Abstract
Aims
In the USA, transthyretin cardiac amyloidosis usually results from ‘wild-type’ transthyretin (senile cardiac amyloidosis [SCA]) or the V122I variant.
Patients & methods
We compared presentations and outcomes among SCA and V122I patients referred to the Center for Advanced Cardiac Care at Columbia University Medical Center (NY, USA) between 2001 and 2012.
Results
V122I patients were younger (mean: 71 years, standard deviation [SD]: 7) than SCA patients (mean: 77, SD: 6; p = 0.0002) and 96% were black compared with 3% of SCA patients (p < 0.0001). Average ejection fraction was lower among V122I patients (mean: 25% [SD: 12] vs mean: 47% [SD: 15]; p = 0.0001), as was mean cardiac index. Median time to death or orthotopic heart transplant was 36.4 months for V122I patients and 66.5 for SCA patients (p = 0.09).
Conclusion
In this study of patients with transthyretin cardiac amyloidosis, V122I patients presented to a tertiary academic medical center at a younger age than SCA patients but had higher levels of cardiac dysfunction, despite genetic screening availability. There was a trend toward shorter time to orthotopic heart transplant or death among V122I patients. Whether this is a result of a different biologic progression or late diagnosis requires further study.
Keywords: African–American, amyloidosis, elderly, heart failure, transthyretin
Cardiac transthyretin (TTR) amyloidosis (ATTR) is a gradually progressive and ultimately fatal condition caused by extracellular cardiac deposition of polymerized TTR fibrils that results in a restrictive cardiomyopathy and congestive heart failure (CHF). TTR circulates as a homotetramer that binds thyroxine and forms a complex with retinol-binding protein [1,2]. Tetramers can dissociate into monomers that can form fibrillar aggregates [3]. Cardiac ATTR results from deposition of either the canonical (‘wild-type’) TTR amino acid sequence (Ensembl transcript ID: ENST00000237014) [101] or an altered TTR sequence. ATTR patients with wild-type TTR have been designated as having senile cardiac amyloidosis (SCA). In the USA, the most common TTR alteration implicated in cardiac amyloidosis is a G-to-A transition (rs76992529) that results in V122I, a substitution of isoleucine for valine at position 122.
The single nucleotide polymorphism underlying V122I is found almost exclusively among people of black African descent, with an allele frequency as high as 4% in the USA [4,5]. V122Idefined reduces the stability of TTR tetramers, causing an enhanced rate of cardiac deposition of TTR fibrils and resulting in an autosomal dominant familial cardiomyopathy [6]. For unclear reasons, clinically apparent ATTR does not generally occur before 50 years of age, based upon previous observational data [7,8] and our own clinical experience. The penetrance of the V122I polymorphism has not been adequately defined but appears to be high, with echocardiographic penetrance perhaps as high as 75% in one study [9].
There are few comparative data regarding clinical presentation and outcomes among patients with SCA and V122I disease. We analyzed a single-center cohort of patients with cardiac ATTR. The aim of the current study was to describe clinical presentations and outcomes among older adult patients referred to an academic medical center with wild-type cardiac ATTR (SCA) or V122I disease.
Patients & methods
Data were collected from patients with cardiac ATTR who received treatment at the Center for Advanced Cardiac Care at Columbia University Medical Center (NY, USA) between 2001 and 2012.
Diagnosis of cardiac ATTR was by histologic documentation of Congo red staining and apple-green birefringence under cross-polarized light on an endomyocardial biopsy or another organ with confirmation that the deposits were of TTR origin, either by immunohistochemistry or mass spectroscopy. Immunoperoxidase staining was used to confirm TTR and exclude serum amyloid A and immunoglobulin light chain deposition. In the absence of an endomyocardial biopsy, cardiac amyloid was defined echocardiographically as end-diastolic thickness of the inter-ventricular septum of >1.2 cm in the absence of any other cause of ventricular hypertrophy and in the presence of a mutant allele (e.g., V122I). All patients had full sequencing of the TTR gene to identify the presence variants. Baseline demographic and clinical data, the results of electrocardiography, echocardiography and right heart catheterization, and serum electrolytes, troponin and B-type natriuretic peptide (BNP) were recorded where available. All patients were treated according to standard of care by their treating physician. Patient follow-up was scheduled as clinically dictated by the treating physician.
