Abstract
The management of full thickness articular cartilage defects is a challenging problem for orthopaedic surgeons. It has limited potential for healing and can be a significant source of pain and loss of function. Multiple cartilage repair strategies have been attempted. Matrix-induced Autologous Chondrocyte Implantation (MACI) has been shown to produce hyaline-like cartilage into chondral defects. The goal of this review is to provide the current principles and technique of the MACI procedure along with reported clinical outcomes with its use.
Keywords: matrix-induced autologous chondrocyte implantation, cartilage repair, surgical technique
INTRODUCTION
Isolated chondral lesions of the knee are common and are often found during arthroscopy.(1) They may be incidentally found or due to trauma, abnormal alignment, or osteochondritis dissecans (OCD).(2,3) These chondral lesions have limited capacity for self repair.(4) Untreated lesions can lead to debilitating pain and degenerative arthritis.(5)
Multiple strategies have been described to repair or preserve articular cartilage lesions. These include debridement, bone marrow stimulation, cell based, and whole-tissue transplantation techniques.(6) Successful early treatment of these lesions can potentially prevent long term morbidity.
Cell based procedures have had promising results for cartilage restoration.(7–10) The first generation cell therapy, autologous chondrocyte implantation (ACI), was introduced in 1987 and published in 1994.(11) This involves implantation of a suspension of cultured autologous chondrocytes into a chondral defect under a periosteal patch. The periosteal patch must be sutured water-tight to the surrounding cartilage to contain the injected suspension. This technique while successful is technically challenging and sometimes cumbersome. It requires an arthrotomy for access to the lesion and, if the lesion is located in a far posterior location on the femoral condyle or on the tibia, may require a large arthrotomy including a takedown of meniscus and collateral ligaments to reach the defect. The periosteal harvest required for the original ACI technique is technically cumbersome and increases operative time and morbidity.(12) Complications associated with the arthrotomy and use of the periosteal patch due to graft hypertrophy have been reported.(13–15) Due to the technical difficulty and associated complications, the second generation of ACI was developed using a collagen scaffold instead of a periosteal patch.(12,16–18) However, this technique still requires an arthrotomy and labor-intensive repair to the surrounding cartilage.
The third generation of chondrocyte implantation involves culturing the chondrocytes on to a scaffold.(19) Specifically, culturing the cells on to a biodegradeable type I/III collagen membrane is commonly referred to as matrix-induced autologous chondrocyte implantation (MACI, Genzyme Biosurgery, Cambridge, Massachusetts).(20) MACI is currently not approved for use in the United States of America but has been widely used in Europe and Australia. MACI is the forerunner of other product lines of three dimensional membrane bound cultured chondrocytes such as Hyalograft-C (Fidia Advanced Biopolomers, Abano Terme, Italy).(21,22)
This paper will provide an overview of the MACI technique and current clinical data with its use.
INDICATIONS
Cell based procedures such as ACI or MACI are typically used for symptomatic full-thickness chondral defects of the femoral articular surface in younger patients. The reported average age range for this type of procedure is 15 to 55 years of age.(23–26) It is not indicated for those patients who have tri-compartmental advanced arthritis. However, it has been used in the setting of early osteoarthritis with radiographic Kellgren-Lawrence grades of 2 to 3.(27) Cell based procedures have been effective for treatment of variable sized lesions from 2 to 10 cm2 on average.(23–26) These lesions may be idiopathic, related to trauma (such as ACL injuries), or due to other reasons such as osteochondritis dissecans.(28) It is important to evaluate the alignment of the lower extremity as well as ligamentous stability. In general, any malalignment or instability must be addressed prior or concomitantly with the ACI procedure. The primary theoretical advantage for use of autologous cell based procedures in younger patients is the development of hyaline-like cartilage as opposed to fibrocartilage.(16) This would presumably lead to better long term outcomes.
This technique has also been advocated for patients with osteochondritis dissecans and subchondral bone loss. Defects greater than 3 to 4 cm2 with bone loss have been treated with MACI along with autologous bone grafting.(10,23,29,30) Good clinical and magnetic imaging results have been reported with this procedure.(30)
CLINICAL AND RADIOGRAPHIC ASSESSMENT
When evaluating patients for possible cell based procedures, it is extremely important to confirm that the patient’s source of pain is indeed due to a chondral lesion. Typically patients will have pain localized to the joint line. They often times will have an associated effusion. There may be crepitus noted with joint range of motion. It is essential to rule out other sources of pathology contributing to the patient’s pain. This would include ligamentous instability, meniscal pathology, and abnormal extremity alignment. Localizing the patient’s pain must be consistent with radiographic evaluation.
