Abstract
Objectives
Livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) emergence is a major public health concern. This study was aimed at assessing risk factors for persistently carrying MRSA in veal calf farmers and their family members. We also evaluate the dynamics of MRSA environmental load during the veal-calf production cycle.
Design
Observational, longitudinal, repeated cross-sectional study.
Setting
52 veal calf farms in the Netherlands.
Participants
From the end of 2010 to the end of 2011, a total of 211 farmers, family members and employees were included in the study.
Primary outcome and secondary outcome measures
Nasal swabs were taken from participants on days 0, 4, 7 and week 12. A persistent MRSA carrier was defined as a person positive for MRSA on days 0, 4 and 7. Participants filled in an extensive questionnaire to identify potential risk factors and confounders. For estimation of MRSA prevalence in calves and environmental contamination, animal nasal swabs and Electrostatic Dust Collectors were taken on day 0 and week 12.
Results
The presence of potential animal reservoirs (free-ranging farm cats and sheep) and the level of contact with veal calves was positively associated with persistent MRSA carriage. Interestingly, at the end of the study (week 12), there was a twofold rise in animal prevalence and a significantly higher MRSA environmental load in the stables was found on farms with MRSA carriers.
Conclusions
This study supports the hypothesis that environmental contamination with MRSA plays a role in the acquisition of MRSA in farmers and their household members and suggests that other animal species should also be targeted to implement effective control strategies.
Keywords: Epidemiology
Article summary.
Strengths and limitations of this study
The longitudinal nature of the data allows dynamic epidemiological inferences to be established.
Data on environmental contamination with MRSA give a more in depth picture of MRSA transmission in farms.
No other animal apart from veal calves were sampled in this study. The self-sampling of noses by individuals might influence the sensitivity for MRSA detection.
Introduction
In recent years, livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA), specifically sequence type (ST) 398, has emerged in food-producing animals and people in contact with these animals.1–4 Illness associated to ST398 in humans is rare and only a small proportion of MRSA infections can be attributed to LA-MRSA.5 6 Nonetheless, invasive infections and hospital outbreaks of MRSA ST398 have been reported in Europe, the USA and Asia.5 7 8
LA-MRSA strains have been found mainly in pigs and veal calves, but they have the capacity to colonise a wide spectrum of hosts, including sheep and poultry.9 Farmers are easily contaminated and in general the carriage prevalence in farmers is high. Frequency of transmission between farmers and their family members and among hospitalised humans appears to be low.2 10 11 However, this belief might be contradicted by recently described LA-MRSA transmission events in Dutch patients with neither risk factors nor livestock contact.12 The potential public health threat posed by these strains is emphasised in a recent metapopulation model in which the likelihood of persistent carriage in the livestock-exposed population was the key parameter for LA-MRSA spreading to the community.13
Previous studies have been mainly based on cross-sectional designs and have shown that intensity of animal contact and MRSA prevalence among animals are positively associated to LA-MRSA human carriage.14 Associations between animal carriage and farm hygiene and antimicrobial use have also been shown.15 16 A longitudinal study including periods of high and low exposure to animals showed that LA-MRSA carriage was mainly transient. It was suggested that LA-MRSA is a poor persistent coloniser in humans, which was confirmed by a study on short-term occupational exposure.10 14 However, risk factors for persistent LA-MRSA carriage and for a possible true colonisation have not been thoroughly assessed. Furthermore, little is still known about the dynamics of environmental contamination with MRSA in the farm and its role in transmission to humans. A recent study showed a steep increase in prevalence among calves and in MRSA air load during the production cycle.17
The aim of the current study was twofold. First, to assess risk factors and dose–response relationships for persistently carrying MRSA over a period of 1 week at the beginning of the production cycle in veal calf farmers and their family members. Second, to evaluate the deposition of MRSA-containing dust inside the farm and its relationship with animal and human MRSA carriage.
Materials and methods
Study design and population
A longitudinal cohort study was performed over a period of 12 weeks in 52 veal calf farms starting at the beginning of the production cycle. All farms were visited from the end of 2010 to the end of 2011. All farms met the following inclusion criteria: implemented all-in-all-out system; no other livestock in large scale apart from veal calves; a unique location for all the stables or farm; veal calf farmers not working in another animal sector (eg, transport of pigs) and not operating in other farms. Preference for selection was given to farms in the proximity of Utrecht, the Netherlands. On each farm there were two sampling periods for animal and environmental samples (day 0 and week 12) and four sampling periods for human samples (days 0, 4, 7 and week 12). Nasal swabs from both anterior nares of calves were taken and analysed in 10 pools of six swabs each (60 animals per farm). Swabs were also collected from farmers, family members and employees (n=211). On day 0, quantitative nasal and throat swabs were taken by field workers in the majority of participants or by self-sampling. On days 4, 7 and on week 12, dry cotton swabs (Copan, Brescia, Italy) were used to self-sample the nose. Swabs were given to participants with instructions including photographs in case of self-sampling. Nasal swabs in animals and humans were introduced in the nostril and rotated once. Throat swabs in humans sampled the area of the inner cheek including the tonsils. The swabs were immediately taken to the laboratory or sent by post and processed within 24 h after arrival. Furthermore, environmental samples were taken by placing four Electrostatic Dust Collectors (EDCs; Zeeman, Utrecht, The Netherlands) on different surfaces inside the stables and one on the highest cupboard in the living room or kitchen of the house. The EDCs were left in place during a period of 2 weeks and sent by post to the laboratory. On arrival, EDC samples were stored at –20°C until quantitative analysis.18
All participants completed an informed consent form and filled in an extensive questionnaire including items related to individual health status, household and farm characteristics, activities performed on the farm and hygiene practices. The protocol of the study was approved by Medical Ethical Committee of Utrecht University. The collection of animal samples was in compliance with the Dutch Law on Animal Health and Welfare.