Statistical analyses were performed with SAS software (NC, USA). Group differences in continuous variables were assessed by the Student’s t-test and in categorical variables by the Fisher’s exact test or χ2 test. Kaplan–Meier plots of time to death and time to a combined end point of death or orthotopic heart transplant (OHT) were created for the V122I and SCA groups. Differences between curves were analyzed by log-rank. Logistic regression was used to screen clinical variables for prediction of death. These variables included TTR mutation status, sex, age, invasive hemodynamic parameters, echocardiographic measures (ejection fraction and estimates of myocardial mass), blood pressure, troponin, BNP, estimated glomerular filtration (eGFR), serum electrolytes, cardiac rhythm and pacemaker implantation. For each continuous variable, the odds ratio was calculated based upon the median of the variable.
Results
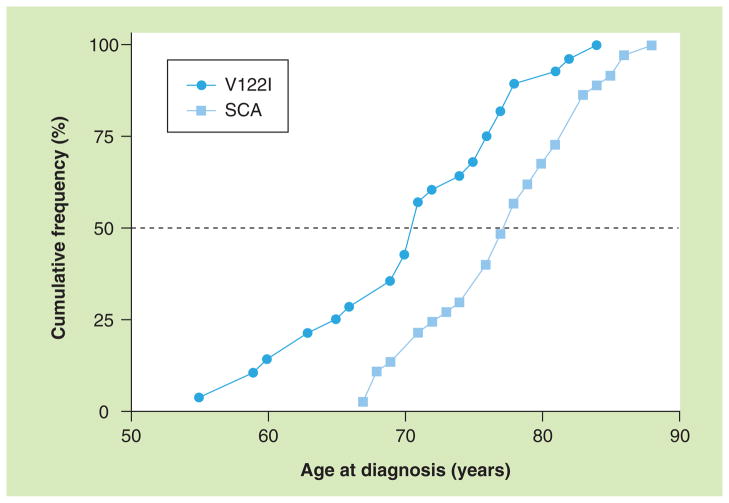
Among 65 patients with cardiac ATTR, 37 had SCA and 28 had V122I. As noted in Table 1 and in Figure 1, patients who had the V122I polymorphism were an average of 6 years younger at diagnosis than SCA patients (71 years, standard deviation [SD]: 7 vs 77 years, SD: 6; p = 0.0002). As expected, there were striking differences in racial distribution between groups: all but one of the V122I patients were African–American or Afro–Latino (96.4%) but there was only one African–American among the SCA patients (2.7%; p < 0.0001). The great majority of patients with either SCA (97%) or V122I (82%) were men (p = 0.08). Other TTR variants encountered in our center during this time included seven patients with Ala60 (T60A), and single patients with Ala120Ser (A120S), Gln89 (E89Q), Val30Gly (V30G), Val30Met (V30M), Phe64Leu (F64L), Ser23Asn (S23N), Arg34Thr (R34T) and Thr59Lys (T59K). There were insufficient numbers of patients carrying these variants to allow meaningful comparisons.
Table 1.
