Abstract
The diagnosis of insomnia is based solely on subjective complaints. This has contributed to the low reliability and validity of the current nosology of insomnia as well as to its lack of firm association with clinically relevant outcomes such as cardiometabolic and neurocognitive morbidity. We review evidence that insomnia with objective short sleep duration is associated with physiological hyperarousal, higher risk for hypertension, diabetes, neurocognitive impairment, and mortality as well as with a persistent course. It also appears that objective short sleep duration in poor sleepers is a biological marker of genetic predisposition to chronic insomnia. In contrast, insomnia with objective normal sleep duration is associated with cognitive-emotional and cortical arousal and sleep misperception but not with signs of physiological hyperarousal or medical complications. Thus, short sleep duration in insomnia may be a reliable marker of the biological severity and medical impact of the disorder. We propose that (a) objective measures of sleep be included in the diagnosis of insomnia and its subtypes, (b) objective measures of sleep obtained in the home environment of the patient would become part of the routine assessment and diagnosis of insomnia in a clinician’s office setting, and (c) insomnia with short sleep duration may respond better to biological treatments, whereas insomnia with normal sleep duration may respond primarily to psychological therapies.
Keywords: Insomnia, short sleep duration, cardiometabolic, neurocognitive, physiological hyperarousal, morbidity, mortality
1. Nosology of Insomnia
The prevalence of insomnia in the general population ranges between 8-40%, depending on the definition used. While 20-30% of the general population has poor sleep (i.e., insomnia symptoms of difficulty initiating or maintaining sleep, early morning awakening, or non-restorative sleep at any given time), another 8-10% of the population suffers from chronic insomnia.1-3 Insomnia is a major public health problem as it is associated with impaired occupational performance, increased absenteeism at work, higher health care costs, and worse quality of life.4-5 However, the connection of insomnia with cardiometabolic risk and cognitive impairment has not been examined until very recently. This has led some clinicians to view insomnia and its associated mental and physical health complaints as obsessions of otherwise healthy individuals.
The diagnostic nosology of insomnia has traditionally faced the dilemma of lumping vs. splitting insomnia subtypes. The former view is represented in the Diagnostic and Statistical Manual of Mental Disorders (DSM) that reflects the predominant view of the American Psychiatric Association, while the latter is represented in the International Classification of Sleep Disorders (ICSD) reflecting the predominant view of the sleep specialists in North America. In 1987, sleep disorders were included for the first time in the 3rd revised edition of the DSM (DSM-III-R) and overall diagnostic criteria for “insomnia disorders” were included based on the subjective complaints of difficulty initiating or maintaining sleep or of non-restorative sleep, occurring at least 3 times a week for at least 1 month, and associated daytime functioning complaints. The DSM-III-R6 allowed the diagnoses of “insomnia related to another mental disorder” or “to a known organic factor”, “primary insomnia”, and “dysomnia NOS” (see Table 1). The DSM-IV-TR eliminated the definition of “insomnia disorders” and included the diagnoses of “primary insomnia”, “dysomnia NOS”, insomnia “related to another mental disorder”, “due to a general medical condition”, and “substance-induced”.7 The DSM-5 is expected to be published in May 2013 and the diagnoses of “primary insomnia”, “sleep disorder related to another mental disorder”, and “sleep disorder due to a general medical condition” will most probably be eliminated in favor of “insomnia disorder” with concurrent specification of clinically comorbid mental and/or physical conditions so that no causal attributions between insomnia and the physical/mental condition are made and the independent clinical attention of insomnia is warranted.8 The DSM-5 is expected to incorporate the use of self-reported severity measures with the aim of identifying sub-threshold sleep disturbances in patients at risk for developing a full-fledged “insomnia disorder” and who may be candidates for preventive interventions.8 Also, it is expected an extension of the duration criterion from 1 month to 3 months. However, no objective measures of the biological severity of the disorder are expected to be included in the forthcoming DSM-5.
Table 1.
Insomnia diagnoses across DSM and ICSD most recent versions
| DSM-III-R (1987) | DSM-IV-TR (2000) | ICSD-R (1997) | ICSD-2 (2005) |
|---|---|---|---|
|
Dysomnias Insomnia Disorders Insomnia related to Another Mental Disorder (nonorganic) Insomnia related to a Known Organic Factor Primary Insomnia Dysomnia Not Otherwise Specified |
Primary Sleep Disorders Dysomnias Primary Insomnia Dysomnia Not Otherwise Specified Sleep Disorder related to Another Mental Disorder Insomnia related to Another Mental Disorder Sleep Disorder Due to a General Medical Condition Insomnia type Substance-Induced Sleep Disorder Insomnia type |
Dysomnias Intrinsic Sleep Disorders Psychophysiologic Insomnia Sleep State Misperception Idiopathic Insomnia Intrinsic Sleep Disorder Not Otherwise Specified Extrinsic Sleep Disorders Inadequate Sleep Hygiene Environmental Sleep Disorder Altitude Insomnia Adjustment Sleep Disorder Limit-setting Sleep Disorder Sleep-onset Association Disorder Food Allergy Insomnia Hypnotic-Dependent Sleep Disorder Stimulant-Dependent Sleep Disorder Alcohol-Dependent Sleep Disorder Toxin-Induced Sleep Disorder Extrinsic Sleep Disorder Not Otherwise Specified Sleep Disorders Associated with Other Disorders Associated with Mental Disorders Associated with Neurologic Disorders Associated with Other Medical Disorders |
Insomnias Adjustment Insomnia Psychophysiological Insomnia Paradoxical Insomnia Idiopathic Insomnia Insomnia Due to Mental Disorder Inadequate Sleep Hygiene Behavioral Insomnia of Childhood Insomnia Due to Drug or Substance Insomnia Due to Medical Condition Insomnia Not Due to Substance or Known Physiological Conditions, Unspecified Physiological (Organic) Insomnia, Unspecified |
The ICSD (1990), and its revised form ICSD-R (1997), also defined insomnia based on subjective sleep and daytime functioning complaints but, in contrast, attempted to identify subtypes based on “intrinsic” factors such as etiology (i.e., “psychophysiological”), age of onset (i.e., “idiopathic insomnia”), degree of discrepancy between objective sleep findings and subjective perception of sleep (i.e., “sleep state misperception”) or “extrinsic” environmental factors such as “inadequate sleep hygiene”, “food-allergy” or “altitude insomnia” (see Table 1). However, these subtypes, even when refined in the ICSD-2,9 have not proven to be clinically useful and their reliability is at best modest.10-12 For example, although the ICSD-2 diagnosis of “psychophysiological insomnia” is based solely on the subjective reports of the patient, it is presumed to be associated with physiological hyperarousal and polysomnographic (PSG) disturbances. The diagnostic subtype of “idiopathic insomnia” has produced very little research since it was first proposed13 and it appears that the proposed differences between “psychophysiological” and “idiopathic insomniacs”14 are not large enough to determine a true differential treatment approach, e.g., medication vs. psychotherapy. Finally, the subtype of “paradoxical insomnia” is estimated to represent as little as 5% of all insomnia sufferers according to the ICSD;9 however, recent epidemiological studies have shown that insomnia sufferers with clinically significant sleep misperception represent about 50% of all insomnia sufferers.15 Consequently, large multicenter studies have shown that the current insomnia diagnoses available have low diagnostic reliability and validity.10-12 Finally, although PSG features are suggested, but not required, for virtually all ICSD subtypes, their usefulness in terms of differential diagnosis or severity assessment have not been demonstrated and are not currently used in clinical practice.
