Abstract
Purpose
During chemotherapy, women with breast cancer not only experience poor quality of life (QOL), they also have little exposure to bright light, which has been shown to be associated with depression, fatigue and poor sleep in other chronic illnesses. This study examined whether increased light exposure would have a positive effect on QOL.
Methods
39 women with Stage I–III breast cancer scheduled to receive ≥4 cycles of chemotherapy were randomized to a bright white light (BWL, n=23) or dim red light (DRL, n=16) treatment group. Data were collected before (baseline) and during cycles 1 and 4 of chemotherapy. Light was administered via a light box (Litebook®, Ltd.). QOL was assessed with the Functional Assessment of Cancer Therapy-Breast (FACT-B) and the Functional Outcomes of Sleep Questionnaire (FOSQ).
Results
Compared to baseline, the DRL group demonstrated significant decline in QOL during the treatment weeks of both cycles (all p’s<0.02), whereas the BWL group had no significant decline (all p’s>0.05). Mixed model analyses revealed that there was a group-by-time interaction for FOSQ at the treatment week of cycle 4 and this interaction was mediated by fatigue.
Conclusion
The data suggest that increased exposure to bright light during chemotherapy may prevent the decline in QOL via preventing the increase of fatigue.
Keywords: breast cancer, chemotherapy, light therapy, quality of life, fatigue, depression
Introduction
Oncological treatments have a strong impact on patients’ QOL [1, 2]. Decreased QOL has been reported in patients undergoing chemotherapy and in some survivors of breast cancer after chemotherapy [3, 4, 5]. However, chemotherapy itself likely is not the sole cause of decreased QOL, as the stress from a diagnosis of cancer and other related problems are also associated with decreased QOL [6]. For example, fatigue and not chemotherapy was reported as the strongest predictor of QOL [7].
Women are exposed to little bright light both before and during chemotherapy for breast cancer, and this reduced light exposure is associated with more fatigue [8]. Bright light is not only one of the strongest synchronizers of circadian rhythms, it also has an alerting effect and improves sleep and depression in other populations [9, 10, 11, 12]. Such symptoms as fatigue, poor sleep and depression usually result in poor QOL.
Our laboratory has shown that bright light therapy keeps fatigue [13] and sleep [14] from deteriorating. This study examined whether increasing bright light exposure might also improve QOL in women with breast cancer undergoing chemotherapy.
Methods
Subjects
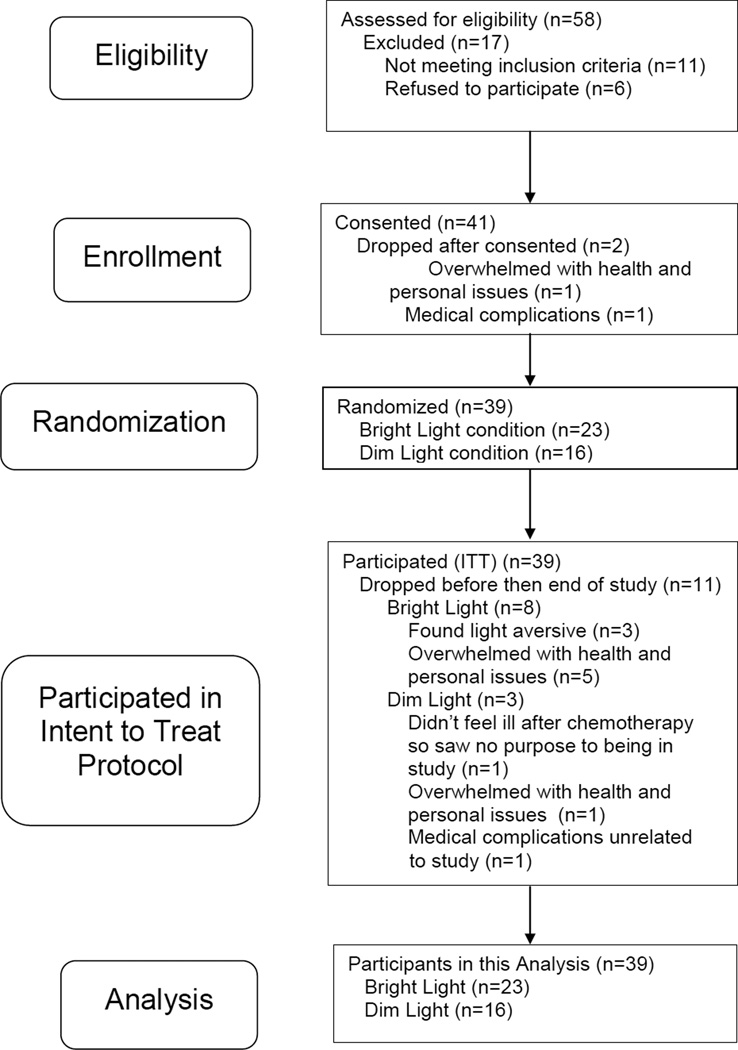
Thirty-nine women participated in this study (Figure 1). Inclusion criteria were: women 18 years or older, stage I–III breast cancer, scheduled for adjuvant/neoadjuvant chemotherapy, able to commit to daily morning self-administered light treatment, and fluent in English. Exclusion criteria were: shift workers, pregnant, or with physical or mental impairments. The study was approved by the UCSD Committee on the Protection of Human Subjects and the UCSD Moores Cancer Center.
Figure 1.
Consort table of participant recruitment and retention