Demographic and clinical comparisons of senile cardiac amyloidosis and V122I patients.
| Parameter | SCA (n = 37) | V122I (n = 28) | p-value |
|---|---|---|---|
| Demographics | |||
| Age at diagnosis (years)† | 77 ± 6 | 71 ± 7 | 0.0002 |
| Gender (% male) | 97 | 82 | 0.08 |
| Race (% black) | 3 | 96 | <0.0001 |
| Electrocardiography (%) | |||
| Atrial fibrillation/flutter | 29 | 36 | 0.5789 |
| Pacemaker | 43 | 36 | 0.5395 |
| Laboratory† | |||
| BNP (pg/ml) | 495 ± 210 | 589 ± 229 | 0.2043 |
| Troponin I (ng/ml) | 0.18 ± 0.44 | 0.27 ± 0.52 | 0.4623 |
| eGFR (ml/min) | 49.4 ± 22.2 | 55.4 ± 21.4 | 0.2953 |
| ECG† | |||
| LVIDd (mm) | 43.0 ± 7.0 | 43.1 ± 5.1 | 0.9554 |
| IVS (mm) | 19.3 ± 4.1 | 17.4 ± 3.8 | 0.0795 |
| EF (%) | 46.8 ± 14.6 | 24.8 ± 12.0 | 0.0001 |
| LV mass index (g/m2) | 232.2 ± 77.7 | 208.3 ± 63.3 | 0.2431 |
| LA size (mm) | 48.3 ± 5.2 | 46.7 ± 5.5 | 0.3160 |
| Right heart catheterization† | |||
| Mean RA pressure (mmHg) | 11.2 ± 7.2 | 9.7 ± 6.3 | 0.4570 |
| Systolic PA pressure (mmHg) | 46.9 ± 12.1 | 43.1 ± 13.7 | 0.3156 |
| Mean PCWP (mmHg) | 18.5 ± 6.8 | 17.3 ± 7.8 | 0.5895 |
| PA saturation (%) | 60.4 ± 7.4 | 56.0 ± 8.5 | 0.0779 |
| Cardiac index (l/min/m2) | 1.8 ± 0.4 | 1.6 ± 0.3 | 0.0299 |
| PVR (dyn × s/cm5) | 240 ± 105 | 376 ± 210 | 0.0179 |
Values represent the mean ± standard deviation.
BNP: B-type natriuretic peptide; EF: Ejection fraction; eGFR: Estimated glomerular filtration rate; IVS: Interventricular septal thickness; LA: Left atrium; LV: Left ventricle; LVIDd: Left ventricular internal dimension at diastole; PA: Pulmonary artery; PCWP: Pulmonary capillary wedge pressure; PVR: Pulmonary vascular resistance; RA: Right atrium; SCA: Senile cardiac amyloidosis.
Figure 1. Cumulative frequency distribution of age at diagnosis of cardiac amyloidosis among patients with senile cardiac amyloidosis and patients with V122I mutation.
SCA: Senile cardiac amyloidosis.
As shown in Table 1, among patients for whom baseline ECG data were available, there were no significant differences between the V122I and SCA groups in the proportions of patients with low QRS voltage (V122I: 13 out of 25, SCA: 8 out of 20), or atrial fibrillation or flutter (V122I: 9 of 25, SCA: 9 out of 31), both frequent findings in ATTR. Permanent artificial pacemakers were implanted into 36% of V122I patients and 43% of SCA patients (p= 0.5). Pacemaker implantation was not associated with a significant change in the likelihood of dying or of time to death among patients who did not receive OHT.
Despite being younger, V122I patients were likely to have a more advanced cardiomyopathy at presentation. The average left ventricular (LV) ejection fraction among V122I patients was 25% (SD: 12) but was 47% (SD: 15) among patients with SCA (p = 0.0001). Of V122I patients, 75% had New York Heart Association (NYHA) class III or IV symptoms compared with only 42% of SCA patients (p = 0.01). There was no significant difference in echocardiographic estimates of myocardial mass, although there was a trend towards higher interventricular septal thickness among SCA patients (19 mm [SD: 4] vs 17 mm [SD: 4]; p = 0.08). Pulmonary vascular resistance, obtained from pulmonary artery catheterization, was higher among V122I patients (376 dyn × s/cm5 [SD: 210] vs 240 dyn × s/cm5 [SD: 105]; p = 0.02). The V122I group had a lower mean cardiac index than the SCA group (1.6 l/min/m2 [SD: 0.3] vs 1.8 l/min/m2 [SD: 0.4]; p = 0.03). Other hemodynamic measures, including pulmonary capillary wedge pressure and pulmonary artery pressure did not differ between groups. There were no significant differences in BNP levels or eGFR. Table 2 details the distributions of major outcomorbidities or major medication classes among SCA and V122I patients; there were no significant differences between the groups.
