Abstract
G protein-coupled receptors (GPCR) are a superfamily of receptors that are vital in a wide array of physiological processes. Modulation of GPCR signaling has been an intensive area of therapeutic study, mainly due to the diverse pathophysiological significance of GPCRs. Pepducins are cell-penetrating lipidated peptides designed to target the intracellular loops of the GPCR of interest. Pepducins can function as agonists or antagonists of their cognate receptor, making them highly useful compounds for the study of GPCR signaling. Pepducins have been used to control platelet-dependent hemostasis and thrombosis, tumor growth, invasion, and angiogenesis, as well as to improve sepsis outcomes in mice. Pepducins have been successfully designed against a wide variety of GPCRs including the protease-activated receptors (PAR1, 2, 4), the chemokine receptors (CXCR1, 2, 4), the sphingosine-1-phosphate receptor (S1P3), the adrenergic receptor (ADRA1B), and have the potential to help reveal the functions of intractable GPCRs. Pharmacokinetic, pharmacodynamic, and biodistribution studies have showed that pepducins are widely distributed throughout the body except the brain and possess appropriate drug-like properties for use in vivo. Here, we discuss the delivery, pharmacology, and biodistribution of pepducins, as well as the effects of pepducins in models of inflammation, cardiovascular disease, cancer, and angiogenesis.
Keywords: Pepducin, GPCR, Inflammation, Sepsis, Thrombosis, Cancer, Angiogenesis, PAR1, PAR4, CXCR1, CXCR2, CXCR4
1. Introduction
G protein-coupled receptors (GPCR) are a superfamily of receptors that are vital in a wide array of physiological processes. GPCRs share a unique seven transmembrane structure that transmits extracellular signals across the plasma membrane and activate intracellular signal transduction pathways through G proteins (1). Modulation of GPCR signaling has been an intensive area of therapeutic study, mainly due to the diverse pathophysiological significance of GPCRs (2, 3). Essentially, all small-molecule drugs directed at GPCRs interact with the ligand binding site on the extracellular surface of the receptor. By comparison, pepducins exploit the importance of the G protein and modulate the interactions of the receptor with the G protein on the intracellular surface (4). Pepducins are cell-penetrating lipidated peptides designed to target the intracellular loops of the GPCR of interest (4). Pepducins can function as agonists or antagonists of their cognate receptor, making them highly useful compounds for the study of GPCR signaling.
Pepducins have been used to target GPCR signaling pathways in many disease models, including inflammation, thrombosis, and cancer. We and others have shown that pepducins can be used to control platelet-dependent hemostasis and thrombosis (5–7), tumor growth, invasion, and angiogenesis (8–10), as well as to improve sepsis outcomes in mice (11, 12). Pepducins have been successfully designed against a wide variety of GPCRs including protease-activated receptors (PAR1, 2, 4), chemokine receptors (CXCR1, 2, 4), the sphingosine-1-phosphate receptor (S1P3) (13), the adrenergic receptor (ADRA1B) (14), and have the potential to help reveal the functions of intractable GPCRs. Here, we discuss the delivery, pharmacology, and biodistribution of pepducins, as well as the effects of pepducins in models of inflammation, cardiovascular disease, cancer, and angiogenesis.
2. Pepducin Delivery, Pharmacology, and Biodistribution
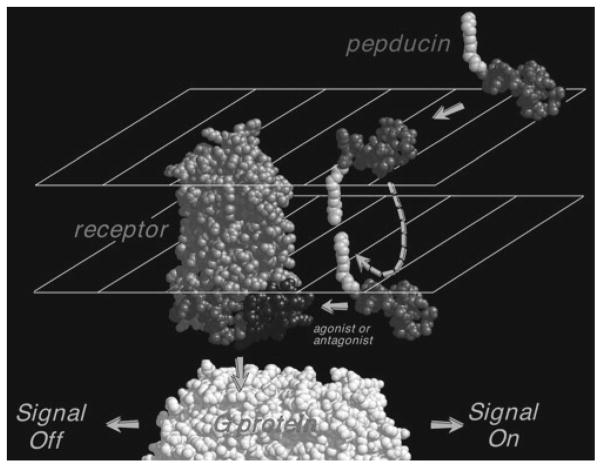
The seven transmembrane domains of GPCRs are joined by intracellular loops (i1–i3) and extracellular loops (e2–e4), and are flanked by an N-terminal e1 extracellular domain and an i4 C-terminal intracellular domain. Pepducins are created by attaching a lipidated group, such as an acyl chain (e.g., C12–C18) or steroid to a peptide corresponding to the i1–i4 loops of the GPCR of interest (2, 4, 11, 13–17). Mechanistic studies suggest that the hydrophobic lipid group partitions into the plasma membrane and “flips” across the bilayer, thus shuttling the attached peptide to the inner leaflet of the plasma membrane in a reversible manner (Fig. 1) (2, 4, 18, 19). Mutagenesis analysis of the receptor and pepducin indicate that the peptide can interact with the intracellular domains of the GPCR of interest (4).
Fig. 1.
Proposed mechanism of modulation of GPCR signaling by its cognate pepducin. Cell-penetrating pepducins with a covalently attached palmitate are shown inserting and flipping across to the intracellular surface of the plasma membrane where they interact with the GPCR and G protein to either turn off or turn on signaling.
The delivery of PAR1- and PAR4-based pepducins to circulating platelets confirmed that pepducins can partition to the plasma membrane of the target cells of animals (2, 18). Fluorescein-tagged palmitoylated or nonpalmitoylated peptides were injected intravenously in mice (2). Flow cytometry of circulating platelets, after using pronase to remove peripherally bound peptides from the platelet surface, revealed significantly higher levels of fluorescence in the mice treated with the palmitoylated peptide as compared to those treated with the nonpalmitoylated peptide. Confirmation that the palmitoylated peptides can flip across the lipid bilayer was provided by Wielders and colleagues, who used a FRET-based assay with differentially labeled phospholipids that were distributed either to the outer leaflet (NBD-phosphocholine as donor) or inner leaflet (NBD-phospho-L-serine as donor) of the plasma membrane (18). They demonstrated that rhodamine-labeled PAR1 pepducins (Rho-P1pal-12 as an acceptor) are present in both inner and outer leaflets of the bilayer. Together, these data support the proposed mechanism in Fig. 1 that palmitoylation is sufficient for delivery of the peptide across the plasma membrane of the cell.