The sleep laboratory is essential for the evaluation of patients with sleep-disordered breathing (SDB), the diagnosis of narcolepsy, and the differential diagnosis of idiopathic vs. psychogenic hypersomnia.16,17 In addition, sleep laboratory measurements provide valuable objective information on the initial effectiveness, continued efficacy or tolerance, and potential withdrawal effects of a hypnotic drug. With the disorder of insomnia, the usefulness of the sleep laboratory has been at best controversial. As mentioned above, the ICSD-2 includes PSG findings as diagnostic criteria, although not required, for virtually all diagnoses.9 The validity and clinical utility of sleep lab testing for diagnosing insomnia has been evaluated in large studies11,18,19 and it has been concluded that (a) PSG is not useful in the evaluation of insomnia except to confirm or exclude other sleep pathologies when there is reasonable evidence from clinical history (e.g., SDB or periodic limb movements)4,20-22 and (b) sleep integrity measures, such as latency to sleep onset, total sleep time, number of arousals and awakenings, and sleep efficiency, are not useful in the diagnosis or differential diagnosis including subtyping of insomnia.4,11,18-23 The current consensus is, therefore, that PSG is not recommended for routine, differential diagnosis, or severity assessment of insomnia in clinical practice.22
However, we are going to present and discuss evidence, accumulated in the recent years, that objective measures of sleep (1) are useful in predicting the biological severity of insomnia (i.e., cardiometabolic morbidity and mortality as well as cognitive impairment) and (2) should be included in the new nosology of insomnia given their clinical utility for severity assessment and choice of treatment based on relevant clinical outcomes.
2. Pathophysiology of Insomnia
In the last two decades, several models have been proposed to understand the etiology and pathophysiology of insomnia.24-30 Most of these models have emphasized the importance of the joint effect of stress and psychological factors in the pathogenesis of insomnia. Kales and Kales early stated that “stressful events . . . when mediated by certain predisposing emotional factors and inadequate coping mechanisms, are indeed closely related to the onset of long-term sleep difficulty”.24 The characteristic psychological profile of insomnia sufferers, consisting of cognitive-emotional hyperarousal (i.e., obsessive, anxious, ruminative, and dysthymic personality traits) and emotion-oriented coping strategies,24,31,32 is thought to be present pre-morbidly and play a key role in the etiology of the disorder.24,33-36 The 3-P model by Spielman et al25 specified each of the predisposing, precipitating, and perpetuating factors involved in the etiopathogensis of insomnia and provided with a framework for therapies targeting the cognitive-behavioral factors that perpetuate insomnia after the resolution of the original stressor.
Stress has been associated with the activation of the hypothalamic-pituitary-adrenal (HPA) and the sympatho-adrenal-medullary axes, whereas corticotropin-releasing hormone (CRH) and cortisol (products of the hypothalamus and adrenals, respectively), and catecholamines (products of the sympathetic system) are known to cause arousal and sleeplessness to humans and animals. On the other hand, sleep and particularly deep sleep has an inhibitory effect on the stress system including its main two components, the HPA axis and the sympathetic system. Given that insomnia is associated with precipitating stressful life events37 and cognitive-emotional arousal,24 it was only natural for researchers to explore the association of this disorder with the stress system. Several studies have shown increased HPA axis, sympathetic, and central activation in insomnia sufferers, including increased cortisol and catecholamine secretion, heart rate and impaired heart rate variability, and 24-hour whole body and brain metabolic rate. While the majority of early studies reported no difference between subjectively defined “poor sleepers” and controls in the levels of 24-h cortisol and 17-hydorxysteroid excretion,38-40 later studies found that 24-h urinary free cortisol, norepinephrine, and catecholamine metabolites levels were either increased in insomnia sufferers with objective sleep disturbances as compared to controls or were correlated with PSG indices of sleep disturbance in insomnia patients.41-47 Only two recent studies have not replicated these findings. However, in one of these studies the objective sleep of insomnia sufferers was very similar to that of controls,48 while in the other study49 cortisol levels and other indices of physiological arousal were increased but not to a statistically significant level due to lack of power and controls not being carefully selected.50 Finally, of interest is a study that demonstrated that middle-aged healthy individuals are more vulnerable to the sleep disturbing effects of CRH, which may explain physiologically the increased prevalence of insomnia in older subjects.51
Furthermore, although two early studies found small differences between subjectively defined good and poor sleepers in heart rate measures,38,52 two more recent studies that included insomnia patients who also met objective criteria for sleep disturbances have found significant changes in nocturnal heart rate and heart rate variability.53,54 Two studies have shown that overall oxygen consumption (VO2), a measure of whole-body metabolic rate, is constantly elevated in insomnia patients with PSG documented sleep disturbance as compared to controls, whereas VO2 is increased in sleep misperception insomnia sufferers but to a lesser degree compared to insomnia patients with PSG documented sleep disturbance.55,56 In a group of insomnia patients with PSG verified sleep disturbance, a significantly increased pupil size, indicative of sympathetic system activation, was observed,57 whereas two other studies, in which insomnia diagnosis was based only on subjective measures, showed opposite results, i.e. decreased pupil size.58,59
Although insomnia sufferers typically complain that they are fatigued and sleepy during the day, and one would expect that during the Multiple Sleep Latency Test (MSLT) they would demonstrate short sleep latencies, they have either similar or increased daytime sleep latencies when compared to controls.59-62 Importantly, several studies have shown that, within insomnia sufferers, those with shorter objective sleep duration show longer sleep latencies in the MSLT61,63-65 and are more alert in vigilance tests.55,56,63 This is in contrast to normal individuals who after modest short-term sleep loss experience significantly reduced sleep latencies on the MSLT and decreased alertness in vigilance tests, i.e., physiological sleepiness.66,67 Thus, long latencies in the MSLT may represent a reliable marker of physiological hyperarousal in insomnia patients.
Finally, evidence about the presence of central nervous system hyperarousal in insomnia comes from studies using human subjects using neuroimaging,68,69 and spectral,70-75 arousal,76,77 and event-related78,79 electroencephalography analyses, and from studies on the neural circuitry of stress-induced insomnia in rats.80 Increased cortical arousal during sleep is present to a variable degree in all insomnia sufferers70-75 and may explain why they perceive their sleep as wake and as non-restorative.15,28,81,82
Collectively, these findings suggest that 1) the activity of the stress system is directly proportional to the degree of objective nighttime sleep disturbance or daytime increased vigilance in insomnia sufferers, 2) physiological hyperarousal, i.e. hyperactivity of both limbs of the stress system, is primarily present in insomnia patients with objective short sleep duration, while cognitive-emotional and cortical arousal is present to a variable degree in all insomnia sufferers, 3) objective short sleep duration appears to be a marker of the physiological hyperarousal in insomnia and not a measure of cumulative chronic sleep loss, and 4) objective sleep measures, i.e., short sleep duration (PSG) or increased daytime sleep latencies (MSLT), can provide a reliable index of the biological severity and medical impact of the disorder.83
3. Insomnia and Cardiometabolic Morbidity
Many studies have established that insomnia is highly comorbid with psychiatric disorders and is a risk factor for the development of depression, anxiety, and suicide.4 However, in contrast to SDB, chronic insomnia has not been linked firmly with significant medical morbidity, such as cardiovascular disease. Several questionnaire-based studies have shown a significant relationship between difficulty falling asleep or poor sleep with cardiometabolic outcomes such as hypertension84-86 and diabetes.87-90 For example, in a prospective study from Japan,85 persistent (>4 years) complaints of difficulty initiating or maintaining sleep were associated with an increased risk of hypertension (OR = 1.96). In another prospective study, difficulty falling asleep (HR =1.4) and difficulty staying asleep (HR = 1.3) were associated with acute myocardial infarction.86 Furthermore, other studies have shown that sleep disturbances or complaints are associated with increased incidence of type 2 diabetes.87-90 However, these studies showed relatively small effect sizes and did not include a PSG evaluation so as to control for SDB or other sleep pathology. The findings of these early studies were dismissed as artifacts or flawed by many clinicians and researchers alike.91,92 In fact, at least one report showed a reduced mortality rate for those individuals complaining of sleep difficulties after 6 years of follow-up.93
Given the well-established association of hypercortisolemia with significant medical morbidity, (i.e., hypertension, diabetes, metabolic syndrome, osteoporosis, and others) and the findings that hypercortisolemia is primarily associated with insomnia with objective short sleep duration,41-47 we hypothesized that this type of insomnia is associated with significant cardiometabolic morbidity and mortality. A series of recent epidemiological studies from the Penn State Adult Cohort,94,95 that used in-lab PSG, have shown that insomnia with objective short sleep duration is associated with a high risk of hypertension,96 diabetes,97 and mortality.98 For example, compared to normal sleepers who slept ≥ 6h per night, the highest odds of hypertension or diabetes was in insomnia sufferers who slept ≤5h (OR = 5.1 and OR = 2.95, respectively) and the second highest in insomnia sufferers who slept 5-6 h (OR = 3.5 and OR = 2.07, respectively), while insomnia sufferers who slept ≥ 6h were not at significantly increased risk of hypertension or diabetes (OR = 1.3 and OR = 1.1, respectively). Recent longitudinal data from the same cohort has shown that insomnia sufferers who slept < 6h were at a significantly higher risk of incident hypertension (OR = 3.75),99 suggesting that it is insomnia that causes hypertension and not vice versa. Furthermore, other longitudinal research showed that mortality risk in men was significantly increased in insomnia sufferers who slept < 6 h compared to normal sleepers (OR = 4.00), and that there was a marginally significant trend toward higher mortality from insomnia with short sleep duration in men with diabetes or hypertension (OR = 7.17) than in those without these comorbid conditions (OR = 1.45). Thus, the impact of insomnia with short sleep duration was much stronger in those with diabetes and hypertension at baseline versus those who were healthy.98 In women, mortality was not associated with insomnia with short sleep duration, a finding that might be related to the fact that women were followed-up for a shorter time period and that a smaller number were deceased at the time of follow-up compared to men (5% vs. 21%, respectively).