Table 2.
Distributions of comorbidities and medication classes among senile cardiac amyloidosis and V122I patients at diagnosis.
| Comorbidities & medications | SCA (%; n = 35) | V122I (%; n = 21) | p-value |
|---|---|---|---|
| Comorbidity | |||
| History of malignancy | 11 | 33 | 0.08 |
| Coronary artery disease | 20 | 14 | 0.73 |
| Hyperlipidemia | 9 | 14 | 0.66 |
| Hypertension | 20 | 43 | 0.12 |
| Diabetes mellitus | 6 | 24 | 0.09 |
| Chronic kidney disease | 43 | 62 | 0.27 |
| Medication class | |||
| Aspirin | 46 | 57 | 0.58 |
| Clopidogrel | 14 | 14 | 1.00 |
| Diuretic | 86 | 100 | 0.15 |
| Nitrate | 6 | 5 | 1.00 |
| ACE inhibitor/angiotensin receptor blocker | 40 | 43 | 1.00 |
| β-blocker | 54 | 62 | 0.78 |
| Aldosterone receptor antagonist | 29 | 52 | 0.09 |
| Statin | 60 | 43 | 0.27 |
| Oral hypoglycemic | 6 | 10 | 0.63 |
| Insulin | 3 | 0 | 1.00 |
| Warfarin | 43 | 62 | 0.27 |
ACE: Angiotensin-converting enzyme; SCA: Senile cardiac amyloidosis.
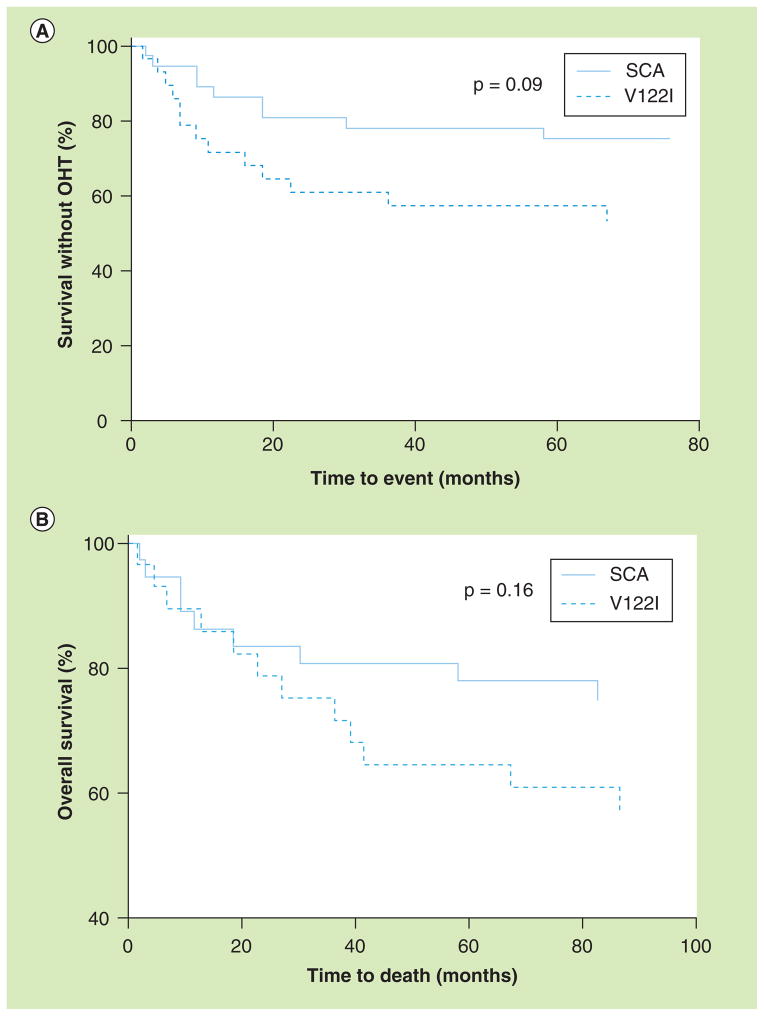
During the period of this study, the median time from diagnosis to death or OHT was 36.4 months for patients with V122I ATTR and 66.5 months for those with SCA (p = 0.09 by log-rank, Figure 2a). The median time to death alone was not significantly different between the groups (Figure 2b). Eight out of 28 V122I patients (28.6%) received an OHT compared with one out of 37 SCA patients (2.7%; p = 0.0038). For V122I patients, the average age at diagnosis for those who received OHT (60.7 years [SD: 4.7]) was significantly lower than for patients who did not receive OHT (73.5 years [SD: 5.5]; p = 0.0001). This difference was nearly identical when only V122I patients who survived the period of study were compared. The lone OHT recipient among SCA patients was diagnosed at age 68 years, which is in the bottom age decile of SCA patients.