Procedure
Once written informed consent was obtained, woman were randomized according to a statistically driven randomization table into either the bright white light (BWL; n=23) or the dim red light (DRL; n=16) group. A larger proportion was randomized to BWL group to allow for a larger sample treated with this potentially beneficial, noninvasive treatment. Each participant completed questionnaires on sleep, fatigue, depression and QOL and wore an actigraphy for 72 hours at five time points: before starting chemotherapy (baseline), during the treatment weeks of cycles 1 and 4 (C1TW, C4TW) and the last weeks (recovery week) of cycles 1 and 4 (C1RW, C4RW).
Both BWL and DRL treatments were administered via identical-appearing light boxes (Litebook®, Ltd. Medicine Hat, Canada), which emit either 10,000 lux of BWL or less than 50 lux of DRL. Detailed information for the light boxes and their applications were reported elsewhere [15].
Measures
Quality of life
QOL was assessed with the Functional Assessment of Cancer Therapy-Breast (FACT-B) and the Functional Outcomes of Sleep Questionnaire (FOSQ).
The 36-item FACT-B measures the effect of having breast cancer on functional status. The total score ranges between 0–144, with lower scores indicating worse breast-cancer-related QOL [16, 17].
The 30-item FOSQ is designed to measure functional status in situations that produce sleepiness. Its weighted total score ranges between 0–20. Lower scores indicate worse sleepiness-related QOL [18].
Depressive symptoms
Depressive symptoms were assessed with the 20-item Center for Epidemiologic Studies-Depression scale (CES-D). The total score ranges between 0–60, with higher scores representing more symptoms of depression [19].
Fatigue and sleep
Detailed information on fatigue and sleep are reported elsewhere [14, 15]. Briefly, fatigue was measured with the Multidimensional Fatigue Symptom Inventory and sleep was objectively measured with actigraphy. For the current analysis, total sleep time (TST) and total nap time (NAPTIME) were analyzed [20].
Statistical Analyses
Summary statistics (means, SEM for continuous variables, and proportions for categorical variables) were computed for all measures. T-tests and Fisher’s exact tests were used to compare those measures between treatment arms.
Mixed effects models were used to assess the effect of light treatment over time. This approach allowed adjustment for potential confounders and also permitted examination of the time-trend of outcomes in the two treatment arms. As indicated by residual plots of goodness of fit, total FOSQ scores were cubic-transformed to improve approximation to a Gaussian distribution. A subject-specific intercept term was included in the models to allow for individual variability in outcome. Treatment group, time, and group-by-time interaction were modeled as fixed effects. A significant group-by-time interaction would indicate that changes of measures over time were different in the two treatment arms. For QOL indices which exhibited a group-by-time interaction, a series of mediation analyses [21, 22] would be conducted to examine if this interaction was mediated by other symptoms.
