Abstract
Sodium channel inhibitor (SCI) insecticides were discovered almost four decades ago but have only recently yielded important commercial products (eg., indoxacarb and metaflumizone). SCI insecticides inhibit sodium channel function by binding selectively to slow-inactivated (non-conducting) sodium channel states. Characterization of the action of SCI insecticides on mammalian sodium channels using both biochemical and electrophysiological approaches demonstrates that they bind at or near a drug receptor site, the "local anesthetic (LA) receptor." This mechanism and site of action on sodium channels differentiates SCI insecticides from other insecticidal agents that act on sodium channels. However, SCI insecticides share a common mode of action with drugs currently under investigation as anticonvulsants and treatments for neuropathic pain. In this paper we summarize the development of the SCI insecticide class and the evidence that this structurally diverse group of compounds have a common mode of action on sodium channels. We then review research that has used site-directed mutagenesis and heterologous expression of cloned mammalian sodium channels in Xenopus laevis oocytes to further elucidate the site and mechanism of action of SCI insecticides. The results of these studies provide new insight into the mechanism of action of SCI insecticides on voltage-gated sodium channels, the location of the SCI insecticide receptor, and its relationship to the LA receptor that binds therapeutic SCI agents.
Keywords: indoxacarb, DCJW, metaflumizone, RH3421, sodium channel, local anesthetic receptor
1. Introduction
Nearly three decades separated the initial discovery of the first sodium channel inhibitor (SCI) insecticides [1] from the registration and availability of the first commercial insecticide from this class [2]. Despite excellent insecticidal activity, these compounds failed repeatedly to yield commercial insecticides due to their photoinstability, unacceptable persistence in the soil, and high mammalian toxicity [3]. Nevertheless, the commercial development of indoxacarb [2] and metaflumizone [4] illustrates the practical value of this class and the potential for the development of new SCI insecticides with favorable environmental and toxicological properties.
Previous reviews considered literature prior to 2004 on the development, toxicology and mode of action of SCI insecticides [5], the properties and actions of indoxacarb in relation to earlier SCI insecticides [6], and the actions of SCI insecticides on insect voltage-gated sodium channels [7]. Here we briefly summarize the historical development of SCI insecticides and the evidence for a common mode of action of this structurally diverse group on voltage-gated sodium channels. We then review research conducted since 2004 in this laboratory using site-directed mutagenesis and heterologous expression of cloned mammalian sodium channels in Xenopus laevis oocytes to further elucidate the site and mechanism of action of SCI insecticides.
2. Discovery, Chemistry, and Nomenclature of SCI Insecticides
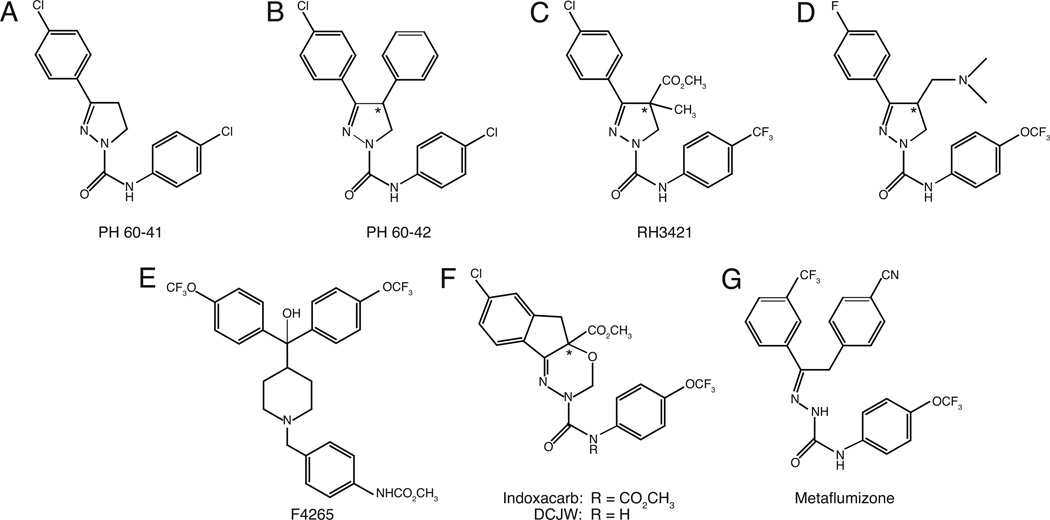
Research at Philips-Duphar B.V. in the Netherlands during the early 1970s identified the first SCI insecticides, exemplified by PH 60-41 [1] (Fig. 1A). PH 60-41 exhibited symptoms of poisoning in insects consistent with action at a target site in the nervous system [1]. Further investigation of 3-phenyl-, 3,4-diphenyl- and 3,5-diphenyldihydropyrazoles [8–10] identified compounds in the 3,4-diphenyl series (e.g., PH 60-42; Fig. 1B) with insecticidal activity 100-fold greater than compounds in the 3-phenyl series. Despite the strong insecticidal activity of the Philips-Duphar dihydropyrazoles, extensive photoaromatization with loss of insecticidal activity [11] and unacceptable persistence in soil [12] prevented the development of commercial products from this series.
Fig. 1.
Structures of SCI insecticides.