For the assessment of MRSA-persistent carriage, we selected the beginning of the veal-calf production cycle, just after the stables were empty and when animal prevalence is lower. In this period, deposition of MRSA-containing dust particles in human nasal cavities and mechanical carriage was assumed to be less likely. Therefore and for the purpose of this study, a person was defined to be a persistent MRSA carrier when each of the nasal swabs collected on days 0, 4 and 7 were positive for MRSA presence.
Laboratory analysis
Swabs in liquid transport medium (ESwab, Copan, Brescia, Italy) were used for quantitative cultivation. Serial dilutions (1:10) of the transport medium (concentration 100) were made by adding 100 µL sample to 900 µL phosphate buffered saline (PBS) to a final concentration of 10−4 of the original sample. Each dilution was cultured on chromID S aureus and chromID MRSA agar plates (BioMérieux, La Balme Les Grottes, France) at 37°C for 18–24 h. Plates with 10–100 colony-forming units (CFU) were used to calculate the original amount of CFU per swab. In order to detect positive samples without bacterial growth in the first day, the remaining transport medium and swab were enriched overnight in Mueller Hinton broth with 6.5% NaCl (MH+), and consequently cultured on chromID S aureus and chromID MRSA agar plates. The theoretical lower limit of quantification (LLOQ) of MRSA CFU was 10. Dry cotton swabs (Copan) were inoculated directly onto chromID S aureus, chromID MRSA and MH+. Confirmation of MRSA presence in the three sampling moments was carried out using real-time (RT) PCR targeting mecA, femA and nuc genes.19 20 Methicillin-susceptible S aureus (MSSA) presence was tested when the bacterial growth on chromID S aureus was higher than on chromID MRSA. For this purpose, 10 colonies were screened for methicillin susceptibility by using the cefoxitin disk diffusion method. Confirmation of MSSA was carried out using RT-PCR. Nasal swabs from calves were analysed in pools following standard procedures previously described.21
To obtain an estimate of exposure in CFU per EDC, EDCs were analysed using RT- quantitative PCR (qPCR). EDC samples were suspended in 10 mL EDTA saline buffer (150 mM NaCl, 1 mM EDTA) and mixed in a Stomacher (Seward Ltd., London, UK) for 10 min. Two millilitre of the resulting suspension was stored at –20°C for the analysis. For DNA isolation, 200 µL of the suspension was incubated at 95°C for 15 min. PBS was added and a Versant kPCR molecular system (Siemens Healthcare Diagnostics, The Hague, The Netherlands) was used for DNA purification with an elution volume of 50 µL. Five microlitre of the purified sample were used for detection of mecA, femA and nuc genes by the means of a LightCycler 480-II system (Roche Diagnostics, Almere, The Netherlands). For MRSA quantification, a standard curve was established for all targets. A standard control sample was included in each run to correct the curve for run-to-run variation. For interpretation of the results, CFU counts per PCR were transformed to CFU counts per EDC (1 CFU/PCR=200 CFU/EDC). The theoretical limit of detection was 20 CFU/EDC.
RT-PCR targeted at C01 gene was carried out for confirmation of ST398 in all MRSA-positive human, animal and environmental samples.
Data analysis
Statistical analysis was performed using SAS software V.9.2 (SAS institute Inc, Cary, North Carolina, USA). Descriptive analysis determined the cross-sectional human prevalences on each of the four sampling moments and the longitudinal carriage patterns (persistent, intermittent or non-carriers).
Risk factors for nasal MRSA-persistent carriage were investigated with univariate and multivariate analysis. PROC GENMOD was used for Generalised Estimating Equations modelling to take clustering of data at farm level into account. The mean response was modelled with a Poisson regression with robust SEs. Crude and age-sex adjusted prevalence ratios (PRs) were obtained. Eligibility criteria for variables to be considered in multivariable analysis included univariate p values below 0.2, less than 10% of missing data in relation with the outcome, and at least two persistent carriers falling in each of the categories of the explanatory categorical variables. Bivariate correlation structure of all eligible variables was studied with PROC CORR, and Spearman correlation coefficients were obtained. Thereafter, eligible variables were added in a stepwise backward selection approach and retained in the final model when p<0.15. A p value <0.05 was considered statistically significant.
The shape of the relationships between MRSA-persistent carriage and numerical variables was studied by means of non-parametric or semiparametric regression modelling (smoothing) using PROC GAM to relax the assumption of linearity. For this purpose, the number of CFU from quantitative nasal swabs positive for MRSA but below LLOQ was set to 5.
To assess the environmental exposure during the first week, farms were classified in three categories: (1) farms with persistent carrier, when there was at least one MRSA-persistent carrier working and/or living on the farm; (2) farms with intermittent carrier, when there was at least one MRSA-intermittent carrier and there was no persistent carrier on the farm; (3) non-carrier farms, when all people at the farm were MRSA negative on the first three sampling periods. On week 12 farms were classified as carrier and non-carrier farms when there was at least one MRSA carrier on the farm, and when all people on the farm were MRSA negative on week 12, respectively. Proportions of MRSA-positive EDCs were calculated per farm category and sampling moment. For calculation of average exposure levels, CFU counts per EDC were log-transformed since they followed a highly right-tailed distribution. PROC LIFEREG was used for left-censored regression (tobit) modelling to obtain an accurate estimate of the mean exposure level accounting for the large proportion of undetectable values. Thereafter geometric means were calculated.