Many studies have been conducted to determine the specificity of various pepducins to their cognate receptors (2, 11–13, 20, 21). Early work with the PAR1 i3 loop antagonist P1pal-12 indicated that the pepducin was highly specific for PAR1. Treatment of human platelets with 5 μM P1pal-12 for 1 min resulted in a 75–95% decrease in SFLLRN (PAR1 agonist)-induced aggregation and complete blockade of aggregation in response to 3 nM thrombin (2). The specificity of P1pal-12 was demonstrated by the lack of an effect in the aggregation of platelets induced by a series of agonists for the thromboxane, ADP, collagen, or GPIb/IX/V receptors. Furthermore, P1pal-12 had no effect in endothelial cells on the responses to IL8, SDF-1α, S1P, thromboxane, MCP-1, RANTES, or the migration of recombinantly transfected HEK293 cells to ligands for PAR2, PAR4, CXCR1, CXCR2, S1P1, S1P3, or CCR5 receptors (12). A PAR4 i3 loop-based pepducin, P4pal-10, completely blocked AYPGKF (PAR4 agonist)-induced aggregation and had the ability to partially block PAR1 activation at higher concentrations but did not affect ADP, thromboxane, and GPIb/IX/V receptors (2). Hollenberg and colleagues verified that P4pal-10, but not the reverse-sequence pepducin rev-P4pal-10, inhibited human platelet aggregation to PAR4 agonists (20). Slofstra et al. demonstrated that P4pal-10 had no effect on migration of human neutrophils to IP-10, SDF-1α, and S1P but completely blocked migration to thrombin (21).
The pharmacokinetics (PK), pharmacodynamics (PD), and bioavailability of pepducins were determined using fluorescent (2) and radioactively labeled pepducins. To perform PK and PD studies, the PAR4 pepducin P4pal-10 was labeled with Alexa Fluor (P4pal-10-Alexafluor) and injected into mice intravenously (19). Bolus intravenous injection of P4pal-10-Alexafluor resulted in high plasma and platelet pepducin levels for 5 h followed by elimination with a half-life of 3.5 h (19). We had previously showed that P4pal-10 inhibits murine platelet function by antagonizing PAR4, a major hemostasis receptor in rodents (2). Therefore, PD studies were carried out by measuring the effect of P4pal-10 on bleeding time in mice (19). Intravenous injection of P4pal-10 increased bleeding time threefold to fivefold after 5 min which was maintained for 1 h after injection. P4pal-10 extended bleeding time approximately twofold at the 4 h point and returned to baseline at the 24 h time point. Subcutaneous injection of 3 mg/kg P4pal-10 resulted in a sixfold prolongation of bleeding time 4 h after injection and gave a more prolonged PD half-life of approximately 14 h.
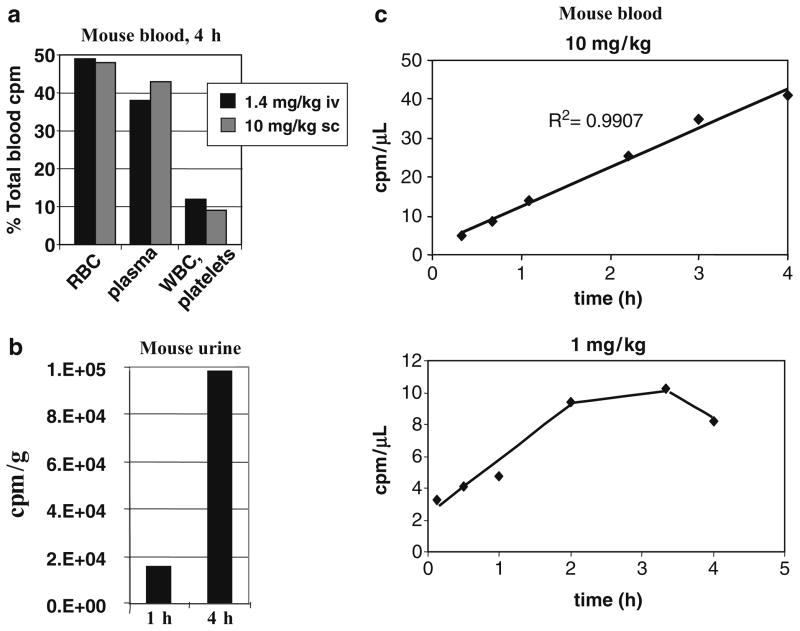
To begin to study the biodistribution of pepducins, P4pal-10C (pal-CGRRYGHALR) was radioactively labeled with (14C)-acetamide on a cysteine residue ([14C]-P4pal-10C) and injected subcutaneously or intravenously into mice at four different doses. After 4 h, the localization of radioactive pepducin was measured in various organs and tissues (Fig. 2). Intravenous injection of [14C]-P4pal-10C with a therapeutic dose of 1.4 mg/kg P4pal-10 resulted in the appearance of radioactivity in the liver, kidney, lungs, and spleen, and lesser amounts to other tissues (Fig. 2a). Intravenous injection with a high dose of 14 mg/kg P4pal-10 resulted in appearance of radioactivity in highly perfused tissues such as the kidney, lungs, liver, and spleen with lesser radioactivity appearing in the heart, blood, muscle, and fat, but not the brain. Subcutaneous injection of [14C]-P4pal-10C with a therapeutic dose of 1 mg/kg P4pal-10 resulted in appearance of radioactivity in the liver, kidney, lungs, and blood. Subcutaneous injection at a higher dose of 10 mg/kg gave a fairly even distribution of radioactivity to the kidney, liver, spleen, blood, heart, and lungs, with lesser amounts to muscle and fat, but not to brain. This pattern is consistent with a biodistribution of [14C]-P4pal-10C to highly vascularized tissues. More detailed examination of blood components revealed that 50% of the [14C]-P4pal-10C radioactivity partitioned to red blood cells, 40% to plasma, and the remaining 10% was detected in white blood cells and platelets 4 h after intravenous or subcutaneous injection (Fig. 3a). By 1 h, radioactivity was detected in urine, with a fivefold increase in urine at 4 h (Fig. 3b). Radioactivity appeared in mouse blood in a linearly increasing manner following subcutaneous injection (Fig. 3c). Together, these findings suggest that the P4pal-10 pepducin is widely distributed throughout the body and blood components, is biologically active, and is excreted into the urine.
Fig. 2.