Consistent with the findings of these population-based studies, other recent studies have shown that nighttime systolic blood pressure is higher and day-to-night systolic blood pressure dipping is reduced in insomnia sufferers and that the magnitude of beta EEG activity correlates with their systolic blood pressure dipping.100 Also, impaired heart rate variability,101 lower cardiac pre-ejection period,102 and poorer indices of glucose metabolism103 are primarily present in insomnia sufferers with objective short sleep duration. Cumulatively, these data indicate that objective short sleep duration may predict the biological severity of chronic insomnia.
4. Insomnia and Cognitive Impairment
Insomnia is associated with complaints of difficulty concentrating, memory problems, and inattention. However, studies using objective neuropsychological testing have produced inconsistent findings. This has led some researchers to question the existence of true cognitive impairments in insomnia104 and attribute daytime complaints to excessive attention to the expected consequences of poor sleep.29
A recent meta-analysis has shown that individuals with insomnia exhibit performance impairments of small to moderate magnitude in several cognitive functions, including working memory, episodic memory, and some aspects of executive functioning.105 However, an important factor that has not been examined in meta-analytic research of the neurocognitive literature is the degree of objective sleep disturbance shown by the insomnia sufferers across studies. For example, most of the studies included in meta-analyses have shown that cognitive performance is impaired in insomnia sufferers with objective sleep disturbances60,106-110 or that it correlates with objective markers of sleep disturbance in insomnia sufferers,110-116 whereas those studies in which performance was not significantly impaired established insomnia diagnoses using solely subjective criteria.56,113,117-119 The role of objective sleep measures in the association of insomnia with cognitive impairment has been addressed in a recent study from the Penn State Adult Cohort.120 This study showed that insomnia sufferers, based solely on a subjective complaint, did not differ significantly from controls on either PSG variables or neurocognitive performance. However, significant interactions between insomnia and objective short sleep duration (i.e., < 6h) on specific neurocognitive tests were found. Specifically, insomnia sufferers with objective short sleep duration showed poorer neuropsychological performance on tests of processing speed, set-switching attention, and number of short-term visual memory errors and omissions compared to control groups with normal or short sleep duration. In contrast, insomnia sufferers with normal sleep duration group showed no significant deficits when compared to controls. Based on these findings, it seems that insomnia with objective short sleep duration is associated with deficits in set-switching attention, a key component of the “executive control of attention”.120 Importantly, the presence of a group of good sleepers with short sleep duration allowed to demonstrate that deficits in executive functions were associated with underlying physiological hyperarousal, a characteristic of chronic insomnia, rather than to short sleep per se.120 Cumulatively, the data from these studies indicate that objective short sleep duration may predict the biological severity of chronic insomnia, including its effect on cognitive functions.
Brain functions such as set-switching attention, working memory, and interference control have been linked to the activation of the prefrontal and anterior cingulate cortices. Tasks of set-switching attention also require the ability to retain both sets so they are ready to be recalled and, in fact, neuroimaging studies show that set-switching attention and working memory cooperate in the same areas of the prefrontal cortex.121,122 Together, these findings suggest that insomnia with objective short sleep duration, via hypercortisolemia, may affect brain structures and associated cognitive functions.123-126 Furthermore, insomnia with objective short sleep duration may be a premorbid risk factor for amnestic and non-amnestic mild cognitive impairment (MCI), an area open to future studies.
5. Natural History of Insomnia
As mentioned in the introduction, about 20% of the general population has poor sleep (i.e., insomnia symptoms at any given time) and about another 10% has chronic insomnia. Natural history studies have shown that chronic insomnia is a highly persistent condition, whereas the course of poor sleep is more variable and has a higher remission rate.33,35,36,127,128 This suggests that insomnia is a disorder while poor sleep is a symptom of underlying mental and physical health problems.35,36,128 Furthermore, objective short sleep duration has been shown to be a risk factor for poor sleep evolving into the more severe form of chronic insomnia36 as well as of chronic insomnia becoming persistent.128 These latter findings suggest that objective short sleep duration may be a biologic marker of genetic predisposition to chronic insomnia36 and of the severity and chronicity of the disorder.128
6. Clinical Implications
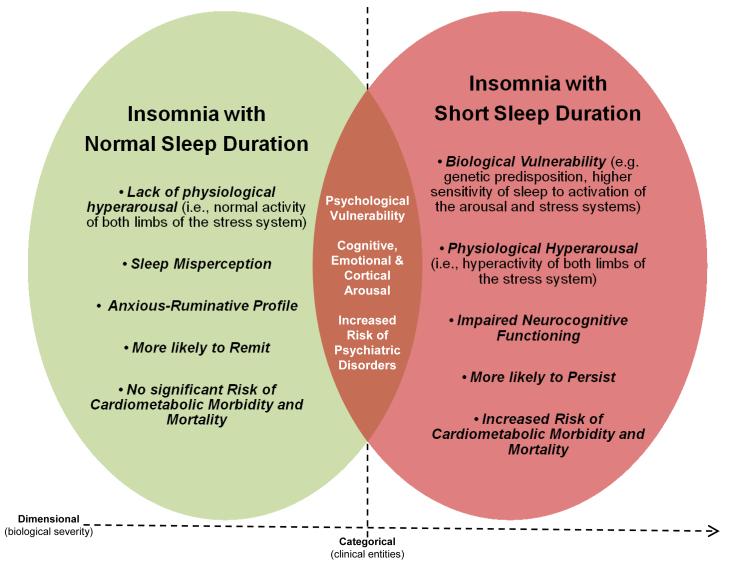
The evidence presented in this paper has led us to suggest two phenotypes of chronic insomnia that are different in terms of etiology, pathophysiology, biological severity, natural course, psychological characteristics, and possibly, treatment response. The first phenotype is primarily associated with physiological hyperarousal (i.e., short sleep duration and activation of both limbs of the stress system), significant medical sequelae (e.g., hypertension, diabetes, cognitive impairment, increased mortality), and a persistent course. The second phenotype is associated with cognitive-emotional and cortical arousal, but not with physiological hyperarousal (i.e., normal sleep duration and normal activity of the stress system) or significant medical sequelae, and is more likely to remit over time. Furthermore, the first phenotype is associated with a psychological profile typical of medical outpatients, whereas the second phenotype is associated with sleep misperception, anxious-ruminative traits, and poor coping resources.83 Table 2 summarizes the findings of key studies, while Figure 1 depicts a heuristic model of the underlying pathophysiological mechanisms and clinical characteristics of the two insomnia phenotypes.
Table 2.