Radiographic assessment should include weight bearing anterior-posterior, lateral, flexed posterior-anterior (PA), and merchant views. Flexed PA views may help localize posterior tibio-femoral OCD lesions. Long leg alignment views are often needed to assess for abnormal alignment of the tibio-femoral joint. This must be addressed concomitantly or prior to cartilage restoration procedures. Magnetic resonance imaging is the diagnostic gold standard and should be utilized when a chondral injury is suspected.(31,32) It may also provide information about the quality of the surrounding articular cartilage, persistent subchondral bone damage such as bone bruising. Subchondral bone edema or bone bruising has become more of a focus due to its effect on overall outcome with cell based procedures. It is the focus of the article on “subchondroplasty”. Addressing bone loss with grafting at the time of surgery may improve results of the cell-based procedure.(30) Adding a T2 Mapping Sequence to routine knee MR imaging protocol has been reported to improve the sensitivity of detecting cartilage lesions within the knee joint from 74.6% to 88.9%.(33)
ARTHROSCOPIC ASSESSMENT AND CARTILAGE BIOPSY
Once a patient has been found to be a potential candidate for a MACI procedure, the next step is direct arthroscopic visualization in order to assess eligibility for the MACI procedure. Factors that need to be considered during arthroscopy include location, depths, ICRS grade, number of lesions, kissing lesions and associated injuries. Another important factor is to obtain an idea of the “character” of the joint environment. Chondral repair requires normal or nearly normal chondral margins to be successful. If the lesion is embedded in a condyle that shows ICRS grade 2 changes throughout, the success of a chondral repair procedure may be compromised. It is also important take into consideration the fact that better outcomes can generally be expected when treating corresponding lesions between the femur and tibia as opposed to the patella-femoral joint.
After a thorough assessment of the chondral lesion is complete and the patient is found to be a good candidate for MACI, it is necessary to obtain a biopsy of articular cartilage. The cartilage biopsy is usually taken from a non-weight bearing portion of the intercondylar notch or trochlear ridge. A full thickness cartilage specimen is harvested using a sharp-edged ring curette or osteotome. Approximately 200 to 300 mg of healthy cartilage is harvested.(19) It is placed into sterile transport medium and sent to the manufacturer for preparation.
The chondrocytes are then enzymatically isolated from the cartilage specimen. Once a sufficient number of cells have been cultured, they are seeded onto a three dimensional porcine type I/III collagen scaffold. It typically takes 3 to 4 weeks before the cells are ready for implantation. This is usually sufficient time for the patient to rehabilitate from the initial arthroscopy. If other procedures are performed at the time of biopsy, additional time may be needed for rehabilitation.
MACI SURGICAL IMPLANTATION
In general, implantation of the chondrocytes can be performed using a mini-open arthrotomy or arthroscopy. This typically depends on the size and location of the lesion. The chondral defect is initially debrided down to the calcified cartilage layer without penetration into the underlying subchondral bone (Figure 1). The surrounding rim of cartilage should be healthy and stable. This is best performed with a non-angulated, round-eyed sharp curette.(34,35) After careful debridement, the lesion is sized with a template. The scaffold is then cut to fit the measured template. It is important that the graft lay within the defect and does not ride over the rim of the surrounding articular margins. This is different than the traditional ACI technique that required the membrane to span the defect. The implant is then placed into the defect, cell-seeded side face down, and secured with fibrin glue (Figure 2). Digital pressure is then applied to the scaffold to ensure fixation to the underlying subchondral bone and surrounding cartilage. It is important to check the stability of the implant with several cycles of motion. The use of sutures or anchors may prevent delamination if the implant is unstable or the lesion is uncontained and large in size.(20) For select lesions this technique can be modified to be performed arthroscopically with the use of an inflatable silastic catheter as described by Cortese et al.(36) This in turn had led to reduced surgical times and reduced the overall surgical morbidity.(12)
Figure 1.
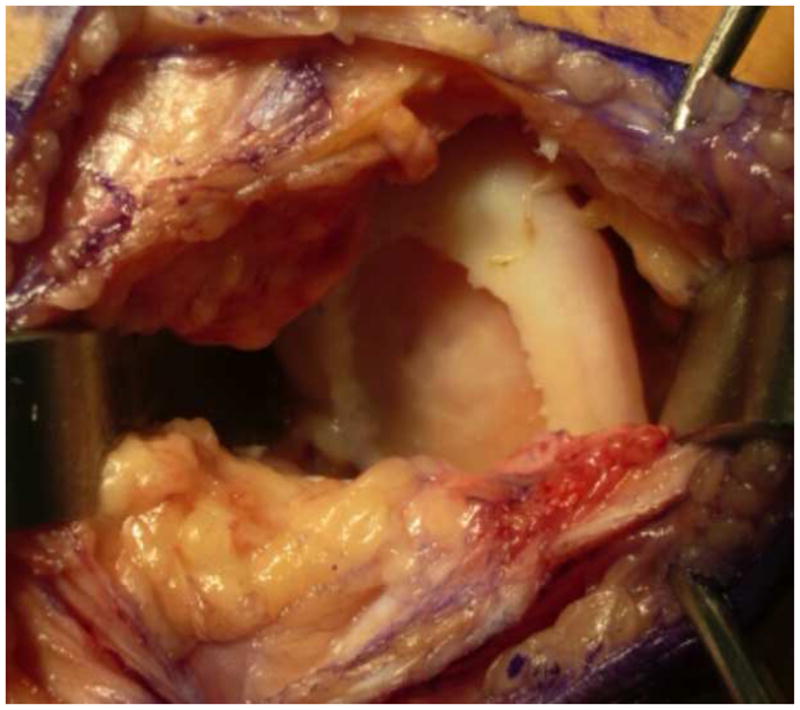
Preparation of the defect: The subchondral bone needs to be prepared carefully with a ring curette to be void of any calcified cartilage. Care must be taken not to violate the subchondral bone. If a previous microfracture has been performed it may be necessary to stop subchondral bleeding with a small amount of fibrin glue that can be pressed into the bleeding surface to stop the bleeding. Stable shoulders need to be created for the repair tissue to have a good attachment point.