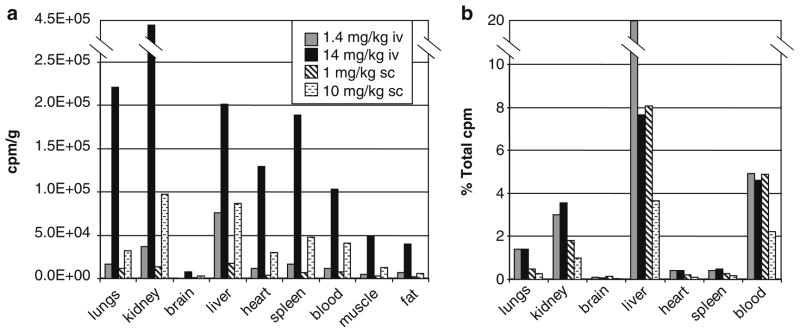
Pepducin biodistribution. Mice were injected intravenously (1.4 mg/kg or 14 mg/kg) or subcutaneously (1 mg/kg or 10 mg/kg) with P4pal-10 radioactively labeled pepducin, [14C]-P4pal-10C. After 4 h the distribution of [14C]-P4pal-10C was measured in various organs and tissues by radioactive count in counts per million (cpm). Data are represented as (a) cpm/g tissue or (b) as a percent of the total cpm.
Fig. 3.
Pharmacokinetics of pepducins in mouse blood and urine. (a) Mice were injected with 1.4 mg/kg intravenously or 10 mg/kg subcutaneously with [14C]-P4pal-10. After 4 h, radioactivity was measured in red blood cells (RBC), plasma, and white blood cells (WBC)/platelets and represented as a percent of the total counts per million (cpm) in whole blood. (b) Mice were injected with [14C]-P4pal-10C. After 1 and 4 h radioactivity was measured in the urine. (c) Mice were injected subcutaneously with 10 mg/kg or 1 mg/kg 14C-P4pal-10C. Radioactivity was measured in the blood over time.
3. Efficacy of Pepducins in Disease Models
3.1. Inflammation
GPCRs have been implicated in many inflammatory diseases, including sepsis and systemic inflammatory response syndrome, rheumatoid arthritis, ulcerative colitis, atherosclerosis, and psoriasis. Leukocytes and other cells, such as endothelial cells, fibroblasts, epithelium, and glial cells, contribute to the inflammatory response by the secretion of proinflammatory mediators. This often results in an overzealous immune response that does more harm than good and is responsible for much of the morbidity and mortality associated with inflammatory conditions. Pepducins targeted against PAR1, PAR2, PAR4, CXCR1, CXCR2, and CXCR4 have been used to study the role of these receptors in specific inflammatory diseases (Table 1) and could potentially be used as therapeutics for these conditions (11, 20–26).
Table 1.
Pepducin applications and outcomes in inflammatory disease models in mice
| Disease model | Pepducin | Sequence | Target | Outcome | References |
|---|---|---|---|---|---|
| Inflammation/paw edema | P4pal-10 | N-pal-SGRRYGHALR-NH2 | PAR4 | Reduced edema and granulocyte recruitment induced by carrageenan | (20, 22) |
|
| |||||
| Sepsis, systemic inflammatory response syndrome (SIRS), and disseminated intravascular coagulation (DIC) | P1pal-12 P4pal-10 |
N-pal-RCLSSSAVANRS-NH2 N-pal-SGRRYGHALR-NH2 |
PAR1 PAR4 |
Reduced organ damage and inflammatory mediators, inhibited neutrophil migration | (21) |
| x1/2pal-i3 x1/2LCA-i1 x4pal-i1 |
N-pal-RTLFKAHMGQKHRAMR-NH2 N-LCA-YSRVGRSVTD-NH2 N-pal-MGYQKKLRSMTD-NH2 |
CXCR1/2 CXCR1/2 CXCR4 |
CXCR1/2 pepducins reduced organ damage, inhibited neutrophil migration, improved survival, and inhibited DIC | (11) | |
| P1pal-12S P1pal-13 |
N-pal-RSLSSSAVANRS-NH2 N-pal-AVANRSKKSRALF-NH2 |
PAR1 PAR1 (agonist) |
Temporally modulated survival, vascular leakage, and DIC | (12) | |
|
| |||||
| Ulcerative colitis | P4pal-10 | N-pal-SGRRYGHALR-NH2 | PAR4 | Decreased epithelial cell permeability in murine colonic strips | (24, 25) |
| Rheumatoid arthritis | P4pal-10 | N-pal-SGRRYGHALR-NH2 | PAR4 | Reduced agonist induced joint vascular conductance and edema | (26) |
pal palmitoyl, LCA lithicholic
Sepsis and systemic inflammatory response syndrome (SIRS) are a leading cause of mortality in intensive care units (27). Sepsis can lead to systemic inflammation and overactivation of the coagulation system – a condition termed disseminated intravascular coagulation (DIC) (28). Pepducins have been used to study the role of neutrophil, platelet, and endothelial cell PAR1, PAR2, PAR4, CXCR1, CXCR2, CXCR4 in sepsis and systemic inflammation (11, 12, 21). Chemokine receptor pepducins for CXCR1 and CXCR2 were found to improve survival and prevent DIC in septic mice (11). Antagonist pepducins were designed against the i1 and i3 loops of CXCR1 and CXCR2 (x1/2pal-i3, x1/2LCA-i1), and CXCR4 (x4pal-i1, x4pal-i2). Treatment with the CXCR1/2 pepducins blocked neutrophil chemotaxis toward IL-8, improved survival, and reversed DIC and liver failure in septic mice. However, treatment with a CXCR4 pepducin had no effect on survival but caused massive leukocytosis consistent with the role of CXCR4 in SDF-1α neutrophil homeostasis. Slofstra and colleagues used the PAR4 antagonist pepducin, P4pal-10, to study the role of PAR4 in sepsis and discovered that PAR4 inhibition of neutrophils protected against systemic inflammation and DIC (21).
PAR1 i3 loop agonist and i3 loop antagonist pepducins were used to study the role of PAR1 at different stages of sepsis in mice (12). Treatment with the PAR1 antagonist pepducin, P1pal-12S, at early time points but not late time points, improved survival and prevented DIC in septic mice. Interestingly, treatment with the PAR1 agonist pepducin, P1pal-13, at late time points improved survival and prevented DIC in septic mice by inhibiting leakage of endothelial cell tight junctions. These findings suggested that PAR1 switches from being a vascular-disruptive receptor to a vascular-protective receptor during sepsis. Further studies demonstrated that transactivation of PAR2 by PAR1 within a PAR1–PAR2 heterodimer mediated the protective effects of the PAR1 agonist pepducin seen in later stages of sepsis which was lost in either the PAR1−/− or PAR2−/− mice. PAR1 antagonist pepducins did not prevent the transactivation of PAR2 by the PAR1 tethered ligand, suggesting that the pepducins did not cause dissociation of the PAR1–PAR2 heterodimer complex. Thus, pepducins revealed a novel transactivation of PAR2 by the PAR1 tethered ligand, which complemented the genetic approaches (12).