Association of insomnia with short sleep duration with physiological hyperarousal, cardiometabolic morbidity, neurocognitive impairment, and a persistent course
| Controls | Insomnia with Normal Sleep Duration |
Insomnia with Short Sleep Duration |
|
|---|---|---|---|
| Physiological Hyperarousal | |||
| Cortisol levels (20:00-8:00h), nmol/L43 | 189.8 | 172.0 | 302.0* |
| Cortisol AUC (Evening 4 h), μg/ml44 | 15.6 | 24.3* | |
| Cortisol AUC (19:00-9:00h), μg/dl48 | 253.7 | 214.4 | |
| Heart rate variability (LHF ratio wake), power54 | 0.8 | 1.2* | |
| Heart rate variability (SDNN wake), ms101 | 63.5 | 64.1 | 50.5* |
| Whole body metabolic rate (VO2), % increased55,56 | --- | 6* | 9* |
| MSLT, minutes55,56 | 9.5 | 10.2 | 13.3* |
| Cardiometabolic Morbidity | |||
| Hypertension, OR96 | 1.0 | 1.1 | 5.1* |
| Incident Hypertension, OR99 | 1.0 | 0.9 | 3.8* |
| Diabetes, OR97 | 1.0 | 1.1 | 2.9* |
| Mortality in Men, OR98 | 1.0 | 0.7 | 4.0* |
| Mortality in Men w/ hypertension/diabetes, OR98 | 1.0 | 0.6 | 7.2* |
| Neurocognitive Impairment | |||
| SDMT, # correct120 | 50.6 | 52.4 | 46.3* |
| TMT-B, seconds120 | 75.2 | 68.0 | 87.8* |
| TMT B–A, seconds120 | 43.6 | 36.0 | 54.6* |
| SAT, error rate130 | 2.5 | 3.2 | 4.5* |
| BVRT, # omissions120 | 0.4 | 0.4 | 0.7* |
| Short-term memory, words55,56 | 7.5 | 7.2 | 5.7* |
| Sleep Misperception | |||
| Subjective sleep time, hours15 | 6.5 | 5.6* | 5.0* |
| Objective sleep time, hours15 | 6.6 | 6.6 | 4.7* |
| Subjective – objective sleep time, hours15 | −0.1 | −1.0* | 0.3 |
| Psychological Profile | |||
| MMPI-2 Psychasthenia, score15 | 49.1 | 54.5* | 51.8 |
| MMPI-2 Anxiety, score15 | 48.2 | 53.8* | 49.4 |
| MMPI-2 Ego Strength, score15 | 48.7 | 44.4* | 47.8 |
| STAI Trait Anxiety, score137 | 30.7 | 37.0* | 34.9 |
| DBAS Effects, score137 | 33.2 | 44.6* | 41.5 |
| DBAS Needs, score137 | 37.2 | 46.3* | 38.3 |
| Natural History | |||
| Persistence vs. Full remission, OR128 | 1.0 | 0.2 | 4.9* |
Value significantly higher or lower as compared to controls; AUC = Area Under the Curve; BVRT = Benton Visual Retention Test; DBAS = Dysfunctional Beliefs and Attitudes about Sleep; MMPI-2 Minnesota Multiphasic Personality Invesntory-2; MSLT = Multiple Sleep Latency Test; OR = odds ratio; SAT = Switching Attention Task; SDMT = Symbol Digit Modalities Test; STAI = State-Trait Anxiety Inventory; TMT-B = Trail Making Test part B; TMT B–A = Trail Making Test part B – part A
Figure 1. Heuristic model of the underlying pathophysiological mechanisms and clinical characteristics of the two insomnia phenotypes based on objective sleep duration.
The common characteristics of the two phenotypes are presented in the overlapping area, while their unique characteristics are presented in the areas of each phenotype that do not overlap.83
Our proposed model for the two insomnia phenotypes has several implications in terms of nosology, diagnostic procedures and specificity of treatment for chronic insomnia. As we have stated earlier, previously proposed subtypes of insomnia have been associated with low diagnostic reliability and have not been associated with clinically relevant outcomes. In addition, the current diagnostic procedures used in insomnia are subjective tools such as clinical interviews, questionnaires, and specific scales. The data reviewed here suggest that objective measures of sleep can be useful in detecting the most severe form of insomnia in terms of its importance on the patient’s health. Thus, we propose the inclusion of objective sleep duration as a criterion in future diagnostic manuals for insomnia in order to differentiate these two clearly different and clinically relevant subtypes of insomnia.
Further, our data suggest that objective measures of sleep, in addition to a thorough clinical evaluation, should become part of the standard diagnostic procedures for insomnia.83 Although our studies have focused on the utility of sleep duration, other studies suggest that other variables of sleep efficiency and continuity or of physiological hyperarousal (i.e., MSLT) may also serve as markers of the biological severity of the disorder.36,42,106,110,112,129,130 However, a potential disadvantage of biomarkers such as stage 1, SWS, or MSLT is that they require a full PSG study or daytime laboratory assessment, whereas sleep duration perhaps could be obtained with simpler methods, e.g., actigraphy. In this regard, several studies suggest the potential usefulness of actigraphy to assess sleep patterns for a period of days or weeks in the “habitual home environment”, to characterize the severity of the insomnia disorder.21,131,132 A similar amount of home sleep monitoring with PSG would be difficult and impractical for clinical venues. However, several problems associated with the use of actigraphy, such as lack of an industry standard for the sleep algorithms used in different actigraphic devices and the propensity to over-or underestimate sleep time, make its current use limited. Future studies using cost-effective methods should examine whether night-to-night variability in sleep duration may be a stronger predictor of cardiometabolic and neurocognitive morbidity compared to average sleep duration.
Finally, our findings may affect the way we treat both chronic insomnia and poor sleep. The insomnia phenotype with short sleep duration may respond better to treatments that primarily aim at decreasing physiological hyperarousal and increasing sleep duration, such as medication or other biological treatments.46 Previous studies have shown that sedative antidepressants such as trazodone or doxepin, used at low dosages, down-regulate the activity of the HPA axis, decrease cortisol levels, and increase sleep duration.46,133,134 We should note that biological treatments should be part of a multidimensional approach that combines behavioral changes, i.e., sleep hygiene, and psychological interventions, i.e., cognitive-behavioral therapy (CBT), when indicated from the clinical evaluation. The second phenotype, i.e., insomnia with normal sleep duration, is not likely associated with physiological hyperarousal and hypnotics or sedative antidepressants may be not be warranted. This phenotype may respond better to treatments that primarily aim at decreasing cognitive-emotional arousal, changing sleep-related beliefs and behaviors, and altering sleep misperception, such as CBT.135,136 In fact, given that insomnia with normal sleep duration is associated with poor coping resources and anxious-ruminative traits that extend beyond sleep-related preoccupation, other psychotherapeutic techniques such as behavioral experiments or emotion regulation techniques may be indicated. Of course use of psychotherapeutic medication may be indicated based on the presence of comorbid psychiatric conditions, i.e., anxiety or depressive disorders. The differential treatment response of these two phenotypes should be tested in future placebo-controlled clinical trials.
In the prevention of insomnia, treatment strategies for persistent poor sleep should focus on treating the underlying physical health conditions (e.g., cardiovascular disorders, ulcer, or allergy/asthma) as well as the associated psychological distress with, for example, CBT. Given that poor sleepers with shorter objective sleep duration and a family history of sleep problems are at greater risk of developing chronic insomnia,36 their appropriate detection with objective sleep measures is warranted and treatment should focus on decreasing physiological hyperarousal and lengthening sleep duration.
Summary
In this article we present evidence that the degree of objective sleep disturbance in insomnia is directly proportional to the activity of both limbs of the stress system and other indices of physiological hyperarousal. Evidence suggests that insomnia with objective short sleep duration is associated with a significant risk of cardiometabolic morbidity and mortality and cognitive impairment. In contrast, insomnia with normal sleep duration is associated with cognitive-emotional and cortical arousal and sleep misperception but not with signs of physiological hyperarousal or cardiometabolic or neurocognitive morbidity. We propose that objective measures of sleep duration may become part of the routine evaluation and diagnosis of chronic insomnia in an office setting. Also we propose that these insomnia phenotypes may respond differentially to treatment, i.e., insomnia with short sleep duration may respond better to biological treatments, whereas insomnia with normal sleep duration may respond better to psychological treatment alone.