Results
Descriptive results
Nasal swabs were collected from 211 participants on 52 farms. The average nasal MRSA prevalence for the four sampling moments was twice as high in farmers (29.7%) as compared with family members (13%). Cross-sectional nasal MRSA prevalences per sampling moment are displayed in online supplementary figure S1.
Nasal carriage patterns for MRSA, MSSA and S aureus in general (including both MSSA and MRSA) were assessed over the 1-week period. The MRSA and MSSA-persistent carrier prevalence followed opposite directions in farmers as compared with family members. For MRSA-persistent carriage the prevalence in farmers (15.5%) was twice as high as in family members (7.6%). MSSA-persistent carriage prevalence was three times higher in family members than in farmers (15.3% and 5.2%, respectively). Regarding S aureus, there were not significant differences between the subpopulations of farmers and family members and 22.8% of all individuals were persistently carrying the bacteria, 29.6% were intermittent carriers and the remaining 47.6% never carried S aureus. Online supplementary table S1 shows these longitudinal carriage patterns in more detail.
Table 1.
Crude and adjusted for sex and age prevalence ratios (PR) for nasal MRSA-persistent carriage in 195 veal calf farmers and household members from 51 farms
| Determinant | Category | N | Number persistent carriers† (prevalence %) | Mean (range) | PR | 95% CI | PR‡ adjusted | 95% CI |
|---|---|---|---|---|---|---|---|---|
| General characteristics | ||||||||
| Sex | Male | 103 | 9 (8.7) | – | 1 | – | – | – |
| Female | 92 | 11 (12.0) | – | 1.4 | 0.6 to 3.2 | – | – | |
| Age | – | 195 | – | 30 (0.1–81) | 1.0 | 1.0 to 1.0** | – | – |
| Per 10 years increase | – | 195 | – | – | 1.3 | 1.1 to 1.6** | – | – |
| Farm and household characteristics | ||||||||
| Presence of sheep in farm | No | 149 | 12 (8.1) | – | 1 | – | 1 | – |
| Yes | 46 | 8 (17.4) | – | 2.2 | 1.1 to 4.5* | 2.4 | 1.2 to 4.8* | |
| Presence of cats on farm | No | 96 | 5 (5.2) | – | 1 | – | 1 | – |
| Yes | 99 | 15 (15.2) | – | 3.0 | 1.2 to 7.1* | 2.7 | 1.1 to 6.6* | |
| Presence of pets | No | 74 | 4 (5.4) | – | 1 | – | 1 | – |
| Yes | 121 | 16 (13.2) | – | 2.7 | 1.0 to 7.4* | 2.6 | 1.0 to 6.7§ | |
| Tasks performed last 7 days¶ | ||||||||
| Sorting calves (stable management) | No | 113 | 5 (4.4) | – | 1 | – | 1 | – |
| Yes | 82 | 15 (18.3) | – | 4.2 | 1.5 to 12.3** | 4.7 | 1.3 to 16.8* | |
| Healthcare/control†† | No | 132 | 9 (6.8) | – | 1 | – | 1 | – |
| Yes | 63 | 11 (17.5) | – | 2.6 | 1.1 to 6.1* | 2.3 | 0.8 to 7.3 | |
| Feeding calves | No | 72 | 2 (2.8) | – | 1 | – | 1 | – |
| Yes | 123 | 18 (14.6) | – | 7.2 | 0.9 to 58.6§ | 5.4 | 0.6 to 52.3 | |
| Work at farm, hygiene cleaning and disinfection | ||||||||
| Administration of antibiotics during | No | 131 | 8 (6.1) | – | 1 | – | 1 | – |
| last month | Yes | 64 | 12 (18.8) | – | 3.2 | 1.4 to 7.1** | 3.4 | 1.3 to 9.1* |
| Number of working hours per week | – | 195 | – | 16.5 (0–80) | 1.0 | 1.0 to 1.0*** | 1.0 | 1.0 to 1.1** |
| Per 20 h increase | – | – | – | – | 1.8 | 1.4 to 2.4*** | 2.5 | 1.4 to 4.2** |
| Clean towel | No | 45 | 7 (16.7) | – | 1 | – | 1 | – |
| Yes | 150 | 13 (8.67) | – | 0.6 | 0.3 to 1.3 | 0.6 | 0.3 to 1.1 | |
| Changing room available | No | 18 | 3 (16.7) | – | 1 | – | 1 | |
| Yes | 177 | 17 (9.7) | – | 0.6 | 0.3 to 1.2 | 0.5 | 0.2 to 1.0§ | |
| Cleaning of baby boxes | No | 184 | 18 (9.8) | – | 1 | – | 1 | – |
| Yes | 11 | 2 (18.2) | – | 1.9 | 1.0 to 3.5* | 1.3 | 0.6 to 2.8 | |
†A person is considered a persistent carrier when all nasal swabs at days 0, 4 and 7 are positive for MRSA.
‡Prevalence ratios adjusted for sex and age.
§Non-significant trend (p Value 0.05–0.10). * p Value 0.01–0.05. **p Value 0.0001–0.01. ***p Value <0.0001.
¶Tasks performed in the week before time 0.
††The task healthcare and control includes the administration of antibiotics.
MRSA, methicillin-resistant Staphylococcus aureus.
The RT-PCR targeted at C01 gene showed that ST398 was present in 90.5% of the human MRSA isolates, in 97.9% of the MRSA-positive animal pools and 90.9% of the MRSA-positive EDCs.
