Abstract
As the nanotechnology field continues to develop, assessing nanoparticle toxicity is very important for advancing nanoparticles for biomedical application. Here we report cytotoxicity of gold nanomaterial of different size and shape using MTT test, absorption spectroscopy and TEM. Spherical gold nanoparticles with different sizes are not inherently toxic to human skin cells, but gold nanorods are highly toxic due to the presence of CTAB as coating material. Due to CTAB toxicity, and aggregation of gold nanomaterials in the presence of cell media, it is a real challenge to study the cytotoxicity of gold nanomaterials individually.
Introduction
The nanoscience revolution that sprouted throughout the 1990s is having promises to great benefits for society 1–24. Since their size scale is similar to that of biological molecules (e.g., proteins, DNA) and structures (e.g., viruses and bacteria), enormous interest is growing to exploit nanoparticles for various biomedical applications 1–24. Due to valuable size and shape dependent properties, gold nanoparticles have been exploited as tools in biology for the last fifteen years 1–24. Their high electron density has made them popular labels in electron microscopy, and because their surface properties enable a great variety of functionalization procedures. Having extremely small size with highest possible surface area enables them to access a variety of biological environments. Those advantages, along with the universal biocompatibility, gold nanoparticles promise as vehicles for drug and gene delivery. Furthermore, gold nanoparticles have recently been demonstrated in cell imaging, targeted drug delivery, and cancer diagnostics and therapeutic applications 4–24. As nanotechnology field continues to develop, studies on the cytotoxicity of nanoparticles, with respect to their size and shape, are required in order to advance nanotechnology for biomedical applications. This will be important for assessing nanoparticle toxicity, for advancing nanoparticles for imaging, drug delivery, and therapeutic applications and for designing multifunctional nanoparticles. Though nanoparticle production has been estimated to increase from 2,300 tons produced today to 58,000 tons by 2020, it is surprising that knowledge on the toxicity effect of nanoparticle exposure is in infancy and also reported results are confusing 25–30. Driven by the need, we report the systematic study of cytotoxicity of gold nanomaterial of different size and shape and demonstrate that it is a real challenge to understand the toxicity of nanomaterials.
Experimental
Gold nanoparticles of different particle sizes were synthesized using the citrate reduction method as reported recently 9–16.
Transmission electron microscope (TEM) images and UV-Vis absorption spectra were used to characterize the nanoparticles, as we reported recently 9–16. The particle concentration was measured by UV-Vis spectroscopy using molar extinction coefficients at the wavelength of maximum absorption of each gold colloid as reported recently [ε(15) 518nm = 3.6 × 108 cm−1 M−1, ε (30) 530nm = 3.0 × 109 cm−1 M−1, ε (40) 533nm = 6.7 × 109 cm−1 M−1, ε (50) 535nm = 1.5 × 1010 cm−1 M−1, ε (60) 540nm = 2.9 × 1010 cm−1 M−1, and ε (80) 550nm = 6.9 × 1010 cm−1 M−1]9–16.
Gold nanorods were synthesized using a seed-mediated, surfactant-assisted growth method in a two-step procedure 10,14,16,23. Colloidal gold seeds (~1.5 nm diameter) were first prepared by mixing aqueous solutions of hexadecylcetyltrimethylammonium bromide (CTAB, 0.1 M, 4.75 mL) and HAuCl4 (0.01 M, 0.2 mL) followed by addition of an aqueous solution of NaBH4 (0.01 M, 0.6 mL). The colloidal gold seeds were then injected into an aqueous growth solution of CTAB (0.1 M, 4.75 mL), silver nitrate (0.01 M, varying amounts of silver nitrate between 20 and 120 mL were used to achieve the desired nanorod aspect ratio), HAuCl4 (0.01 M, 0.2 mL), and ascorbic acid (0.1 M, 0.032 mL). Nanorods were purified by several cycles of suspension in ultrapure water, followed by centrifugation. Nanorods were isolated in the precipitate, and excess CTAB was removed in the supernatant. Nanorods were characterized by TEM and absorption spectroscopy as shown in Figure 1.
Figure 1.