Results
There were no significant differences between the two groups in terms of the demographic and medical characteristics listed in Table 1, so no confounders were adjusted in the mixed model analyses.
Table 1.
Demographic and medical characteristics at baseline
| Bright Light Group (N=23) |
Dim Light Group (N=16) |
p-values | |
|---|---|---|---|
| Age (mean yrs [SD]) | 54.3 (9.3) | 53.5 (9.0) | 0.8 |
| Ethnicity/Race (n [%]) | 0.9 | ||
| Caucasian | 15 (65.2) | 13 (81.3) | |
| African American Black | 4 (17.4) | 2 (12.4) | |
| Asian | 2 (8.7) | 1 (6.3) | |
| Other | 2 (8.7) | ||
| Education (n [%]) | 0.9 | ||
| Some high school or less | 1 (4.4) | 0 | |
| Completed high school | 1 (4.4) | 0 | |
| Some college | 6 (26.1) | 6 (37.5) | |
| College degree | 8 (34.7) | 4 (25) | |
| Graduate degree | 7 (30.4) | 6 (37.5) | |
| Annual Family Income (n [%]) | 0.2 | ||
| ≤ $15,000 | 5 (21.7) | 3 (18.8) | |
| ≤ $30,000 | 6 (26.1) | 0 | |
| ≤ $50,000 | 1 (4.4) | 2 (12.5) | |
| ≤ 100,000 | 4 (17.4) | 2 (12.5) | |
| > 100,000 | 5 (21.7) | 6 (37.4) | |
| Did not answer | 2 (8.7) | 3 (18.8) | |
| Marital Status (n [%]) | 0.9 | ||
| Never Married | 1 (4.4) | 1 (6.3) | |