Microbiological status and persistent MRSA nasal carriage
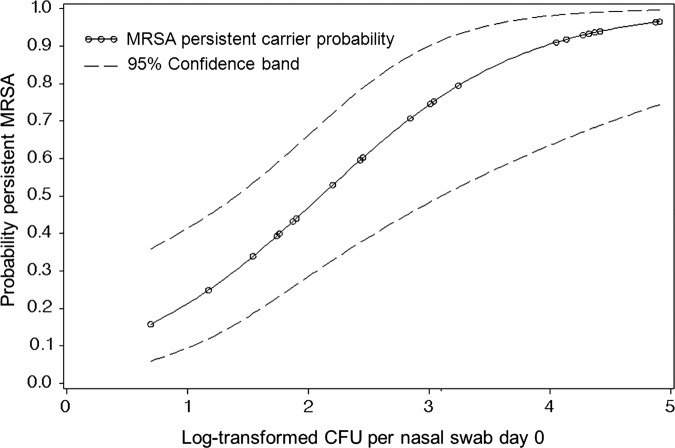
CFU counts were determined in 42 participants from quantitative nasal swabs on day 0. Figure 1 shows the shape of the relationship between the probability of being a persistent MRSA nasal carrier and the log-transformed MRSA concentration (CFU/swab suspension). The median CFU count was 43.65 with an IQR 5.01–1096.48. In addition, the univariate logistic regression analysis in this population resulted in 1.68 times higher risk (95% CI 1.34 to 2.10, p<0.001) for persistent MRSA carriage per 10 CFU increase.
Figure 1.
Probability of nasal methicillin-resistant Staphylococcus aureus (MRSA) persistent carriage and its relationship with the log-transformed colony-forming units from MRSA-positive nasal swabs at day 0. Non-parametric regression modelling.
No MSSA was found in MRSA-positive samples at day 0. In order to obtain an estimation of the PR for the outcome when MSSA is present at day 0, data were manipulated by placing an MSSA-positive result for one of the persistent carriers. This way an adjusted PR of 0.14 (95% CI 0.02 to 1.06, p=0.06) was obtained.
People found positive for MRSA in throat swabs at day 0 were at higher risk for being persistent nasal carriers (adjusted PR=12.2, 95% CI 5.2 to 28.8, p<0.0001). The spearman correlation coefficient between this variable and the outcome was 0.6 (p<0.0001).
A sensitivity analysis restricted to ST398 was carried out and it yielded similar results as described above.
Univariate and multivariate analysis for persistent MRSA nasal carriage
Crude and age-sex-adjusted PRs in determinants meeting the specified criteria are presented in table 1. Gender and smoking habits were not clearly associated with the outcome (p>0.2). Although these variables together with age are considered potential confounders, sensitivity analysis was performed with smoking habits added to gender and age for adjustment. This did not result in significant changes in estimates (results not shown) when compared with adjustment without smoking habits.
Statistically significant risk factors for persistent MRSA carriage were identified (table 2). Pet ownership showed a PR of 2.7 (p=0.05). The number of working hours per week in the farm was positively associated with the outcome (adjusted PR=2.5 expressed per 20 h/week increase, p=0.001). An increasing probability for MRSA-persistent carriage with number of working hours in the farm was also demonstrated through semiparametric regression modelling (see online supplementary figure S2). Administration of antimicrobials to calves through milk and injection in the past month preceding sampling was also a significant risk factor (adjusted PR=3.4, p=0.01). Other associations with the outcome did not show statistical significance. These include protective factors such as people living on farms with a changing room available (adjusted PR=0.5, p=0.07) or on farms where clean towels are used after work (adjusted PR=0.6, p=0.11) and risk factors, such as people living in farms where baby boxes are cleaned at the beginning of the production cycle (adjusted PR=1.3, p=0.54). Other determinants such as the prevalence of MRSA in animals at the farm level did not show an association with persistent human MRSA carriage (PR=1.0, 95% CI 1.0 to 1.0, p=0.96). There was also no association found with variables regarding individual health status.
Table 2.
Results from multiple logistic regression analysis for nasal MRSA-persistent carriage in veal calf farmers and their household members (N=195)
| Determinant | Category | PR | 95% CI | p Value |
|---|---|---|---|---|
| Model A | ||||
| Number of working hours per week | – | 1.03 | 1.02 to 1.04 | 0.000* |
| per 20 h increase | 1.81 | 1.49 to 2.19 | – | |
| Presence of cats on farm | No | 1 | – | – |
| Yes | 2.80 | 1.23 to 6.36 | 0.014* | |
| Presence of sheep in farm | No | 1 | – | – |
| Yes | 1.83 | 0.89 to 3.77 | 0.100 | |
| Changing room available | No | 1 | – | – |
| Yes | 0.48 | 0.20 to 1.13 | 0.094 | |
| Cleaning of baby boxes | No | 1 | – | – |
| Yes | 3.96 | 1.59 to 9.90 | 0.003* | |
| Model B | ||||
| Age | – | 1.02 | 1.00 to 1.05 | 0.037* |
| per 10 years increase | – | 1.26 | 1.01 to 1.56 | – |
| Presence of cats on farm | No | 1 | – | – |
| Yes | 2.57 | 1.05 to 6.33 | 0.040* | |
| Presence of sheep in farm | No | 1 | – | – |
| Yes | 1.78 | 0.88 to 3.59 | 0.107 | |
| Sorting calves | No | 1 | – | – |
| Yes | 3.10 | 1.14 to 8.47 | 0.027* | |
Model A: final model in which all variables meeting eligibility criteria were added to the automatic selection. Model B: final model in which all the variables in model A were added to the automatic selection except number of working hours.
*p Value statistically significant (ie, <0.05).
MRSA, methicillin-resistant Staphylococcus aureus; PR, prevalence ratio.
