Abstract
Tumor suppressor p53 maintains genome stability by regulating diverse cellular functions including cell cycle arrest, apoptosis, senescence and metabolic homeostasis. Mutations in the p53 gene occur in almost all human cancers with a frequency up to 80%. However, it is only 20% in breast cancers, 18% in endometrial cancers and 1.5% in cervical cancers. Estrogen receptor alpha (ERα) plays a pivotal role in hormonedependent cancer development and the status of ERα is used for designing treatment strategy and for prognosis. A closer look at the cross-talk between p53 and ERα has revealed that their activities are mutually regulated. This review will summarize the current body of knowledge on p53, ERα and ERβ in cancer. Clinical correlations between estrogen receptors and p53 status have also been reported. Thus, this review will discuss the relationship between p53 and ERs at both the molecular and clinical levels.
Keywords: Estrogen receptor, hormone-dependent cancer, p53, transcription factors
INTRODUCTION
Stress signals, such as DNA damage, hypoxia, oncogene activation and ribosomal stress, activate the p53 tumor suppressor mainly through posttranslational modifications. Activated p53 acts as a sequence-specific transcription factor that regulates a plethora of downstream target genes involved in diverse cellular processes including cell cycle arrest, DNA repair, apoptosis, and cellular senescence. For example, in response to DNA damage, p53 induces expression of p21, a cyclin-dependent kinase inhibitor, which binds to and inhibits the activity of cyclinD-CDK4/6 and cyclinE-CDK2 complexes and then arrests cells at G1 phase [1, 2]. Cell cycle arrest allows for the cell to repair the damaged DNA before it is replicated in S phase. If the cell cannot repair the DNA damage, p53 then induces expression of apoptotic target genes, such as PUMA and Bax, for programmed cell death [3]. Therefore, loss of p53 leads to aberrant cell proliferation and cell death and eventually tumor formation. Indeed, p53 inactivation occurs in almost all human tumors with a rate up to 80% depending on the type, stage, and etiology of cancers [4, 5]. Loss of p53 activity causes early onset of multiple tumors in p53-knockout mice and Li-Fraumeni syndrome patients [6, 7]. However, p53 mutation is not common in estrogen-responsive tumors. It is only 20% in breast cancers, 18% in endometrial cancers and 1.5% in cervical cancers [8, 9]. In the case of cervical cancer, loss of p53 activity is due to its inactivation and degradation by human papillomavirus E6 protein [10, 11].
Estrogen is an essential hormone for mammary gland development and reproductive organ function. Estrogen also regulates other diverse physiological functions associated with the cardiovascular, central nervous, immune, and skeletal systems. The estrogen activities are mediated by estrogen receptors (ER), including ERα and ERβ. Both ERα and ERβ are intracellular nuclear hormone receptors and mediate estrogen signaling primarily through transcriptional activation of target genes. Estrogen can also be recognized by G-protein coupled receptor 30 (GPR30), an integral membrane protein. In response to estrogen, GPR30 initiates non-genomic functions such as intracellular calcium mobilization, stimulation of adenylyl cyclase, synthesis of nuclear phosphatidylinositol (3,4,5)-trisphosphate (PIP3), and activation of the mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K) signaling pathways [13]. GPR30 acts independently of ERα and ERβ and its role in hormone-dependent cancer is not well characterized. Thus, this review will focus on ERα and ERβ.
In estrogen-regulated tissues such as the breast, endometrium and colon, tumors can be induced by deregulated ERα expression and signaling in cell proliferation, survival, and migration [14]. About 70% of breast cancers express ERα and are classified as estrogen receptor positive (ER-positive) and anti-estrogen, such as tamoxifen, has been developed to treat ER-positive breast cancer patients. In addition, chemotherapy is used as adjuvant treatment for anti-estrogen or main treatment for metastatic breast cancers. p53 is a major mediator for chemotherapy, and therefore understanding the crosstalk between p53 and ER signaling will provide important clues to improve current breast cancer treatment strategies. In this review, we provide an overview of the relationship between p53 and ERα in estrogen-responsive tumors at both the molecular and clinical levels.
THE ESTROGEN RECEPTOR SIGNALING PATHWAYS
ERα and ERβ are located on different chromosomes, but share 97% identity in their DNA-binding domains and 55% identity in their ligand-binding domains. ERα and ERβ are divided into six functional domains (Fig. 1A). The N-terminal A/B domains interact with coactivators and contain activation function-1 (AF-1), which is necessary for transcriptional activity. The C domain contains the DNA binding region. The D domain, also called the hinge region, contains a nuclear localization signal. The E domain contains a ligand binding region, a dimerization domain, AF-2, and an additional nuclear localization signal. The F domain is the C-terminal ligand binding region, which is highly regulated by agonists and antagonists [15].
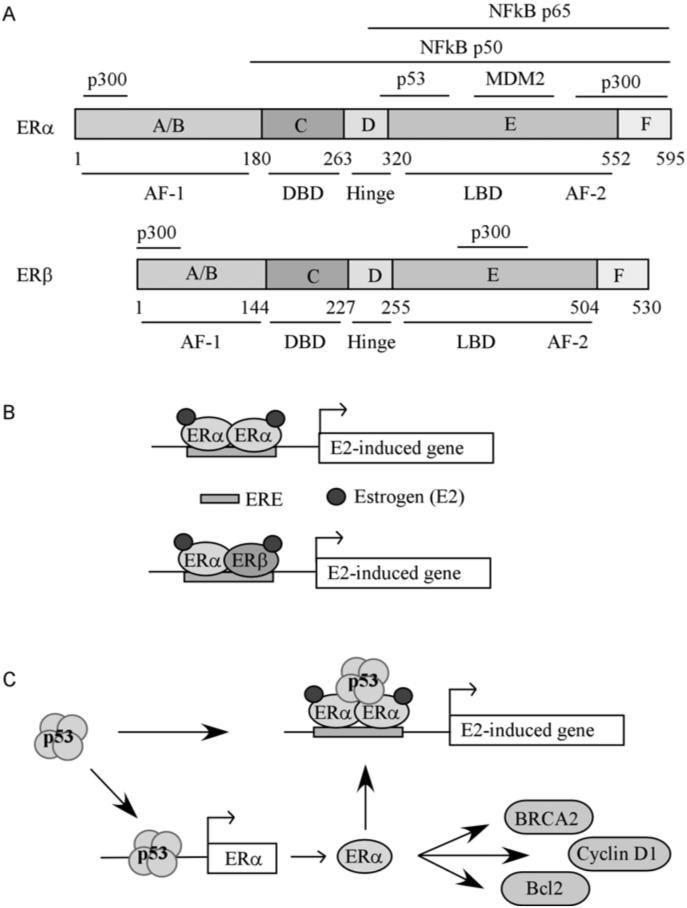
Fig. (1). Regulation of estrogen-induced genes.
(A) ERα and ERβ functional domains. Top panel: ERα functional domains. A/B, ligand-independent activation function (AF-1: aa 1-180). C, DNA binding domain (DBD: aa 181-263). D, dimerization/hinge domain (Hinge: aa 264-302). E, the ligand binding domain/activation function (LBD/AF-2: aa 303-552). F, the C terminal end of ERα (F: aa 553-595). Marked above ERα functional domains are NFκB p65 binding region (domains E-F), NFκB p50 binding region (domains C-F), p300 binding region (aa 56-72 and aa 534-595), p53 binding region (aa 283-395), and MDM2 binding region (the C-terminal end of the LBD). Bottom panel: ERβ functional domains. A/B, ligand-independent activation function (AF-1: aa 1-144). C, DNA binding domain (DBD; aa 145-227). D, dimerization/hinge domain (Hinge: aa 227-255). E, the ligand binding domain/activation function (LBD/AF-2: aa 256-504). F, the C terminal end of ERα (F: aa 505-530). The location of p300 binding sites (aa 1-72 and aa 432-530) is marked above ERβ functional domains. (B) Schematic diagram of how estrogen-induced genes are regulated by ERα homodimers and ERα/ ERβ heterodimers. (C) Schematic diagram of how estrogen-induced genes are regulated by ERα and p53.
Human ERα is expressed as two main isoforms, ERα and ERα46, the latter of which lacks the N-terminal AF-1. ERα46 can form a heterodimer with full length ERα, resulting in suppression of ERα transactivation activity [16]. A third ERα isoform, ERα36, which lacks both transactivation domains AF-1 and AF-2, has been identified [17]. Unlike full length ERα and ERα46, ERα36 is localized in the cytoplasm and the plasma membrane, where it is thought to mediate non-genomic estrogen signaling [18]. Human ERβ is expressed as more than five isoforms through alternative splicing [19]. ERβ1 is the full length receptor and referred to as ERβ. ERβ2-5 have a truncated AF-2 ligand binding domain and therefore cannot bind ligand. Although ERβ2-5 are unable to form functional homodimers, they can form heterodimers with ERα or ERβ1 and modulate their function [20, 21].
Upon binding to 17β-estradiol (estrogen), ERα and ERβ form homo- and hetero-dimers that function as transcription factors. These nuclear hormone receptors regulate gene expression either by binding directly to an estrogen response element (ERE) in their target gene promoters (Fig. 1B) or through secondary interactions with other transcription factors such as Activator Protein 1 (AP1), NFκB, or Sp1 [22, 23]. EREs can be either a full consensus sequence of 5'-GGTCAnnnTGACC-3' (where "n" is any nucleotide), a half consensus sequence of 5'-GGTCA-3', or a non-consensus sequence [24, 25]. Interestingly, both estrogen receptors have different tissue expression profiles and regulate common as well as unique sets of genes [26]. ERα is the main estrogen receptor in the female reproductive system, including the mammary gland and uterus [27]. ERβ is the main estrogen receptor in the central nervous system, lung, cardiovascular system, prostate and colon [28].