PAR4 pepducins have been used to study ulcerative colitis, irritable bowel syndrome, and rheumatoid arthritis (24–26). Dabek and colleagues (25) used the PAR4 antagonist pepducin, P4pal-10, to study the role of PAR4 and its activator cathepsin G in colonic epithelial barrier function and neutrophil activity in ulcerative colitis. Treatment of mice with fecal supernatants from ulcerative colitis patients increased epithelial cell permeability, which was blocked by P4pal-10. Furthermore, P4pal-10 was used to study joint pain and inflammation in a model of rheumatoid arthritis (26). McDougall et al. (26) treated mice with P4pal-10 and found that the pepducin could block the proinflammatory and pronociceptive effects of a PAR4 agonist in the mouse knee joint and alleviate acute joint inflammation. Together, these animal studies demonstrate that pepducins designed for a specific GPCR target can be successfully used to study a wide variety of inflammatory diseases.
3.2. Cardiovascular Disease
Major cardiovascular diseases include atherosclerosis, coronary artery disease, thrombosis, restenosis, hypertension, and heart failure. Cardiovascular disease is the major cause of death in the developed world, and significant resources have been invested in finding new therapies to treat these diseases. The technology of pepducins has helped elucidate the role of various GPCRs in the pathophysiology of arteriothrombosis, myocardial ischemia, and blood vessel inflammation (Table 2).
Table 2.
Pepducin applications and outcomes in cardiovascular disease in animal models
| Pepducin | Sequence | Target | Outcome | References |
|---|---|---|---|---|
| P1pal-7 | N-pal-KKSRALF-NH2 | PAR1 antagonist | Inhibition of guinea pigs platelet aggregation Prolonged collagen-induced arterial occlusion time in guinea pigs |
(7) |
|
| ||||
| P1pal-12 | N-pal-RCLSSSAVANRS-NH2 | PAR1 antagonist | Inhibition of collagen-induced platelet aggregation | (7) |
| Delayed thrombin generation | (18) | |||
| Decreased collagen-induced calcium signal | (5) | |||
| Decreased thrombin-induced relaxation in rat aortic rings | (33) | |||
|
| ||||
| P1pal-12S | N-pal-RSLSSSAVANRS-NH2 | PAR1 antagonist | Increased transendothelial migration of murine monocytes | (32) |
|
| ||||
| P1pal-19 | N-pal-RCLSSSAVANRSKKSRALF-NH2 | PAR1 agonist | Promotes Ca2+ signal, relaxation of rat aortic rings, and prostaglandin E2 release | (33) |
|
| ||||
| P4pal-10 | N-pal-SGRRYGHALR-NH2 | PAR4 antagonist and PAR1 partial antagonist | Delayed thrombin generation | (18) |
| Delayed platelet accumulation | (18, 20) | |||
| Prolonged tail bleeding time in mice | (2, 19) | |||
| Reduced infarct size in ischemia/reperfusion in rats | (34) | |||
|
| ||||
| Rev-P4pal-10 | N-pal-RLAHGYRRGS-NH2 | Inactive | (20) | |
|
| ||||
| P4pal-i1 | N-pal-ATGAPRLPST-NH2 | PAR4 antagonist | Inhibition of arterial occlusion in guinea pigs, when combined with bivalirudin or P1pal-7 | (6) |
| Inhibition of epinephrine-induced Ca2+ flux | (30) | |||
pal palmitoyl
The first in vivo studies using pepducins explored the contribution of thrombin receptor signaling to hemostasis, thrombosis, and systemic platelet activation. In accordance with the tail-bleeding phenotype observed in PAR4 knockout mice (29), mice infused with P4pal-10 exhibited prolonged tail-bleeding times and unstable thrombi formation as compared to P1pal-12 or vehicle-treated mice (2). Infusion of mice with P4pal-10 also protected against systemic thrombus formation induced by the PAR4-agonist peptide AYPGKF plus epinephrine (2). Wielders and colleagues (18) showed that P4pal-10 delayed the generation of thrombin. Fluorescent platelets were monitored by real-time accumulation at the site of wire injury to the carotid artery of mice. P4pal-10-inhibited platelets had a significant delay in accumulation at the site of vascular injury (18).
To study the distinct functions of PAR1 versus PAR4 in human platelets, a PAR4 pepducin based on the i1 loop, P4pal-i1, was developed (6). P4pal-i1 proved to be selective for PAR4 without affecting PAR1-induced platelet aggregation. Using P4pal-i1 in a carotid injury model in guinea pigs, which express PAR1 and PAR4 on their platelets, it was shown that blocking PAR4 decreases arterial occlusion by approximately 50%. Inhibition of PAR1 with P1pal-7 (0.3 mg/kg i.v.) in the same injury model (7) also gave approximately 50% prolongation of the occlusion time. When PAR1 and PAR4 inhibitors were combined, it resulted in a great increase in the arterial occlusion time in the guinea pig model (7). Also, when P4pal-i1 was combined with bivalirudin, a direct thrombin inhibitor widely used in patients with acute coronary syndromes, there was a significant inhibition of human platelet aggregation and suppression of arterial thrombosis in guinea pigs to a much higher degree than bivalirudin alone (6). The P4pal-i1 pepducin was also used by Grenegard et al. (30) to study the activation of human platelets. In thrombin-preactivated platelets, P4pal-i1 inhibited the epinephrine-induced increase of calcium concentration [Ca2+] and aggregation. The above observations support the role of both PAR1 and PAR4 in platelet-driven arterial thrombosis (6, 31).