Results from the multiple logistic regression analysis are presented in table 2. In model A, all variables meeting the described criteria were eligible to entry. In this model, number of working hours per week showed the most significant association with persistent MRSA carriage (PR=1.8 expressed per 20 h/week increase, p<0.0001). As this variable was a very strong determinant, the result of which potential tasks were not retained, a model was explored (model B) without the number of working hours. In consequence, stable management (sorting calves) was retained in the final model B with a statistically significant PR of 3.1 (p=0.03). In both multivariate models, the presence of cats on the farm was significantly associated with the outcome (PR=2.8, p=0.01 in model A and PR=2.6, p=0.04 in model B).
Specific tasks on the farm were adjusted for number of working hours in a bivariate analysis and the estimates obtained were not statistically significant. Only stable management remained positively associated with the outcome with a PR of 2.5 (95% CI 0.7 to 9.6; p=0.17); however, administration of antibiotics in the month before sampling showed no association with a PR of 1.1 (95% CI 0.2 to 5.9; p=0.91).
A sensitivity analysis restricted to ST398 was carried out and it yielded similar univariate and multivariate results.
Contamination of the environment with MRSA
At the beginning of the production cycle, MRSA was detected in only 4.6% of all EDCs placed in stables and on six farms. Differences in environmental exposure across persistent, intermittent and non-carrier farms were not significant (table 3). None of the EDCs placed inside the houses were found to be positive for MRSA.
Table 3.
Environmental MRSA samples (EDCs) taken in stables at the beginning of the production cycle in 51 farms with persistent, intermittent or non-MRSA carrying veal calf farmers and household members
| Persistent* | Intermittent* | Non-carrier* | p Value† | |
|---|---|---|---|---|
| Number of farms with MRSA-positive EDCs/total number of farms (%) | 2/18 (11.11) | 2/12 (16.67) | 2/21 (9.52) | 0.86 |
| Number of MRSA positive EDCs/total number of EDCs (%) | 2/69 (2.90) | 4/47 (8.51) | 3/78 (3.85) | 0.38 |
| GM MRSA CFU/EDC (p Value) ‡ | <1 (0.75) | <1 (0.29) | <1 (ref.) | – |
*A farm was categorised as persistent when there was at least one persistent carrier living and/or working on the farm, non-carrier farms had no individual positive for MRSA in nasal swabs on days 0, 4, 7 and intermittent farms were the remaining.
†p Values among proportions were calculated with Fisher's exact test. Mean values had not an overall assigned p value since they could not be tested with non-parametric tests.
‡Geometric mean (antilogged results from tobit regression). p Values indicate the difference with the reference category (non-carrier farm).
CFU, colony-forming units; EDC, Electrostatic Dust Collector; GM, geometric means; MRSA, methicillin-resistant Staphylococcus aureus.
In week 12, MRSA was detected in 50.6% of all EDCs placed in the stables and on 39 farms. There was a significantly higher proportion of EDCs positive for MRSA and a trend for higher CFU counts per EDC in farms where MRSA carriers were found in week 12 (table 4). Stratified analysis was performed in farmers and family members. The same trends for higher MRSA environmental load were found only in farmers, however not statistically significant (results not shown). MRSA was found in EDCs from 10 houses (table 4).
Table 4.
Environmental MRSA samples (EDCs) taken in stables and houses on week 12 in 49 farms with MRSA carriers and non-carriers
| Location EDC | Carrier farms* | Non-carrier farms* | p Value† |
|---|---|---|---|
| Number of farms with MRSA-positive EDCs/total number of farms (%) | |||
| Stable | 22/25 (88.00) | 17/24 (70.83) | 0.14 |
| House | 3/25 (12.00) | 7/24 (29.17) | 0.17 |
| Number of MRSA-positive EDCs/total number of EDCs (%) | |||
| Stable | 54/90 (60.00) | 35/86 (40.70) | 0.01 |
| House‡ | – | – | – |
| GM§ MRSA CFU/EDC | |||
| Stable | 27.54 | 16.98 | 0.06 |
| House | 2.29 | 5.50 | 0.29 |
*A farm was categorised as carrier when there was at least one carrier on week 12 living and/or working on the farm, non-carrier farms were the remaining.
†p Values among proportions were calculated with χ2 test and Fisher's exact test when 20% of the expected cell values were <5. p Values for the GM indicate the difference with the reference category (non-carrier farms).
‡There was one EDC per house, thus the values in this line are the same as the ones in ‘Number of farms with MRSA-positive EDCs/total number of farms (%)’.
§Geometric mean (antilogged results from tobit regression).
CFU, colony-forming units; EDC, Electrostatic Dust Collector; GM, geometric means; MRSA, methicillin-resistant Staphylococcus aureus;
The mean pooled MRSA prevalence in calves rose from 18.7% at day 0 to 46% in week 12. A simple linear regression between the EDC MRSA levels (maximum log-transformed MRSA CFU/EDC per farm) and animal prevalence showed a positive and significant association (β=0.006, p=0.0014). Furthermore, there was a 60% increased probability for detecting an MRSA-positive EDC in farms where animal prevalence in week 12 was above the mean (PR=1.6, 95% CI 1.09 to 2.38, p=0.02). With regard to human carriage in relation to animal prevalence, no association between being an MRSA carrier and the prevalence in calves was found on day 0. On week 12 there was a slight increase in prevalence among farmers as compared with the previous sampling period (see online supplementary figure S1) and individuals from farms with MRSA prevalence in calves above the mean were at two times higher risk of carrying MRSA (PR=2.12, 95% CI 1.12 to 4.01, p=0.02).