In the late 1980s, Rohm and Haas disclosed the discovery of a second generation of dihydropyrazole insecticides derived from the original Philips-Duphar compounds [13]. This work yielded compounds in the dihydropyrazole series (e.g., RH3421; Fig. 1C), with excellent insecticidal activity and reduced photodegradation and soil persistence compared to PH 60-42. Asymmetric 4-disubstitution of the dihydropyrazole ring, as in RH3421, introduced a chiral center into the molecule. For an analog of RH3421 carrying the same substituents at C-4 of the dihydropyrazole ring, the S enantiomer was 10- to 100-fold more active as an insecticide than the R enantiomer [14]. This result implies that chiral dihydropyrazoles interact stereoselectively with their neuronal target site.
During the same period research at FMC Corporation sought to develop new dihydropyrazoles with reduced lipophilicity that would exhibit contact insecticidal activity. Insertion of novel aliphatic substituents at C-4 of the dihydropyrazole ring yielded insecticides (Fig. 1D) with reduced lipophilicity, but these compounds did not achieve the level of insecticidal potency exhibited by the corresponding 4-phenyl-substituted analogs of PH 60-42. [3].
The development of commercial dihydropyrazole insecticides was ultimately limited by their unacceptable mammalian toxicity. The acute oral toxicities of dihydropyrazoles to mammals are low, giving acute oral LD50 values in rats greater than 1000 mg/kg [8,10,13]. However, daily administration in the diet revealed that dihydropyrazoles cause delayed-onset neurotoxicity at doses much lower than those producing acute intoxication [3].
Further research at FMC Corporation identified a novel series of insecticidal arylalkylbenzhydrolpiperidines (BZPs; Fig. 1F) based on natural product leads (10,23-dihydro-24,25-dehydroflavinine and nominine) and the antihistamine cinnarizine [15]. Iterative structural optimization led to compounds (e.g., F4265; Fig. 1E) with excellent insecticidal activity and low mammalian toxicity (acute oral LD50 values >1000 mg/kg) [16] but did not yield commercial insecticides. Despite their structural divergence from insecticidal dihydropyrazoles, the BZPs exhibit functional and pharmacological properties consistent with their inclusion in the SCI insecticide class [17,18].
Efforts at DuPont to overcome the toxicological limitations of the dihydropyrazoles led to the development of a series of insecticidal oxadiazines including indoxacarb (Fig. 1F), the first SCI insecticide to achieve commercial registration [2]. Indoxacarb is a proinsecticide that undergoes efficient bioactivation in insects to an insecticidal metabolite, DCJW (Fig. 1F) [19]. Indoxacarb is also bioactivated in mammals, but N-decarbomethoxylation to DCJW is less efficient than in insects and detoxication is achieved by alternative biochemical pathways [6]. The selective conversion of indoxacarb to DCJW in insects underlies its favorable selective toxicity, thereby overcoming one of the principal shortcomings of the dihydropyrazoles.
Research at Nihon Noyaku Company also attempted to improve the environmental and toxicological profile of the dihydropyrazoles by modification of the core ring structure. This effort yielded a series of semicarbazones, conceived as ring-opened analogs of the dihydropyrazoles, and led to the discovery of the second registered SCI insecticide, metaflumizone (Fig. 1G) [4]. Metaflumizone has a broad spectrum of insecticidal activity [4] and very low acute and chronic toxicity to mammals [20].
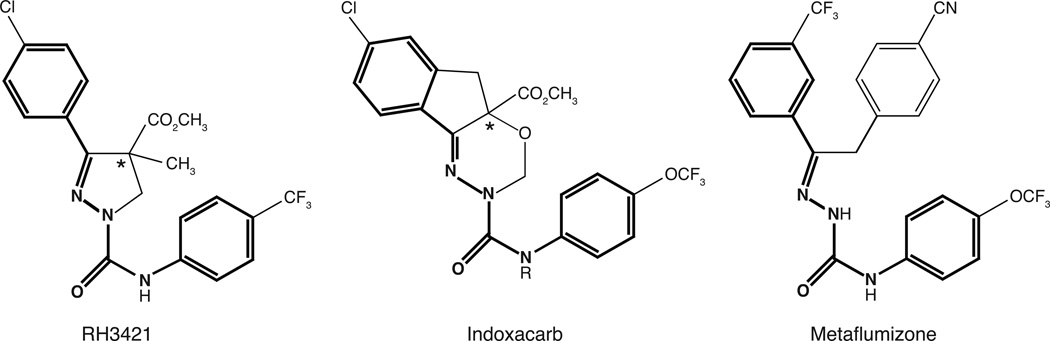
Takagi et al. [4] identified a common structural backbone for SCI insecticides that encompasses the dihydropyrazoles, oxadiazines and semicarbazones (Fig. 2) and suggested that this core structure may constitute the toxophore for SCI insecticide-like activity. The conserved structural features among these SCI insecticide series are two aromatic rings connected by five atoms including three nitrogens and one carbonyl at conserved positions. It is noteworthy that the BZP series of SCI insecticides does not exhibit this core structure. However, we and others include them in the SCI insecticide family based on their pharmacological properties, which are summarized in the following section of this review.
Fig. 2.
The common core structure of SCI insecticides identified by Takagi et al [4] (bold) exemplified in the structures of RH3421, indoxacarb and metaflumizone.