| Divorced | 7 (30.3) | 3 (18.7) | |
| Widowed | 2 (8.7) | 1 (6.3) | |
| Married | 13 (56.6) | 11 (68.7) | |
| Cancer Stage | 0.6 | ||
| Stage I | 4 (17.4) | 5 (31.3) | |
| Stage II | 10 (43.5) | 6 (37.5) | |
| Stage III | 4 (17.4) | 2 (12.5) | |
| Unknown | 5 (21.7) | 3 (18.7) | |
| Chemotherapy regimen | 0.5 | ||
| AC | 3 (15.0) | 5 (20.0) | |
| AC + docetaxel | 8 (35.0) | 4 (26.7) | |
| AC + paclitaxel | 6 (30.0) | 3 (13.3) | |
| Other | 3 (20.0) | 4 (40.0) | |
| Not available | 3 | 0 | |
Note: AC = Doxorubicin + Cyclophosphamide
Quality of life
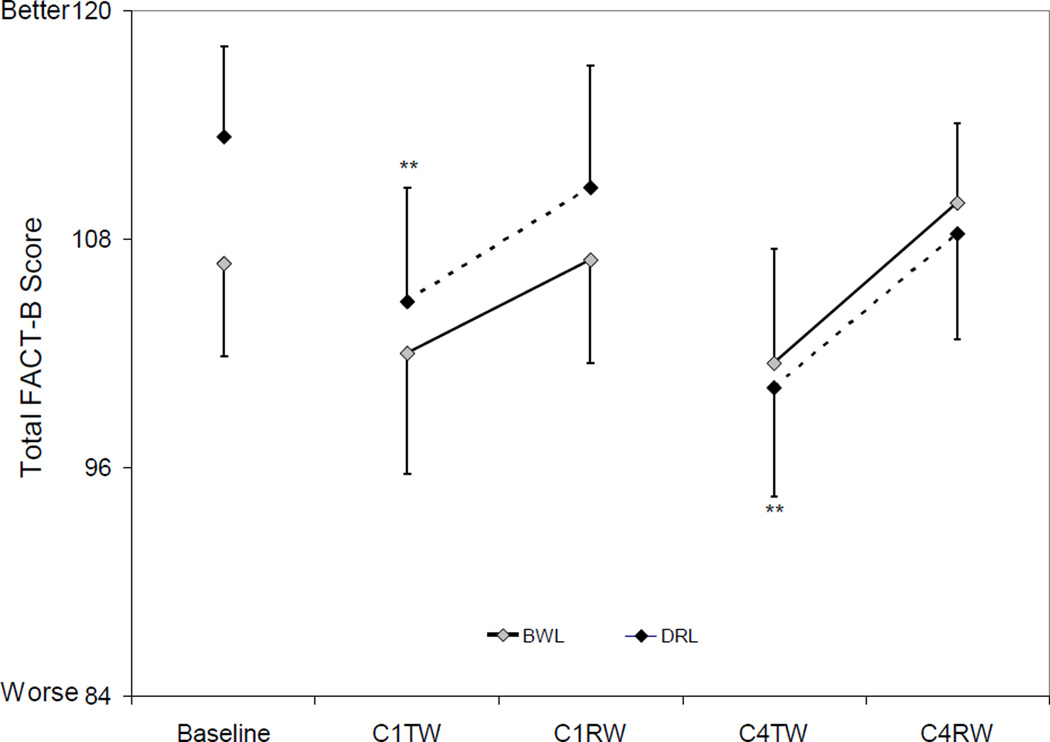
FACT-B (Figure 2)
Figure 2. Total FACT-B scores by group and time.
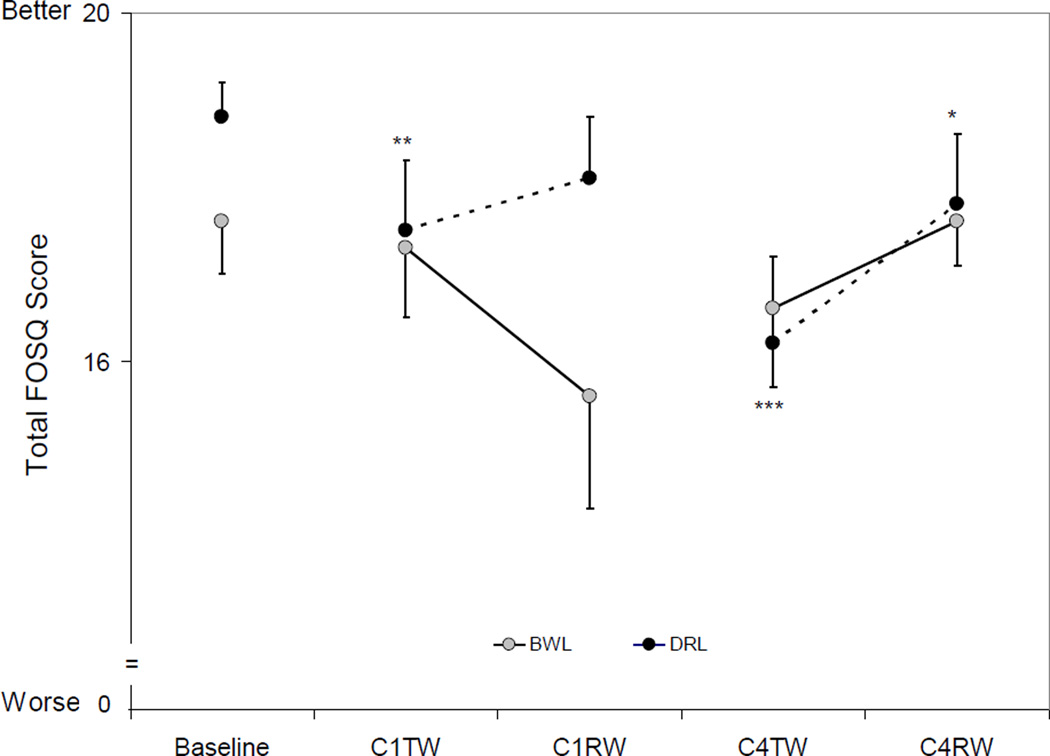
Total FOSQ Scores (mean ± SEM) at Baseline and During Chemotherapy. The error bars are depicted as being one tailed to avoid overlap across measures. There was an overall time effect for the DRL group (p<0.0001) but not for the BWL group. There was a group-by-time interaction at C4TW (p=0.03). Compared to baseline, total cubic-transformed FOSQ scores decreased significantly at C1TW, C4TW and C4RW in the DRL group. Compared to baseline: * p<0.05, ** p<0.01, *** p<0.0001.