Discussion
The associations found during the first week after arrival of the animals on the farm show that the level of exposure to veal calves and the presence of potential animal reservoirs (pets, free-ranging farm cats and sheep) are risk factors for persistent MRSA carriage in farmers and household members. Additionally, persistent MRSA carriers seem to have a different microbiological profile when compared with intermittent and non-carriers, which is characterised by higher MRSA load in nose, presence of MSRA in throat and absence of MSSA. This study shows that as the production cycle advances, there is a rise in MRSA prevalence in calves that leads to higher contamination of the air and higher probability for human MRSA carriage.
Descriptive results confirm that high MRSA carriage prevalence (17.6%) is observed among individuals living on farms, as seen in other studies.2 16 This percentage represents a carriage burden in countries where estimated MRSA prevalence in the community is below 1% such as the Netherlands and Scandinavian countries. The large difference in prevalence between farmers and family members can be attributed to the different intensity of animal contact and is again an indication of a low LA-MRSA human-to-human transmission.16 22 Swabs in liquid transport medium were used only on day 0 for the purpose of quantification. The fact that higher prevalences are observed on day 0 as compared with days 4 and 7 might be due to highest sensitivity for MRSA detection as compared with dry cotton swabs (see online supplementary figure S1). The carriage patterns of S aureus presented are similar to those described by Wertheim et al,23 in which they found percentages of 20%, 30% and 50% for persistent, intermittent and non-carriers, respectively, among healthy individuals. The lower MRSA-persistent carrier prevalence in the total study population (9.7%) as compared with the average cross-sectional MRSA prevalence (17.6%) indicates that carriage of LA-MRSA is fleeting and varies within individuals.
Confirmation of only ST398 was carried out in the laboratory and it was predominant (higher than 90%) among the MRSA isolates from humans, animal pools and EDC samples. MRSA-positive subjects negative for ST398 did not visit a hospital during the previous 12 months of the study and there were other elements than ST398 MRSA present in animal and environmental samples. All MRSA was considered to be circulating and transmitted in the farm since it is very likely that other livestock-associated STs were present as in previous studies.14 16
Owing to culturing techniques, MSSA was detected with difficulty when there was a predominant MRSA growth. The possible underestimation of MSSA asks for a cautious interpretation of the results. Nevertheless it is remarkable that no persistent MRSA carrier was positive for MSSA at day 0. This suggests that the presence of MSSA in the nose might be a protective factor for MRSA-persistent carriage. Moreover, a negative association between MSSA and MRSA has been recently found in a study.14
In the first week of the production cycle the MRSA environmental load was lower and it can be assumed that nasal contamination with MRSA-containing dust particles and transient mechanical carriage was less likely to occur as compared with further time points in the production cycle. As shown in figure 1, there is an increased probability for persistent MRSA carriage associated with higher MRSA CFU counts in nasal swabs. Moreover, isolation of MRSA in throat swabs at day 0 was significantly associated to the outcome (PR=12.2). These findings suggest that there might be a true colonisation in persistent MRSA carriers as defined here. Furthermore a recent study has shown that ST398 is capable of adequately competing for a niche with a human strain and survives in the human nose for longer periods.24
Direct association between administration of antibiotics and MRSA-persistent carriage in farmers and their family members, as defined in our study, was shown in univariate results (PR=3.2). It is known that when antimicrobials are administered to animals, substantial quantities of these drugs can be present in manure, on surfaces of animal houses and in dust as a potential risk source.25 We could hypothesise that respiration of dust containing antibiotics, either from a contaminated environment or directly from a powder formulation, would exert a selective pressure in the anterior nares leading to higher risk for MRSA-persistent carriage in people occupationally exposed. However, this association was not confirmed in multivariate models and it needs further exploration. Number of working hours and other tasks were correlated and may have more influence on persistent carriage. This was also shown when adjustment for number of working hours was carried out in a bivariate fashion.
This study supports that close contact with animals is a major risk factor for persistent LA-MRSA carriage in humans. This is made clear by the final set of variables retained in the multivariate models. The number of working hours was most strongly associated with persistent carriage as indicated by the model A and by the smoothed exposure–response relation shown in online supplementary figure S3. Moreover, when the number of working hours was removed for model B, another variable representing close contact with animals (stable management) was retained by the backward procedure.
In recent years, several reports have suggested a potential role for pet animals, specifically cats and dogs, in household MRSA transmission and relapse of human MRSA infections. This transmission seems to be of anthropozoonotic origin. Thus, pets can acquire human strains from humans and they can cause colonisation or infection in human cohabitants.26–31 In most cases, the distribution of the clones in pet animals has mirrored the epidemiology of human clones and mainly shared hospital-associated and community-associated MRSA strains have been reported. It is remarkable that in this study, having a pet in the household was strongly associated with MRSA carriage in veal farmers and household members. Moreover, there is a demonstrated spread of LA-MRSA between animal species, humans and the farm environment.32 In this study no other animal apart from veal calves were sampled; however, the presence of free-ranging farm cats and sheep were significantly associated and retained in multivariate models. A previous large cross-sectional study sampled 35 cats from 25 farms, 26 of them came frequently in the veal stables. Only one of these cats was found to be MRSA positive with a spa type t011 (ST398).33 Cats might act as reservoirs but this is more suggestive of cats acting as mechanical vectors. These animals might represent an intermittent source of LA-MRSA that might contribute to LA-MRSA-persistent carriage in humans.
Other farm characteristics and hygiene practices were also associated with persistent MRSA carriage, although not significantly. Having a changing room in the farm and using a clean towel after working in the stables were found as protective factors. This might give a direction to specific preventive strategies. On the other hand, cleaning of baby boxes at the beginning of the production cycle was a risk factor for the outcome (PR=4 in multivariate model A and PR=1.9 in univariate analysis). This hygiene practice could give rise to transitory spread in the air of accumulated MRSA.