Figure 2. Orthotopic heart transplant and survival by TTR genotype.
Kaplan–Meier presentation of time from diagnosis to (A) death or OHT or to (B) death alone among SCA and V122I patients.
OHT: Orthotopic heart transplant; SCA: Senile cardiac amyloidosis.
Of patients who did not receive an OHT, seven out of 22 V122I patients and eight out of 36 SCA patients died during the study period (p = 0.4). The mean age at death among V122I and SCA nonrecipients was 72.8 years (SD: 6.1) and 80.9 years (SD: 4.9), respectively (p = 0.007). Univariate predictors of death among all nontransplanted patients included serum potassium ≥4.2mmol/l, eGFR <48.7 ml/min, serum BNP ≥735 ng/ml, serum troponin I level ≥0.10 pg/ml and NYHA class III or IV (Table 3). The association of potassium level with death was not explained by confounding due to eGFR. V122I status did not predict death among nontransplanted patients (odds ratio: 0.96, 95% CI: 0.31–3.01; p = 0.94), neither did African–American race, given its near synonymity with V122I carriage. Among OHT recipients, there was a single death, a V122I carrier died 1 year after a successful OHT from complications of liver transplantation.
Table 3.
Univariate predictors of death among nontransplanted V122I and senile cardiac amyloidosis patients.
| Variable | Deceased | Alive or censored | OR (95% CI) | p-value |
|---|---|---|---|---|
| NYHA class III or IV | 14/18 | 13/37 | 6.46 (1.76–23.71) | 0.0030 |
| BNP ≥735 pg/ml | 14/18 | 13/36 | 6.19 (1.68–22.78) | 0.0039 |
| Troponin I ≥0.10 pg/ml | 14/16 | 16/35 | 8.31 (1.64–42.17) | 0.0049 |
| eGFR <48.7 ml/min | 12/16 | 15/37 | 4.40 (1.19–16.28) | 0.0212 |
| Serum potassium ≥4.2 mmol/l | 14/18 | 17/35 | 3.71 (1.02–13.52) | 0.0410 |
| V122I positivity | 7/19 | 14/37 | 0.96 (0.31–3.01) | 0.9419 |
BNP: B-type natriuretic peptide; eGFR: Estimated glomerular filtration rate; NYHA: New York Heart Association; OR: Odds ratio.