Figure 2.
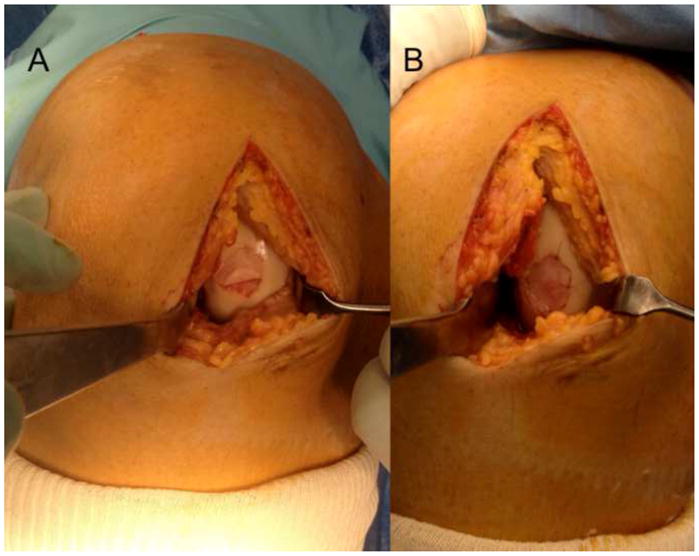
After preparation of the subchondral bone and sizing of the defect, the membrane is cut to size and carefully lifted onto the defect. The membrane is glued into place using a thin layer of fibrin glue and kept in place using digital pressure. The graft should be contained inside the defect and not ride over the rim of the prepared defect. Some authors will choose to secure the edges of the graft with a 6-0 resorbable suture, however, this is not required for the technique. (Courtesy of Peter Verdonk, Antwerp, Belgium)
REHABILITATION
All patients will undergo rehabilitation with goals of restoring normal motion and strength while protecting the implanted graft. This process should include protected weight bearing for generally 6 weeks with progression to full weight bearing by 12 weeks after surgery to prevent delamination of the graft.(37,38) Depending on the size and location of the repair, weight bearing can be adjusted accordingly. Trochlear and patellar repairs may initiate weight bearing earlier with the leg locked in extension for ambulation. Each patient’s progress is different and should be guided by their symptoms.
The use of continuous passive motion (CPM) has been shown to enhance chondrocyte regeneration and decrease the potential for intra-articular adhesions.(39,40) The majority of studies have reported CPM use initiated within 12 to 24 hours of implantation and for 6 to 8 hours a day for approximately 6 weeks.(41–43) Range of motion may vary depending on the size and location of the repair. In general for weight bearing repairs, higher impact activities such as running are not permitted until graft hardening has occurred. Typically after 9 to 12 months the repair cartilage has similar consistency to the surrounding cartilage but may be up to 18 months for larger lesions.(41)
CPM use for trochlear and patellar repairs is typically used with range of motion from 0° to 40°. CPM from 40° to 70° is not recommended due to increased patella-femoral contact pressures within this range.(41) The remainder of motion may be obtained with physical therapy and home exercises such as prone hangs and heel slides. The focus of rehabilitation for these repairs is centered on decreasing patella-femoral contact pressures.
MACI OUTCOMES
MACI promised to improve the technical aspects of cell based chondral repair by improving surgical morbidity and ease of application without compromising clinical results. It appears that MACI has been able to achieve this goal as clinical outcomes have been similar to those seen with first and second generation ACI. It has been reported to successfully improve pain and function due to symptomatic isolated chondral defects. Even though literature is limited to short and medium-term follow-up the results are encouraging.(29,42,44–48) A review of relevant clinical results of MACI and comparative studies are summarized in Table 1.
Table 1.