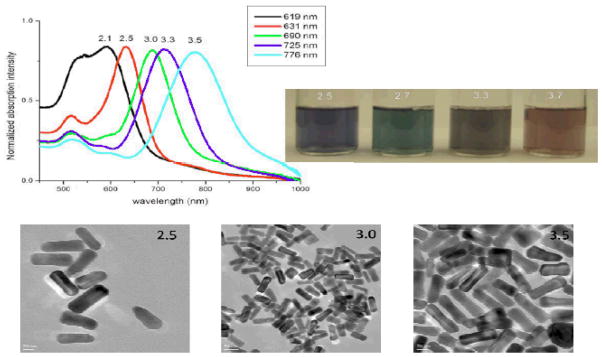
Absorption spectra, photograph and TEM images of gold nanorod of different aspect ratios
To explore the cytotoxicity of gold nonmaterials, we have used human skin cell line, HaCaT keratinocytes, a transformed human epidermal cell line obtained from Dr. Norbert Fusening of the Germany Cancer Research Center, Heidelberg, Germany. The HaCaT cells were grown in Dulbecco’s Modified Eagle’s Medicum (DMEM) with 10% FBS in 25 cm2 culture flasks cultured in a humidified incubator with 5% CO2 at 37°C. After cells grew to the desired concentration, they were centrifuged at 2000 rpm for 5 min. The cells were washed twice with 1× PBS and resuspended in DMEM to reach a final cell concentration of 1 × 105 cells/mL for further treatment. After treating the cells with gold nanomaterials at different time intervals, the cell viability was determined using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Viable cells are capable of metabolizing MTT while dead cells are not. 10 μL of nanomaterial were added in 90 μL cell media and incubated for 24 hours at 37°C with 5% CO2. After treatment, the medium was removed and the culture wells were washed in PBS buffer. Then we added 50 μL of 5 mg/mL MTT solution and incubated for 60 min. The cells were then centrifuged and the suspension was discarded. 200 μL DMSO was added to each well and incubated for 10 min. The absorbance at 540 nm was recorded using Multiskan Ascent Plate Reader with the Ascent software (Labsystems).
Results and Discussion
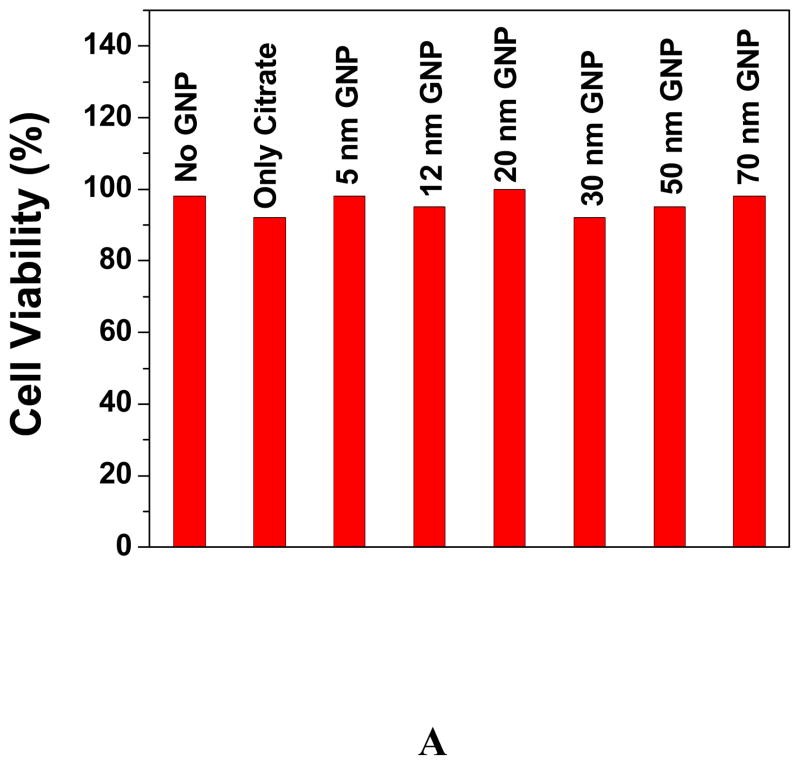
The cell viabilities for gold nanoparticle with different sizes are shown in Figure 2. Chithrani et. al.29 have measured the intracellular uptake of different sizes and shapes of colloidal gold nanoparticles. Their results show that gold nanoparticles and nanorods are capable of entering the cells. The cellular uptake increases with incubation time until it is saturated at eight hours.
Figure 2.