Pepducins have been used to help elucidate the mechanisms of platelet procoagulant activity. Keuren and colleagues used the pepducins P1pal-12 and P4pal-10 to study the synergistic action of thrombin and collagen in generating procoagulant platelet surfaces (5). P1pal-12 significantly decreased the thrombin plus collagen-induced calcium signal and decreased the prothrombinase activity to levels induced by collagen alone. These data suggest that PAR1 activation is a prerequisite for both sustained elevations in [Ca2+] and that procoagulant activity is induced by a combination of collagen and thrombin through PAR1 (5). A slightly modified version of P1pal-12, P1pal-12S (12), has been recently used as a PAR1 antagonist in leukocyte inflammatory studies in mice (32). Monocytes derived from hypercoagulable mice (TMPro/Pro) treated with P1pal-12S showed increased transendothelial migration towards human complement factor 5a, suggesting a role of PAR1 in monocyte inflammatory responses.
The pepducins P1pal-12 and P1pal-7 were also used to study collagen-dependent aggregation in human platelets (7). These PAR1 pepducins led to inhibition of collagen-induced platelet aggregation, blocked p38 MAPK activation, and significantly reduced the propagation of platelet–platelet thrombi in human whole blood, under arterial flow conditions. Intravenous administration of P1pal-7 in guinea pigs protected from collagen-induced thrombocytopenia and prolonged the mean occlusion time in a carotid artery FeCl3 injury model.
PAR1-based pepducins have been valuable in studying other cell types involved in cardiovascular diseases. Kubo et al. (33) investigated the activity of PAR1 pepducins P1pal-19 and P1pal-12 in vascular tissue preparations and compared their activity to that of soluble activating peptides. They found that the P1pal-19 agonist can promote Ca2+ signals, prostaglandin E2 release, and persistent relaxation of rat aortic rings in a concentration-dependent manner. When using P1pal-12 in precontracted rat aortic rings, there was decreased relaxation induced by TFLLRN or P1pal-19, in agreement with the contractile role of PAR1 in cells of the blood vessel wall.
The role of PAR4 in ischemic injury of the heart was the focus of Strande and colleagues (34). P4pal-10 was administered as an intravenous bolus of 10 μg/kg to rats, and it significantly reduced the myocardium infarct size by approximately 20% in an ischemia/reperfusion (I/R) injury model. According to this study, by blocking PAR4 with P4pal-10, they further revealed the protective effects of adenosine signaling in the myocardium.
Other GPCRs that play key roles in the cardiovascular system have also been targeted with pepducins. A high-throughput functional assay developed by Edwards et al. (14) identified a number of palmitoylated cell-permeable oligopeptides that acted either as agonists or antagonists of GPCRs involved in platelet function including prostaglandin, LPA, and adrenergic receptors.
3.3. Cancer and Angiogenesis
GPCRs such as PAR1 and PAR2 play critical roles in cancer progression, invasion, and metastasis (35). In this section, we discuss the utility of pepducins targeted against the intracellular loops of PAR1, PAR2, Smoothened (SMO), S1P3, and CXCR4 in cancer and angiogenesis.
In addition to its well-recognized roles in platelet and vascular biology, PAR1 has been proposed to be involved in the invasive and metastatic processes of breast cancer (36, 37), pancreatic cancer (38), and melanoma (39–41) and has been identified as an oncogene in the transformation of NIH3T3 mouse fibroblasts (42, 43). PAR1 can stimulate Gαi, Gαq, and Gα12/13 pathways, which contribute to various processes involved in the regulation of tumor cell biology.
We have extensively tested the efficacy of the i3 loop-derived PAR1 pepducin, P1pal-7, as a monotherapy and in combination with taxotere in breast (9, 44) and ovarian (45) xenograft mouse models (Table 3). The effective pepducin therapeutic dose range in several xenograft cancer efficacy models is 3–10 mg/kg. To examine the in vivo efficacy of pepducins in tumor growth, the mammary fat pads of mice were injected with two different PAR1 expressing cell lines: PAR1-MCF7/N55 or MDA-MB-231 cells. Inhibition of PAR1 with P1pal-7 significantly reduced tumor growth of both PAR1-MCF7/N55 and MDA-MB-231 breast tumors by 62% (p < 0.01) as monotherapy (9), and 95% (p < 0.01) as dual therapy (44), respectively. In another set of studies, P1pal-7 gave a highly significant 88% reduction in metastasis to lung with tail vein injected breast cancer GFP/MDA-MB-231 cells (p < 0.001) as monotherapy (44) and significant reduction of ovarian OVCAR4 peritoneal dissemination (p < 0.005) as dual therapy with docetaxel (45). There were no obvious toxicities associated with multiday dosing of P1pal-7 up to 70 days in mice. These data provide an in vivo validation that targeting PAR1 may be a novel therapeutic approach in the treatment of breast and ovarian carcinomas.
Table 3.
Pharmacology of P1pal-7 (C15H31CONH-KKSRALF-NH2) in human tumor xenograft models
| Tumor type | Cell line | Dosing regimen | Efficacy | References |
|---|---|---|---|---|
| Breast carcinoma | PAR1-MCF7/N55 (s.q.) | 10 mg/kg (s.c. q2d), single agent | Tumor burden, significant 62% reduction (p < 0.01) in tumor growth | (9) |
| Breast carcinoma | MDA-MB-231 (s.q.) | 10 mg/kg (i.p. q2d) combination with docetaxel (10 mg/kg i.p. once weekly) | Tumor burden, significant 95% reduction (p < 0.01) in tumor growth | (44) |
| Ovarian carcinoma | OVCAR4 (i.p.) | 3.6 mg/kg (i.p. q2d) combination with docetaxel (10 mg/kg i.p. once weekly) | Significant reduction in diaphragm metastasis (p < 0.005) | (45) |
| Breast carcinoma | GFP/MDA-MB-231 (i.v.) | 10 mg/kg (s.c. 5 days per week), single agent | Metastasis, significant 88% reduction (p < 0.001) in metastasis to lung | (44) |
| Breast carcinoma | PAR1-MCF7/N55 (s.q.) | 10 mg/kg (s.c. q2d), single agent | Vascularity, significant 75% inhibition (p < 0.006) in angiogenesis | (9) |
| Ovarian carcinoma | OVCAR4 (i.p.) | 3.6 mg/kg (i.p. q2d) combination with docetaxel (10 mg/kg i.p. once weekly) | Vascularity, significant 75% inhibition (p < 0.0001) in angiogenesis | (45) |
| Ovarian carcinoma | SKOV3 (i.p.) | 10 mg/kg (s.c. 5 days per week), single agent | Ascites formation, complete inhibition of ascites | (45) |
| Ovarian carcinoma | OVCAR4 (i.p.) | 10 mg/kg (i.p. q2d), single agent | Ascites formation, significant 60% inhibition (p = 0017) in ascites formation | (45) |
s.q. subcutaneous, i.p. intraperitoneal, i.v. intravenous
Pepducins have also been successfully used to study angiogenesis and proangiogenic activities in endothelial cell migration and proliferation. P1pal-7 almost completely blocked angiogenesis of peritoneal ovarian (45) and breast cancers (9). Moreover, P1pal-7 significantly inhibited ascites production in peritoneal ovarian cancer (45) due to blockade of PAR1-dependent endothelial barrier function (12). P1pal-7 monotherapy gave complete inhibition of ascites formation with SKOV3 peritoneal carcinomatosis and 60% (p < 0.005) reduction with OVCAR4 ovarian cells. Licht et al. developed a pepducin to the i2 loop of S1P3 called KRX-725 (13), using a myristoyl–glycine attached to the N-terminus of the peptide. KRX-725 was found to have agonist activity for S1P3 and mimicked the effects of sphingosine 1-phosphate, the ligand for S1P3, in endothelial cells. KRX-725 induced angiogenesis in vitro and in vivo in the mouse corneal pocket assay.