Discussion
Cardiac TTR deposition causes a restrictive cardiomyopathy that may evolve to marked systolic dysfunction. Other potential features include atrial fibrillation, low QRS voltage on ECG, ventricular arrhythmias and bradyarrhythmias requiring pacemaker placement. Extracardiac TTR deposition causes autonomic dysfunction and carpal tunnel syndrome [1,2]. Delays in diagnosing ATTR may reflect the difficulties of differentiating structural and physiologic effects of ATTR from changes associated with aging or hypertensive heart disease. Prominent findings in ATTR, such as CHF, myocardial thickening on echocardiography and atrial arrhythmias, are common among elderly patients who do not have ATTR. Indications of ATTR include right heart failure (suggested by anorexia, hepatomegaly, ascites and leg edema), right ventricle thickening or myocardial granular speckling on echocardiography and a low ratio of ECG voltage to echocardiographic LV mass. Suspicion may be heightened with a patient of west African decent (for V122I), a history of bilateral carpal tunnel syndrome or a family history of late-onset heart failure. Other findings may include development of hypotension in a previously hypertensive patient or the absence of a hypertensive history for a patient with LV hypertrophy [1,2]. Genetic testing and newer nuclear imaging and echocardiographic techniques, such as tissue Doppler and strain-rate imaging, may allow earlier diagnosis. How best to use these complementary methods to delineate the genotype and phenotype of ATTR and their relationship requires further study.
In our single-center observational study of ATTR patients, SCA patients were older on average than V122I patients, were much less likely to be African–American and had a higher mean LV ejection fraction. Survival did not differ between cohorts. There was a trend towards shorter time to death or OHT among V122I patients, which was primarily driven by a difference in use of OHT between the groups. Younger patients were more likely to receive OHT. The systolic dysfunction among our V122I patients is greater than that seen in other studies [9]. The absence of a statistically significant survival detriment due to advanced dysfunction is not easily explained, but may potentially reflect a benefit of surgical and nonsurgical therapy.
Our study has several limitations. It is a retrospective assessment of patient data gathered in the course of usual treatment at a tertiary referral center for advanced heart disease. As such, there is potential for ascertainment bias. At a tertiary referral center such as ours, patients are certain to have more advanced disease on average than would be seen in other centers, thus, potentially limiting the generalizability of our data. It is not clear whether this limitation affects comparisons between V122I and SCA patients. The sizes of the V122I and SCA groups limit power for advanced statistical assessment of intergroup differences. The near-absence of African–Americans from the SCA group prohibits disentanglement of race and V122I status.
Our study adds to a small body of literature comparing V122I ATTR with other cardiac amyloidoses. Connors et al. compared African–Americans with cardiac amyloidosis due to V122I ATTR or immunoglobulin light chain deposition [10]. V122I patients had longer median survival (27 vs 5 months) despite being 14 years older on average at diagnosis (70 vs 56 years) compared with immunoglobulin light chain deposition patients. There were no significant presentation differences between V122I heterozygous (n = 27) or homozygous (n = 5) patients. Homozygous patients trended towards lower median survival (14 vs 41 months; p = 0.07). Median survival among V122I patients in our study was approximately 36 months, similar to that in the Connors et al study.
Jacobson et al. compared elderly African–American male V122I carriers (n = 23) with age- and race-matched noncarriers (n = 46, mean age per group: 73 years) [9]. None had an ATTR diagnosis. Hypertension was less common in the V122I cohort and CHF was more common. The V122I group had a higher average heart rate, and V122I subjects were more likely to have atrial premature complexes and Q waves indicative of a pseudoinfarct pattern on ECG, a common finding in cardiac amyloidosis. Echocardiography revealed a higher mean interventricular septal thickness, and higher frequencies of right ventricular hypertrophy and granular sparkling. There was a trend towards lower mitral deceleration time, a marker of diastolic dysfunction, among the V122I subjects. Long-term outcomes were not reported. These data suggest high clinical, ECG and echocardiographic penetrance of V122I.
Buxbaum et al. found higher mortality and frequency of CHF for V122I carriers compared with noncarriers among African–Americans, who were older than 65 years, in the Cardiovascular Health Study; none were known to have ATTR. No similar effects were seen among participants in the Atherosclerosis Risk in Communities study, who were all younger than 65 years, highlighting age-dependent V122I penetrance [11]. Ruberg et al. conducted a prospective multicenter analysis of SCA and V122I patients, finding lower median survival for V122I patients (25.6 vs 43.0 months) and a higher rate of cardiac hospitalizations [12]. Other major mortality predictors were time since diagnosis, heart rate ≥70 beats per minute, lower stroke volume, and LV ejection fraction <50%. All of the African–Americans were V122I carriers and vice versa.