There was an overall time effect for the DRL group (p=0.001) but not for the BWL group (p=0.2). Total FACT-B scores in the DRL group decreased significantly from baseline to C1TW (p=0.004) and C4TW (p=0.0004), while the BWL group had no significant changes from baseline (all p’s>0.08).
There was no significant group-by-time interaction in total FACT-B scores at any time point (all p’s>0.2).
FOSQ (Figure 3)
Figure 3. Total FOSQ scores by group and time.
Total FACT-B Scores (mean ± SEM) at Baseline and During Chemotherapy. The error bars are depicted as being one tailed to avoid overlap across measures. There was an overall time effect for the DRL group (p=0.001) but not for the BWL group. Compared to baseline, total FACT-B scores decreased significantly at C1TW and C4TW in the DRL group while no significant changes in the BWL group. Compared to baseline: ** p<0.01.
There was an overall time effect for the DRL group (p<0.0001) but not for the BWL group (p=0.6). Total FOSQ scores in the DRL group decreased significantly from baseline to C1TW (p=0.0006), C4TW (p<0.0001) and C4RW (p=0.04), while scores in the BWL group did not show any significant changes.
Mixed model analysis revealed a significant group-by-time interaction at C4TW (p=0.04) for total FOSQ scores, indicating that decreases from baseline in the DRL group was significantly higher than that in the BWL group at C4TW. There was no significant group-by-time effect at any other time point (all p’s>0.1).
Since we had previously observed a bright light treatment effect on fatigue [13] and actigraphy-measured TST and NAPTIME [14], a series of secondary mixed model analyses were conducted to examine if the light-treatment associated changes of total FOSQ scores were mediated by changes of fatigue or sleep. The results showed that changes of FOSQ from baseline to C4TW were significantly associated with changes in fatigue (p<0.0001); when fatigue was added to the mixed model, the group-by-time interaction of FOSQ at C4TW was no longer significant (p=0.6). These results suggest that the mechanism of light therapy on QOL was primarily through its impact in preventing worse fatigue.
When objectively measured sleep was examined, changes of FOSQ from baseline to C4TW were not significantly associated with changes in TST and NAPTIME, so no further mediation analyses were conducted.
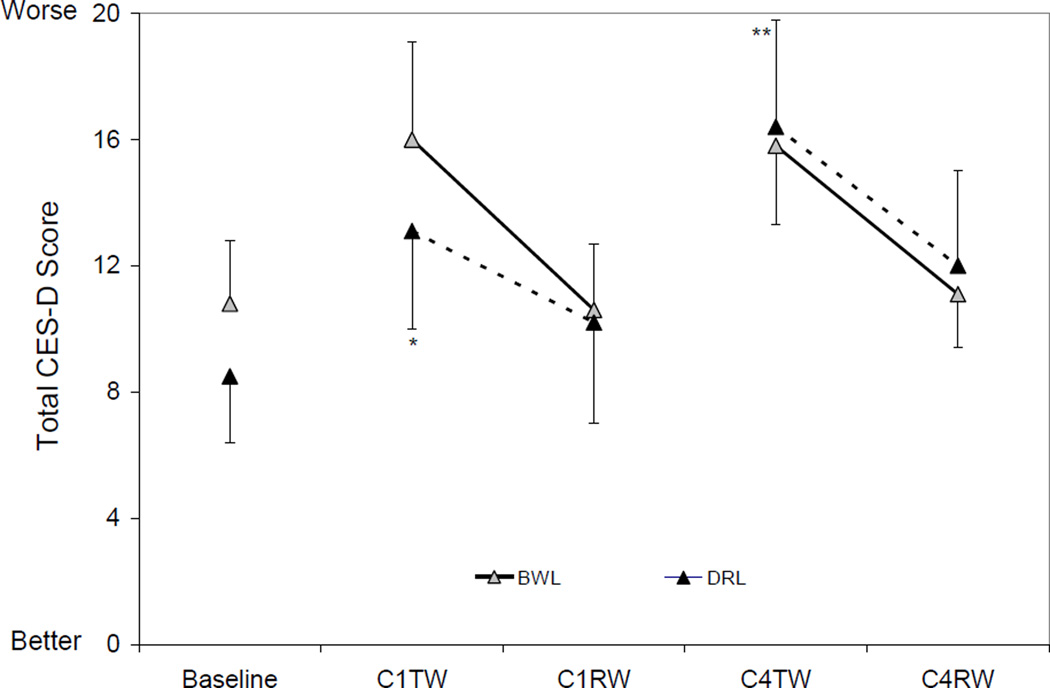
Depressive symptoms (Figure 4)
Figure 4. Total CES-D scores by group and time.