Environmental contamination with dust particles containing MRSA is much lower in veal calf farming as compared with pig farming and associations are less evident.34 As shown in table 3, no difference in the environmental MRSA load was found across persistent, intermittent and non-carrier farms at the beginning of the production cycle. However, the twofold rise in animal prevalence at the end of the study was associated with a considerably higher environmental MRSA load and a significantly higher proportion of MRSA-positive EDCs were found on farms with MRSA carriers on week 12. This finding supports that contamination of the environment plays a role in the acquisition of MRSA in people living or working in the farm.
A possible limitation of the study is the self-sampling of nose and throat by individuals which might be lacking in accuracy for MRSA detection. This is however believed to be a minor bias. A recent pilot study has shown high degree of agreement between self-samples and investigator samples (93% agreement, κ 0.85 for nasal swabs and 83% agreement, κ 0.60 for throat swabs).35 Another limitation is the previously described underestimation of MSSA presence but this is of negligible impact in the results because detection of MRSA and S aureus remains unaffected. Finally, there were many missing values in some variables and they were excluded from the analysis. There were five individuals out of the 211 with missing nasal samples but sensitivity analysis did not reveal significant changes in estimates.
In conclusion, people living and/or working in veal calf farms who persistently carry MRSA seem to be defined by a differential microbiological profile. The associations found here with the presence of free-ranging farm cats and multispecies farming ask for improved internal and external biosecurity measures. Detailed molecular-epidemiological analysis of MRSA specimens on the farm in various animal species and humans is also essential to identify reservoirs and transmission routes for LA-MRSA. Finally, environmental contamination with MRSA has to be thoroughly studied to assess the extent of its importance in the transmission of MRSA within the veal-calf farming community.
Supplementary Material
Acknowledgments
The authors wish to thank all farmers for their participation and all field workers and laboratory assistants, especially Isabella Oosting-Van Schothorst, Arjen Timmerman and Marian Broekhuizen-Stins.
Footnotes
Contributors: AD-G performed the statistical analyses and interpretation of the data, and drafted the manuscript. MEHB collected the data, contributed to the interpretation of the data, and contributed to the critical revision of the manuscript. HG participated in the conception and design of the study, collected the data and contributed to the critical revision of the manuscript. BAGLVC and JAJWK contributed to the critical revision of the manuscript. KMV carried out the laboratory analysis. JAW conceived the study and contributed to the critical revision of the manuscript. DJJH conceived the study and contributed to the interpretation of the data and the critical revision of the manuscript. All authors read and approved the final manuscript.
Funding: The POM project was funded by The Netherlands Organisation for Health Research and Development (ZonMw) (grant number 125020009), the Product Boards for Livestock Meat and Eggs and the Dutch Ministry of Agriculture Nature and Food Quality.
Competing interests: None.
Ethics approval: Medical Ethical Committee of Utrecht University.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Fitzgerald JR. Livestock-associated Staphylococcus aureus: origin, evolution and public health threat. Trends Microbiol 2012;20:192–8 [DOI] [PubMed] [Google Scholar]
- 2.Graveland H, Duim B, Van Duijkeren E, et al. Livestock-associated methicillin-resistant Staphylococcus aureus in animals and humans. Int J Med Microbiol 2011;301:630–4 [DOI] [PubMed] [Google Scholar]
- 3.Van Duijkeren E, Box AT, Heck ME, et al. Methicillin-resistant staphylococci isolated from animals. Vet Microbiol 2004;103:91–7 [DOI] [PubMed] [Google Scholar]
- 4.Tiemersma EW, Bronzwaer SL, Lyytikainen O, et al. Methicillin-resistant Staphylococcus aureus in Europe, 1999–2002. Emerg Infect Dis 2004;10:1627–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Cleef BA, Monnet DL, Voss A, et al. Livestock-associated methicillin-resistant Staphylococcus aureus in humans, Europe. Emerg Infect Dis 2011;17:502–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van den Broek IV, Van Cleef BA, Haenen A, et al. Methicillin-resistant Staphylococcus aureus in people living and working in pig farms. Epidemiol Infect 2009;137:700–8 [DOI] [PubMed] [Google Scholar]
- 7.Van der Mee-Marquet N, Francois P, Domelier-Valentin AS, et al. Emergence of unusual bloodstream infections associated with pig-borne-like Staphylococcus aureus ST398 in France. Clin Infect Dis 2011;52:152–3 [DOI] [PubMed] [Google Scholar]
- 8.Rasigade JP, Laurent F, Hubert P, et al. Lethal necrotizing pneumonia caused by an ST398 Staphylococcus aureus strain. Emerg Infect Dis 2010;16:1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catry B, Van Duijkeren E, Pomba MC, et al. Reflection paper on MRSA in food-producing and companion animals: epidemiology and control options for human and animal health. Epidemiol Infect 2010;138:626–44 [DOI] [PubMed] [Google Scholar]