The structural diversity of SCI insecticides complicates the naming of this insecticide class. By convention classes of insecticides are typically named according to a shared (and usually toxophoric) chemical feature (e.g., organophosphorus esters) or a shared template molecule (e.g., neonicotinoids, pyrethroids). Designation of the first SCI insecticides as “dihydropyrazoles” or “pyrazolines,” by this convention failed to accommodate the subsequent development of functionally related but structurally distinct series such as the BZPs and oxadiazines. The name “pyrazoline-type insecticides” [2] encompassed this diversification of SCI insecticide structure, whereas the name “sodium channel blocker insecticides (SCBIs)” [6] grouped structurally diverse compounds by their common action. We consider the latter approach to be the most appropriate. However, in recent publications [21,22] and in this review we employ the name “sodium channel inhibitor (SCI) insecticides” because these compounds inhibit sodium channel function by binding to and stabilizing inactivated, nonconducting channel states rather than physically occluding the channel. Our preference for the term “sodium channel inhibitor” is consistent with the recent use of this term as a collective name for the diverse therapeutic agents (e.g., local anesthetics, anticonvulsants, antiarrhythmics and analgesics) that also bind to and stabilize inactivated sodium channel states [23]. The pharmacological convergence between SCI insecticides and drugs provides a conceptual framework for understanding both the mode of action and the distinctive properties of SCI insecticides.
3. Action of SCI Insecticides on Voltage-Gated Sodium Channels
The rationale for considering SCI insecticides as a single class despite their structural diversity depends on evidence that they share a common mechanism of insecticidal action. In the following sections we briefly review the evidence for common a mechanism based on signs of intoxication in poisoned insects and electrophysiological assays of SCI insecticide action in various nerve preparations in vitro. More detailed summaries of data published prior to 2004 are available in previous reviews [5,6].
3.1. Symptoms of poisoning in insects
Adult cockroaches (Periplaneta Americana) and larval tobacco hornworms (Manduca sexta) and houseflies (Musca domestica) injected with the dihydropyrazole RH3421 exhibited consistent and distinctive intoxication symptoms [24]. In each species, initial disruption of motor coordination and locomotion was followed by irreversible quiescent “pseudoparalysis” punctuated by bouts of convulsive movement in response to mechanical stimulus. Indoxacarb produced the same pseudoparalysis intoxication symptoms following injection into P. americana adults and M. sexta larvae [6,25]. Similarly, injection of BZPs into tobacco budworm (Heliothis zea) larvae [15] and metaflumizone into fall armyworm (Spodoptera frugiperda) larvae [26] also produced pseudoparalysis. Whereas the symptoms of SCI insecticide intoxication in insects clearly implicate an action in the nervous system, the pseudoparalysis caused by all members of this class distinguishes them from other insecticides known to act at neuronal target sites.
3.2. Actions of SCI insecticides on spontaneous and evoked neural activity
Electrophysiological studies of spontaneous nerve activity and evoked potentials in insect nerve preparations provide further insight into the mechanism of action of SCI insecticides. Recordings of spontaneous activity in preparations from poisoned insects show that pseudoparalysis by SCI insecticides is accompanied by silencing of spontaneous, “background” activity arising from pacemaker cells in the central nervous system and tonic sensory receptors. First reported in studies with the dihydropyrazole RH3421 in P. americana, M. sexta and M. domestica [24], this effect was subsequently also found in comparable assays with insects paralyzed by BZPs [17], indoxacarb [6], and metaflumizone [26]. Despite the silencing of endogenous activity during pseudoparalysis, stimulation of motor and sensory neurons produced action potential responses [24]. Thus, SCI insecticides selectively block endogenous action potential generation in both the central nervous system and sense organs during paralysis at doses in vivo and concentrations in vitro that do not otherwise compromise evoked action potentials in axons.
Consistent with their selective block of endogenous activity, RH3421 and related dihydropyrazoles blocked stretch receptors in preparations from P. americana, M. sexta, and the crayfish (Procambarus clarkii) [24]. Similarly, BZPs and metaflumizone blocked stretch receptors in preparations from H. zea and S. frugiperda, respectively [17,26]. In crayfish preparations RH5529, a dihydropyrazole analog of RH3421, increased the threshold for spike generation without altering passive membrane properties [24]. In these preparations, depolarization of the membrane potential promoted block whereas hyperpolarization relieved block.
SCI insecticides also block evoked neural activity in invertebrate axons and neurons. Depolarization of crayfish giant axons rendered them susceptible to block by dihydropyrazoles [24]. In P. americana dorsal unpaired median neurons, the indoxacarb metabolite blocked evoked action potentials and also caused hyperpolarization of the resting membrane potential, an effect not reported in other preparations [27].
3.3. State-dependent inhibition of voltage-gated sodium channels by SCI insecticides
The depolarization-dependent block of action potentials in stretch receptors and axons implied that SCI insecticides acted by blocking voltage-gated sodium channels in a voltage-dependent manner. To test this hypothesis directly Salgado [28] determined the effects of RH3421 and related dihydropyrazoles on sodium currents in crayfish giant axons under voltage-clamp conditions. In this study, dihydropyrazoles blocked crayfish sodium channels only at depolarized membrane holding potentials that promoted conversion of resting channels to slow-inactivated states. Moreover, repolarization of the membrane during dihydropyrazole perfusion both removed slow inactivation and relieved block. Consistent with this finding, RH3421 caused a hyperpolarizing shift in the voltage dependence of slow sodium channel inactivation in these preparations. SCI insecticides caused the reversible (indoxacarb) or irreversible (DCJW) inhibition of two functionally and pharmacologically distinct sodium currents recorded from cultured P. americana ganglionic neurons [29]. Indoxacarb and DCJW also inhibited the tetrodotoxin (TTX)-sensitive and TTX-resistant sodium currents found in rat dorsal root ganglion (DRG) neurons [30,31]. Thus, the depolarization-dependent block of nerve action potentials by SCI insecticides involves a selective interaction with slow-inactivated sodium channels, the relative abundance of which in a given cell type is determined by the specific sodium channel isoforms or splice variants expressed in that cell, the level of cellular electrical activity, and the extent of steady-state membrane depolarization.