In other studies, pepducins were used to delineate the contribution of PAR1 to the Akt survival pathways in breast cancers. P1pal-7 inhibited the viability of PAR1-expressing breast cancer cells through Akt (44). Phosphorylation of Akt was significantly inhibited in established tumors treated with P1pal-7 for 5 days and gave significant attenuation of the survival pathway. The P1pal-7 protective effect was rescued by constitutively active Akt, suggesting that P1pal-7 and PAR1 act upstream of Akt.
Majumdar et al. (8) used the PAR1 pepducin P1pal-12 (4) to demonstrate that plasmin induced migration of α9 integrin-expressing CHO cells through PAR1. The PAR4 pepducin served as a negative control and had no effect on plasmin-induced migration of α9–CHO cells. A recent publication by Kaufmann and colleagues (46) investigated the role of PAR2 in the proliferation, spread, and invasion of hepatocellular carcinoma (HCC). The PAR2 pepducin antagonist P2-pal-21, which has an IC50 of 1 μM for PAR2 (2), completely blocked the PAR2-dependent invasion of HCCs.
Hedghog signaling is regulated by the seven-transmembrane receptor, Smoothened (SMO). The aberrant regulation of the Hedghog-SMO pathway has been implicated in tumor progression (47, 48). Remsberg and colleagues (10) synthesized N-palmitoylated peptides spanning the intracellular loops of SMO (i1, i2, and i3). A series of N-terminal and C-terminal truncations of the loops were generated and tested for inhibition of growth of breast MCF-7 cells and melanoma SK-Mel2 cells. The most potent peptide was derived from the i2 loop SMO-i2-12 (Pal-LTYAWHTSFK) with an IC50 of 0.06 μM. Substitution of the palmitoyl lipid with a myristoyl resulted in a loss of potency. Synthesis of metabolically stable retroinverse derivatives using all D-amino acids were found to improve the potency over the parent compound.
4. Conclusion
GPCRs play diverse roles in many physiological processes, yet relatively few have been successfully targeted. Pepducins can function as antagonists or agonists of their cognate receptor and prove to be useful compounds for the study of GPCRs that are difficult to target with small-molecule approaches. Pepducins provide a useful complement to genetic approaches and may help uncover novel functions of GPCRs, such as the transactivation of PAR2 by PAR1 within a PAR1–PAR2 heterodimer. Pepducins have been extensively tested in animal models of systemic inflammation, sepsis, thrombosis, atherosclerosis, cancer, and angiogenesis. Pharmacodynamic, pharmacokinetic, and biodistribution studies in mice have demonstrated that pepducins are widely distributed throughout the body and suggest that pepducins possess appropriate drug-like properties for use in vivo. Toxicity studies have also been performed and have found pepducins to be well tolerated in animals. Together, pepducins are a promising new class of compounds for the study of GPCRs and may ultimately be developed as therapeutics in a variety of diseases.
Acknowledgments
This research was supported by NIH grants CA104406 (L. Covic) and CA122992, HL64701, and HL57905 (A. Kuliopulos).
References
- 1.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: a G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 2.Covic L, Misra M, Badar J, Singh C, Kuliopulos A. Pepducin-based intervention of thrombin-receptor signaling and systemic platelet activation. Nat Med. 2002;8:1161–1165. doi: 10.1038/nm760. [DOI] [PubMed] [Google Scholar]
- 3.Kuliopulos A, Covic L. Blocking receptors on the inside: pepducin-based intervention of PAR signaling and thrombosis. Life Sci. 2003;74:255–262. doi: 10.1016/j.lfs.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Covic L, Gresser AL, Talavera J, Swift S, Kuliopulos A. Activation and inhibition of G protein-coupled receptors by cell-penetrating membrane-tethered peptides. Proc Natl Acad Sci USA. 2002;99:643–648. doi: 10.1073/pnas.022460899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keuren JF, Wielders SJ, Ulrichts H, Hackeng T, Heemskerk JW, Deckmyn H, Bevers EM, Lindhout T. Synergistic effect of thrombin on collagen-induced platelet procoagulant activity is mediated through protease-activated receptor-1. Arterioscler Thromb Vasc Biol. 2005;25:1499–1505. doi: 10.1161/01.ATV.0000167526.31611.f6. [DOI] [PubMed] [Google Scholar]
- 6.Leger AJ, Jacques SL, Badar J, Kaneider NC, Derian CK, Andrade-Gordon P, Covic L, Kuliopulos A. Blocking the protease-activated receptor 1–4 heterodimer in platelet-mediated thrombosis. Circulation. 2006;113:1244–1254. doi: 10.1161/CIRCULATIONAHA.105.587758. [DOI] [PubMed] [Google Scholar]
- 7.Trivedi V, Boire A, Tchernychev B, Kaneider NC, Leger AJ, O’Callaghan K, Covic L, Kuliopulos A. Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell. 2009;137:332–343. doi: 10.1016/j.cell.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majumdar M, Tarui T, Shi B, Akakura N, Ruf W, Takada Y. Plasmin-induced migration requires signaling through protease-activated receptor 1 and integrin alpha(9) beta(1) J Biol Chem. 2004;279:37528–37534. doi: 10.1074/jbc.M401372200. [DOI] [PubMed] [Google Scholar]