Total CES-D Scores (mean ± SEM) at Baseline and During Chemotherapy. The error bars are depicted as being one tailed to avoid overlap across measures. There was an overall time effect for the DRL group (p=0.005) but not for the BWL group. Compared to baseline, the total CES-D scores decreased significantly at C1TW and at C4TW in the DRL group. Compared to baseline: * p<0.05, ** p<0.01.
There was an overall time effect for the DRL group (p=0.005) but not for the BWL group (p=0.1). Total CES-D scores in the DRL group increased significantly from baseline to C1TW (p=0.02) and C4TW (p=0.0007) while that in the BWL group had no significant changes (all p’s>0.05).
There was no significant group-by-time interaction in total CES-D scores at any time point during treatment (all p’s>0.3), thus, depression was not tested as a mediator for QOL.
Discussion
This pilot study showed that women exposed to BWL did not demonstrate a significant decline in QOL, whereas those women exposed to DRL had significantly worse QOL during chemotherapy. Furthermore, exploratory mediation analyses suggested that the effect of light therapy on QOL occurred via the effect of bright light on preventing deterioration of fatigue, rather than the effect of bright light on depressive symptoms or sleep.
The results also show that compared to baseline, women exposed to BWL did not report low breast-cancer-related QOL (total FACT-B scores) or more symptoms of depression (total CES-D scores) during chemotherapy, while those exposed to DRL reported lower QOL and more depressive symptoms. However, there was no significant group-by-time interaction, hence we cannot conclude a definitive impact of light therapy on these two measures.
Various treatment modalities have been studied in women with breast cancer to improve quality of life [23, 24, 25], but light therapy to date has not been studied or implemented as a means of improving QOL in these women. Findings from this study suggest that bright light may be useful in protecting women from decline in QOL during chemotherapy by protecting women from experiencing more fatigue. The possible mechanism might be that bright light breaks the association between low light exposure and increased fatigue [8, 13].
Although data from this study did not identify a mediation effect of depression and sleep problems on QOL, given that light therapy has been reported to be effective in the treatment of depression [26, 27] and sleep problems [28, 29], studies exploring weather light therapy could improve QOL via the effect of improving depression or sleep in cancer patients are warranted.
Limitations of this study include a small sample size, which could have limited study power to detect more significant findings. This small sample-size also limited our ability to examine multiple meditational paths; hence these mediation results should be interpreted cautiously. Follow-up data were not collected in this study, so long term effect of bright light on QOL could not be determined.
In summary, women with breast cancer undergoing chemotherapy have a decline in overall QOL. Exposure to bright light therapy thwarts this decline by preventing fatigue from deteriorating. Future studies with larger sample sizes should be conducted to further assess the effects of light treatment during and after chemotherapy. In the meantime, oncologists might suggest that patents undergoing chemotherapy to increase their exposure to bright light either by going outside in the morning or by using a light box to improve QOL, as this treatment is safe, inexpensive, easy to use, and may be less time-consuming and more convenient than other interventions, such as attending social support groups or exercising.