4. Action of SCI Insecticides on Mammalian Sodium Channels Expressed in Xenopus Oocytes
4.1. The Xenopus oocyte expression system
Heterologous expression and electrophysiological characterization in oocytes of the frog Xenopus laevis has provided extensive information on the functional properties of mammalian and insect sodium channels [32–34]. The oocyte expression system offers several clear advantages for studies of SCI insecticides. First, oocytes are the only in vitro system in which both insect and mammalian sodium channels can be expressed, thus permitting direct comparisons of insecticide action and channel sensitivity in a common cellular environment. Second, oocytes typically survive and yield stable recordings under voltage-clamp conditions for up to 2 hours, thereby facilitating studies with SCI insecticides that require prolonged profusion to permit the slow equilibration typical of these compounds and the long pulse protocols required to characterize effects on slow-inactivated channel states. Third, transient expression in oocytes, coupled with site-directed mutagenesis on cloned cDNAs to generate modified channels, provides ready access to structure-function studies involving amino acid replacement in defined locations in the sodium channel sequence. The following sections of this review summarize our research using this system to define and compare the action of SCI insecticides on mammalian sodium channels and to characterize further the mechanism and site of action of SCI insecticides by site-directed mutagenesis.
4.2 Actions of SCI insecticides on rat Nav1.4 sodium channels
We chose the rat Nav1.4 sodium channel isoform as our principal model for studies of SCI insecticides on mammalian sodium channels in the Xenopus oocyte system for three reasons. First, among the eight rat sodium channel isoforms available to us only the Nav1.4 isoform gave highly reproducible expression of large-amplitude (10–20 µA) sodium currents in voltage-clamped oocytes. Experimental protocols to study SCI insecticide action involve depolarization to induce partial slow inactivation followed by insecticide-dependent inhibition of the residual (non-inactivated) component of the current. Large-amplitude initial currents are required in order to retain adequate signal-to-noise levels for the small residual currents remaining after fractional slow inactivation and insecticide inhibition. Second, the well-established pharmacology of SCI drug action on Nav1.4 sodium channels allowed us to place the state-dependent inhibition of channels by SCI insecticides firmly in the larger context of state-dependent channel inhibition by other agents. Third, prior mutagenesis experiments identified specific amino acid determinants of SCI drug binding and slow inactivation in the Nav1.4 channel sequence that we could employ directly to probe the action of SCI insecticides.
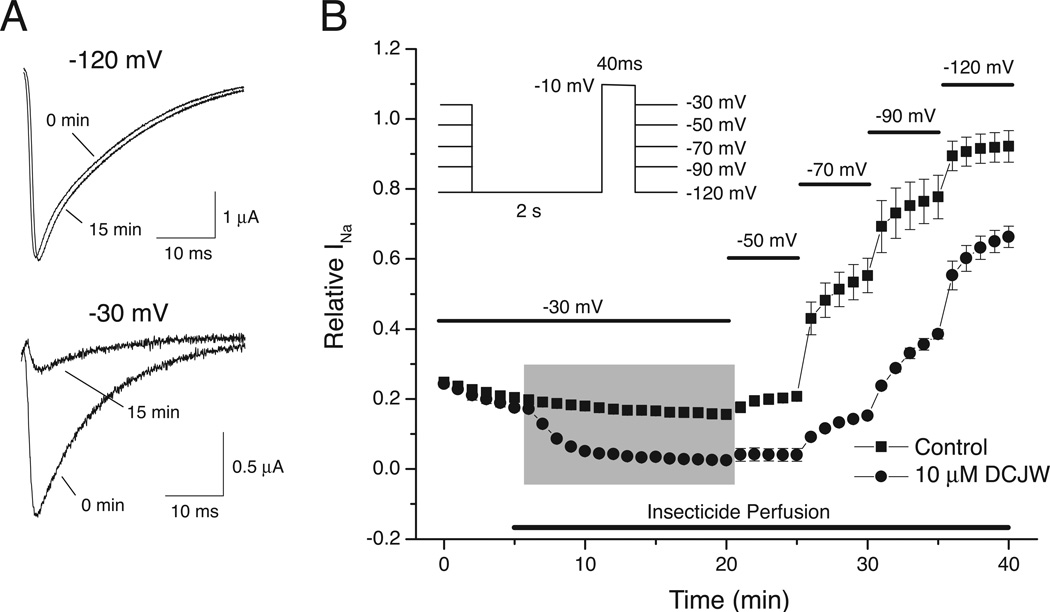
Figure 3 illustrates the characteristic effects of SCI insecticides on Nav1.4 sodium channels under voltage clamp conditions, exemplified here with data for DCJW [35]. Perfusion with 10 µM DCJW for 15 min at a holding potential of −120 mV had no effect on sodium currents, whereas perfusion at a holding potential of −30 mV caused significant inhibition of the sodium current (Fig. 3A). Figure 3B illustrates the effect of depolarization to −30 mV and stepwise repolarization on currents measured in the absence or presence of 10 µM DCJW. Depolarization to −30 mV, followed by a hyperpolarizing prepulse to permit the recovery of fast-inactivated channels, reduced the available sodium current by ~75% by placing the majority of channels in non-conducting slow-inactivated states. Application of DCJW caused channel inhibition, further reducing the available current (Fig. 3B, shaded area). Stepwise hyperpolarization in the absence of DCJW progressively removed slow inactivation, recovering all of the available current under control conditions, but DCJW impeded the voltage-dependent recovery from slow inactivation. Unlike membrane repolarization, perfusion with DCJW-free medium at −30 mV failed to reverse the inhibitory effects of DCJW (not shown). RH3421 and metaflumizone produced effects similar to those illustrated in Fig. 3 for DCJW [21,35].