Summary of relevant MACI clinical outcomes
| Reference (year) | Study Design | Follow-up (months) | Patients and Defect Location | Major Findings | Clinical Scores |
|---|---|---|---|---|---|
| D’Anchise et al26 (2005) | Case series | 24 | Patients: 35 MFC: 19 LFC: 7 Trochlea: 6 Patella: 9 Med tibial plateau: 2 |
Significant improvements in IKDC, VAS, Lysholm and Tegner scores. Cartilage biopsies with histologic characteristics of hyaline cartilage. | IKDC: 3.31 ± 0.45 to 1.13 ± 0.31 VAS: 5.52 ± 1.53 to 0.20 ± 0.40 Lysholm: 70.94 ± 0.66 to 99.54 ± 0.97 Tegner: 2.78 ± 1.71 to 4.61 ± 0.52 |
| Behrens et al25 (2006) | Prospective case series | 34.5 | Patients: 25 MFC: 11 LFC: 1 Retropatellar: 7 Multiple Lesions: 6 |
Significant improvements in ICRS, Lysholm-Gillquist, and Meyer scores at 2 and 5 years. Tegner-Lysholm score with non-significant improvements. | ICRS: 45.2 ± 18.3 to 58.8 ± 22.9 Lysholm-Gillquist: 59.1 ± 21.7 to 74.1 ± 26.8 Meyer: 12.0 ± 3.5 to 15.3 ± 3.3 Tegner-Lysholm: 3.2 ± 2.2 to 3.7 ± 1.9 All scores averaged from initial to final follow-up |
| Ebert et al45 (2011) | Case series | 60 | Patients: 35 MFC: 22 LFC: 11 Patella: 11 Trochlea: 9 |
Significant improvements in KOOS, SF-36, 6 minute walk test, and knee range of motion. Significant improvement in MRI composite scores. | KOOS (mean): 45.58 to 74.78 SF-36 (mean): 44.47 to 53.6 6 minute walk test (meters): 519.1 ± 30.7 to 583.7 ± 22.4 (P < 0.0001) Knee flexion range of motion (degrees): 136.1 ± 4 to 137.7 ± 1.9 (P = 0.128) Knee extension range of motion (degrees): 1.1 ± 0.9 to −1.0 ± 0.4 (P = 0.012) |
| Marvolitz et al42 (2012) | Case series | 60 | Patients: 21 MFC: 13 LFC: 4 Patella: 7 |
Significant improvements in KOOS, IKDC, Tegner-Lysholm, and modified Cincinnati scores at 5 years. Subchondral bone edema on MRI for 47% of patients at 5 years. | KOOS (mean): 29.6 ± 9.15 to 77.7 ± 21.2 (P < 0.05) IKDC (mean): 30.1 ± 6.6 to 74.3 ± 20.4 (P < 0.05) Tegner-Lysholm and modified Cincinnati scores with significant improvements at 5 years (P < 0.05) |
| MACI vs Microfracture | |||||
| Basad et al44 (2010) | Prospective, randomized trial | 24 |
MACI: 40 Condylar: 29 Patellar-trochlear: 11 Microfracture: 20 Condylar: 16 Patellar-trochlear: 4 |
Significant improvements over 24 months for both groups (Lysholm, Tegner, patient ICRS, and surgeon ICRS scores). MACI had significantly better results when compared directly to microfracture group. | Lysholm mean (MACI): 52 ± 26 to 92 ± 9 Lysholm mean (Microfracture): 55 ± 25 to 69 ± 26 Tegner median level: (MACI): Level 2 to Level 4 Tegner median level: (Microfracture): Level 2 to Level 3 ICRS patient scores at baseline and 24 months significant for both groups (P < 0.0001) and MACI more effective over time (P = 0.03) ICRS surgeon scores at baseline and 24 months significant for both groups (P < 0.0001) and MACI more effective over time (P = 0.02) |
| MACI vs. ACI | |||||
| Bartlett et al29 (2005) | Prospective, randomized trial | 12 |
MACI: 47 MFC: 25 LFC: 6 Patella-trochlear: 22 ACI: 44 MFC: 25 LFC: 5 Patella-trochlear: 29 (Multiple lesions for some patients) |
Significant improvements in modified Cincinnati knee score for both groups. Clinical, arthroscopic, and histological outcomes similar for both groups. | Modified Cincinnati score mean (MACI): 44.5 to 64.1 (P = 0.002) Modified Cincinnati score mean (ACI): 41.4 to 59 (P = 0.01) At 1 year, good to excellent ICRS score in 79.2% ACI grafts and 66.6% MACI grafts |
| Zeifang et al48 (2010) | Randomized controlled trial | 24 |
MACI: 11 ACI: 10 MFC: 18 LFC: 3 (Group specifics not provided) |
Significant improvements noted for both groups at 12 and 24 months for IKDC score and Tegner Activity score but not between groups. ACI group significantly more effective for Lysholm and Gillquist scores. | IKDC (MACI): 51.1 ± 22.8 to 70.1 ± 28.6 (P = 0.635) IKDC (ACI): 52.0 ± 13.5 to 77.1 ± 22.7 (P = 0.0098) Tegner activity score (MACI): 4.1 ± 2.8 to 4.7 ± 2.9 (P = 0.7832) Tegner activity score (ACI): 3.7 ± 1.9 to 5.3 ± 1.9 (P = 0.0625) Lysholm and Gillquist score (MACI): 71.4 ± 23.8 to 72.5 ± 28.0 (P = 0.915) Lysholm and Gillquist score (ACI): 61.3 ± 14.3 (P = 0.0273) ACI group more effective for Lysholm and Gillquist score (P = 0.0487) |
| MACI for Subchondral Bone Loss | |||||
| Ochs et al30 (2011) | Case series | 60 | Patients: 26 MFC: 22 LFC: 4 |
Significant improvements for Tegner, Lysholm-Gillquist, Cincinnati knee rating, and IKDC scores. Simultaneous remodeling of the articular cartilage and subchondral lamina was noted. | Tegner activity score: 3.5 ± 0.8 to 4.6 ± 1.2 (P = 0.002) Lysholm-Gillquist score: 53.2 ± 18 to 88.5 ± 9.5 (P < 0.001) Cincinnati knee rating: 51.7 ± 13 to 84.6 ± 11.7 (P < 0.001) IKDC score: 50.5% ± 16.1% to 78.4% ± 13.4% (P < 0.001) |