70% of breast tumors express ERα and are classified as estrogen receptor positive (ER-positive) [29]. ER-positive breast cancer generally is an indication of good patient prognosis and treatment responsiveness with anti-estrogens such as tamoxifen [30]. However, half of tamoxifen responsive tumors develop resistance to treatment [31, 32]. ER-positive breast cancer patients with de novo resistance to tamoxifen are generally responsive to ICI 182,780 as are patients who develop resistance to tamoxifen treatment [33]. ICI 182,780 (Fulvestrant) is a pure ER antagonist and inhibits dimerization following binding to ER [34]. In addition, ICI 182,780 reduces ERα levels by increasing receptor degradation via the ubiquitin-proteasome pathway [35]. Treatment resistance can be due to a reduction in the amount of ERα, which leads to an ER-negative tumor status. ER-negative tumors are associated with anti-estrogen treatment resistance, aggressive tumor growth, increased invasiveness and poor patient prognosis [30]. Some evidence suggests that ERα downregulation could be achieved by hypermethylation of CpG islands in the ERα promoter region [36], by hyperactive MAPK (ERK1/2) signaling [37], or by elevated expression of its transcription repressors, such as Her2/neu [38] and TWIST [39]. However, the mechanism for loss of ERα expression in ER-positive breast cancers is poorly defined.
While ERα expression is associated with tumor growth, ERβ expression may play an inhibitory role in tumorigenesis [40]. It has been shown that ERβ expression is low in breast cancer and ectopic expression of ERβ leads to reduced proliferation and/or invasion of several breast cancer cells, including MCF7, T47D, and MDA-MB-231 cells [41-44]. However, reports also showed that ERα-negative invasive breast cancers express ERβ and overexpression ERβ stimulated growth and/or metastasis of MDA-MB-231 and MDA-MB- 435 breast cancer cells [45-47]. Thus, it is likely that ERβ is capable of either promoting or inhibiting proliferation/metastasis of breast cancer cells. In future studies, characterization of the role of ERβ in breast tumorigenesis and the correlation between ERβ expression in clinical breast tumor samples and patient prognosis will be of great interest.
EFFECT OF ESTROGEN AND ESTROGEN RECEPTOR ON P53
Estrogen Regulates p53 Expression
An early study showed that in serum starved mouse 3T3 cells, p53 mRNA and protein levels were decreased whereas addition of serum increased p53 mRNA and protein levels [48]. This effect was particularly profound to estrogen compared to other steroids in the serum. Similarly, in T47D ER-positive breast cancer cells, the level of mutant p53 (L194F) was decreased to 10% after 4-5 days of culturing with hormone-free medium. However, normal levels of p53 were restored by 24 hours of treatment with 100 pM estradiol, a level well below the physiological level of estradiol. Conversely, anti-estrogen ICI 164,384 decreased p53 expression in both normal and hormone stripped medium [49, 50]. In addition, ER-positive and p53 wild-type MCF-7 breast cancer cells responded to estrogen treatment with an increase in p53 levels [51]. Moreover, in YAMC cells, addition of estrogen induced p53 downstream targets PUMA, Bcl-2-associated X protein (Bax), and Noxa [52]. Consistently, MCF-7 cells treated with doxorubicin, a chemotherapeutic drug that induces DNA damage and p53 activation, had a significantly lower number of cells undergoing apoptosis in estrogen-free conditions compared to cells treated in complete medium [53]. In addition, mRNA levels of PUMA, 14-3-3σ and especially GADD45 were also significantly reduced in doxorubicin-treated cells grown in estrogen-free media compared to cells treated with doxorubicin in complete media [53]. Moreover, overexpression of ERα sensitizes, whereas knockdown of ERα desensitizes, MCF-7 cells to DNA damage-induced growth suppression in a p53-dependent manner [54]. These together suggest that the estrogen receptor pathway is implicated in p53 regulation as well as DNA damage-induced p53 activation.
Reports showed that estrogen can regulate p53 transcription (Fig. 2A). For example, estrogen is found to induce p53 gene expression through CCAAT-binding transcription factor-1 and NFκB-binding motifs located between nt −106 and −40 upstream of the p53 transcriptional start site [55]. Because the proximal p53 promoter contains no consensus ERE site, it suggests that estrogen could induce p53 transcription through binding to the p65 subunit of NFκB at its C-terminal transactivation domain [55]. In addition, it showed that estrogen induces c-Myc, which then leads to subsequent activation of p53 through the Myc/Max Ebox response element [56]. A recent report showed that the GC-rich Sp1 site on the proximal p53 promoter is activated by ERα/Sp1 complex [57]. Interestingly, we found that ERα binds to and activates the p53 promoter via two distal ERE half-sites [54].
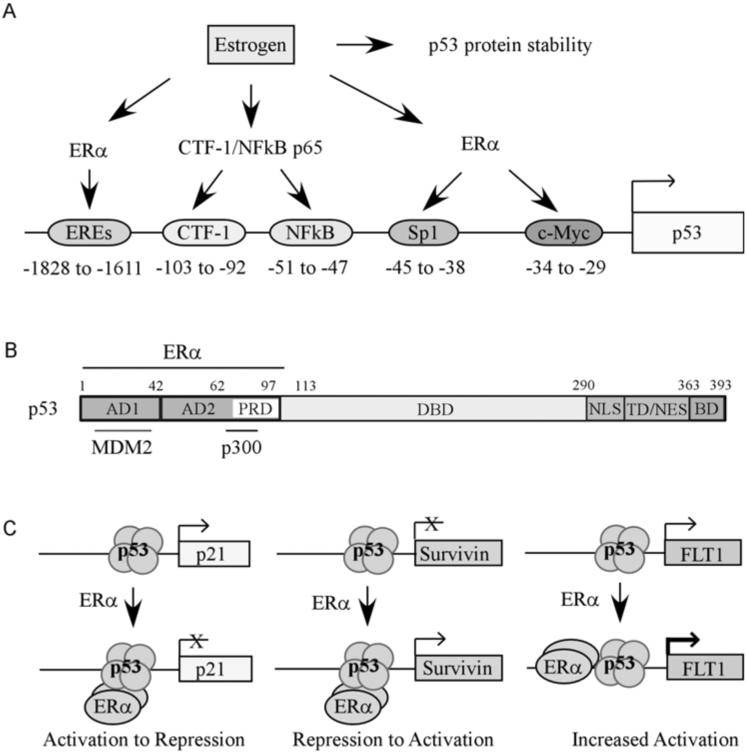
Fig. (2). The effect of estrogen and ER on the p53 pathway.
(A) Estradiol increases p53 via enhancing p53 protein stability or promoting p53 gene transcription through multiple transcription factors, including ERα and NFκB (see details in the text). (B) Schematic presentation of p53 functional domains. AD1, activation domain 1 (aa 1-42); AD2, activation domain 2 (aa 43-92); DBD, DNA binding domain (aa 102-292); NLS, nuclear localization sequence (aa 293-325); TD, tetramerization domain (aa 326-363); BD, C-terminal basic domain (aa 364-393). Marked above p53 functional domains is ERα binding region (aa 1-102). Marked below p53 functional domains is Mdm2 binding region (aa 13-41) and p300 binding region (aa 71-90). ERα binding region overlap with the MDM2 and p300 binding sites. (C) ERα modulates p53 target gene expression. ERα binds to p53 on the promoters of p53 target genes, and thus represses p21 expression, relieves the repression of Survivin expression, or cooperatively induces FLT1 expression.
To date, knowledge of the post-transcriptional regulation of p53 by estrogen is limited. One report showed that estrogen prolonged p53 protein half-life from a basal time of 5-30 minutes to 90 minutes in MCF-7 cells, but had no effect on mutant p53 stability in T47D or MDA-MD-231 cells [58]. Another report showed that estrogen promoted nuclear export of p53 in MCF-7 cells cultured in serum-free medium, and thus prevented p53 from inducing target genes, such as p21[59].
ERα Modulates p53 to Regulate its Target Genes
ERα and p53 bind to each other directly (Fig. 2B) [60-62]. Initial observations reported that ERα interacts mainly with the N-terminal 102 amino acids of p53 and some of residues beyond 103 amino acid may be also involved in this protein-protein interaction [62]. However, another report showed that the C-terminal aa 319-393 of p53 is necessary for p53 interaction with the AF-2 domain (aa 283-395) of ERα [60]. Although ERα associates with p53 at the region which is important for interacting with p300 and MDM2 [63, 64], ERα interaction with p53 is not affected by MDM2 or p300 [62, 65]. Indeed, MDM2 exists in the p53- ERα complex and has less ability to downregulate p53 in the presence of increased amounts of ERα, suggesting that ERα has protective properties over p53 [62].