Fig. 3.
Voltage-dependent inhibition of rat Nav1.4 sodium channels expressed in Xenopus oocytes by DCJW. (A) Example current traces recorded before (0 min) or after perfusion for 15 min with 10 µM DCJW at holding potential of either −120 mV (top) or −30 mV (bottom). Currents were recorded during a 40-ms test pulse to −10 mV; oocytes held at −30 mV were given a 2-s repolarization pulse to −120 mV prior to the test pulse to convert fast-inactivated channels to resting channels. (B) Voltage-dependent inhibition and recovery of sodium currents in the presence of DCJW. Inset: pulse protocol employed to sample sodium currents once per minute at each holding potential. The figure is redrawn from the data of Silver and Soderlund [35].
Consistent with these results and previous studies of SCI insecticides using invertebrate nerve preparations, RH3421, DCJW and metaflumizone also caused hyperpolarizing shifts in the voltage dependence of slow inactivation and recovery from slow inactivation but did not affect the voltage dependence of steady-state fast inactivation (not shown) [21,35]. Whereas RH3421 and DCJW also did not affect the voltage dependence of Nav1.4 channel activation in this system [35], metaflumizone caused a small (~5 mV) but statistically significant hyperpolarizing shift in the voltage dependence of activation [21]. This result implies that metaflumizone, unlike the other SCI insecticides examined, is able to bind to sodium channels in the resting state and affect the activation of resting channels.
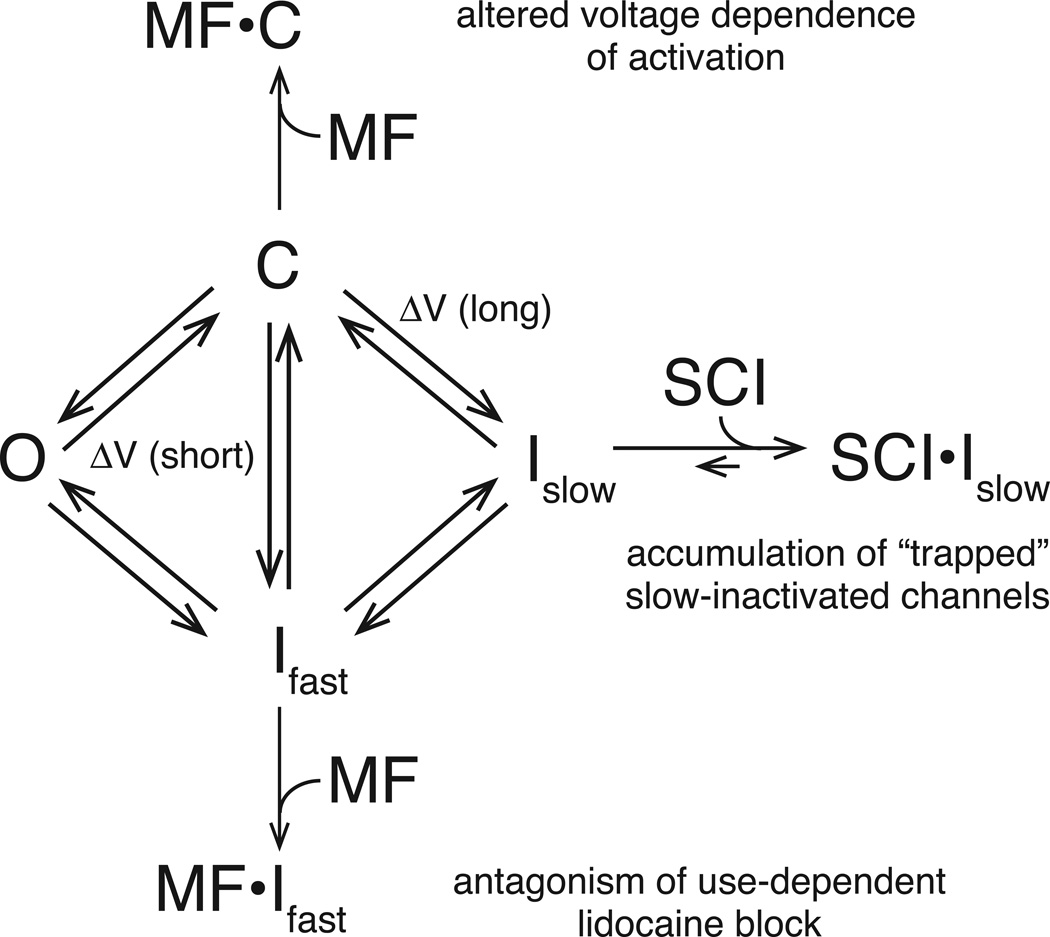
Figure 4 provides a simplified state transition model that summarizes the state-dependent effects of SCI insecticides on rat Nav1.4 sodium channels in the Xenopus oocyte system. This model depicts transitions between four functionally distinct channel states: closed (resting) channels available for voltage-dependent activation (C); open channels (O); fast-inactivated channels (Ifast); and slow-inactivated channels (Islow). In practice, each of the states represented in Fig. 4 may exist as a family of substates. When the cell membrane is clamped at a hyperpolarized potential, the majority of channels are in the resting state and available for activation. Short depolarizations (ΔV) to membrane potentials above the channel activation threshold produce open channels, which undergo inactivation on a millisecond time scale to the fast-inactivated state. Alternatively, short subthreshold depolarizations may lead directly to fast inactivated states without channel activation. By contrast, sustained depolarization drives channels predominantly to slow-inactivated states. Channels in both inactivated states return to the closed state, albeit with different voltage dependence and kinetics, upon repolarization of the cell membrane. SCI insecticides (SCI) bind selectively and persistently to slow-inactivated channels, preventing the removal of slow inactivation and trapping populations of channels in stable, insecticide-modified slow-inactivated states. The insecticide-dependent sequestration of channels in nonconducting states inhibits macroscopic sodium currents by reducing the pool of closed channels available for activation. The model shown in Fig. 4 also depicts the unique interaction of metaflumizone (MF) with resting channels that is evident in its ability to alter the voltage dependence of channel activation. The functional consequences of this effect of metaflumizone have not been established.