Abbreviations: Med, Medial; IKDC, International Knee Documentation Committee; VAS, Visual Analog Scale; ICRS, International Cartilage Repair Society; KOOS, Knee Injury and Osteoarthritis Outcome Score; MRI, Magnetic Resonance Imaging; MACI, Matrix-Induced Autologous Chondrocyte Implantation; ACI, Autologous Chondrocyte Implantation; MFC, Medial Femoral Condyle; LFC: Lateral Femoral Condyle
Ebert et al treated chondral lesions with MACI and evaluated them clinically and with MRI at 5 years after transplantation.(45) A significant improvement was noted with both the Knee Injury and Osteoarthritis Outcome Score (KOOS) (average of 45.6 to 74.8) as well as SF-36 subscales (average of 44.5 to 53.55) in the 41 patients treated. MRI composite scores were significantly improved with 67% of grafts showing complete infill, and 89% showing good to excellent infill. Similarly, Marlovitz et al reported 5 year follow-up of clinical and radiological outcomes for chondral defects treated with MACI.(42) Significant improvements (P < 0.05) were noted for all 5 KOOS subcategories at year 1 and maintained through year 5 in 19 of the 21 patients (90.5%) treated. Significant improvements were also noted for the International Knee Documentation Committee score (IKDC) (30.1 to 74.3), the modified Cincinnati score (38.1 to 79.6), and the Tegner-Lysholm activity score (1.8 to 4.3). After 5 years, complete filling (83%) and integration (82%) of the graft were seen in the majority of patients. Subchondral bone edema was still present in 47% of patients at 5 years.
Several prospective studies have compared cell based procedures to marrow stimulation techniques. Basad et al performed a randomized control trial and found superior results of MACI over microfracture within the first 2 years after the procedure.(44) Sixty patients were randomized according to a 2;1 scheme into two groups with 40 patients receiving the MACI procedure and 20 patients receiving the microfracture procedure. Significant improvements were noted for both groups with respect to the Lysholm, Tegner, patient ICRS and surgeon ICRS scores (all P < 0.0001). However, at 2 years time MACI was significantly more effective than microfracture according to the Lysholm (P = 0.005), Tegner (P = 0.04), ICRS patient (P = 0.03) and ICRS surgeon (P = 0.02) scores. Similarly, Kon et al performed a prospective cohort study comparing arthroscopic third-generation cell based repair using Hyalograft C with microfracture.(47) Good and excellent results were noted 2–5 years after implantation with the Hyalograft C group over time whereas the microfracture group progressively deteriorated after 2 years. In another study, similar results were found in high level male soccer players over time. The microfracture group returned to sport earlier but had declines in results over time. The authors concluded that the cell based membrane cultured repair (Hyalograf-C) procedure offered more durable clinical results.(46)
Zeifang et al performed a randomized control trial investigating differences between the original periosteum-covered ACI and MACI technique.(48) Improvements were noted in both groups but no difference was found between the two techniques at 12 and 24 months regarding the IKDC score (P = 0.5573, P = 0.4994) and Tegner Activity Score (P = 0.4063, P = 0.1043). Similarly, Bartlett et al performed a prospective, randomized study comparing ACI with a collagen sheet covering and MACI for treatment of symptomatic chondral defects.(29) The authors concluded that both procedures were similar with regards to clinical, arthroscopic, and histological results at short-term follow-up. Although clinical results are similar between the two techniques, a theoretical advantage of the use of a membrane based ACI is that the scaffold provides support for cell adhesion and production of chondrocyte matrix.(49) Use of a scaffold has been shown to maintain chondrocyte differentiation as opposed to dedifferentiation lost during liquid culture.(50) Additionally, Gomoll et al recently reported a significant decrease in reoperation rates for symptomatic hypertrophy using a type I/III bilayer collagen membrane.(13) The author noted a decrease in reoperation rate from 25.7% to 5% (P < 0.0001) for graft hypertrophy when comparing ACI using a periosteal patch to the type I/III bilayer collagen membrane, which is also used for the MACI technique.