Acknowledgments
Supported by California Breast Cancer Research Program11IB-0034, a grant from Litebook, Inc, NCI CA112035, UL1RR031980 (CTRI), The UCSD Moores Cancer Center (NCI P30 CA23100), Department of Veterans Affairs Center of Excellence for Stress and Mental Health (CESAMH), the Stein Institute for Research on Aging, .and the National Institute on Aging T35 grant AG026757.
Abbreviations
- QOL
Quality of Life
- UCSD
University of California, San Diego
- BWL
Bright White Light
- DRL
Dim Red Light
- FACT-B
Functional Assessment of Cancer Therapy-Breast
- FOSQ
Functional Outcomes of Sleep Questionnaire
- CES-D
Center for Epidemiologic Studies Depression Scale
- TST
Total Sleep Time
- NAPTIME
Total Nap Time
- C1TW
Chemotherapy Treatment Week of cycle 1
- C1RW
Last Week (recovery week) of cycle 1
- C4TW
Chemotherapy Treatment Week of cycle 4
- C4RW
Last Week (recovery week) of cycle 4
References
- 1.Greimel E, Thiel I, Peintinger F, Cegnar I, Pongratz E. Prospective assessment of quality of life of female cancer patients. Gynecologic oncology. 2002;85(1):140–147. doi: 10.1006/gyno.2002.6586. [DOI] [PubMed] [Google Scholar]
- 2.Lutgendorf SK, Anderson B, Rothrock N, Buller RE, Sood AK, Sorosky JI. Quality of life and mood in women receiving extensive chemotherapy for gynecologic cancer. Cancer. 2000;89(6):1402–1411. [PubMed] [Google Scholar]
- 3.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J. Clin. Oncol. 2000;18(4):743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 4.Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. Journal of the National Cancer Institute. 2002;94(1):39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 5.Watters JM, Yau JC, OÆrourke K, Tomiak E, Gertler SZ. Functional status is well maintained in older women during adjuvant chemotherapy for breast cancer. Annals of Oncology. 2003;14(12):1744–1750. doi: 10.1093/annonc/mdg497. [DOI] [PubMed] [Google Scholar]
- 6.Yen JY, Ko CH, Yen CF, Yang MJ, Wu CY, Juan CH, et al. Quality of life, depression, and stress in breast cancer women outpatients receiving active therapy in Taiwan. Psychiatry Clin Neurosci. 2006;60(2):147–153. doi: 10.1111/j.1440-1819.2006.01479.x. [DOI] [PubMed] [Google Scholar]
- 7.Arndt V, Stegmaier C, Ziegler H, Brenner H. A population based study of the impact of specific symptoms on quality of life in women with breast cancer 1 year after diagnosis. Cancer. 2006;107(10):2496–2503. doi: 10.1002/cncr.22274. [DOI] [PubMed] [Google Scholar]
- 8.Liu L, Marler M, Parker BA, Jones V, Johnson S, Cohen-Zion M, et al. The relationship between fatigue and light exposure during chemotherapy. Support. Care Cancer. 2005;13(12):1010–1017. doi: 10.1007/s00520-005-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dijk DJ, Boulos Z, Eastman CI, Lewy AJ, Campbell SS, Terman M. Light Treatment for Sleep Disorders: Consensus report. II. Basic properties of circadian physiology and sleep regulation. Journal of Biological Rhythms. 1995;10(2):113–125. doi: 10.1177/074873049501000204. [DOI] [PubMed] [Google Scholar]
- 10.Terman M. Light treatment for sleep disorders: Consensus report. J Biol Rhythms. 1995;10:99–176. doi: 10.1177/074873049501000208. [DOI] [PubMed] [Google Scholar]
- 11.Eastman CI, Boulos Z, Terman M, Campbell SS, Dijk DJ, Lewy AJ. Light Treatment for Sleep Disorders: Consensus report. VI. Shift work. Journal of Biological Rhythms. 1995;10(2):157–164. doi: 10.1177/074873049501000208. [DOI] [PubMed] [Google Scholar]
- 12.Boulos Z, Campbell SS, Lewy AJ, Terman M, Dijk DJ, Eastman CI. Light Treatment for Sleep Disorders: Consensus report. VII. Jet lag. Journal of Biological Rhythms. 1995;10(2):167–176. doi: 10.1177/074873049501000209. [DOI] [PubMed] [Google Scholar]