ERα is found to interact with p53 on the promoters of p53 target genes, such as p21 and PCNA, and represses p53 transcriptional activity (Fig. 2C) [60]. In addition, estrogen increases the p53- ERα interaction, consistent with the observation that p21 transcription is decreased following estrogen treatment [66]. Moreover, ERα relieved p53 repression of target genes Survivin and MDR-1 (multidrug resistance gene 1) (Fig. 2C) [67]. In contrast, γ-irradiation, on the other hand, disrupted the p53- ERα interaction while anti-estrogens, tamoxifen and ICI 164,384, had no effect [60]. This result was validated in an animal model by exposing mice with xenografted MCF-7 tumors to ionizing radiation. The tumor growth was significantly reduced and a ChIP assay showed a disrupted p53-ERα interaction on the promoter of Survivin in the xenografted cells [68]. Interestingly, a recent report showed that ERα antagonizes p53-mediated cell death by suppressing several doxorubicin-induced proapoptotic p53 target genes, including ATF3, BTG2, and TRAF4, without altering the access of p53 to these gene promoters [69].
Reports showed that ERα regulates some p53 target genes which contain ERE sites (Fig. 2C). For example, FLT1, an angiogenesis-related gene, contains two ERE half sites which are located 225 nt upstream and 145 nt downstream from a half p53 response element. Synergistic transactivation of FLT1 occured in the presence of increased levels of ERα with both wild-type p53 and some p53 mutants [70, 71]. Since p53 is a direct target of ERα activation [54], it is possible that ERα is able to modulate p53 target gene expression. Indeed, we showed that knockdown of ERα leads to decreased expression of p53 along with its targets including p21, MDM2, PolH, PUMA, and MIC-1 [54]. However, ERα has little or no effect on the expression of these genes in p53-deficient cells [54]. Taken together, it suggests that ERα modulates p53 target gene expression through multiple mechanisms. For example, ERα reduces p21 expression either by physical association with p53 and hence inhibiting p53 transactivation at the p21 promoter or by regulating p53 expression and consequently controlling p21 expression.
In ER-positive breast cancers, the increase of ERα and subsequent increase of p53 could lead to an abnormal balance between the two proteins. ERα induces Bcl-2, an anti-apoptotic gene, while p53 inhibits Bcl-2 through Noxa [72, 73]. If more ERα is present in tumor cells than activated p53, apoptosis of cancer cells can be avoided. However, ERα can block MDM2 inhibition of p53, thus activating the tumor suppressor and possibly promoting the cancer cells to undergo apoptosis. If this delicate balance of ERα and p53 is disturbed, it may result in deregulated signaling pathways as well as changes in ERα- and/or p53-dependent gene expression that promotes tumor growth and survival.
ERβ and p53
Unlike ERα, ectopic expression of ERβ has no effect on the levels of p53 and p21 regardless of DNA-damage treatment in ERα-knockdown MCF10a cells [74]. Consistently, we found that ERβ does not activate the p53 promoter [54]. These indicate that ERβ has no direct effect on the expression of p53. However, emerging evidence suggested that ERβ may contribute to p53 levels in some circumstances. For examples, ERβ-specific agonists induce p53 and apoptosis in MC4-L2 mouse mammary adenocarcinoma cells, which express both ERα and ERβ [75]. In addition, expression of ERβ led to increased expression of mutant p53 in SW480 cells, but decreased expression of wild-type p53 in HCT116 cells [76]. Moreover, overexpression of ERβ inhibited cell survival by enhancing p53-mediated apoptosis and growth suppression in LoVo cells in an estrogen-dependent manner [77]. Furthermore, it showed that ERβ is able to antagonize estrogen-induced cytoplasmic translocation of p53, and thus increases p53 transcriptional activity in MCF7 cells [78]. Therefore, regulation of p53 by ERβ is achieved through multiple mechanisms which need to be further explored.
REGULATION OF ESTROGEN RECEPTOR BY p53
p53 transcriptionally regulates a vast array of target genes, including ERα and a subset of estrogen-responsive genes. There are two potential mechanisms by which ER target genes are regulated by p53. First, protein-protein interaction between ERα and p53 can lead to alterations in ER target gene expression. To suppress hormone-induced cancer cell growth, p53 interferes with ERα binding to EREs in estrogen-responsive target gene promoters instead of interfering with ERα dimerization (Fig. 1C) [65]. This may explain why some estrogen-responsive genes, such as BRCA2, Bcl2, IL-6 and tissue plasminogen activator [79], are repressed by p53 [80-84]. Second, ERα is a p53 target. In MCF-7 cells, ectopic expression of p53 increased ERα expression whereas knockdown of p53 decreased ERα expression [85]. DNA damage treatment with doxorubicin or ionizing radiation increased p53 and subsequently ERα protein and mRNA levels. Additionally, the effect of DNA damage on ERα expression can be further enhanced by overexpression of p53. Treatment with doxorubicin recruited p53 to the ERα promoter at nt −128 to −40 while at a non-stress condition, p53 was recruited to the ERα promoter at nt −2094 to −1941 and −350 to −298 [86].
A positive feedback regulatory loop between ERα and p53 may explain why tumors with mutated p53 tend to be ER-negative and ER-negative breast cancers lose wild-type p53 expression, and consequently become an aggressive tumor. However, there are still a significant number of ER-positive breast cancer cases with mutant p53 tumor status, which would not be explained by this hypothesis.
ER AND p53 IN HORMONE-DEPENDENT CANCERS
ERα and p53 in Breast Cancer
It is well-known that women whose first full term pregnancy occurs under the age of 18 have one third the lifetime risk of breast cancer than women whose first birth occurs over the age of 30 [87]. Similarly, parous rodents were shown to be less likely to develop cancer after exposure to chemical carcinogens than virgin rodents [88-90]. In addition, short term treatment with ovarian hormones estrogen and progesterone enhanced p53-dependent responses and reduced the incidence of mammary cancer in rats and mice [89, 91-93]. In a study of p53-deficient nulliparous mice, those with deregulated ERα expression had higher mammary epithelial cell proliferation and reduced rates of apoptosis compared to mice with normal ERα expression [94]. Interestingly, p53-null mice with estrogen treatment developed mostly ERα-positive mammary tumors with a short latency, but those without estrogen treatment developed mostly ERα-negative mammary tumors [95]. While these observations of p53 and estrogen interaction in animal models and human clinical cases appear to correlate with results from molecular studies, much is left unexplained in the tumor behavior after treatment with anti-estrogen therapy, chemotherapy or radiation therapy as well as development of resistance to these therapies.
To date, many studies of clinical breast cancer cases have given conflicting results, but overall, a correlation between ERα and p53 is observed: that is, p53 is primarily wild-type in ER-positive breast cancer and mutant in ER-negative breast cancer. Many of the inconsistencies stem from small sample size and method of p53 status determination. Immunohisto-chemistry detects increased amounts of p53, which generally indicates mutant p53 status. Wild-type p53 has a much shorter lifespan than mutant p53 and is not easily detected by immunohistochemistry. Moreover, 30% of tumors with mutant p53 cannot be detected by immunostaining [96].
A consortium study on 1,280 breast carcinomas looking at the allele loss on chromosome 17 found that 52.4% of primary breast carcinomas had loss of heterozygosity [97] on 17p, an area where p53 resides (17p13.1) [98]. Previous studies also reported LOH on 17p in 50-60% of breast cancer cases [99, 100]. Furthermore, abnormalities on chromosome 17 were statistically associated with increased aggressiveness of breast tumors, larger tumor size, ER-negative status, and early age of onset [98]. Studies focusing on the p53 locus found that the rate of p53 mutations is in the range of 16-58% using immunohistochemistry and 14-40% using DNA-based methods [101-105]. In 1,794 breast cancer patients, those with p53 mutations in exons 5-8 had a higher risk of breast cancer-specific death as compared to patients with wild-type p53. For patients with ER-positive tumors, mutant p53 status reduced survival to 60% after 10 years [106].
Tamoxifen is the leading drug for all stages of ER-positive breast cancer in pre- and post-menopausal women. Treatment of ER-positive breast cancer patients with tamoxifen resulted in a 31% reduction in mortality rate compared to patients not treated with tamoxifen. As expected, treatment of ER-negative breast cancer patients with tamoxifen showed little to no benefit [107]. While treatment of ER-negative breast cancers with a combination of chemotherapeutic agents (doxorubicin, cyclophosphamide and paclitaxel) increased disease-free survival by 22.8% after five years, addition of these chemotherapy to tamoxifen treatment of ER-positive patients only increased disease-free survival by 7% [108].
Tamoxifen is classified as a selective estrogen receptor modulator (SERM) for its ability to act as an antagonist of estrogen in some tissues such as breast and act as an agonist in other tissues including heart and bone [109]. As a prodrug, tamoxifen is metabolized in the liver to form the active metabolite 4-hydroxytamoxifen (OHT), which competes with estrogen for binding at the ligand-binding domain of ERα in the breast tissue. Once bound, OHT induces a conformational change by displacing helix 12 to block the coactivator binding groove in the AF-2 domain. As a result, corepressors, such as nuclear receptor corepressor (NCoR) and silencing mediator for retinoid and thyroid hormone receptors (SMRT), bind and influence ERα to regulate a different set of genes [109-111]. Treatment with tamoxifen also leads to a rapid decrease of cells in S-phase with a concurrent accumulation of cells in the G1-fraction. Though the mechanism of tamoxifen-induced cell cycle arrest is still uncertain, it is thought to be mediated by ERα based on the observation that ER-negative cells are not as sensitive to tamoxifen as ER-positive cells [112]. Treatment of ER-positive, p53 wild-type cells with tamoxifen resulted in a dose-dependent increase of p53 and p21 proteins suggesting that a p53-dependent G1 cell cycle arrest pathway is activated [1, 113]. Addition of estrogen to tamoxifen-treated G1 arrested cells induced the cells to progress into S phase along with an increase in protein levels of cyclin D1 and the kinase activity of cyclin D1/CDK4 and cyclin D1/CDK2 [114-118]. Together, these observations suggest that p53-mediated cell cycle arrest plays a role in the hormone-therapy targeting ERα.