MACI has also been used in the setting of subchondral bone loss associated with osteochondritis dissecans. Ochs et al evaluated 26 patients with symptomatic condylar lesions. These defects were treated using yet another matrix-associated cell based repair technique similar to the MACI technique. In this study the authors used Novocart 3D (TETEC AG, Reutlingen, Germany) combined with bone grafting using monocortical cancellous cylinders and cortical graft.(30) After an average follow up of 39.8 months, all scores improved significantly. Nineteen patients (73%) obtained good/excellent results with the Lysholm-Gillquist score (average 53.2 to 88.5), Cincinnati knee rating score (51.7 to 84.6), and IKDC score (50.5 to 78.4). The authors concluded that addressing subchondral bone loss combined with a cell based procedure was feasible in one step. Simultaneous remodeling of the articular cartilage and subchondral lamina was noted. However, this synchronization in not yet completely understood.
CONCLUSION
The management of articular lesions of the knee remains a challenging problem for orthopaedic surgeons. Significant improvements have been made with cell based repair strategies. The MACI technique provides hyaline-like cartilage repair with potential decreased operative times and arthroscopic implantation. The potential for reduced long term morbidity and the available mid to long term follow up in the literature makes this an attractive procedure. In general, short and medium-term clinical outcomes suggest symptomatic improvement in patients. Additional long term studies are needed to further validate the efficacy of the MACI procedure. Nonetheless, the MACI procedure appears to be a promising cell based technique at restoring hyaline-like cartilage.
Acknowledgments
We thank Dr. Peter Verdonk for graciously providing us with the image for Figure 2.
Footnotes
Conflict of Interest:
- Brad S Dunkin: None
-
Christian Lattermann:
- Sanofi/Genzyme: consultant
- Zimmer: consultant, member of DSMB (Denovo ET)
- icartilage.com: consultant
- Smith & Nephew: Institutional Support
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hjelle K, Solheim E, Strand T, et al. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18:730–734. doi: 10.1053/jars.2002.32839. [DOI] [PubMed] [Google Scholar]
- 2.Noyes FR, Bassett RW, Grood ES, et al. Arthroscopy in acute traumatic hemarthrosis of the knee. Incidence of anterior cruciate tears and other injuries. J Bone Joint Surg Am. 1980;62:687–695. [PubMed] [Google Scholar]
- 3.Jacobi M, Wahl P, Bouaicha S, et al. Association between mechanical axis of the leg and osteochondritis dissecans of the knee: radiographic study on 103 knees. Am J Sports Med. 2010;38:1425–1428. doi: 10.1177/0363546509359070. [DOI] [PubMed] [Google Scholar]
- 4.Widuchowski W, Lukasik P, Kwiatkowski G, et al. Isolated full thickness chondral injuries. Prevalance and outcome of treatment. A retrospective study of 5233 knee arthroscopies. Acta Chir Orthop Traumatol Cech. 2008;75:382–386. [PubMed] [Google Scholar]
- 5.Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. [PubMed] [Google Scholar]
- 6.Bedi A, Feeley BT, Williams RJ. Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:994–1009. doi: 10.2106/JBJS.I.00895. [DOI] [PubMed] [Google Scholar]
- 7.Browne JE, Anderson AF, Arciero R, et al. Clinical outcome of autologous chondrocyte implantation at 5 years in US subjects. Clin Orthop Relat Res. 2005;436:237–245. doi: 10.1097/00003086-200507000-00036. [DOI] [PubMed] [Google Scholar]
- 8.Farr J. Autologous chondrocyte implantation improves patellofemoral cartilage treatment outcomes. Clin Orthop Relat Res. 2007;463:187–194. [PubMed] [Google Scholar]
- 9.Marlovits S, Zeller P, Singer P, et al. Cartilage repair: generations of autologous chondrocyte transplantation. Eur J Radiol. 2006;57:24–31. doi: 10.1016/j.ejrad.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Peterson L, Brittberg M, Kiviranta I, et al. Autologous chondrocyte transplantation: biomechanics and long-term durability. Am J Sports Med. 2002;30:2–12. doi: 10.1177/03635465020300011601. [DOI] [PubMed] [Google Scholar]
- 11.Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 12.Bartlett W, Krishnan SP, Skinner JA, et al. Collagen-covered versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a comparison of tourniquet times. Eur J Orthop Surg Traumatol. 2006;16:315–317. [Google Scholar]