Fig. 4.
Conceptual model illustrating state transitions between closed (C), open (O), fast-inactivated (Ifast) and slow-inactivated (Islow) sodium channels, the selective interaction of SCI insecticides (SCI) with slow-inactivated channels, and the unique interactions of metaflumizone (MF) with closed and fast-inactivated channels. Further explanation is provided in the text.
4.3. Differential sensitivity of mammalian sodium channel isoforms to SCI insecticides
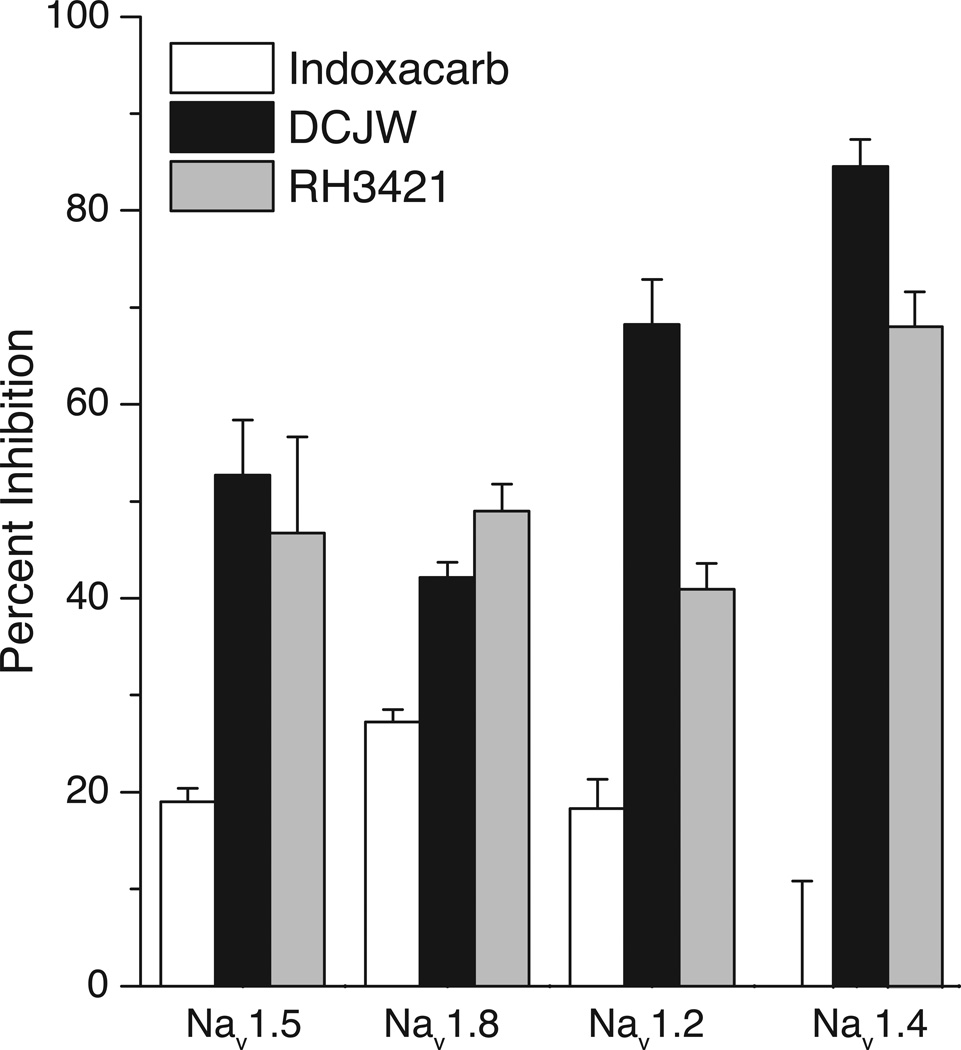
Mammalian voltage-gated sodium channel isoforms differ in their relative sensitivity to inhibition by SCI insecticides. Rat DRG neurons exhibit two functionally and pharmacologically distinct sodium currents that are carried by different combinations of isoforms: a TTX-sensitive current that is associated with the expression of various combinations of the Nav1.2, Nav1.3, Nav1.6 and Nav1.7 isoforms and a TTX-resistant current associated primarily with the Nav1.8 isoform [36]. The TTX-sensitive current of DRG neurons is also more sensitive to inhibition by indoxacarb and DCJW than the TTX-resistant current [31]. Consistent with these data, rat sodium channel isoforms expressed in Xenopus oocytes also differed in their sensitivity to inhibition by indoxacarb, DCJW and RH3421 (Fig. 5) [37]. Specifically, the TTX-sensitive Nav1.2 and Nav1.4 isoforms were more sensitive to inhibition by RH3421 and DCJW than the TTX-resistant Nav1.5 and Nav1.8 isoforms. Paradoxically the Nav1.8 isoform, which was least sensitive to inhibition by DCJW, was the isoform most sensitive to inhibition by indoxacarb. This study also assessed the effect of coexpressing either the Nav1.2 or Nav1.4 isoforms with the auxiliary β1 subunit (data not shown). The only statistically significant effect of the β1 subunit on the SCI insecticide sensitivity of either isoform was a modest reduction in the sensitivity of Nav1.2 channels to RH3421.