Patients with node-positive tumors containing mutant p53 had a significantly lower overall survival rate upon treatment with tamoxifen and loco-regional radiotherapy as compared to patients with node-positive wild-type p53 status [114]. A similar study, which examined 243 ER-positive breast cancer cases, found that patients with p53 mutation had a lower response to tamoxifen (31%) as compared to those with wild-type p53 (66%) [119]. ER-negative tumors with p53 mutation had a worse overall response to tamoxifen (22%) than ER-positive tumors with wild-type p53 (73%). These studies suggest that mutant p53 has a negative effect on tamoxifen treatment. However, others have found no such correlation [102, 120].
Breast cancer patients with mutant p53 tend to have worse disease free survival rates regardless of estrogen receptor status [121]. Molecular studies showed that tamoxifen increased levels of p53 and activation of downstream p53 target genes in ER-positive, p53 wild-type cells, but not in ER-positive cells with mutant p53 [111]. Because p53 regulates ERα expression, mutations in p53 would inhibit ERα expression, decreasing the effects of tamoxifen [86]. It is not clear whether direct interaction between p53 and ERα has an effect on treatment or if treatment outcome results from other pathway interactions.
ERα and p53 in Cervical Cancer
A majority of cervical cancer cases are positive in human papilloma virus (HPV). Women infected with HPV16 are 38 times more at risk of developing cervical cancer than uninfected women [122]. High-risk HPVs, such as HPV16 and HPV18, express two oncogenes, E6 and E7, which inactivate p53 and pRb (retinoblastoma protein) tumor suppressor proteins, respectively [10, 11]. Loss of p53 and pRb is known to be responsible for increased cervical tumor cell proliferation. However, many patients infected with HPV alone do not develop cervical cancer [123], indicating the involvement of other cofactors. Indeed, the estrogen signaling contributes to HPV-induced cervical tumorigenesis. Analysis showed that long-term use (five years or more) of oral contraceptives in HPV-infected women resulted in a four-fold increase in cervical cancer risk compared to HPV-infected women not exposed to long-term estrogens [124]. This is confirmed in several mouse models. It has been shown that HPV16 transgenic mice treated with estrogen for nine months developed larger, more aggressive tumors than mice treated with estrogen for six months [125]. In addition, the expression of E6/E7 was increased by estrogen and thus potentiates cervical cell transformation in HPV18 E6/E7 transgenic mice [126]. Moreover, ERα is thought to mediate this effect as ERα-null HPV transgenic mice failed to develop cervical cancer after exposure to estrogen [127] and blocking ERα by dominant negative ERα inhibits the growth of cervical cancer cells [128]. Furthermore, 88% of conditional p53 knockout mice with 6-month exposure to estrogen developed a wide spectrum of high-grade cervical tumors [95]. However, E6 transgenic mice do not develop tumor in the absence of estrogen or develop low-grade cervical cancers with estrogen treatment for 6 or 9 months [95, 129, 130]. This suggests that E6 is unable to fully inactivate p53.
ERα and p53 in Endometrial Cancer
Studies showed that long-term tamoxifen treatment increases the risk for developing endometrial cancer by 50% in ER-positive breast cancer patients [131-133]. However, much fewer cases and diseased-related death of endometrial cancer occur each year compared to breast cancer [134]. While tamoxifen shows strong anti-estrogenic activity in breast tissue, it has weak proestrogenic effects in endometrial tissue [135]. Indeed, tamoxifen users with endometrial cancer had a less favorable prognosis and worse overall survival compared to endometrial cancer patients not exposed to tamoxifen [136]. In addition, longer exposure to tamoxifen (60 vs 30 months) correlated with mutant p53 status. Tumors arising in women previously treated with tamoxifen were also more likely to be ER-negative [137]. Like breast cancer, 60-70% of endometrial cancers express ERα with a favorable prognosis compared to ER-negative endometrial cancers. In addition, the level of ERα is decreased, whereas the level of p53 (likely mutant p53) is increased, as the disease is progressed from stage I to stage II-III and recurrent tumors [138]. However, there has been no significant difference in the abnormality of the p53 pathway between ER-negative and ER-positive patients [139]. Similar to breast cancer cases, ER-negative endometrial tumors with a mutant p53 status strongly correlate with aggressive growth and poor patient prognosis [140].
ERβ and p53 in Cancer
The lifetime risk of developing colon cancer is significantly lower in females than in males [141]. In addition, females with colon cancer have better survival rates than male patients [142]. Similar to breast cancer risks, women who have had multiple children have a lower occurrence of colon cancer than women with no children [143]. Studies also report a correlation between postmenopausal women on estrogen replacement therapy and lower incidences of colon cancer [144, 145]. These indicate that estrogen has been implicated in a protective role against colon cancer. ERβ is predominantly expressed over ERα in the colorectal epithelium and mediates the effects of estrogen in the colon [146]. Therefore, although ERβ may not be a major factor for patient survival in breast cancer, it might be critical in colon cancer. Several studies showed that ERβ protein, but not mRNA, is decreased in colon cancer cells compared to normal colon cells and further decreased in poorly differentiated tumors compared to well-differentiated tumors [147, 148]. In addition, a mouse study showed that ERβ-deficiency leads to an accelerated progression of colitis-associated colorectal cancer and is associated with increased cell proliferation [149]. Consistently, ectopic expression of ERβ not only inhibits cell proliferation in multiple colon cancer cells, such as HCT8, SW480 and HCT116, but also suppresses the growth of SW480 cell transplants in mice [76, 150]. It has been shown that c-Myc expression is decreased whereas p21 and p27 are increased by ectopic expression of ERβ [76, 150]. However, whether p53 is necessary for ERβ to exert its activity in colorectal tumorigenesis is not clear.
CONCLUSION REMARKS
In normal cells, estrogen promotes cell proliferation. The concordant increase of p53 may counter the enhanced level of proliferation following estrogen stimulation. In cells where p53 and ER levels are deregulated, the balance between estrogen-stimulated growth via ER and p53-mediated growth suppression is disrupted, leading to uncontrolled tumor growth despite the presence of elevated p53 levels.
While there are many other factors contributing to tumor formation, ER-positive cancers make up the majority of breast, cervical and endometrial cancer cases. It is unknown how ER-positive tumors with wild-type p53 progress to become ER-negative tumors with mutant p53 although it is hypothesized that mutant p53 is able to transcriptionally repress ERα transcription. Furthermore, it is largely unexplained why half of patients with ER-positive tumors initially responding to tamoxifen develop resistance to treatment. Fully characterizing the interaction between p53 and ER and their signaling pathways in normal versus cancer cells will provide an insight into hormone-dependent cancer development and progression. Additional molecular and clinical analysis of how tumors respond to treatment with anti-estrogens in the case of ER-positive cancers or DNA damage-induced chemotherapies in the case of ER-negative cancers is needed to improve patient treatment.
