Scholar]
- 38.Qian X, Li J, Shuming N. J Am Chem Soc. 2009;131:7540–7541. doi: 10.1021/ja902226z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laurence TA, Braun G, Talley C, Schwartzberg A, Moskovits M, Reich N, Huser T. J Am Chem Soc. 2009;131:162–169. doi: 10.1021/ja806236k. [DOI] [PubMed] [Google Scholar]
- 40.Lal S, Clare SE, Halas NJ. Acc Chem Res. 2008;41:1842–1851. doi: 10.1021/ar800150g. [DOI] [PubMed] [Google Scholar]
- 41.Jain PK, Huang X, El-Sayed IH, El-Sayed MA. Acc Chem Res. 2008;41:1578–1586. doi: 10.1021/ar7002804. [DOI] [PubMed] [Google Scholar]
- 42.Stoeva SI, Lee JS, Smith JE, Rosen ST, Mirkin CA. J Am Chem Soc. 2006;128:8378–8379. doi: 10.1021/ja0613106. [DOI] [PubMed] [Google Scholar]
- 43.Huang X, El-Sayed IH, Qian W, El-Sayed MA. J Am Chem Soc. 2006;128:2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 44.Maeda T, Nakamura R, Kadckam K, Ohawa HI. Environmental Toxicology and Chemistry. 2007;25:237–241. doi: 10.1897/06-019r1.1. [DOI] [PubMed] [Google Scholar]