Key Points.
The nosology of insomnia is associated with unsatisfactory reliability and validity, whereas current clinical practice guidelines do not recommend the use of objective sleep measures in the diagnosis, evaluation, and treatment of insomnia.
Recent evidence shows that objective measures of sleep are useful in predicting the biological severity of insomnia, i.e., activation of both limbs of the stress system, cardiometabolic morbidity and mortality as well as cognitive impairment.
We propose two phenotypes of insomnia, based on objective sleep duration, that differ in terms of physiological hyperarousal, medical morbidity, psychological profiles, and natural course.
Objective sleep measures, i.e., sleep duration, should be included in the diagnostic criteria for subtyping insomnia.
Objective measures of sleep duration obtained with cost-effective and simpler methods than polysomnography may become part of the routine diagnosis and treatment of chronic insomnia in an office setting.
These two phenotypes may respond differentially to treatment, i.e., insomnia with short sleep duration may respond better to biological treatments, whereas insomnia with normal sleep duration may respond primarily to psychological treatments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bixler EO, Vgontzas AN, Lin HM, Vela-Bueno A, Kales A. Insomnia in central Pennsylvania. J Psychosom Res. 2002;53(1):589–92. doi: 10.1016/s0022-3999(02)00450-6. [DOI] [PubMed] [Google Scholar]
- 2.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 3.Morin CM, LeBlanc M, Daley M, Gregoire JP, Mérette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123–30. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 4.National Institutes of Health NIH state of the science statement on manifestations and management of chronic insomnia in adults. J Clin Sleep Med. 2005;1:412–421. [PubMed] [Google Scholar]
- 5.Léger D, Bayon V. Societal costs of insomnia. Sleep Med Rev. 2010;14(6):379–89. doi: 10.1016/j.smrv.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds CF, 3rd, Kupfer DJ, Buysse DJ, Coble PA, Yeager A. Subtyping DSM-III-R primary insomnia: a literature review by the DSM-IV Work Group on Sleep Disorders. Am J Psychiatry. 1991;148:432–8. doi: 10.1176/ajp.148.4.432. [DOI] [PubMed] [Google Scholar]
- 7.American Psychiatric Association . Diagnostic and statistical manual of mental disorders, text revision. 4th ed American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 8.Reynolds CF, 3rd, Redline S. DSM-V Sleep-Wake Disorders Workgroup and Advisors. The DSM-V sleep-wake disorders nosology: an update and an invitation to the sleep community. Sleep. 2010;33(1):10–1. doi: 10.1093/sleep/33.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Academy of Sleep Medicine . The International Classification of Sleep Disorders (ICSD-2): Diagnostic and Coding Manual. 2nd ed American Academy of Sleep Medicine; Westchester: 2005. [Google Scholar]
- 10.Soldatos CR, Lugaresi E. Nosology and prevalence of sleep disorders. Semin Neurol. 1987;7(3):236–42. doi: 10.1055/s-2008-1041423. [DOI] [PubMed] [Google Scholar]
- 11.Buysse DJ, Reynolds CF, III, Hauri PJ, Roth T, Stepanski EJ, Thorpy MJ, et al. Diagnostic concordance for insomnia patients among sleep specialists using proposed DSM-IV, proposed ICD-10, and ICSD diagnostic systems: report from the APA/NIMH DSM-IV field trial. In: Widiger TA, et al., editors. DSM-IV Sourcebook. volume 4. American Psychiatric Association; Washington, DC: 1998. pp. 869–889. [Google Scholar]
- 12.Edinger JD, Wyatt JK, Stepanski EJ, Olsen MK, Stechuchak KM, Carney CE, et al. Testing the reliability and validity of DSM-IV-TR and ICSD-2 insomnia diagnoses. Results of a multitrait-multimethod analysis. Arch Gen Psychiatry. 2011;68(10):992–1002. doi: 10.1001/archgenpsychiatry.2011.64. [DOI] [PubMed] [Google Scholar]
- 13.Hauri P, Olmstead E. Childhood-onset insomnia. Sleep. 1980;3(1):59–65. doi: 10.1093/sleep/3.1.59. [DOI] [PubMed] [Google Scholar]
- 14.Espie CA, Barrie LM, Forgan GS. Comparative investigation of the psychophysiologic and idiopathic insomnia disorder phenotypes: psychologic characteristics, patients’ perspectives, and implications for clinical management. Sleep. 2012;35(3):385–93. doi: 10.5665/sleep.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez-Mendoza J, Calhoun SL, Bixler EO, Karataraki M, Liao D, Vela-Bueno A, et al. Sleep misperception and chronic insomnia in the general population: role of objective sleep duration and psychological profiles. Psychosom Med. 2011;73(1):88–97. doi: 10.1097/PSY.0b013e3181fe365a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vgontzas AN, Kales A. Sleep and its disorders. Annu Rev Med. 1999;50:387–400. doi: 10.1146/annurev.med.50.1.387. [DOI] [PubMed] [Google Scholar]
- 17.Vgontzas AN, Bixler EO, Kales A, Criley C, Vela-Bueno A. Differences in nocturnal and daytime sleep between primary and psychiatric hypersomnia: diagnostic and treatment implications. Psychosom Med. 2000;62:220–226. doi: 10.1097/00006842-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Vgontzas AN, Bixler EO, Kales A, Manfredi RL, Tyson K. Validity and clinical utility of sleep laboratory criteria for insomnia. Int J Neurosci. 1994;77(1-2):11–21. doi: 10.3109/00207459408986015. [DOI] [PubMed] [Google Scholar]
- 19.Vgontzas AN, Kales A, Bixler EO, Manfredi RL, Vela-Bueno A. Usefulness of polysomnographic studies in the differential diagnosis of insomnia. Int J Neurosci. 1995;82:47–60. doi: 10.3109/00207459508994289. [DOI] [PubMed] [Google Scholar]
- 20.Chesson A, Jr, Hartse K, Anderson WM, Davila D, Johnson S, Littner M, et al. Practice parameters for the evaluation of chronic insomnia. An American Academy of Sleep Medicine Report Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep. 2000;23:237–241. [PubMed] [Google Scholar]
- 21.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 22.Littner M, Hirshkowitz M, Kramer M, Kapen S, Anderson WM, Bailey D, et al. Practice parameters for using polysomnography to evaluate insomnia: an update. Sleep. 2003;26:754–760. doi: 10.1093/sleep/26.6.754. [DOI] [PubMed] [Google Scholar]
- 23.Reite M, Buysse D, Reynolds C, Mendelson W. The use of polysomnography in the evaluation of insomnia. Sleep. 1995;18:58–70. doi: 10.1093/sleep/18.1.58. [DOI] [PubMed] [Google Scholar]
- 24.Kales A, Kales JD. Evaluation and Treatment of Insomnia. Oxford University Press; New York: 1984. [Google Scholar]
- 25.Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10:541–53. [PubMed] [Google Scholar]
- 26.Morin CM. Insomnia: Psychological Assessment and Management. The Guilford Press; New York: 1993. [Google Scholar]
- 27.Bonnet MH, Arand DL. Hyperarousal and insomnia. Sleep Med Rev. 1997;1:97–108. doi: 10.1016/s1087-0792(97)90012-5. [DOI] [PubMed] [Google Scholar]
- 28.Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6:179–88. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 29.Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40:869–93. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 30.Espie CA. Insomnia: conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annu Rev Psychol. 2002;53:215–43. doi: 10.1146/annurev.psych.53.100901.135243. [DOI] [PubMed] [Google Scholar]