ACKNOWLEDGEMENTS
Declared none.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.El-Deiry WS, Tokino T, Velculescu VE, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75(4):817–25. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 2.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366(6456):701–4. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 3.Takaoka A, Hayakawa S, Yanai H, et al. Integration of interferon-[alpha]/[beta] signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424(6948):516–23. doi: 10.1038/nature01850. [DOI] [PubMed] [Google Scholar]
- 4.Petitjean A, Mathe E, Kato S, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28(6):622–9. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- 5.Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2(1):a001008. doi: 10.1101/cshperspect.a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malkin D, Li FP, Strong LC, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250(4985):1233–8. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- 7.Lavigueur A, Maltby V, Mock D, Rossant J, Pawson T, Bernstein A. High incidence of lung, bone, and lymphoid tumors in transgenic mice overexpressing mutant alleles of the p53 oncogene. Mol Cell Biol. 1989;9(9):3982–91. doi: 10.1128/mcb.9.9.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pharoah PDP, Day NE, Caldas C. Somatic mutations in the p53 gene and prognosis in breast cancer: a meta-analysis. Br J Cancer. 1999;80(12):1968–73. doi: 10.1038/sj.bjc.6690628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westhof G, Bader W, Greiner-Mai E, Hatzmann W. Comparison of cytosolic p53 protein levels in the female genital tract and breast, and their tumors. Tumour Biol. 2000;3(21):123–34. doi: 10.1159/000030118. [DOI] [PubMed] [Google Scholar]
- 10.Dyson N, Hawley R, Miinger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243(4893):934–7. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 11.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63(6):1129–36. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 12.Enmark E, Pelto-Huikko M, Grandien K, et al. Human Estrogen Receptor {beta}-Gene Structure, Chromosomal Localization, and Expression Pattern. J Clin Endocrinol Metab. 1997;82(12):4258–65. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- 13.Mosselman S, Polman J, Dijkema R. ER[beta]: Identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392(1):49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 14.Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors {alpha} and {beta} Endocrinology. 1997;138(3):863–70. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 15.Green S, Walter P, Kumar V, et al. Human oestrogen receptor cDNA: sequence, expression and homology to verb-A. Nature. 1986;320(6058):134–9. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 16.Flouriot G, Brand H, Denger S, et al. Identification of a new isoform of the human estrogen receptor-alpha (hER-[alpha]) that is encoded by distinct transcripts and that is able to repress hER-[alpha] activation function 1. EMBO J. 2000;19(17):4688–700. doi: 10.1093/emboj/19.17.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. Identification, cloning, and expression of human estrogen receptor-alpha36, a novel variant of human estrogen receptor-alpha66. Biochem Biophys Res Commun. 2005;336(4):1023–7. doi: 10.1016/j.bbrc.2005.08.226. [DOI] [PubMed] [Google Scholar]
- 18.Shi L, Dong B, Li Z, et al. Expression of ER-{alpha}36, a novel variant of estrogen receptor {alpha}, and resistance to tamoxifen treatment in breast cancer. J Clin Oncol. 2009;27(21):3423–9. doi: 10.1200/JCO.2008.17.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore JT, McKee DD, Slentz-Kesler K, et al. Cloning and characterization of human estrogen receptor beta isoforms. Biochem Biophys Res Commun. 1998;247(1):75–8. doi: 10.1006/bbrc.1998.8738. [DOI] [PubMed] [Google Scholar]
- 20.Leung YK, Mak P, Hassan S, Ho SM. Estrogen receptor (ER)-beta isoforms: a key to understanding ER-beta signaling. Proc Natl Acad Sci USA. 2006;103(35):13162–7. doi: 10.1073/pnas.0605676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omoto Y, Eguchi H, Yamamoto-Yamaguchi Y, Hayashi S. Estrogen receptor (ER) beta1 and ERbetacx/beta2 inhibit ERalpha function differently in breast cancer cell line MCF7. Oncogene. 2003;22(32):5011–20. doi: 10.1038/sj.onc.1206787. [DOI] [PubMed] [Google Scholar]
- 22.Cerillo G, Rees A, Manchanda N, et al. The oestrogen receptor regulates NF[kappa]B and AP-1 activity in a cell-specific manner. J Steroid Biochem Mol Biol. 1998;67(2):79–88. doi: 10.1016/s0960-0760(98)00078-8. [DOI] [PubMed] [Google Scholar]
- 23.Krishnan V, Wang X, Safe S. Estrogen receptor-Sp1 complexes mediate estrogen-induced cathepsin D gene expression in MCF-7 human breast cancer cells. J Biol Chem. 1994;269(22):15912–7. [PubMed] [Google Scholar]
- 24.Walker P, Germond J- E, Brown-Luedi M, Givel Fo, Wahli W. Sequence homologies in the region preceding the transcription initiation site of the liver estrogen-responsive vitellogenin and apo-VLDLII genes. Nucleic Acids Res. 1984;12(22):8611–26. doi: 10.1093/nar/12.22.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Ashry D, Chrysogelos SA, Lippman ME, Kern FG. Estrogen induction of TGF-alpha is mediated by an estrogen response element composed of two imperfect palindromes. J Steroid Biochem Mol Biol. 1996;59(3-4):261–9. doi: 10.1016/s0960-0760(96)00118-5. [DOI] [PubMed] [Google Scholar]
- 26.Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ER beta) of the human estrogen receptor modulates ER alpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140(12):5566–78. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 27.Hanstein B, Eckner R, DiRenzo J, et al. p300 is a component of an estrogen receptor coactivator complex. Proc Natl Acad Sci USA. 1996;93(21):11540–5. doi: 10.1073/pnas.93.21.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor AH, Al-Azzawi F. Immunolocalisation of oestrogen receptor beta in human tissues. J Mol Endocrinol. 2000;24(1):145–55. doi: 10.1677/jme.0.0240145. [DOI] [PubMed] [Google Scholar]
- 29.Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet. 1998;351(9114):1451–67. [PubMed] [Google Scholar]
- 30.Bentzon N, During M, Rasmussen BB, Mouridsen H, Kroman N. Prognostic effect of estrogen receptor status across age in primary breast cancer. Int J Cancer. 2008;122(5):1089–94. doi: 10.1002/ijc.22892. [DOI] [PubMed] [Google Scholar]
- 31.Clarke R, Liu MC, Bouker KB, et al. Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene. 2003;22(47):7316–39. doi: 10.1038/sj.onc.1206937. [DOI] [PubMed] [Google Scholar]
- 32.Hurtado A, Holmes KA, Geistlinger TR, et al. Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature. 2008;456(7222):663–6. doi: 10.1038/nature07483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howell A, DeFriend D, Robertson J, Blamey R, Walton P. Response to a specific antioestrogen (ICI 182780) in tamoxifen-resistant breast cancer. Lancet. 1995;345(8941):29–30. doi: 10.1016/s0140-6736(95)91156-1. [DOI] [PubMed] [Google Scholar]
- 34.Dauvois S, White R, Parker MG. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci. 1993;106(4):1377–88. doi: 10.1242/jcs.106.4.1377. [DOI] [PubMed] [Google Scholar]
- 35.Long X, Nephew KP. Fulvestrant (ICI 182,780)-dependent interacting proteins mediate immobilization and degradation of estrogen receptor-alpha. J Biol Chem. 2006;281(14):9607–15. doi: 10.1074/jbc.M510809200. [DOI] [PubMed] [Google Scholar]
- 36.Ottaviano YL, Issa J- P, Parl FF, Smith HS, Baylin SB, Davidson NE. Methylation of the estrogen receptor gene CpG island marks loss of estrogen receptor expression in human breast cancer cells. Cancer Res. 1994;54(10):2552–5. [PubMed] [Google Scholar]
- 37.Oh AS, Lorant LA, Holloway JN, Miller DL, Kern FG, El-Ashry D. Hyperactivation of MAPK Induces Loss of ER{alpha} Expression in Breast Cancer Cells. Mol Endocrinol. 2001;15(8):1344–59. doi: 10.1210/mend.15.8.0678. [DOI] [PubMed] [Google Scholar]
- 38.Guo S, Sonenshein GE. Forkhead box transcription factor FOXO3a regulates estrogen receptor alpha expression and is repressed by the Her-2/neu/phosphatidylinositol 3-kinase/Akt signaling pathway. Mol Cell Biol. 2004;24(19):8681–90. doi: 10.1128/MCB.24.19.8681-8690.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu J, Zhang L, He T, et al. TWIST represses estrogen receptor-alpha expression by recruiting the NuRD protein complex in breast cancer cells. Int J Biol Sci. 2012;8(4):522–32. doi: 10.7150/ijbs.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas C, Gustafsson JA. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer. 2011;11(8):597–608. doi: 10.1038/nrc3093. [DOI] [PubMed] [Google Scholar]
- 41.Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004;64(1):423–8. doi: 10.1158/0008-5472.can-03-2446. [DOI] [PubMed] [Google Scholar]