- 13.Ancoli-Israel S, Rissling M, Neikrug AB, Trofimenko V, Natajaran L, Parker BA, et al. Light treatment prevents fatigue in women undergoing chemotherapy for breast cancer. Support. Care Cancer. 2011;20:1211–1219. doi: 10.1007/s00520-011-1203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ancoli-Israel S, Rissling M, Trofimenko V, Parker BA. Preliminary effects of bright light on sleep in women with breast cancer. Journal of Clinical Oncology. 2007;25:9094. [Google Scholar]
- 15.Neikrug AB, Rissling M, Trofimenko V, Liu L, Natajaran L, Lawton S, et al. Bright light therapy protects women from circadian rhythm desynchronization during chemotherapy for breast cancer. Behavioral Sleep Medicine. 2012;10(3):202–216. doi: 10.1080/15402002.2011.634940. [DOI] [PubMed] [Google Scholar]
- 16.Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-B (FACT-B) quality of life instrument. Journal of Clinical Oncology. 1997;15(3):974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 17.Cella DF. Quality of life outcomes: measurement and validation. Oncology. 1996;10(11) Suppl:233–246. [PubMed] [Google Scholar]
- 18.Weaver TE, Laizner AM, Evans LK, Maislin G, Chugh DK, Lyon K, et al. An instrument to measure functional status outcome for disorders of excessive sleepiness. Sleep. 1997;20(10):835–843. [PubMed] [Google Scholar]
- 19.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychol. Measurement. 1977;1:385–401. [Google Scholar]
- 20.Liu L, Rissling M, Natajaran L, Fiorentino L, Mills PJ, Dimsdale JE, et al. The Longitudinal Relationship between Fatigue and Sleep in Breast Cancer Patients Undergoing Chemotherapy. Sleep. 2012;35(2):237–245. doi: 10.5665/sleep.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociological Methodology. 1982;13(1982):290–312. [Google Scholar]
- 22.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers. Soc. Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro SL, Lopez AM, Schwartz GE, Bootzin R, Figueredo AJ, Braden CJ, et al. Quality of life and breast cancer: relationship to psychosocial variables. Journal of clinical psychology. 2001;57(4):501–519. doi: 10.1002/jclp.1026. [DOI] [PubMed] [Google Scholar]
- 24.Bradt J, Goodill SW, Dileo C. Dance/movement therapy for improving psychological and physical outcomes in cancer patients. Cochrane. Database. Syst. Rev. 2011;(10):CD007103. doi: 10.1002/14651858.CD007103.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Galantino ML, Desai K, Greene L, Demichele A, Stricker CT, Mao JJ. Impact of Yoga on Functional Outcomes in Breast Cancer Survivors With Aromatase Inhibitor-Associated Arthralgias. Integr. Cancer Ther. 2011 doi: 10.1177/1534735411413270. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 26.Golden RN, Gaynes BN, Ekstrom RD. The Efficacy of Light Therapy in the Treatment of Mood Disorders: A Review and Meta-Analysis of the Evidence. Am J Psychiatry. 2005;162:656–662. doi: 10.1176/appi.ajp.162.4.656. [DOI] [PubMed] [Google Scholar]
- 27.Terman M. Evolving applications of light therapy. Sleep Med Rev. 2008;11(6):497–507. doi: 10.1016/j.smrv.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Chesson A, Littner M, Davila DG, Anderson WM, Grigg-Damberger M, Hartse KM, et al. Practice parameters for the use of light therapy in the treatment of sleep disorders. Sleep. 1999;22(5):641–660. doi: 10.1093/sleep/22.5.641. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi R, Kohsaka M, Fukuda N, Sakakibara S, Honma H, Koyama T. Effects of morning bright light on sleep in healthy elderly women. Psychiatry Clin Neurosci. 1999;53(2):237–238. doi: 10.1046/j.1440-1819.1999.00486.x. [DOI] [PubMed] [Google Scholar]