Fig. 5.
Relative sensitivity of four rat sodium channel isoforms expressed in Xenopus oocytes to inhibition by indoxacarb, DCJW and RH3421. The figure is re-drawn from the data of Silver and Soderlund [37].
5. Mutational Analysis of SCI Insecticide Interactions with the Local Anesthetic (LA) Receptor
5.1. Evidence for involvement of the LA receptor in SCI insecticide action
SCI drugs (local anesthetics, anticonvulsants, antiarrhythmics) inhibit sodium channel function through state-dependent binding to an unique sodium channel domain called the LA receptor [23]. Pharmacological evidence suggests that SCI insecticides and SCI drugs may share a common site of action. Radiosodium uptake studies show that RH3421 inhibits veratridine-stimulated uptake of sodium into mouse brain synaptosomes [38,39] in a manner similar to local anesthetics, class I anticonvulsants, and class I antiarrhythmics [40]. Radioligand binding studies show that RH3421 and therapeutic sodium channel blockers are competitive allosteric inhibitors of batrachotoxinin-A-20-α-benzoate (BTX-B) binding to mammalian brain preparations [41,42].
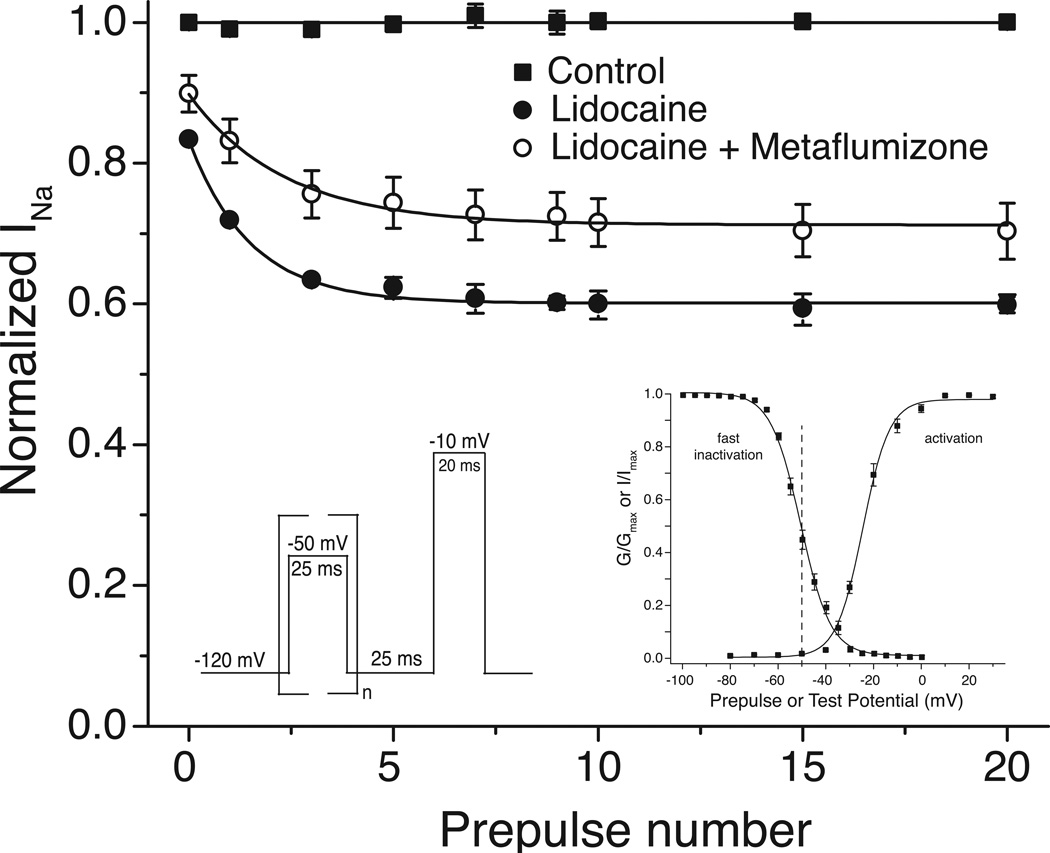
Other studies have identified competitive interactions between SCI insecticides and SCI drugs. RH3421 and the local anesthetic dibucaine are mutually competitive inhibitors of BTX-B binding to mouse brain sodium channels [39]. Phenytoin, an anticonvulsant, interferes with the ability of DCJW and RH3421 to block cloned rat Nav1.4 sodium channels expressed in Xenopus oocytes [35]. Recently, we found that metaflumizone antagonized the use-dependent inhibition of rat Nav1.4 sodium channels expressed in Xenopus oocytes by the local anesthetic lidocaine (Fig. 6) [21]. This study employed trains of brief subthreshold depolarizing prepulses to convert resting channels to fast-inactivated channels without significant channel activation. In the absence of drug, repetitive depolarization had no effect on sodium currents because the fast inactivation caused by each depolarization decayed completely prior to the subsequent depolarization. In the presence of lidocaine sodium currents were reduced by the trapping of lidocaine-modified channels in nonconducting fast-inactivated states. The ability of metaflumizone to antagonize this effect identifies an interaction of this compound with the LA receptor on fast-inactivated sodium channel states (see Fig. 4). The failure of other SCI insecticides to antagonize lidocaine block (not shown) implies that metaflumizone’s ability to bind to fast-inactivated channels is apparently not shared with other SCI insecticides.