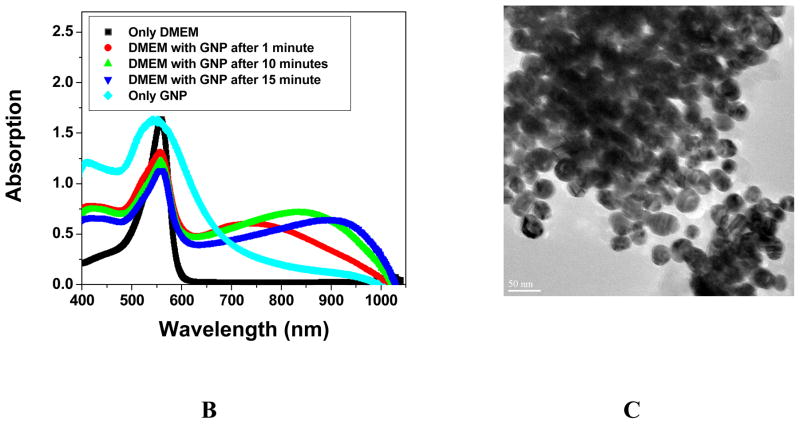
Figure 2A: Viability of cells incubated with gold nanoparticle of different sizes, 2B: Time dependents absorption change in the presence DMEM of 30 nm gold nanoparticle and 2C: TEM image for gold nanoparticle in the presence of cell media.
To make sure that cellular uptake reaches maximum, all our results are for 24 hr incubation. As shown in Figure 2A, no difference was found in cell viability between cell with and without gold nanoparticles of different sizes. This indicates that spherical gold nanoparticles of different sizes are not inherently toxic to human cells at our test concentration. To further confirm this, we also continuously exposed the cell for 48 hr and no toxicity was observed. Connor et. al. 28 and Takahasi et. al. 30 have shown that nanoparticles or nanorods are usually clustered inside the cell. They concluded that cell death might be the results of a harmful influence by the aggregates. To understand whether gold nanomaterials are only aggregated inside the cell or may be aggregated outside the cell and then entered as aggregate, we did a time dependence study of gold nanoparticle’s surface plasmon resonance band (SPB) between 510–570 nm. The SPB origin is attributed to the collective oscillation of the free conduction electrons induced by an interacting electromagnetic field. These resonances are also denoted as surface plasmons. Our experiments indicate that the shift in plasmon band energy to longer wavelengths by about 150–400 nm (Figure 2B) in the presence of DMEM, which indicated strong aggregation of gold nanoparticles (TEM image, Figure 2C). This aggregation is due to the presence of high concentration of sodium salt in DMEM. To understand whether other components of DMEM media are also responsible for aggregation, we performed aggregation experiment with simulated media without sodium salt. We have not observed any aggregation even after 24 hours. Even experiments with different sodium salt concentration indicate that for simulated media with sodium salt concentrations less than 0.1 M, there was no aggregation. Our experiments show that the plasmon band shifted to longer wavelengths with time, indicating bigger cluster formation. Therefore, our results show that nanoparticles aggregated outside the cell and may enter the cell as aggregated form. It is known that unique properties of nanoparticles changed as it formed bigger clusters and, as a result, our experiments indicate that it is really challenging to study the gold nanoparticle toxicity individually in cell media.