- 10.Van Cleef BA, Graveland H, Haenen AP, et al. Persistence of livestock-associated methicillin-resistant Staphylococcus aureus in field workers after short-term occupational exposure to pigs and veal calves. J Clin Microbiol 2011;49:1030–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bootsma MC, Wassenberg MW, Trapman P, et al. The nosocomial transmission rate of animal-associated ST398 meticillin-resistant Staphylococcus aureus. J R Soc Interface 2011;8:578–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lekkerkerk WS, Van de Sande-Bruinsma N, Van der Sande MA, et al. Emergence of MRSA of unknown origin in the Netherlands. Clin Microbiol Infect 2012;18:656–61 [DOI] [PubMed] [Google Scholar]
- 13.Porphyre T, Giotis ES, Lloyd DH, et al. A metapopulation model to assess the capacity of spread of meticillin-resistant Staphylococcus aureus ST398 in humans. PLoS ONE 2012;7:e47504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graveland H, Wagenaar JA, Bergs K, et al. Persistence of livestock associated MRSA CC398 in humans is dependent on intensity of animal contact. PLoS ONE 2011;6:e16830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bos ME, Graveland H, Portengen L, et al. Livestock-associated MRSA prevalence in veal calf production is associated with farm hygiene, use of antimicrobials, and age of the calves. Prev Vet Med 2012;105:155–9 [DOI] [PubMed] [Google Scholar]
- 16.Graveland H, Wagenaar JA, Heesterbeek H, et al. Methicillin resistant Staphylococcus aureus ST398 in veal calf farming: human MRSA carriage related with animal antimicrobial usage and farm hygiene. PLoS ONE 2010;5:e10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graveland H, Wagenaar JA, Verstappen KM, et al. Dynamics of MRSA carriage in veal calves: a longitudinal field study. Prev Vet Med 2012;107:180–6 [DOI] [PubMed] [Google Scholar]
- 18.Noss I, Wouters IM, Visser M, et al. Evaluation of a low-cost electrostatic dust fall collector for indoor air endotoxin exposure assessment. Appl Environ Microbiol 2008;74:5621–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilic A, Muldrew K, Tang Y, et al. Triplex real-time polymerase chain reaction assay for simultaneous detection of Staphylococcus aureus and coagulase-negative staphylococci and determination of methicillin resistance directly from positive blood culture bottles. Diagn Microbiol Infect Dis 2010;66:349–55 [DOI] [PubMed] [Google Scholar]
- 20.Francois P, Pittet D, Bento M, et al. Rapid detection of methicillin-resistant Staphylococcus aureus directly from sterile or nonsterile clinical samples by a new molecular assay. J Clin Microbiol 2003;41:254–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graveland H, Van Duijkeren E, Van Nes A, et al. Evaluation of isolation procedures and chromogenic agar media for the detection of MRSA in nasal swabs from pigs and veal calves. Vet Microbiol 2009; 139:121–5 [DOI] [PubMed] [Google Scholar]
- 22.Wassenberg MW, Hopmans TE, Troelstra A, et al. Methicillin-resistant Staphylococcus aureus of livestock origin in Dutch hospitals: high-risk patients need only to be investigated if admitted to hospital. Ned Tijdschr Geneeskd 2008;152:2681–8 [PubMed] [Google Scholar]
- 23.Wertheim HF, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 2005;5:751–62 [DOI] [PubMed] [Google Scholar]
- 24.Slingerland BC, Tavakol M, McCarthy AJ, et al. Survival of Staphylococcus aureus ST398 in the human nose after artificial inoculation. PLoS ONE 2012;7:e48896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamscher G, Pawelzick HT, Sczesny S, et al. Antibiotics in dust originating from a pig-fattening farm: a new source of health hazard for farmers? Environ Health Perspect 2003;111:1590–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bender JB, Waters KC, Nerby J, et al. Methicillin-resistant Staphylococcus aureus (MRSA) isolated from pets living in households with MRSA-infected children. Clin Infect Dis 2012;54:449–50 [DOI] [PubMed] [Google Scholar]
- 27.Haenni M, Saras E, Chatre P, et al. A USA300 variant and other human-related methicillin-resistant Staphylococcus aureus strains infecting cats and dogs in France. J Antimicrob Chemother 2012;67:326–9 [DOI] [PubMed] [Google Scholar]
- 28.Bramble M, Morris D, Tolomeo P, et al. Potential role of pet animals in household transmission of methicillin-resistant Staphylococcus aureus: a narrative review. Vector Borne Zoonotic Dis 2011;11:617–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coughlan K, Olsen KE, Boxrud D, et al. Methicillin-resistant Staphylococcus aureus in resident animals of a long-term care facility. Zoonoses Public Health 2010;57:220–6 [DOI] [PubMed] [Google Scholar]
- 30.Sing A, Tuschak C, Hörmansdorfer S. Methicillin-resistant Staphylococcus aureus in a family and its pet cat. N Engl J Med 2008;358:1200–1 [DOI] [PubMed] [Google Scholar]
- 31.Cefai C, Ashurst S, Owens C. Human carriage of methicillin-resistant Staphylococcus aureus linked with pet dog. Lancet 1994;344:539–40 [DOI] [PubMed] [Google Scholar]
- 32.Pletinckx LJ, Verhegghe M, Crombe F, et al. Evidence of possible methicillin-resistant Staphylococcus aureus ST398 spread between pigs and other animals and people residing on the same farm. Prev Vet Med 2012;109:293–303 [DOI] [PubMed] [Google Scholar]
- 33.Wagenaar JA, Giessen AW. Veegerelateerde MRSA: epidemiologie in dierlijke productieketens, transmissie naar de mens en karakterisatie van de kloon. RIVM-rapport 330224001, 2009. (in Dutch). [PubMed]
- 34.Van Cleef BA, Van Benthem B, Verkade E, et al. 2013. Dynamics of MRSA carriage in pig farmers: a prospective cohort study. Submitted.
- 35.Van Cleef BA, Van Rijen M, Ferket M, et al. Self-sampling is appropriate for detection of Staphylococcus aureus: a validation study. Antimicrob Resist Infect Control 2012;1:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.