- 9.Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Remsberg JR, Lou H, Tarasov SG, Dean M, Tarasova NI. Structural analogues of smoothened intracellular loops as potent inhibitors of Hedgehog pathway and cancer cell growth. J Med Chem. 2007;50:4534–4538. doi: 10.1021/jm0705657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaneider NC, Agarwal A, Leger AJ, Kuliopulos A. Reversing systemic inflammatory response syndrome with chemokine receptor pepducins. Nat Med. 2005;11:661–665. doi: 10.1038/nm1245. [DOI] [PubMed] [Google Scholar]
- 12.Kaneider NC, Leger AJ, Agarwal A, Nguyen N, Perides G, Derian C, Covic L, Kuliopulos A. ‘Role reversal’ for the receptor PAR1 in sepsis-induced vascular damage. Nat Immunol. 2007;8:1303–1312. doi: 10.1038/ni1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Licht T, Tsirulnikov L, Reuveni H, Yarnitzky T, Ben-Sasson SA. Induction of pro-angiogenic signaling by a synthetic peptide derived from the second intracellular loop of S1P3 (EDG3) Blood. 2003;102:2099–2107. doi: 10.1182/blood-2002-12-3634. [DOI] [PubMed] [Google Scholar]
- 14.Edwards RJ, Moran N, Devocelle M, Kiernan A, Meade G, Signac W, Foy M, Park SD, Dunne E, Kenny D, Shields DC. Bioinformatic discovery of novel bioactive peptides. Nat Chem Biol. 2007;3:108–112. doi: 10.1038/nchembio854. [DOI] [PubMed] [Google Scholar]
- 15.Shpakov AO, Pertseva MN, Guryanov IA, Vlasov GP. Influence of synthetic peptides derived from the third cytoplasmic loop of the type 1 relaxin receptor on the stimulation of G-protein GTP-binding activity by relaxin. Biol Membrany. 2005;22:450–457. [Google Scholar]
- 16.Swift S, Leger AJ, Talavera J, Zhang L, Bohm A, Kuliopulos A. Role of the PAR1 receptor 8th helix in signaling: the 7-8-1 receptor activation mechanism. J Biol Chem. 2006;281:4109–4116. doi: 10.1074/jbc.M509525200. [DOI] [PubMed] [Google Scholar]
- 17.Shpakov AO, Gur’yanov IA, Kuznetsova LA, Plesneva SA, Shpakova EA, Vlasov GP, Pertseva MN. Studies of the molecular mechanisms of action of relaxin on the adenylyl cyclase signaling system using synthetic peptides derived from the LGR7 relaxin receptor. Neurosci Behav Physiol. 2007;37:705–714. doi: 10.1007/s11055-007-0071-y. [DOI] [PubMed] [Google Scholar]
- 18.Wielders SJ, Bennaghmouch A, Reutelingsperger CP, Bevers EM, Lindhout T. Anticoagulant and anti-thrombotic properties of intracellular protease-activated receptor antagonists. J Thromb Haemost. 2007;5:571–576. doi: 10.1111/j.1538-7836.2007.02364.x. [DOI] [PubMed] [Google Scholar]
- 19.Covic L, Tchernychev B, Jacques S, Kuliopulos A. Pharmacology and In Vivo Efficacy of Pepducins in Hemostasis and Arterial Thrombosis. In: Langel U, editor. Handbook of Cell-Penetrating Peptides. CRC Press; Boca Raton, FL: 2007. pp. 245–257. [Google Scholar]
- 20.Hollenberg MD, Saifeddine M, Sandhu S, Houle S, Vergnolle N. Proteinase-activated receptor-4: evaluation of tethered ligand-derived peptides as probes for receptor function and as inflammatory agonists in vivo. Br J Pharmacol. 2004;143:443–454. doi: 10.1038/sj.bjp.0705946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slofstra SH, Bijlsma MF, Groot AP, Reitsma PH, Lindhout T, ten Cate H, Spek CA. Protease-activated receptor-4 inhibition protects from multiorgan failure in a murine model of systemic inflammation. Blood. 2007;110:3176–3182. doi: 10.1182/blood-2007-02-075440. [DOI] [PubMed] [Google Scholar]
- 22.Houle S, Papez MD, Ferazzini M, Hollenberg MD, Vergnolle N. Neutrophils and the kallikrein–kinin system in proteinase-activated receptor 4-mediated inflammation in rodents. Br J Pharmacol. 2005;146:670–678. doi: 10.1038/sj.bjp.0706371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang G, Kernan KA, Collins SJ, Cai X, Lopez-Guisa JM, Degen JL, Shvil Y, Eddy AA. Plasmin(ogen) promotes renal interstitial fibrosis by promoting epithelial-to-mesenchymal transition: role of plasmin-activated signals. J Am Soc Nephrol. 2007;18:846–859. doi: 10.1681/ASN.2006080886. [DOI] [PubMed] [Google Scholar]
- 24.Annahazi A, Gecse K, Dabek M, Ait-Belgnaoui A, Rosztoczy A, Roka R, Molnar T, Theodorou V, Wittmann T, Bueno L, Eutamene H. Fecal proteases from diarrheic-IBS and ulcerative colitis patients exert opposite effect on visceral sensitivity in mice. Pain. 2009;144:209–217. doi: 10.1016/j.pain.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Dabek M, Ferrier L, Roka R, Gecse K, Annahazi A, Moreau J, Escourrou J, Cartier C, Chaumaz G, Leveque M, Ait-Belgnaoui A, Wittmann T, Theodorou V, Bueno L. Luminal cathepsin g and protease-activated receptor 4: a duet involved in alterations of the colonic epithelial barrier in ulcerative colitis. Am J Pathol. 2009;175:207–214. doi: 10.2353/ajpath.2009.080986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDougall JJ, Zhang C, Cellars L, Joubert E, Dixon CM, Vergnolle N. Triggering of proteinase-activated receptor 4 leads to joint pain and inflammation in mice. Arthritis Rheum. 2009;60:728–737. doi: 10.1002/art.24300. [DOI] [PubMed] [Google Scholar]
- 27.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 28.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 29.Sambrano GR, Weiss EJ, Zheng YW, Huang W, Coughlin SR. Role of thrombin signalling in platelets in haemostasis and thrombosis. Nature. 2001;413:74–78. doi: 10.1038/35092573. [DOI] [PubMed] [Google Scholar]