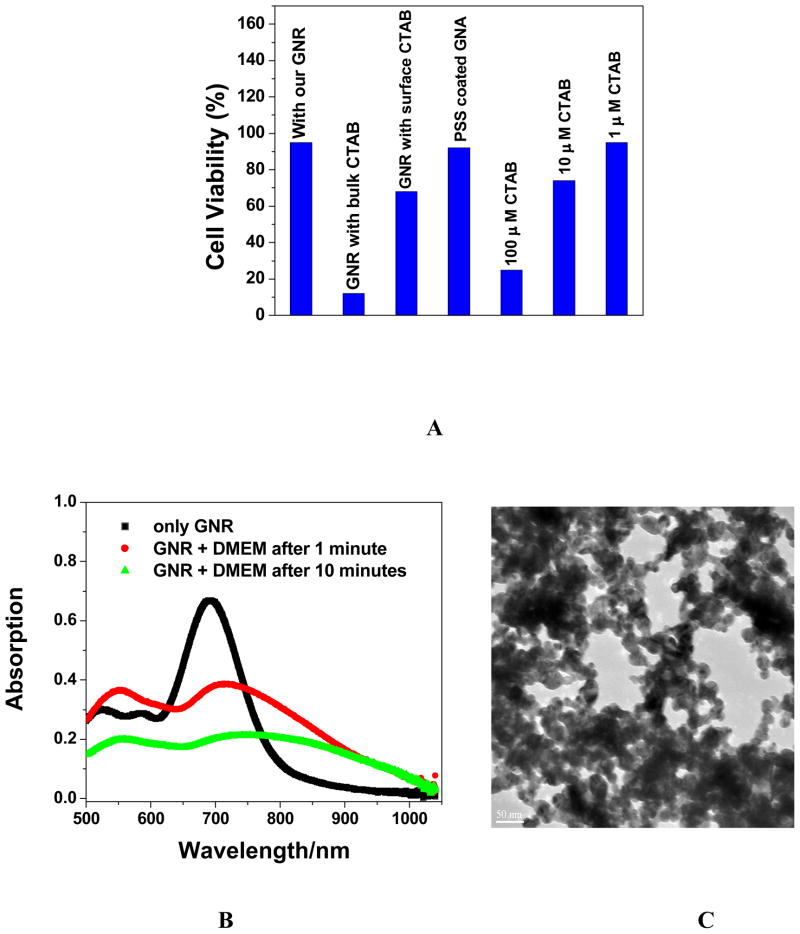
Figure 3A shows the cell viabilities for gold nanorods of aspect ratio 3. Our experiment indicates that gold nanorod is highly toxic. It was surprising that gold nanoparticles are not toxic, whereas gold nanorods exhibit very high toxicity, though gold nanorods are synthesized from gold nanoparticles of small size using a seed-mediated, surfactant-assisted growth method. CTAB is a unique surfactant towards the synthesis of nanorods and is widely used. CTAB selectively forms a tightly packed bilayer on the side faces of nanorods, which leads the ends of the rods to be more exposed to facilitate anisotropic growth along the longitudinal axis. Since the extra chemical present in gold nanorod that is not in gold nanoparticle is CTAB, we studied the toxicity of CTAB (Figure 3A).
Figure 3.
Figure 3A: Viability of cells incubated with gold nanorod (GNR, aspect ratio 3.0) with CTAB, only CTAB and PSS coated GNR, 3B: Time dependents absorption change in the presence of DMEM for gold nanorod with aspect ratio 3.0 and 3C: TEM data for gold nanorod in presence of cell media.
Our results indicate that CTAB alone shows similar toxicity at the concentration of 100 μM. Concentration dependent study shows that CTAB is toxic even at 10 μM concentration (data not shown in Figure 3A). In gold nanorods, there are nanorod-bound CTAB as well as excess free CTAB in solution, which have not been used during nanorod formation. The excess CTAB left in the solution can be removed by centrifugation. Our data indicate that cell viability increases with centrifugation. As we discussed before we started with 0.1 M, 4.75 ml, CTAB for gold nanorod synthesis. After nanorod formed the total volume was 120 ml (CTAB diluted 25 times). To remove the excess CTAB from the solution, we centrifuged several times. For each centrifugation we have diluted nanorod solution 10 times. So after three centrifugations, we have diluted CTAB around 25000 times. Since we do not know how much CTAB has been used for nanorod formation, we estimated maximum CTAB in solution is < .1 μM. CTAB-coated nanorods are positively charged. Their mutual repulsion prevents aggregation so that gold nanorods are stable in solution even for several months.
As shown in Figure 3A, 1 μM CTAB is not toxic. Therefore, we do not expect toxicity after three centrifugations. Our experimental data indicate there is 40% cell death even after three centrifugations (Figure 3A). We believe this is due to the fact that gold nanorod-bound CTAB layer will eventually enter solution in the presence of cell media due to aggregation of nanorods as shown in Figure 3B and 3C. This is due to the screening effect of the sodium salts present in DMEM, leading to more linked particles and hence larger damping of the surface plasmon absorption of Au nanorod surfaces. The aggregated gold nanorods in cell media make it difficult to monitor their toxicity. We tried to remove further CTAB from the nanorod-bound CTAB layer by performing 4–5 times centrifugation, and our experimental results shows that nanorods are not stable if CTAB is removed further by centrifugation more than 3 times. We believe that this is due to the lack of repulsive interaction among individual nanorods and, as a result, nanorods break to nanoparticles and some nanorods undergo aggregation.