- 13.Gomoll AH, Probst C, Farr J, et al. Use of a type I/III bilayer collagen membrane decreases reoperation rates for symptomatic hypertrophy after autologous chondrocyte implantation. Am J Sports Med. 2009;37(suppl 1):20S–23S. doi: 10.1177/0363546509348477. [DOI] [PubMed] [Google Scholar]
- 14.Kreuz PC, Steinwachs M, Erggelet C, et al. Classification of graft hypertrophy after autologous chondrocyte implantation of full-thickness chondral defects in the knee. Osteoarthritis Cartilage. 2007;15:1339–1347. doi: 10.1016/j.joca.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 15.Marlovits S, Striessnig G, Kutscha-Lissberg F, et al. Early postoperative adherence of matrix-induced autologous chondrocyte implantation for the treatment of full-thickness cartilage defects of the femoral condyle. Knee Surg Sports Traumatol Arthrosc. 2005;13:451–457. doi: 10.1007/s00167-004-0535-3. [DOI] [PubMed] [Google Scholar]
- 16.Cherubino P, Grassi FA, Bulgheroni P, et al. Autologous chondrocyte implantation using a bilayer collagen membrane: a preliminary report. J Orthop Surg. 2003;11:10–15. doi: 10.1177/230949900301100104. [DOI] [PubMed] [Google Scholar]
- 17.Gooding CR, Bartlett W, Bentley G, et al. A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: periosteum covered versus type I/III collagen covered. Knee. 2006;13:203–210. doi: 10.1016/j.knee.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Haddo O, Mahroof S, Higgs D, et al. The use of chondrogide membrane in autologous chondrocyte implantation. Knee. 2004;11:51–55. doi: 10.1016/S0968-0160(03)00041-3. [DOI] [PubMed] [Google Scholar]
- 19.Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med. 2010;38:1259–1271. doi: 10.1177/0363546509346395. [DOI] [PubMed] [Google Scholar]
- 20.Jacobi M, Villa V, Magnussen RA, et al. MACI - a new era? Sports Med Arthrosc Rehabil Ther Technol. 2011;3:10. doi: 10.1186/1758-2555-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gobbi A, Kon E, Berruto M, et al. Patellofemoral full-thickness chondral defects treated with Hyalograft-C: a clinical, arthroscopic, and histologic review. Am J Sports Med. 2006;34:1763–73. doi: 10.1177/0363546506288853. [DOI] [PubMed] [Google Scholar]
- 22.Marcacci M, Berruto M, Brocchetta D, et al. Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin Orthop Relat Res. 2005;435:96–105. doi: 10.1097/01.blo.0000165737.87628.5b. [DOI] [PubMed] [Google Scholar]
- 23.Bartlett W, Gooding CR, Carrington RWJ, et al. Autologous chondrocyte implantation at the knee using a bilayer collagen membrane with bone graft: a preliminary report. J Bone Joint Surg Br. 2005;87:330–332. doi: 10.1302/0301-620x.87b3.15552. [DOI] [PubMed] [Google Scholar]
- 24.Behrens P, Bitter T, Kurz B, et al. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI): 5-year follow-up. Knee. 2006;13:194–202. doi: 10.1016/j.knee.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 25.D’Anchise R, Manta N, Prospero E, et al. Autologous implantation of chondrocytes on a solid collagen scaffold: clinical and histological outcomes after two years of follow-up. J Orthop Traumatol. 2005;6:36–43. [Google Scholar]
- 26.Zhang Z, Ye Q, Yang Z, et al. Matrix-induced autologous chondrocyte implantation for treatment of chondral defects of knee: a preliminary report. J Musculoskelet Res. 2006;10:95–101. [Google Scholar]
- 27.Filardo G, Vannini F, Marcacci M, et al. Matrix-assisted autologous chondrocyte transplantation for cartilage regeneration in osteoarthritic knees: resutls and failures at midterm follow-up. Am J Sports Med. doi: 10.1177/0363546512463675. (in press) [DOI] [PubMed] [Google Scholar]
- 28.Noyes FR, Bassett RW, Grood ES, et al. Arthroscopy in acute traumatic hemarthrosis of the knee. Incidence of anterior cruciate tears and other injuries. J Bone Joint Surg Am. 1980;62:687–695. [PubMed] [Google Scholar]
- 29.Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87:640–645. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 30.Ochs BG, Muller-Horvat C, Albrecht D, et al. Remodeling of articular cartilage and subchondral bone after bone grafting and matrix-associated autologous chondrocyte implantation for osteochondritis dissecans of the knee. Am J Sports Med. 2011;39:764–773. doi: 10.1177/0363546510388896. [DOI] [PubMed] [Google Scholar]
- 31.Potter HG, Chongle R. Magnetic resonance imaging assessment of chondral lesions and repair. J Bone Joint Surg Am. 2009;91(suppl 1):126–131. doi: 10.2106/JBJS.H.01386. [DOI] [PubMed] [Google Scholar]