- 31.LeBlanc M, Beaulieu-Bonneau S, Mérette C, Savard J, Ivers H, Morin CM. Psychological and health-related quality of life factors associated with insomnia in a population-based sample. J Psychosom Res. 2007;63:157–66. doi: 10.1016/j.jpsychores.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65:259–67. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- 33.LeBlanc M, Mérette C, Savard J, Ivers H, Baillargeon L, Morin CM. Incidence and risk factors of insomnia in a population-based sample. Sleep. 2009;32:1027–37. doi: 10.1093/sleep/32.8.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernández-Mendoza J, Vela-Bueno A, Vgontzas AN, Ramos-Platón MJ, Olavarrieta-Bernardino S, Bixler EO, et al. Cognitive-emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomnia. Psychosom Med. 2010;72:397–403. doi: 10.1097/PSY.0b013e3181d75319. [DOI] [PubMed] [Google Scholar]
- 35.Singareddy R, Vgontzas AN, Fernandez-Mendoza J, Liao D, Calhoun S, Shaffer ML, et al. Risk factors for incident chronic insomnia: a general population prospective study. Sleep Med. 2012;13(4):346–53. doi: 10.1016/j.sleep.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez-Mendoza J, Vgontzas AN, Bixler EO, Singareddy R, Shaffer ML, Calhoun SL, et al. Clinical and polysomnographic predictors of the natural history of poor sleep in the general population. Sleep. 2012b;35(5):689–697. doi: 10.5665/sleep.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Healey ES, Kales A, Monroe LJ, Bixler EO, Chamberlin K, Soldatos CR. Onset of insomnia: role of life-stress events. Psychosom Med. 1981;43(5):439–51. doi: 10.1097/00006842-198110000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Monroe LJ. Psychological and physiological differences between good and poor sleepers. J Abnorm Psychol. 1967;72(3):255–64. doi: 10.1037/h0024563. [DOI] [PubMed] [Google Scholar]
- 39.Johns MW, Gay TJA, Masterton JP, Bruce DW. Relationship between habits, adrenocortical activity and personality. Psychosom Med. 1971;33:499–508. doi: 10.1097/00006842-197111000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Frankel BL, Buchbinder R, Coursey R, Snyder F. Sleep patterns and psychological test characteristics of chronic primary insomniacs. Sleep Res. 1973;2:149. [Google Scholar]
- 41.Adam K, Tomeny M, Oswald I. Physiological and psychological differences between good and poor sleepers. J Psychiatric Res. 1986;20(4):301–316. doi: 10.1016/0022-3956(86)90033-6. [DOI] [PubMed] [Google Scholar]
- 42.Vgontzas AN, Tsigos C, Bixler EO, Stratakis CA, Zachman K, Kales A, et al. Chronic insomnia and activity of the stress system: a preliminary study. J Psychosom Res. 1998;45:21–31. doi: 10.1016/s0022-3999(97)00302-4. [DOI] [PubMed] [Google Scholar]
- 43.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Mastorakos G, Vela-Bueno A, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86:3787–3794. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 44.Rodenbeck A, Huether G, Ruether E, Hajak G. Interactions between evening and nocturnal cortisol secretion and sleep parameters in patients with severe chronic primary insomnia. Neurosci Lett. 2002;324:163–459. doi: 10.1016/s0304-3940(02)00192-1. [DOI] [PubMed] [Google Scholar]
- 45.Shaver JL, Johnston SK, Lentz MJ, Landis CA. Stress exposure, psychological distress, and physiological stress activation in midlife women with insomnia. Psychosom Med. 2002;64:793–802. doi: 10.1097/01.psy.0000024235.11538.9a. [DOI] [PubMed] [Google Scholar]
- 46.Rodenbeck A, Cohrs S, Jordan W, Huether G, Rüther E, Hajak G. The sleep-improving effects of doxepin are paralleled by a normalized plasma cortisol secretion in primary insomnia. A placebo-controlled, double-blind, randomized, cross-over study followed by an open treatment over 3 weeks. Psychopharmacology (Berl) 2003;170(4):423–8. doi: 10.1007/s00213-003-1565-0. [DOI] [PubMed] [Google Scholar]
- 47.Irwin M, Clark C, Kennedy B, Christian Gillin J, Ziegler M. Nocturnal catecholamines and immune function in insomniacs, depressed patients, and controls subjects. Brain Behav Immun. 2003;17(5):365–372. doi: 10.1016/s0889-1591(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 48.Riemann D, Klein T, Rodenbeck A, Feige B, Horney A, Hummel R, et al. Nocturnal cortisol and melatonin secretion in primary insomnia. Psychiatry Res. 2002;113:17–27. doi: 10.1016/s0165-1781(02)00249-4. [DOI] [PubMed] [Google Scholar]
- 49.Varkevisser M, Van Dongen HP, Kerkhof GA. Physiologic indexes in chronic insomnia during a constant routine: evidence for general hyperarousal? Sleep. 2005;28:1588–1596. [PubMed] [Google Scholar]
- 50.Bonnet MH. Hyperarousal as the basis for insomnia: effect size and significance. Sleep. 2005;28:1500–1501. doi: 10.1093/sleep/28.12.1500. [DOI] [PubMed] [Google Scholar]
- 51.Vgontzas AN, Bixler EO, Wittman AM, Zachman K, Lin HM, Vela-Bueno A, Kales A, Chrousos GP. Middle-aged men show higher sensitivity of sleep to the arousing effects of corticotropin-releasing hormone than young men: clinical implications. J Clin Endocrinol Metab. 2001;86(4):1489–95. doi: 10.1210/jcem.86.4.7370. [DOI] [PubMed] [Google Scholar]
- 52.Freedman RR, Sattler HL. Physiological and psychological factors in sleep-onset insomnia. J Abnorm Psychol. 1982;91(5):380–389. doi: 10.1037//0021-843x.91.5.380. [DOI] [PubMed] [Google Scholar]
- 53.Stepanski E, Glinn M, Zorick F, Roehrs T, Roth T. Heart rate changes in chronic insomnia. Stress Med. 1994;10(4):261–266. [Google Scholar]
- 54.Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60(5):610–615. doi: 10.1097/00006842-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 55.Bonnet MH, Arand DL. 24-hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18(7):581–588. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 56.Bonnet MH, Arand DL. Physiological activation in patients with sleep state misperception. Psychosom Med. 1997;59(5):533–540. doi: 10.1097/00006842-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 57.Lichstein K, Johnson RS. Pupillometric discrimination of insomniacs. Behav Res Ther. 1994a;32(1):123–129. doi: 10.1016/0005-7967(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 58.Lichstein KL, Johnson RS, Sen Gupta S, O’Laughlin DL, Dykstra TA. Are insomniacs sleepy during the day? A pupillometric assessment. Behav Res Ther. 1992;30(3):283–292. doi: 10.1016/0005-7967(92)90074-q. [DOI] [PubMed] [Google Scholar]
- 59.Lichstein KL, Wilson NM, Noe SL, Aguillard RN, Bellur SN. Daytime sleepiness in insomnia: behavioral, biological and subjective indices. Sleep. 1994b;17(8):693–702. doi: 10.1093/sleep/17.8.693. [DOI] [PubMed] [Google Scholar]
- 60.Seidel WF, Ball S, Cohen S, Patterson N, Yost D, Dement WC. Daytime alertness in relation to mood, performance, and nocturnal sleep in chronic insomniacs and noncomplaining sleepers. Sleep. 1984;7(3):230–8. doi: 10.1093/sleep/7.3.230. [DOI] [PubMed] [Google Scholar]
- 61.Stepanski E, Zorick F, Roehrs T, Young D, Roth T. Daytime alertness in patients with chronic insomnia compared with asymptomatic control subjects. Sleep. 1988;11(1):54–60. doi: 10.1093/sleep/11.1.54. [DOI] [PubMed] [Google Scholar]
- 62.Bonnet MH, Arand DL. Activity, arousal, and the MSLT in patients with insomnia. Sleep. 2000;23(2):205–12. [PubMed] [Google Scholar]
- 63.Sugerman JL, Stern JA, Walsh JK. Daytime alertness in subjective and objective insomnia: some preliminary findings. Biol Psychiatry. 1985;20(7):741–50. doi: 10.1016/0006-3223(85)90153-2. [DOI] [PubMed] [Google Scholar]
- 64.Dorsey CM, Bootzin RR. Subjective and psychophysiologic insomnia: an examination of sleep tendency and personality. Biol Psychiatry. 1997;41(2):209–16. doi: 10.1016/0006-3223(95)00659-1. [DOI] [PubMed] [Google Scholar]
- 65.Roehrs TA, Randall S, Harris E, Maan R, Roth T. MSLT in primary insomnia: stability and relation to nocturnal sleep. Sleep. 2011;34(12):1647–52. doi: 10.5665/sleep.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonnet MH, Arand DL. Clinical effects of sleep fragmentation versus sleep deprivation. Sleep Med Rev. 2003;7(4):297–310. doi: 10.1053/smrv.2001.0245. [DOI] [PubMed] [Google Scholar]