- 30.Grenegard M, Vretenbrant-Oberg K, Nylander M, Desilets S, Lindstrom EG, Larsson A, Ramstrom I, Ramstrom S, Lindahl TL. The ATP-gated P2X1 receptor plays a pivotal role in activation of aspirin-treated platelets by thrombin and epinephrine. J Biol Chem. 2008;283:18493–18504. doi: 10.1074/jbc.M800358200. [DOI] [PubMed] [Google Scholar]
- 31.Leger AJ, Covic L, Kuliopulos A. Protease-activated receptors in cardiovascular diseases. Circulation. 2006;114:1070–1077. doi: 10.1161/CIRCULATIONAHA.105.574830. [DOI] [PubMed] [Google Scholar]
- 32.Seehaus S, Shahzad K, Kashif M, Vinnikov IA, Schiller M, Wang H, Madhusudhan T, Eckstein V, Bierhaus A, Bea F, Blessing E, Weiler H, Frommhold D, Nawroth PP, Isermann B. Hypercoagulability inhibits monocyte transendothelial migration through protease-activated receptor-1-, phospholipase-Cbeta-, phosphoinositide 3-kinase-, and nitric oxide-dependent signaling in monocytes and promotes plaque stability. Circulation. 2009;120:774–784. doi: 10.1161/CIRCULATIONAHA.109.849539. [DOI] [PubMed] [Google Scholar]
- 33.Kubo S, Ishiki T, Doe I, Sekiguchi F, Nishikawa H, Kawai K, Matsui H, Kawabata A. Distinct activity of peptide mimetic intracellular ligands (pepducins) for proteinase-activated receptor-1 in multiple cells/tissues. Ann N Y Acad Sci. 2006;1091:445–459. doi: 10.1196/annals.1378.087. [DOI] [PubMed] [Google Scholar]
- 34.Strande JL, Hsu A, Su J, Fu X, Gross GJ, Baker JE. Inhibiting pro-tease-activated receptor 4 limits myocardial ischemia/reperfusion injury in rat hearts by unmasking adenosine signaling. J Pharmacol Exp Ther. 2008;324:1045–1054. doi: 10.1124/jpet.107.133595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 36.Even-Ram S, Uziely B, Cohen P, Grisaru-Granovsky S, Maoz M, Ginzburg Y, Reich R, Vlodavsky I, Bar-Shavit R. Thrombin receptor overexpression in malignant and physiological invasion processes. Nat Med. 1998;4:909–914. doi: 10.1038/nm0898-909. [DOI] [PubMed] [Google Scholar]
- 37.Henrikson KP, Jazin EE, Greenwood JA, Dickerman HW. Prothrombin levels are increased in the estrogen-treated immature rat uterus. Endocrinology. 1990;126:167–175. doi: 10.1210/endo-126-1-167. [DOI] [PubMed] [Google Scholar]
- 38.Rudroff C, Seibold S, Kaufmann R, Zetina CC, Reise K, Schafer U, Schneider A, Brockmann M, Scheele J, Neugebauer EA. Expression of the thrombin receptor PAR-1 correlates with tumour cell differentiation of pancreatic adenocarcinoma in vitro. Clin Exp Metastasis. 2002;19:181–189. doi: 10.1023/a:1014598904644. [DOI] [PubMed] [Google Scholar]
- 39.Nierodzik ML, Chen K, Takeshita K, Li JJ, Huang YQ, Feng XS, D’Andrea MR, Andrade-Gordon P, Karpatkin S. Protease-activated receptor 1 (PAR-1) is required and rate-limiting for thrombin-enhanced experimental pulmonary metastasis. Blood. 1998;92:3694–3700. [PubMed] [Google Scholar]
- 40.Even-Ram SC, Maoz M, Pokroy E, Reich R, Katz BZ, Gutwein P, Altevogt P, Bar-Shavit R. Tumor cell invasion is promoted by activation of protease activated receptor-1 in cooperation with the alpha vbeta 5 integrin. J Biol Chem. 2001;276:10952–10962. doi: 10.1074/jbc.M007027200. [DOI] [PubMed] [Google Scholar]
- 41.Nierodzik ML, Kajumo F, Karpatkin S. Effect of thrombin treatment of tumor cells on adhesion of tumor cells to platelets in vitro and tumor metastasis in vivo. Cancer Res. 1992;52:3267–3272. [PubMed] [Google Scholar]
- 42.Whitehead I, Kirk H, Kay R. Expression cloning of oncogenes by retroviral transfer of cDNA libraries. Mol Cell Biol. 1995;15:704–710. doi: 10.1128/mcb.15.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin CB, Mahon GM, Klinger MB, Kay RJ, Symons M, Der CJ, Whitehead IP. The thrombin receptor, PAR-1, causes transformation by activation of Rho-mediated signaling pathways. Oncogene. 2001;20:1953–1963. doi: 10.1038/sj.onc.1204281. [DOI] [PubMed] [Google Scholar]
- 44.Yang E, Boire A, Agarwal A, Nguyen N, O’Callaghan K, Tu P, Kuliopulos A, Covic L. Blockade of PAR1 signaling with cell-penetrating pepducins inhibits Akt survival pathways in breast cancer cells and suppresses tumor survival and metastasis. Cancer Res. 2009;69:6223–6231. doi: 10.1158/0008-5472.CAN-09-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agarwal A, Covic L, Sevigny LM, Kaneider NC, Lazarides K, Azabdaftari G, Sharifi S, Kuliopulos A. Targeting a metalloprotease-PAR1 signaling system with cell-penetrating pepducins inhibits angiogenesis, ascites, and progression of ovarian cancer. Mol Cancer Ther. 2008;7:2746–2757. doi: 10.1158/1535-7163.MCT-08-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaufmann R, Oettel C, Horn A, Halbhuber KJ, Eitner A, Krieg R, Katenkamp K, Henklein P, Westermann M, Bohmer FD, Ramachandran R, Saifeddine M, Hollenberg MD, Settmacher U. Met receptor tyrosine kinase transactivation is involved in proteinase-activated receptor-2-mediated hepato-cellular carcinoma cell invasion. Carcinogenesis. 2009;30:1487–1496. doi: 10.1093/carcin/bgp153. [DOI] [PubMed] [Google Scholar]
- 47.Bailey JM, Mohr AM, Hollingsworth MA. Sonic hedgehog paracrine signaling regulates metastasis and lymphangiogenesis in pancreatic cancer. Oncogene. 2009;28:3513–3525. doi: 10.1038/onc.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Theunissen JW, de Sauvage FJ. Paracrine Hedgehog signaling in cancer. Cancer Res. 2009;69:6007–6010. doi: 10.1158/0008-5472.CAN-09-0756. [DOI] [PubMed] [Google Scholar]