- 42.Roger P, Sahla ME, Makela S, Gustafsson JA, Baldet P, Rochefort H. Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Res. 2001;61(6):2537–41. [PubMed] [Google Scholar]
- 43.Strom A, Hartman J, Foster JS, Kietz S, Wimalasena J, Gustafsson JA. Estrogen receptor beta inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci USA. 2004;101(6):1566–71. doi: 10.1073/pnas.0308319100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lazennec G, Bresson D, Lucas A, Chauveau C, Vignon F. ER beta inhibits proliferation and invasion of breast cancer cells. Endocrinology. 2001;142(9):4120–30. doi: 10.1210/endo.142.9.8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tonetti DA, Rubenstein R, DeLeon M, et al. Stable transfection of an estrogen receptor beta cDNA isoform into MDA-MB-231 breast cancer cells. J Steroid Biochem Mol Biol. 2003;87(1):47–55. doi: 10.1016/j.jsbmb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Hou YF, Yuan ST, Li HC, et al. ERbeta exerts multiple stimulative effects on human breast carcinoma cells. Oncogene. 2004;23(34):5799–806. doi: 10.1038/sj.onc.1207765. [DOI] [PubMed] [Google Scholar]
- 47.Shaaban AM, O'Neill PA, Davies MP, et al. Declining estrogen receptor-beta expression defines malignant progression of human breast neoplasia. Am J Surg Pathol. 2003;27(12):1502–12. doi: 10.1097/00000478-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Reich N, Levine AJ. Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature. 1984;5955(308):199–201. doi: 10.1038/308199a0. [DOI] [PubMed] [Google Scholar]
- 49.Hurd C, Khattree N, Alban P, et al. Hormonal Regulation of the p53 Tumor Suppressor Protein in T47D Human Breast Carcinoma Cell Line. J Biol Chem. 1995;270(48):28507–10. doi: 10.1074/jbc.270.48.28507. [DOI] [PubMed] [Google Scholar]
- 50.Hurd C, Khattree N, Dinda S, Alban P, Moudgil V. Regulation of tumor suppressor proteins, p53 and retinoblastoma, by estrogen and antiestrogens in breast cancer cells. Oncogene. 1997;15(8):991–5. doi: 10.1038/sj.onc.1201233. [DOI] [PubMed] [Google Scholar]
- 51.Fernandez-Cuesta L, Anaganti S, Hainaut P, Olivier M. p53 status influences response to tamoxifen but not to fulvestrant in breast cancer cell lines. Int J Cancer. 2010;128(8):1813. doi: 10.1002/ijc.25512. [DOI] [PubMed] [Google Scholar]
- 52.Weige CC, Allred KF, Armstrong CM, Allred CD. P53 mediates estradiol induced activation of apoptosis and DNA repair in non-malignant colonocytes. J Steroid Biochem Mol Biol. 2012;128(3-5):113–20. doi: 10.1016/j.jsbmb.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 53.Fernández-Cuesta L, Anaganti S, Hainaut P, Olivier M. Estrogen levels act as a rheostat on p53 levels and modulate p53-dependent responses in breast cancer cell lines. Breast Cancer Res Treat. 2011;125(1):35–42. doi: 10.1007/s10549-010-0819-x. [DOI] [PubMed] [Google Scholar]
- 54.Berger CE, Qian Y, Liu G, Chen H, Chen X. p53, a Target of Estrogen Receptor (ER) alpha, Modulates DNA Damage-induced Growth Suppression in ER-positive Breast Cancer Cells. J Biol Chem. 2012;287(36):30117–27. doi: 10.1074/jbc.M112.367326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qin C, Nguyen T, Stewart J, Samudio I, Burghardt R, Safe S. Estrogen up-regulation of p53 gene expression in MCF-7 breast cancer cells is mediated by calmodulin kinase IVdependent activation of a nuclear factor kappaB/CCAATbinding transcription factor-1 complex. Mol Endocrinol. 2002;16(8):1793–809. doi: 10.1210/me.2002-0006. [DOI] [PubMed] [Google Scholar]
- 56.Hurd C, Dinda S, Khattree N, Moudgil VK. Estrogendependent and independent activation of the P1 promoter of the p53 gene in transiently transfected breast cancer cells. Oncogene. 1999;18(4):1067–72. doi: 10.1038/sj.onc.1202398. [DOI] [PubMed] [Google Scholar]
- 57.Gu G, Barone I, Gelsomino L, et al. Oldenlandia diffusa extracts exert antiproliferative and apoptotic effects on human breast cancer cells through ERalpha/Sp1-mediated p53 activation. J Cell Physiol. 2012;227(10):3363–72. doi: 10.1002/jcp.24035. [DOI] [PubMed] [Google Scholar]
- 58.Okumura N, Saji S, Eguchi H, Hayashi S, Saji S, Nakashima S. Estradiol stabilizes p53 protein in breast cancer cell line, MCF-7. Jpn J Cancer Res. 2002;93(8):867–73. doi: 10.1111/j.1349-7006.2002.tb01331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Molinari AM, Bontempo P, Schiavone EM, et al. Estradiol induces functional inactivation of p53 by intracellular redistribution. Cancer Res. 2000;60(10):2594–7. [PubMed] [Google Scholar]
- 60.Liu W, Konduri SD, Bansal S, et al. Estrogen receptor-alpha binds p53 tumor suppressor protein directly and represses its function. J Biol Chem. 2006;281(15):9837–40. doi: 10.1074/jbc.C600001200. [DOI] [PubMed] [Google Scholar]
- 61.Yu C- L, Driggers P, Barrera-Hernandez G, Nunez SB, Segars JH. Cheng S-y. The tumor suppressor p53 is a negative regulator of estrogen receptor signaling pathways. Biochem Biophys Res Commun. 1997;239(2):617–20. doi: 10.1006/bbrc.1997.7522. [DOI] [PubMed] [Google Scholar]
- 62.Liu G, Schwartz JA, Brooks SC. Estrogen receptor protects p53 from deactivation by human double minute-2. Cancer Res. 2000;60(7):1810–4. [PubMed] [Google Scholar]
- 63.Lill NL, Grossman SR, Ginsberg D, DeCaprio J, Livingston DM. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997;387(6635):823–7. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
- 64.Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90(4):595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 65.Liu G, Schwartz JA, Brooks SC. p53 Down-Regulates ER Responsive Genes by Interfering with the Binding of ER to ERE. Biochem Biophys Res Commun. 1999;264(2):359–64. doi: 10.1006/bbrc.1999.1525. [DOI] [PubMed] [Google Scholar]
- 66.Konduri SD, Medisetty R, Liu W, et al. Mechanisms of estrogen receptor antagonism toward p53 and its implications in breast cancer therapeutic response and stem cell regulation. Proc Natl Acad Sci USA. 2010;107(34):15081–6. doi: 10.1073/pnas.1009575107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sayeed A, Konduri SD, Liu W, Bansal S, Li F, Das GM. Estrogen receptor alpha inhibits p53-mediated transcriptional repression: implications for the regulation of apoptosis. Cancer Res. 2007;67(16):7746–55. doi: 10.1158/0008-5472.CAN-06-3724. [DOI] [PubMed] [Google Scholar]
- 68.Liu W, Ip MM, Podgorsak MB, Das GM. Disruption of estrogen receptor α-p53 interaction in breast tumors: a novel mechanism underlying the anti-tumor effect of radiation therapy. Breast Cancer Res Treat. 2008;115(1):43–50. doi: 10.1007/s10549-008-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bailey ST, Shin H, Westerling T, Liu XS, Brown M. Estrogen receptor prevents p53-dependent apoptosis in breast cancer. Proc Natl Acad Sci USA. 2012;109(44):18060–5. doi: 10.1073/pnas.1018858109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Menendez D, Inga A, Resnick MA. Estrogen receptor acting in cis enhances WT and mutant p53 transactivation at canonical and noncanonical p53 target sequences. Proc Natl Acad Sci USA. 2010;107(4):1500–5. doi: 10.1073/pnas.0909129107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ciribilli Y, Andreotti V, Menendez D, et al. The Coordinated P53 and Estrogen Receptor Cis-Regulation at an FLT1 Promoter SNP Is Specific to Genotoxic Stress and Estrogenic Compound. PLoS One. 2010;5(4):e10236. doi: 10.1371/journal.pone.0010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perillo B, Sasso A, Abbondanza C, Palumbo G. 17beta-estradiol inhibits apoptosis in MCF-7 cells, inducing bcl-2 expression via two estrogen-responsive elements present in the coding sequence. Mol Cell Biol. 2000;20(8):2890–901. doi: 10.1128/mcb.20.8.2890-2901.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perillo B, Sasso A, Abbondanza C, Palumbo G. 17beta Estradiol Inhibits Apoptosis in MCF-7 Cells, Inducing bcl-2 Expression via Two Estrogen-Responsive Elements Present in the Coding Sequence. Mol Cell Biol. 2000;20(8):2890–901. doi: 10.1128/mcb.20.8.2890-2901.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thomas CG, Strom A, Lindberg K, Gustafsson JA. Estrogen receptor beta decreases survival of p53-defective cancer cells after DNA damage by impairing G(2)/M checkpoint signaling. Breast Cancer Res Treat. 2011;127(2):417–27. doi: 10.1007/s10549-010-1011-z. [DOI] [PubMed] [Google Scholar]
- 75.Cotrim CZ, Fabris V, Doria ML, et al. Estrogen receptor beta growth-inhibitory effects are repressed through activation of MAPK and PI3K signalling in mammary epithelial and breast cancer cells. Oncogene. 2013;32(19):2390–402. doi: 10.1038/onc.2012.261. [DOI] [PubMed] [Google Scholar]
- 76.Hartman J, Edvardsson K, Lindberg K, et al. Tumor repressive functions of estrogen receptor beta in SW480 colon cancer cells. Cancer Res. 2009;69(15):6100–6. doi: 10.1158/0008-5472.CAN-09-0506. [DOI] [PubMed] [Google Scholar]