The bulk of the assembled evidence suggests V122I ATTR to be more virulent than SCA; it appears at an earlier age, has a worse phenotype at presentation and may cause higher mortality or need for OHT. The extent to which differences in disease severity, resulting from the aggressiveness of the underlying processes, unappreciated environmental or genetic issues, access to care, or delays in diagnosis, cannot be discerned from the available data. Further study of ATTR due to V122I and wild-type TTR presents a unique opportunity to disentangle the roles of aging, genetics and ethnic differences.
The magnitude of the problem posed by V122I and SCA has been under-recognized. The 2010 US Census reported an African–American population of nearly 39 million people [102], suggesting more than 1.5 million V122I carriers given a frequency of 4%. With an aging and diversifying population, the numbers of SCA and V122I patients will grow. Given the mortality and morbidity associated with advanced heart failure, the expense of advanced treatments and the perpetual shortage of donor hearts, there is a need for more research to identify patients with early disease who may benefit from new therapies to stall the progress of TTR deposition.
Conclusion
In comparison with patients with SCA, patients with ATTR due to V122I are susceptible to an earlier disease onset and may have a more aggressive disease course. We currently lack sufficient understanding of the contributions of genetic and extragenetic factors in presentation and outcome, especially in light of the stark racial asymmetry between the groups. More research is needed to determine the best methods to identify patients before the development of advanced disease and to slow disease progression.
Future perspective
Over the next decade, we expect there to be better understanding of the pathophysiology of wild-type and variant ATTR through studies of the formation of TTR fibrils and their interactions with a complex network of other molecules. These advances will come from a variety of molecular approaches and collaborative efforts. We have already witnessed the emergence of small molecule TTR stabilizers, such as tafamidis [13,14] and diflunisal[15], that may be able to stall and potentially even reverse the progression of early amyloid diseases. On the horizon there may also be inhibitory RNA therapies and oligonucleotides that can decrease the synthesis of wild-type and variant TTR [16,103]. Being able to effectively use emerging therapies will depend upon increasing awareness of ATTR and determining how best to use screening tests for variant TTR so that early disease can be diagnosed. Multicenter prospective studies will help to define the natural histories and optimal treatments for ATTRs.
Executive summary.
Aims
We retrospectively compared the presentations and outcomes of senile cardiac amyloidosis (SCA) and V122I patients.
Patients & methods
Data were gathered from patients who received care for cardiac transthyretin amyloidosis at the Columbia Center for Advanced Cardiac Care (NY, USA) between 2001 and 2012. All data were generated in the context of usual clinical care.
Results
V122I patients were younger on average at diagnosis than SCA patients.
V122I patients had more advanced cardiac dysfunction at diagnosis on average than SCA patients.
There was greater orthotopic heart transplant use among V122I patients compared with SCA patients.
The median time to transplant or death was lower for V122I patients compared with SCA patients, but did not reach statistical significance.