- 32.Potter HG, Foo LF. Magnetic resonance imaging of articular cartilage: trauma, degeneration, and repair. Am J Sports Med. 2006;34:661–677. doi: 10.1177/0363546505281938. [DOI] [PubMed] [Google Scholar]
- 33.Kijowski R, Blankenbaker DG, Munoz Del Rio A, et al. Evaluation of the articular cartilage of the knee joint: value of adding a T2 mapping sequence to a routine MR imaging protocol. Radiology. doi: 10.1148/radiol.12121413. (in press) [DOI] [PubMed] [Google Scholar]
- 34.Abelow SP, Guillen P, Ramos T. Arthroscopic technique for matrix-induced autologous chondrocyte implantation for the treatment of large chondral defects in the knee and ankle. Oper Tech Orthop. 2006;16:257–261. [Google Scholar]
- 35.Bachmann G, Basad E, Lommel D, et al. MRI in the follow-up of matrix-supported autologous chondrocyte transplantation (MACI) and microfracture. Radiologe. 2004;44:773–782. doi: 10.1007/s00117-004-1084-y. [DOI] [PubMed] [Google Scholar]
- 36.Cortese F, McNicholas M, Janes G, et al. Arthroscopic delivery of the matrix-induced autologous chondrocyte implant: international experience and technique recommendations. Cartilage. 2012;3:156–164. doi: 10.1177/1947603511435271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minas T, Peterson L. Advanced techniques in autologous chondrocyte transplantation. Clin Sports Med. 1999;18:13–44. doi: 10.1016/s0278-5919(05)70128-9. [DOI] [PubMed] [Google Scholar]
- 38.Gillogly S, Voight M, Blackburn B. Treatment of articular cartilage defects of the knee with autologous chondrocyte implantation. J Orthop Sports Phys Ther. 1998;28:241–251. doi: 10.2519/jospt.1998.28.4.241. [DOI] [PubMed] [Google Scholar]
- 39.O’Driscoll SW, Keeley FW, Salter RB, et al. Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion. J Bone Joint Surg Am. 1988;70:595–606. [PubMed] [Google Scholar]
- 40.O’Driscoll SW, Salter R. The induction of neochondrogenesis in free intra-articular periosteal autografts under the influence of continuous passive motion. J Bone Joint Surg Am. 1984;66:1248–1257. [PubMed] [Google Scholar]
- 41.Minas T, Peterson L. Autologous Chondrocyte Implantation. Oper Tech Sports Med. 2000;8:144–157. [Google Scholar]
- 42.Marlovits S, Aldrian S, Wondrasch B, et al. Clinical and radiological outcomes 5 years after matrix-induced autologous chondrocyte implantation in patients with symptomatic, traumatic chondral defects. Am J Sports Med. 2012;40:2273–2280. doi: 10.1177/0363546512457008. [DOI] [PubMed] [Google Scholar]
- 43.Rodrigo JJ, Steadman RJ, Fulstone HA. Improvement of full-thickness chondral defect healing in the human knee after debridement and microfracture using continuous passive motion. Am J Knee Surg. 1994;7:109–116. [Google Scholar]
- 44.Basad E, Ishaque B, Bachmann G, et al. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010;18:519–527. doi: 10.1007/s00167-009-1028-1. [DOI] [PubMed] [Google Scholar]
- 45.Ebert JR, Robertson WB, Woodhouse J, et al. Clinical and magnetic resonance imaging-based outcomes to 5 years after matrix-induced autologous chondrocyte implantation to address articular cartilage defects in the knee. Am J Sports Med. 2011;39:753–763. doi: 10.1177/0363546510390476. [DOI] [PubMed] [Google Scholar]
- 46.Kon E, Filardo G, Berruto M, et al. Articular cartilage treatment in high-level male soccer players: a prospective comparative study of arthroscopic second-generation autologous chondrocyte implantation versus microfracture. Am J Sports Med. 2011;39:2549–2557. doi: 10.1177/0363546511420688. [DOI] [PubMed] [Google Scholar]
- 47.Kon E, Gobbi A, Filardo G, et al. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med. 2009;37:33–41. doi: 10.1177/0363546508323256. [DOI] [PubMed] [Google Scholar]
- 48.Zeifang F, Oberle D, Nierhoff C, et al. Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am J Sports Med. 2010;38:924–933. doi: 10.1177/0363546509351499. [DOI] [PubMed] [Google Scholar]
- 49.Freed LE, Marquis JC, Nohria A, et al. Neocartilage formation in vitro and in vivo using cells cultured on synthetic biodegradable polymers. J Biomed Mater Res. 1993;27:11–23. doi: 10.1002/jbm.820270104. [DOI] [PubMed] [Google Scholar]
- 50.Grigolo B, Lisignoli G, Piacentini A, et al. Evidence for redifferentiation of human chondrocytes grown on a hyaluronan-based biomaterial (HYAff 11): molecular, immunohistochemical and ultrastructural analysis. Biomaterials. 2002;23:1187–1195. doi: 10.1016/s0142-9612(01)00236-8. [DOI] [PubMed] [Google Scholar]