- 77.Hsu HH, Cheng SF, Wu CC, et al. Apoptotic effects of over-expressed estrogen receptor-beta on LoVo colon cancer cell is mediated by p53 signalings in a ligand-dependent manner. Chin J Physiol. 2006;49(2):110–6. [PubMed] [Google Scholar]
- 78.Lewandowski SA, Thiery J, Jalil A, Leclercq G, Szczylik C, Chouaib S. Opposite effects of estrogen receptors alpha and beta on MCF-7 sensitivity to the cytotoxic action of TNF and p53 activity. Oncogene. 2005;24(30):4789–98. doi: 10.1038/sj.onc.1208595. [DOI] [PubMed] [Google Scholar]
- 79.Klein-Hitpass L, Ryffel GU, Heitlinger E, Cato ACB. A 13 bp palindrome is a functional estrogen responsive element and interacts specifically with estrogen receptor. Nucleic Acids Res. 1988;16(2):647–63. doi: 10.1093/nar/16.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haldar S, Negrini M, Monne M, Sabbioni S, Croce CM. Down-Regulation of bcl-2 by p53 in Breast Cancer Cells. Cancer Res. 1994;54(8):2095–7. [PubMed] [Google Scholar]
- 81.Jin W, Chen Y, Di GH, et al. Estrogen receptor (ER) beta or p53 attenuates ERalpha-mediated transcriptional activation on the BRCA2 promoter. J Biol Chem. 2008;283(44):29671–80. doi: 10.1074/jbc.M802785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kunz C, Pebler S, Otte, Ahe von der D. Differential regulation of plasminogen activator and inhibitor gene transcription by the tumor suppressor p53. Nucleic Acids Res. 1995;23(18):3710–7. doi: 10.1093/nar/23.18.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Santhanam U, Ray A, Sehgal PB. Repression of the interleukin 6 gene promoter by p53 and the retinoblastoma susceptibility gene product. Proc Natl Acad Sci USA. 1991;88(17):7605–9. doi: 10.1073/pnas.88.17.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Teixeira C, Reed JC, Pratt MAC. Estrogen Promotes Chemotherapeutic Drug Resistance by a Mechanism Involving Bcl-2 Proto-Oncogene Expression in Human Breast Cancer Cells. Cancer Res. 1995;55(17):3902–7. [PubMed] [Google Scholar]
- 85.Angeloni SV, Martin MB, Garcia-Morales P, Castro-Galache MD, Ferragut JA, Saceda M. Regulation of estrogen receptor-alpha expression by the tumor suppressor gene p53 in MCF-7 cells. J Endocrinol. 2004;180(3):497–504. doi: 10.1677/joe.0.1800497. [DOI] [PubMed] [Google Scholar]
- 86.Shirley SH, Rundhaug JE, Tian J, et al. Transcriptional regulation of estrogen receptor-alpha by p53 in human breast cancer cells. Cancer Res. 2009;69(8):3405–14. doi: 10.1158/0008-5472.CAN-08-3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.MacMahon B, Cole P, Lin TM, et al. Age at first birth and breast cancer risk. Bull World Health Organ. 1970;43(2):209–21. [PMC free article] [PubMed] [Google Scholar]
- 88.Marchant J. Influence of pregnancy and lactation on the incidence of mammary carcinoma induced with methylcholanthrene in female mice of the "IF" strain. J Pathol Bacteriol. 1955;70(2):415–8. doi: 10.1002/path.1700700218. [DOI] [PubMed] [Google Scholar]
- 89.Medina D, Smith GH. Chemical Carcinogen-Induced Tumorigenesis in Parous, Involuted Mouse Mammary Glands. J Natl Cancer Inst. 1999;91(11):967–9. doi: 10.1093/jnci/91.11.967. [DOI] [PubMed] [Google Scholar]
- 90.Moon R. Relationship between previous reproductive history and chemically induced mammary cancer in rats. Int J Cancer. 1969;4(3):312–7. doi: 10.1002/ijc.2910040308. [DOI] [PubMed] [Google Scholar]
- 91.Becker KA, Lu S, Dickinson ES, et al. Estrogen and progesterone regulate radiation-induced p53 activity in mammary epithelium through TGF-[beta]-dependent pathways. Oncogene. 2005;24(42):6345–53. doi: 10.1038/sj.onc.1208787. [DOI] [PubMed] [Google Scholar]
- 92.Huggins C, Moon RC, Morii S. Extinction of experimental mammary cancer, I. estradiol-17β and progesterone. Proc Natl Acad Sci USA. 1962;48(3):379–86. doi: 10.1073/pnas.48.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sivaraman L, Conneely OM, Medina D, O'Malley BW. p53 is a potential mediator of pregnancy and hormone-induced resistance to mammary carcinogenesis. Proc Natl Acad Sci USA. 2001;98(22):12379–84. doi: 10.1073/pnas.221459098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Díaz-Cruz ES, Furth PA. Deregulated Estrogen Receptor alpha and p53 Heterozygosity Collaborate in the Development of Mammary Hyperplasia. Cancer Res. 2010;70(10):3965–74. doi: 10.1158/0008-5472.CAN-09-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shai A, Pitot HC, Lambert PF. p53 Loss synergizes with estrogen and papillomaviral oncogenes to induce cervical and breast cancers. Cancer Res. 2008;68(8):2622–31. doi: 10.1158/0008-5472.CAN-07-5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lønning PE, Knappskog S, Staalesen V, Chrisanthar R, Lillehaug JR. Breast cancer prognostication and prediction in the postgenomic era. Ann Oncol. 2007;18(8):1293–306. doi: 10.1093/annonc/mdm013. [DOI] [PubMed] [Google Scholar]
- 97.Banin S, Moyal L, Shieh SY, et al. Enhanced Phosphorylation of p53 by ATM in Response to DNA Damage. Science. 1998;281(5383):1674–7. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 98.Phelan CM, Borg A, Cuny M, et al. Consortium Study on 1280 Breast Carcinomas: Allelic Loss on Chromosome 17 Targets Subregions Associated with Family History and Clinical Parameters. Cancer Res. 1998;58(5):1004–12. [PubMed] [Google Scholar]
- 99.Callahan R, Cropp C, Merlo G, et al. Genetic and molecular heterogeneity of breast cancer cells. Clin Chim Acta. 1993;217(1):63–73. doi: 10.1016/0009-8981(93)90238-y. [DOI] [PubMed] [Google Scholar]
- 100.Osborne RJ, Hamshere MG. A Genome-wide Map Showing Common Regions of Loss of Heterozygosity/Allelic Imbalance in Breast Cancer. Cancer Res. 2000;60(14):3706–12. [PubMed] [Google Scholar]
- 101.Cunningham JM, Ingle JN, Jung SH, et al. p53 Gene Expression in Node-Positive Breast Cancer: Relationship to DNA Ploidy and Prognosis. J Natl Cancer Inst. 1994;86(24):1871–3. doi: 10.1093/jnci/86.24.1871. [DOI] [PubMed] [Google Scholar]
- 102.Elledge RM, Green S, Howes L, et al. bcl-2, p53, and response to tamoxifen in estrogen receptor-positive metastatic breast cancer: a Southwest Oncology Group study. J Clin Oncol. 1997;15(5):1916–22. doi: 10.1200/JCO.1997.15.5.1916. [DOI] [PubMed] [Google Scholar]
- 103.Gretarsdottir S, Tryggvadottir L, Jonasson J, et al. TP53 mutation analyses on breast carcinomas: a study of paraffin embedded archival material. Br J Cancer. 1996;74(4):555–61. doi: 10.1038/bjc.1996.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lipponen P, Ji H, Aaltomaa S, Syrjänen S, Syrjänen K. p53 protein expression in breast cancer as related to histopathological characteristics and prognosis. Int J Cancer. 1993;55(1):51–6. doi: 10.1002/ijc.2910550110. [DOI] [PubMed] [Google Scholar]
- 105.MacGrogan G, Bonichon F, de Mascarel I, et al. Prognostic value of p53 in breast invasive ductal carcinoma: an immunohistochemical study on 942 cases. Breast Cancer Res Treat. 1995;36(1):71–81. doi: 10.1007/BF00690187. [DOI] [PubMed] [Google Scholar]
- 106.Olivier M, Langerød A, Carrieri P, et al. The clinical value of somatic TP53 gene mutations in 1,794 patients with breast cancer. Clin Cancer Res. 2006;12(4):1157–67. doi: 10.1158/1078-0432.CCR-05-1029. [DOI] [PubMed] [Google Scholar]
- 107.Love RR, Mazess RB, Barden HS, et al. Effects of Tamoxifen on Bone Mineral Density in Postmenopausal Women with Breast Cancer. N Engl J Med. 1992;326(13):852–6. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 108.Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor Dynamics and Sufficiency in Estrogen Receptor-Regulated Transcription. Cell. 2000;103(6):843–52. doi: 10.1016/s0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 109.Jackson TA, Richer JK, Bain DL, Takimoto GS, Tung L, Horwitz KB. The Partial Agonist Activity of Antagonist-Occupied Steroid Receptors Is Controlled by a Novel Hinge Domain-Binding Coactivator L7/SPA and the Corepressors N-CoR or SMRT. Mol Endocrinol. 1997;11(6):693–705. doi: 10.1210/mend.11.6.0004. [DOI] [PubMed] [Google Scholar]
- 110.Frasor J, Chang EC, Komm B, et al. Gene Expression Preferentially Regulated by Tamoxifen in Breast Cancer Cells and Correlations with Clinical Outcome. Cancer Res. 2006;66(14):7334–40. doi: 10.1158/0008-5472.CAN-05-4269. [DOI] [PubMed] [Google Scholar]
- 111.Love RR. Tamoxifen therapy in primary breast cancer: biology, efficacy, and side effects. J Clin Oncol. 1989;7(6):803–15. doi: 10.1200/JCO.1989.7.6.803. [DOI] [PubMed] [Google Scholar]
- 112.Ichikawa A, Ando J, Suda K. G1 arrest and expression of cyclin-dependent kinase inhibitors in tamoxifen-treated MCF-7 human breast cancer cells. Hum Cell. 2008;21(2):28–37. doi: 10.1111/j.1749-0774.2008.00048.x. [DOI] [PubMed] [Google Scholar]
- 113.Varma H, Conrad SE. Reversal of an antiestrogen-mediated cell cycle arrest of MCF-7 cells by viral tumor antigens requires the retinoblastoma protein-binding domain. Oncogene. 2000;19(41):4746–53. doi: 10.1038/sj.onc.1203827. [DOI] [PubMed] [Google Scholar]