To protect from CTAB which is bound with gold nanorod, we have exposed the nanoparticles to poly(styrenesulfonate) (PSS) polyelectrolyte solution. The extra PSS in solution was separated by centrifugation of the nanorod solution at 8000 rpm. The pellet was redispersed in N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES) solution. The nanorods prepared by seed-mediated surfactant (CTAB) methods are positively charged. Our zeta potential measurement using Zetasizer NanoZS shows that positively charged surface (Zeta potential 58.7 mV) of the nanorods changed to a negatively charged surface (Zeta potential −69.8 mV) when exposed to PSS. Absorption spectra measurement indicate that λmax remains about same after PSS coating, which indicate there are no aggregation during coating process, which also confirmed by TEM experiment. Our TEM data indicated that the PSS coated outside the CTAB bilayer (as shown in figure 4A)
Figure 4.
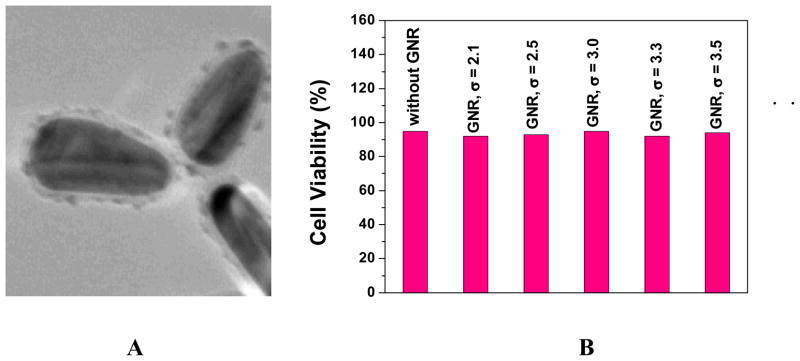
Figure 4A: TEM data for PSS-coated gold nanorod, 4B: Viability of cells incubated with PSS-coated gold nanorod (3.2 aspect ration) of different aspect ratios
Figure 4 shows the cell viability for PSS-coated gold nanorods with different aspect ratios. Our experiment indicates that PSS coated gold nanorod is not toxic. Therefore, the toxicity of gold nanorod is due to the presence of CTAB and replacing CTAB with biocompatible and functionalization friendly stabilizing agents like PSS is essential for the use of gold nanorod in living cells. Due to the toxicity of CTAB, which is essential for stability of gold nanorod and also aggregation of gold nanorod in the presence of cell media, it appears to be challenging to study the cytotoxicity of gold nanorods individually.
In conclusion, the cytotoxicity of gold nanomaterials of different sizes and shapes is explored in this letter. Our data demonstrated that spherical gold nanoparticles with different sizes are not inherently toxic to human skin cells, but gold nanorods are highly toxic due to the presence of CTAB as coating material. However, further PSS coated gold nanorods coated with CTAB is not toxic. So replacing CTAB with biocompatible and functionalization friendly stabilizing agents is essential for using of gold nanorods in living cells. Due to CTAB toxicity, which is essential for stability of gold nanorod and for preventing aggregation of gold nanorods and gold nanoparticles in the presence of cell media, it is a real challenge to study the cytotoxicity of gold nanomaterials individually.
Acknowledgments
We wish to thank NSF-PREM grant # DMR-0611539, NSF-MRI grant #0421406 for their generous funding. We thank Sara H. Bayley, MRSEC Instrumentation Facilities Coordinator, University of Southern Mississippi for helping to acquire TEM data. We also thank reviewers whose valuable suggestion improved the quality of the manuscript.