Death among patients who did not receive orthotopic heart transplant was predicted by lower estimated glomerular filtration, higher serum potassium level, higher B-type natriuretic peptide, elevated troponin I and a higher NYHA class. V122I status did not predict death.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
M Maurer is a recipient of a K24 award from the National Institute on Aging (K 24 AG036778). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
- 1.Dharmarajan K, Maurer MS. Transthyretin cardiac amyloidoses in older North Americans. J Am Geriatr Soc. 2012;60:765–774. doi: 10.1111/j.1532-5415.2011.03868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126:1286–1300. doi: 10.1161/CIRCULATIONAHA.111.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai Z, Colon W, Kelly JW. The acid-mediated denaturation pathway of transthyretin yields a conformational intermediate that can self-assemble into amyloid. Biochemistry. 1996;35:6470–6482. doi: 10.1021/bi952501g. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson DR, Pastore R, Pool S, et al. Revised transthyretin Ile 122 allele frequency in African–Americans. Hum Genet. 1996;98:236–238. doi: 10.1007/s004390050199. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita T, Hamidi Asl K, Yazaki M, Benson MD. A prospective evaluation of the transthyretin Ile122 allele frequency in an African–American population. Amyloid. 2005;12:127–130. doi: 10.1080/13506120500107162. [DOI] [PubMed] [Google Scholar]
- 6.Jiang X, Buxbaum JN, Kelly JW. The V122I cardiomyopathy variant of transthyretin increases the velocity of rate-limiting tetramer dissociation, resulting in accelerated amyloidosis. Proc Natl Acad Sci USA. 2001;98:14943–14948. doi: 10.1073/pnas.261419998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buck FS, Koss MN, Sherrod AE, Wu A, Takahashi M. Ethnic distribution of amyloidosis: an autopsy study. Mod Pathol. 1989;2:372–377. [PubMed] [Google Scholar]
- 8.Dungu J, Sattianayagam PT, Whelan CJ, et al. The electrocardiographic features associated with cardiac amyloidosis of variant transthyretin isoleucine 122 type in Afro–Caribbean patients. Am Heart J. 2012;164:72–79. doi: 10.1016/j.ahj.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson D, Tagoe C, Schwartzbard A, Shah A, Koziol J, Buxbaum J. Relation of clinical, echocardiographic and electrocardiographic features of cardiac amyloidosis to the presence of the transthyretin V122I allele in older African–American men. Am J Cardiol. 2011;108:440–444. doi: 10.1016/j.amjcard.2011.03.069. [DOI] [PubMed] [Google Scholar]
- 10.Connors LH, Prokaeva T, Lim A, et al. Cardiac amyloidosis in African Americans: comparison of clinical and laboratory features of transthyretin V122I amyloidosis and immunoglobulin light chain amyloidosis. Am Heart J. 2009;158:607–614. doi: 10.1016/j.ahj.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Buxbaum J, Alexander A, Koziol J, Tagoe C, Fox E, Kitzman D. Significance of the amyloidogenic transthyretin Val 122 Ile allele in African Americans in the Arteriosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Am Heart J. 2010;159:864–870. doi: 10.1016/j.ahj.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruberg FL, Maurer MS, Judge DP, et al. Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: the Transthyretin Amyloidosis Cardiac Study (TRACS) Am Heart J. 2012;164:222–228.e1. doi: 10.1016/j.ahj.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Bulawa CE, Connelly S, Devit M, et al. Tafamid is, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci USA. 2012;109:9629–9634. doi: 10.1073/pnas.1121005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lartigue J. Tafamidis for transthyretin amyloidosis. Drugs Today. 2012;48:331–337. doi: 10.1358/dot.2012.48.5.1808486. [DOI] [PubMed] [Google Scholar]
- 15.Castano A, Helmke S, Alvarez J, Delisle S, Maurer MS. Diflunisal for ATTR cardiac amyloidosis. Congest Heart Fail. 2012;18(6):315–319. doi: 10.1111/j.1751-7133.2012.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ackermann EJ, Guo S, Booten S, et al. Clinical development of an antisense therapy for the treatment of transthyretin-associated polyneuropathy. Amyloid. 2012;19(Suppl 1):43–44. doi: 10.3109/13506129.2012.673140. [DOI] [PubMed] [Google Scholar]
Websites
- 101. [Accessed 15 January 2013];Ensembl genome browser. www.ensembl.org.
- 102.Rastogi SJ, Johnson TD, Hoeffel EM, Drewery MP. The black population: 2010. US Census Bureau; [Accessed 15 January 2013]. www.census.gov/prod/cen2010/briefs/c2010br-06.pdf. [Google Scholar]
- 103.Alnylam Pharamceuticals. [Accessed 21 January 2013]; www.alnylam.com.