- 114.Bergh J, Norberg T, Sjögren S, Lindgren A, Holmberg L. Complete sequencing of the p53 gene provides prognostic information in breast cancer patients, particularly in relation to adjuvant systemic therapy and radiotherapy. Nat Med. 1995;1(10):1029–34. doi: 10.1038/nm1095-1029. [DOI] [PubMed] [Google Scholar]
- 115.Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-Receptor Status and Outcomes of Modern Chemotherapy for Patients With Node-Positive Breast Cancer. JAMA. 2006;295(14):1658–67. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Planas-Silva MD, Weinberg RA. Estrogen-dependent cyclin E-cdk2 activation through p21 redistribution. Mol Cell Biol. 1997;17(7):4059–69. doi: 10.1128/mcb.17.7.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Prall OWJ, Sarcevic B, Musgrove EA, Watts CKW, Sutherland RL. Estrogen-induced Activation of Cdk4 and Cdk2 during G1-S Phase Progression Is Accompanied by Increased Cyclin D1 Expression and Decreased Cyclin-dependent Kinase Inhibitor Association with Cyclin E-Cdk2. J Biol Chem. 1997;272(16):10882–94. doi: 10.1074/jbc.272.16.10882. [DOI] [PubMed] [Google Scholar]
- 118.Watts CK, Brady A, Sarcevic B, deFazio A, Musgrove EA, Sutherland RL. Antiestrogen inhibition of cell cycle progression in breast cancer cells in associated with inhibition of cyclin-dependent kinase activity and decreased retinoblastoma protein phosphorylation. Mol Endocrinol. 1995;9(12):1804–13. doi: 10.1210/mend.9.12.8614416. [DOI] [PubMed] [Google Scholar]
- 119.Berns EMJJ Foekens JA, Vossen R, et al. Complete Sequencing of TP53 Predicts Poor Response to Systemic Therapy of Advanced Breast Cancer. Cancer Res. 2000;60(8):2155–62. [PubMed] [Google Scholar]
- 120.Archer S, Eliopoulos A, Spandidos D, et al. Expression of ras p21, p53 and c-erbB-2 in advanced breast cancer and response to first line hormonal therapy. Br J Cancer. 1995;72(5):1259–66. doi: 10.1038/bjc.1995.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lim LY, Vidnovic N, Ellisen LW, Leong CO. Mutant p53 mediates survival of breast cancer cells. Br J Cancer. 2009;101(9):1606–12. doi: 10.1038/sj.bjc.6605335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Castle PE, Solomon D, Schiffman M, Wheeler CM. Human Papillomavirus Type 16 Infections and 2-Year Absolute Risk of Cervical Precancer in Women With Equivocal or Mild Cytologic Abnormalities. J Natl Cancer Inst. 2005;97(14):1066–71. doi: 10.1093/jnci/dji186. [DOI] [PubMed] [Google Scholar]
- 123.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16(1):1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Moreno V, Bosch FX, Muñoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359(9312):1085–92. doi: 10.1016/S0140-6736(02)08150-3. [DOI] [PubMed] [Google Scholar]
- 125.Brake T, Lambert PF. Estrogen contributes to the onset, persistence, and malignant progression of cervical cancer in a human papillomavirus-transgenic mouse model. Proc Natl Acad Sci USA. 2005;102(7):2490–5. doi: 10.1073/pnas.0409883102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Park JS, Rhyu JW, Kim CJ, et al. Neoplastic change of squamo-columnar junction in uterine cervix and vaginal epithelium by exogenous estrogen in hpv-18 URR E6/E7 transgenic mice. Gynecol Oncol. 2003;89(3):360–8. doi: 10.1016/s0090-8258(02)00106-3. [DOI] [PubMed] [Google Scholar]
- 127.Chung SH, Wiedmeyer K, Shai A, Korach KS, Lambert PF. Requirement for estrogen receptor alpha in a mouse model for human papillomavirus-associated cervical cancer. Cancer Res. 2008;68(23):9928–34. doi: 10.1158/0008-5472.CAN-08-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Au WW, Abdou-Salama S, Al-Hendy A. Inhibition of growth of cervical cancer cells using a dominant negative estrogen receptor gene. Gynecol Oncol. 2007;104(2):276–80. doi: 10.1016/j.ygyno.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Riley RR, Duensing S, Brake T, Munger K, Lambert PF, Arbeit JM. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003;63(16):4862–71. [PubMed] [Google Scholar]
- 130.Shai A, Brake T, Somoza C, Lambert PF. The human papillomavirus E6 oncogene dysregulates the cell cycle and contributes to cervical carcinogenesis through two independent activities. Cancer Res. 2007;67(4):1626–35. doi: 10.1158/0008-5472.CAN-06-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bernstein L, Deapen D, Cerhan JR, et al. Tamoxifen therapy for breast cancer and endometrial cancer risk. J Natl Cancer Inst. 1999;91(19):1654–62. doi: 10.1093/jnci/91.19.1654. [DOI] [PubMed] [Google Scholar]
- 132.Fisher B, Costantino JP, Redmond CK, Fisher ER, Wickerham DL, Cronin WM. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994;86(7):527–37. doi: 10.1093/jnci/86.7.527. [DOI] [PubMed] [Google Scholar]
- 133.Bergman L, Beelen ML, Gallee MP, Hollema H, Benraadt J, van Leeuwen FE. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres' ALERT Group. Assessment of Liver and Endometrial cancer Risk following Tamoxifen. Lancet. 2000;356(9233):881–7. doi: 10.1016/s0140-6736(00)02677-5. [DOI] [PubMed] [Google Scholar]
- 134.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 135.Stygar D, Muravitskaya N, Eriksson B, Eriksson H, Sahlin L. Effects of SERM (selective estrogen receptor modulator) treatment on growth and proliferation in the rat uterus. Reprod Biol Endocrinol. 2003;1:40. doi: 10.1186/1477-7827-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hoogendoorn WE, Hollema H, van Boven HH, et al. Prognosis of uterine corpus cancer after tamoxifen treatment for breast cancer. Breast Cancer Res Treat. 2008;112(1):99–108. doi: 10.1007/s10549-007-9823-1. [DOI] [PubMed] [Google Scholar]
- 137.Fujiwara K, Enomoto T, Fujita M, et al. Alterations of the K-ras and p53 genes in Tamoxifen-associated endometrial carcinoma. Oncol Rep. 2008;19(5):1293–8. [PubMed] [Google Scholar]
- 138.Niwa K, Morishita S, Hashimoto M, et al. Effects of Tamoxifen on Endometrial Carcinogenesis in Mice. Jpn J Cancer Res. 1998;89(5):502–9. doi: 10.1111/j.1349-7006.1998.tb03290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Maeda K, Tsuda H, Hashiguchi Y, et al. Relationship between p53 pathway and estrogen receptor status in endometrioid-type endometrial cancer. Hum Pathol. 2002;33(4):386–91. doi: 10.1053/hupa.2002.124720. [DOI] [PubMed] [Google Scholar]
- 140.Niwa K, Murase T, Morishita S, Hashimoto M, Itoh N, Tamaya T. p53 overexpression and mutation in endometrial carcinoma: inverted relation with estrogen and progesterone receptor status. Cancer Detect Prev. 1999;23(2):147–54. doi: 10.1046/j.1525-1500.1999.09909.x. [DOI] [PubMed] [Google Scholar]
- 141.Froggatt NJ, Green J, Brassett C, et al. A common MSH2 mutation in English and North American HNPCC families: origin, phenotypic expression, and sex specific differences in colorectal cancer. J Med Genet. 1999;36(2):97–102. [PMC free article] [PubMed] [Google Scholar]
- 142.English MA, Stewart PM, Hewison M. Estrogen metabolism and malignancy: analysis of the expression and function of 17beta-hydroxysteroid dehydrogenases in colonic cancer. Mol Cell Endocrinol. 2001;171(1-2):53–60. doi: 10.1016/s0303-7207(00)00418-4. [DOI] [PubMed] [Google Scholar]
- 143.Weiss N, Daling J, Chow W. Incidence of cancer of the large bowel in women in relation to reproductive and hormonal factors. J Natl Cancer Inst. 1981;67(1):57–60. [PubMed] [Google Scholar]
- 144.Kampman E, Potter JD, Slattery ML, Caan BJ, Edwards S. Hormone replacement therapy, reproductive history, and colon cancer: a multicenter, case-control study in the United States. Cancer Causes Control. 1997;8(2):146–58. doi: 10.1023/a:1018459911147. [DOI] [PubMed] [Google Scholar]
- 145.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350(10):991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 146.Fiorelli G, Picariello L, Martineti V, Tonelli F, Brandi ML. Functional estrogen receptor beta in colon cancer cells. Biochem Biophys Res Commun. 1999;261(2):521–7. doi: 10.1006/bbrc.1999.1062. [DOI] [PubMed] [Google Scholar]
- 147.Campbell-Thompson M, Lynch IJ, Bhardwaj B. Expression of Estrogen Receptor (ER) Subtypes and ERbeta Isoforms in Colon Cancer. Cancer Res. 2001;61(2):632–40. [PubMed] [Google Scholar]
- 148.Konstantinopoulos PA, Kominea A, Vandoros G, et al. Oestrogen receptor beta (ER[beta]) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour's dedifferentiation. Eur J Cancer. 2003;39(9):1251–8. doi: 10.1016/s0959-8049(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 149.Saleiro D, Murillo G, Benya RV, Bissonnette M, Hart J, Mehta RG. Estrogen receptor-beta protects against colitis-associated neoplasia in mice. Int J Cancer. 2012;131(11):2553–61. doi: 10.1002/ijc.27578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Martineti V, Picariello L, Tognarini I, et al. ERbeta is a potent inhibitor of cell proliferation in the HCT8 human colon cancer cell line through regulation of cell cycle components. Endocr Relat Cancer. 2005;12(2):455–69. doi: 10.1677/erc.1.00861. [DOI] [PubMed] [Google Scholar]