- 67.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89(5):2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 68.Nofzinger EA, Nissen C, Germain A, Moul D, Hall M, Price JC, et al. Regional cerebral metabolic correlates of WASO during NREM sleep in insomnia. J Clin Sleep Med. 2006;2(3):316–322. [PubMed] [Google Scholar]
- 69.Winkelman JW, Buxton OM, Jensen JE, Benson KL, O’Connor SP, Wang W, et al. Reduced brain GABA in primary insomnia: preliminary data from 4T proton magnetic resonance spectroscopy (1H-MRS) Sleep. 2008;311(11):1499–1506. doi: 10.1093/sleep/31.11.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hall M, Buysse DJ, Nowell PD, Nofzinger EA, Houck P, Reynolds CF, 3rd, et al. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosom Med. 2000;62(2):227–30. doi: 10.1097/00006842-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 71.Hall M, Thayer JF, Germain A, Moul D, Vasko R, Puhl M, et al. Psychological stress is associated with heightened physiological arousal during NREM sleep in primary insomnia. Behav Sleep Med. 2007;5(3):178–93. doi: 10.1080/15402000701263221. [DOI] [PubMed] [Google Scholar]
- 72.Perlis ML, Smith MT, Andrews PJ, Orff H, Giles DE. Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24(1):110–7. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- 73.Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25:630–40. [PubMed] [Google Scholar]
- 74.Corsi-Cabrera M, Figueredo-Rodríguez P, del Río-Portilla Y, Sánchez-Romero J, Galán L, Bosch-Bayard J. Enhanced frontoparietal synchronized activation during the wake-sleep transition in patients with primary insomnia. Sleep. 2012;35(4):501–11. doi: 10.5665/sleep.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spiegelhalder K, Regen W, Feige B, Holz J, Piosczyk H, Baglioni C, Riemann D, Nissen C. Increased EEG sigma and beta power during NREM sleep in primary insomnia. Biol Psychol. 2012;91(3):329–33. doi: 10.1016/j.biopsycho.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 76.Feige B, Al-Shajlawi A, Nissen C, Voderholzer U, Hornyak M, Spiegelhalder K, et al. Does REM sleep contribute to subjective wake time in primary insomnia? A comparison of polysomnographic and subjective sleep in 100 patients. J Sleep Res. 2008;17:180–90. doi: 10.1111/j.1365-2869.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 77.Parrino L, Milioli G, De Paolis F, Grassi A, Terzano MG. Paradoxical insomnia: the role of CAP and arousals in sleep misperception. Sleep Med. 2009;10:1139–45. doi: 10.1016/j.sleep.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 78.Turcotte I, St-Jean G, Bastien CH. Are individuals with paradoxical insomnia more hyperaroused than individuals with psychophysiological insomnia? Event-related potentials measures at the peri-onset of sleep. Int J Psychophysiol. 2011;81(3):177–90. doi: 10.1016/j.ijpsycho.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 79.Bastien CH, Turcotte I, St-Jean G, Morin CM, Carrier J. Information processing varies between insomnia types: measures of N1 and P2 during the night. Behav Sleep Med. 2013;11(1):56–72. doi: 10.1080/15402002.2012.660896. [DOI] [PubMed] [Google Scholar]
- 80.Cano G, Mochizuki T, Saper CB. Neural circuitry of stress-induced insomnia in rats. J Neurosci. 2008;28(40):10167–84. doi: 10.1523/JNEUROSCI.1809-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harvey AG, Tang NK. (Mis)perception of sleep in insomnia: a puzzle and a resolution. Psychol Bull. 2012;138(1):77–101. doi: 10.1037/a0025730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stone KC, Taylor DJ, McCrae CS, Kalsekar A, Lichstein KL. Nonrestorative sleep. Sleep Med Rev. 2008;12(4):275–88. doi: 10.1016/j.smrv.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 83.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: The most biologically severe phenotype of the disorder. Sleep Med Rev. 2013 Feb 15; doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bonnet MH, Arand DL. Cardiovascular implications of poor sleep. Sleep Med Clin. 2007;2:529–538. [Google Scholar]
- 85.Suka M, Yoshida K, Sugimori H. Persistent insomnia is a predictor of hypertension in Japanese male workers. J Occup Health. 2003;45:344–50. doi: 10.1539/joh.45.344. [DOI] [PubMed] [Google Scholar]
- 86.Laugsand LE, Vatten LJ, Platou C, Janszky I. Insomnia and the risk of acute myocardial infarction: a population study. Circulation. 2011;124:2073–2081. doi: 10.1161/CIRCULATIONAHA.111.025858. [DOI] [PubMed] [Google Scholar]
- 87.Kawakami N, Takatsuka N, Shimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care. 2004;27:282–283. doi: 10.2337/diacare.27.1.282. [DOI] [PubMed] [Google Scholar]
- 88.Nilsson PM, Rööst M, Engström G, Hedblad B, Berglund G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care. 2004;27:2464–2469. doi: 10.2337/diacare.27.10.2464. [DOI] [PubMed] [Google Scholar]
- 89.Meisinger C, Heier M, Loewel H, MONICA/KORA Augsburg Cohort Study Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia. 2005;48:235–241. doi: 10.1007/s00125-004-1634-x. [DOI] [PubMed] [Google Scholar]
- 90.Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28:2762–2767. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- 91.Phillips B, Mannino DM. Do insomnia complaints cause hypertension or cardiovascular disease? J Clin Sleep Med. 2007;3:489–94. [PMC free article] [PubMed] [Google Scholar]
- 92.Bonnet MH. Evidence for the pathophysiology of insomnia. Sleep. 2009;32:441–442. doi: 10.1093/sleep/32.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 94.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 95.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 96.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32(4):491–7. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: A population-based study. Diabetes Care. 2009b;32(11):1980–5. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Basta M, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33(9):1159–64. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fernandez-Mendoza J, Vgontzas AN, Liao D, Shaffer ML, Vela-Bueno A, Basta M, et al. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension. 2012;60:00–00. doi: 10.1161/HYPERTENSIONAHA.112.193268. (doi: 10.1161/HYPERTENSIONAHA.112.193268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lanfranchi PA, Pennestri MH, Fradette L, Dumont M, Morin CM, Montplaisir J. Nighttime blood pressure in normotensive subjects with chronic insomnia: implications for cardiovascular risk. Sleep. 2009;32:760–766. doi: 10.1093/sleep/32.6.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spiegelhalder K, Fuchs L, Ladwig J, Kyle SD, Nissen C, Voderholzer U, et al. Heart rate and heart rate variability in subjectively reported insomnia. J Sleep Res. 2011;20(1 Pt 2):137–45. doi: 10.1111/j.1365-2869.2010.00863.x. [DOI] [PubMed] [Google Scholar]
- 102.De Zambotti M, Covassin N, De Min Tona G, Sarlo M, Stegagno L. Sleep onset and cardiovascular activity in primary insomnia. J Sleep Res. 2011;20(2):318–25. doi: 10.1111/j.1365-2869.2010.00871.x. [DOI] [PubMed] [Google Scholar]
- 103.Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care. 2011;34(5):1171–6. doi: 10.2337/dc10-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Riedel BW, Lichstein KL. Insomnia and daytime functioning. Sleep Med Rev. 2000;4(3):277–298. doi: 10.1053/smrv.1999.0074. [DOI] [PubMed] [Google Scholar]
- 105.Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med Rev. 2012;16(1):83–94. doi: 10.1016/j.smrv.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 106.Edinger JD, Glenn DM, Bastian LA, Marsh GR. Slow-wave sleep and waking cognitive performance II: Findings among middle-aged adults with and without insomnia complaints. Physiol Behav. 2000;70(1-2):127–34. doi: 10.1016/s0031-9384(00)00238-9. [DOI] [PubMed] [Google Scholar]