References
- 1.Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller R. Chem Rev. 2008;108:2064. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 2.Rosi NL, Mirkin CA. Chem Rev. 2005;105:1547. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Vemula PK, Ajayan PM, John G. Nature Materials. 2008;7:236. doi: 10.1038/nmat2099. [DOI] [PubMed] [Google Scholar]
- 4.Jiang W, Kim BYS, Rutka JT, Chan WCW. Nature Nanotechnology. 2008;3:145. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 5.Alivisatos AP. Nat Biotechnol. 2004;22:47. doi: 10.1038/nbt927. [DOI] [PubMed] [Google Scholar]
- 6.Xiang J, Lu W, Hu Y, Wu Y, Yan H, Lieber CM. Nature. 2006;441:441. doi: 10.1038/nature04796. [DOI] [PubMed] [Google Scholar]
- 7.Nam JM, Thaxton CS, Mirkin CA. Science. 2003;301:1884. doi: 10.1126/science.1088755. [DOI] [PubMed] [Google Scholar]
- 8.Gao X, Cui Y, Levenson RM, Chung LWK, Nie S. Nat Biotechnol. 2004;22:969. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 9.Darbha GK, Singh AK, Rai US, Yu E, Yu H, Ray PC. J Am Chem Soc. 2008;130:8038. doi: 10.1021/ja801412b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darbha GK, Rai US, Singh AK, Ray PC. Chem Eur J. 2008;14:3896. doi: 10.1002/chem.200701850. [DOI] [PubMed] [Google Scholar]
- 11.Dasary SR, Rai US, Yu H, Anjaneyulu Y, Dubey M, Ray PC. Chem Phys Lett. 2008 doi: 10.1016/j.cplett.2008.05.082. ASAP Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ray PC. Angew Chem. 2006;45:1151. doi: 10.1002/anie.200503114. [DOI] [PubMed] [Google Scholar]
- 13.Darbha GK, Ray A, Ray PC. ACS Nano. 2007;1:208. doi: 10.1021/nn7001954. [DOI] [PubMed] [Google Scholar]
- 14.Tiwari VS, Oleg T, Darbha GK, Hardy W, Singh JP, Ray PC. Chem Phys Lett. 2007;446:77. [Google Scholar]
- 15.Jennings TL, Singh MP, Strouse GF. J Am Chem Soc. 2006;128:5462. doi: 10.1021/ja0583665. [DOI] [PubMed] [Google Scholar]
- 16.Ray PC, Fortner A, Darbha GK. J Phys Chem B. 2006;110:20745. doi: 10.1021/jp065121l. [DOI] [PubMed] [Google Scholar]
- 17.Jenning TL, Schlatterer JC, Singh MP, Greenbaum NL, Strouse GF. Nano Lett. 2006;6:1318. doi: 10.1021/nl052458a. [DOI] [PubMed] [Google Scholar]
- 18.Seferos DS, Giljohann DA, Hill DH, Progodich AE, Mirkin CA. J Am Chem Soc. 2007;129:15477–15479. doi: 10.1021/ja0776529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skewis LR, Reinhard MB. Nano Lett. 2008;8:208. doi: 10.1021/nl0725042. [DOI] [PubMed] [Google Scholar]
- 20.Huang X, El-Sayed IH, Qian W, El-Sayed MA. J Am Chem Soc. 2006;128:2115. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 21.Bonham AJ, Braun G, Pavel I, Moskovits M, Reich NO. J Am Chem Soc. 2007;129:14572. doi: 10.1021/ja0767837. [DOI] [PubMed] [Google Scholar]
- 22.Lan S, Chenxu Y, Joseph I. Ananl Chem. 2008;80:3342. [Google Scholar]
- 23.Huang X, El-Sayed IH, Qian W, El-Sayed MA. Nano Lett. 2007;7:1591. doi: 10.1021/nl070472c. [DOI] [PubMed] [Google Scholar]
- 24.Chan GH, Zhao J, Hicks EM, Schatz GC, Van Duyne RP. Nano Lett. 2007;7:1947. doi: 10.1021/nl050873x. [DOI] [PubMed] [Google Scholar]
- 25.Lewinski N, Colvin V, Drezek R. Small. 2008;4:26. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 26.Haunk TS, Chazani AA, Chan WCW. Small. 2008;4:153. doi: 10.1002/smll.200700217. [DOI] [PubMed] [Google Scholar]
- 27.Huff TB, Hansen MN, Zhao Y, Cheng Ji-X, Wei A. Langmuir. 2007;23:1596. doi: 10.1021/la062642r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Small. 2005;1:325. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 29.Chithrani BD, Ghazani AA, Chan WCW. Nano Lett. 2006;6:662. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi H, Niidome Y, Niidome T, Kaneko K, Kawasaki H, Yamada S. Langmuir. 2006;22:2. doi: 10.1021/la0520029. [DOI] [PubMed] [Google Scholar]