- 107.Szelenberger W, Niemcewicz S. Severity of insomnia correlates with cognitive impairment. Acta Neurobiol Exp (Wars) 2000;60(3):373. doi: 10.55782/ane-2000-1356. [DOI] [PubMed] [Google Scholar]
- 108.Varkevisser M, Kerkhof GA. Chronic insomnia and performance in a 24-h constant routine study. J Sleep Res. 2005;14(1):49–59. doi: 10.1111/j.1365-2869.2004.00414.x. [DOI] [PubMed] [Google Scholar]
- 109.Haimov I, Hanuka E, Horowitz Y. Chronic insomnia and cognitive functioning among older adults. Behav Sleep Med. 2008;6(1):32–54. doi: 10.1080/15402000701796080. [DOI] [PubMed] [Google Scholar]
- 110.Edinger JD, Means MK, Carney CE, Krystal AD. Psychomotor performance deficits and their relation to prior nights’ sleep among individuals with primary insomnia. Sleep. 2008;31(5):599–607. doi: 10.1093/sleep/31.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rosa RR, Bonnet MH. Reported chronic insomnia is independent of poor sleep as measured by electroencephalography. Psychosom Med. 2000;62(4):474–82. doi: 10.1097/00006842-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 112.Bastien CH, Fortier-Brochu E, Rioux I, LeBlanc M, Daley M, Morin CM. Cognitive performance and sleep quality in the elderly suffering from chronic insomnia. Relationship between objective and subjective measures. J Psychosom Res. 2003;54(1):39–49. doi: 10.1016/s0022-3999(02)00544-5. [DOI] [PubMed] [Google Scholar]; J Psychosom Res. 2003;55(5):475. Erratum in. [Google Scholar]
- 113.Orff HJ, Drummond SP, Nowakowski S, Perils ML. Discrepancy between subjective symptomatology and objective neuropsychological performance in insomnia. Sleep. 2007;30(9):1205–11. doi: 10.1093/sleep/30.9.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fang SC, Huang CJ, Yang TT, Tsai PS. Heart rate variability and daytime functioning in insomniacs and normal sleepers: preliminary results. J Psychosom Res. 2008;65(1):23–30. doi: 10.1016/j.jpsychores.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 115.Backhaus J, Junghanns K, Born J, Hohaus K, Faasch F, Hohagen F. Impaired declarative memory consolidation during sleep in patients with primary insomnia: influence of sleep architecture and nocturnal cortisol release. Biol Psychiatry. 2006;60(12):1324–30. doi: 10.1016/j.biopsych.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 116.Nissen C, Kloepfer C, Feige B, Piosczyk H, Spiegelhalder K, Voderholzer U, et al. Sleep-related memory consolidation in primary insomnia. J Sleep Res. 2011;20(1 Pt 2):129–36. doi: 10.1111/j.1365-2869.2010.00872.x. [DOI] [PubMed] [Google Scholar]
- 117.Broman JE, Lundh LG, Aleman K, Hetta J. Subjective and objective performance in patients with primary insomnia. Scand J Behav Ther. 1992;21:115e26. [Google Scholar]
- 118.Lundh LG, Froding A, Gyllenhammar L, Broman JE, Hetta J. Cognitive bias and memory performance in patients with persistent insomnia. Scand J Behav Ther. 1997;26:27e35. [Google Scholar]
- 119.Varkevisser M, Van Dongen HP, Van Amsterdam JG, Kerkhof GA. Chronic insomnia and daytime functioning: an ambulatory assessment. Behav Sleep Med. 2007;5(4):279e96. doi: 10.1080/15402000701557425. [DOI] [PubMed] [Google Scholar]
- 120.Fernandez-Mendoza J, Calhoun S, Bixler EO, Pejovic S, Karataraki M, Liao D, et al. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010b;33(4):459–65. doi: 10.1093/sleep/33.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ríos M, Periáñez JA, Muñoz-Céspedes JM. Attentional control and slowness of information processing after severe traumatic brain injury. Brain Inj. 2004;18(3):257–72. doi: 10.1080/02699050310001617442. [DOI] [PubMed] [Google Scholar]
- 122.Sánchez-Cubillo I, Periáñez JA, Adrover-Roig D, Rodríguez-Sánchez JM, Ríos-Lago M, Tirapu J, et al. Construct validity of the Trail Making Test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J Int Neuropsychol Soc. 2009;15(3):438–50. doi: 10.1017/S1355617709090626. [DOI] [PubMed] [Google Scholar]
- 123.Riemann D, Voderholzer U, Spiegelhalder K, Hornyak M, Buysse DJ, Nissen C, Hennig J, Perlis ML, van Elst LT, Feige B. Chronic insomnia and MRI-measured hippocampal volumes: a pilot study. Sleep. 2007;30(8):955–8. doi: 10.1093/sleep/30.8.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Altena E, Van Der Werf YD, Sanz-Arigita EJ, Voorn TA, Rombouts SA, Kuijer JP, Van Someren EJ. Prefrontal hypoactivation and recovery in insomnia. Sleep. 2008;31(9):1271–6. [PMC free article] [PubMed] [Google Scholar]
- 125.Altena E, Vrenken H, Van Der Werf YD, van den Heuvel OA, Van Someren EJ. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol Psychiatry. 2010;67(2):182–5. doi: 10.1016/j.biopsych.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 126.Winkelman JW, Benson KL, Buxton OM, Lyoo IK, Yoon S, O’Connor S, Renshaw PF. Lack of hippocampal volume differences in primary insomnia and good sleeper controls: an MRI volumetric study at 3 Tesla. Sleep Med. 2010;11:576–82. doi: 10.1016/j.sleep.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 127.Morin CM, Bélanger L, LeBlanc M, Ivers H, Savard J, Espie CA, Mérette C, Baillargeon L, Grégoire JP. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169(5):447–53. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 128.Vgontzas AN, Fernandez-Mendoza J, Bixler EO, Singareddy R, Shaffer ML, Calhoun SL, et al. Persistent insomnia: the role of objective short sleep duration and mental health. Sleep. 2012;35(1):61–8. doi: 10.5665/sleep.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fung MM, Peters K, Redline S, Ziegler MG, Ancoli-Israel S, Barrett-Connor E, et al. Decreased slow wave sleep increases risk of developing hypertension in elderly men. Hypertension. 2011;58(4):596–603. doi: 10.1161/HYPERTENSIONAHA.111.174409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Edinger JD, Means MK, Krystal AS. Does Physiological Hyperarousal Enhance Error Rates Among Insomnia Sufferers? Sleep. doi: 10.5665/sleep.2882. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sánchez-Ortuño MM, Edinger JD, Means MK, Almirall D. Home is where sleep is: an ecological approach to test the validity of actigraphy for the assessment of insomnia. J Clin Sleep Med. 2010;6(1):21–9. [PMC free article] [PubMed] [Google Scholar]
- 132.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollack CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 133.Deuschle M, Schmider J, Weber B, Standhardt H, Körner A, Lammers CH, et al. Pulse-dosing and conventional application of doxepin: effects on psychopathology and hypothalamus-pituitary-adrenal (HPA) system. J Clin Psychopharmacol. 1997;17(3):156–60. doi: 10.1097/00004714-199706000-00003. [DOI] [PubMed] [Google Scholar]
- 134.Monteleone P. Effects of trazodone on plasma cortisol in normal subjects. A study with drug plasma levels. Neuropsychopharmacology. 1991;5(1):61–4. [PubMed] [Google Scholar]
- 135.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;281(11):991–9. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 136.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. JAMA. 2001;285(14):1856–64. doi: 10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]
- 137.Edinger JD, Fins AI, Glenn DM, Sullivan RJ, Jr, Bastian LA, Marsh GR, Dailey D, Hope TV, Young M, Shaw E, Vasilas D. Insomnia and the eye of the beholder: are there clinical markers of objective sleep disturbances among adults with and without insomnia complaints? J Consult Clin Psychol. 2000;68(4):586–93. [PubMed] [Google Scholar]