Fig. 6.
Antagonism by metaflumizone (10 µM) of the use-dependent inhibition of rat Nav1.4 sodium channels expressed in Xenopus oocytes by lidocaine (200 µM). Left inset: pulse protocol used. Right inset: Boltzmann plots of the voltage dependence of activation and fast inactivation of rat Nav1.4 sodium channels; dashed line indicates the prepulse potential (−50 mV) used to induce fast inactivation without channel activation. The figure is re-drawn from the data of von Stein and Soderlund [21].
5.2. Models of the sodium channel LA receptor
The pore-forming α subunits of voltage-gated sodium channels are large proteins containing 24 transmembrane helical segments organized into four internally homologous domains (DI-DIV), each containing six transmembrane segments (S1-S6) [32,43]. Three- dimensional models of the α subunit protein [44–50], based on the crystal structures of homologous potassium channels, show homology domains DI-DIV arranged as “pseudosubunits” around a central ion pore with the four S6 transmembrane domains lining the intracellular side of the pore. The principal features of these homology models were recently confirmed in the crystal structure of a bacterial sodium channel [51].
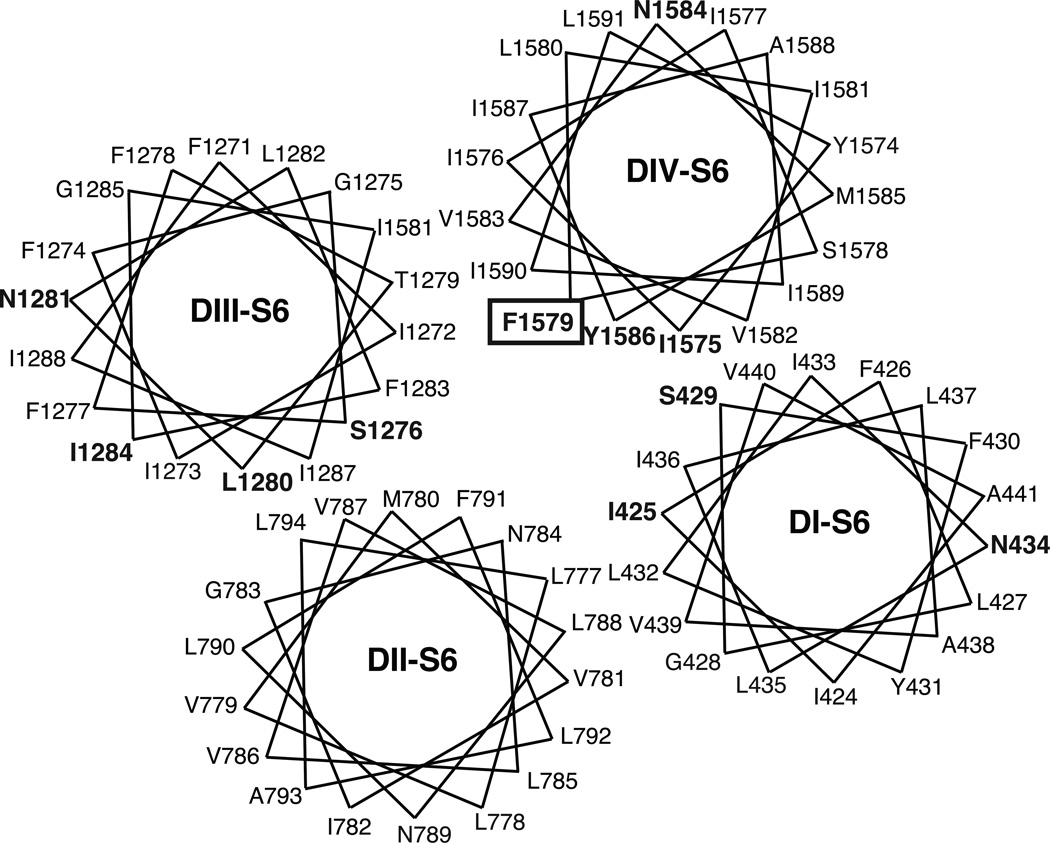
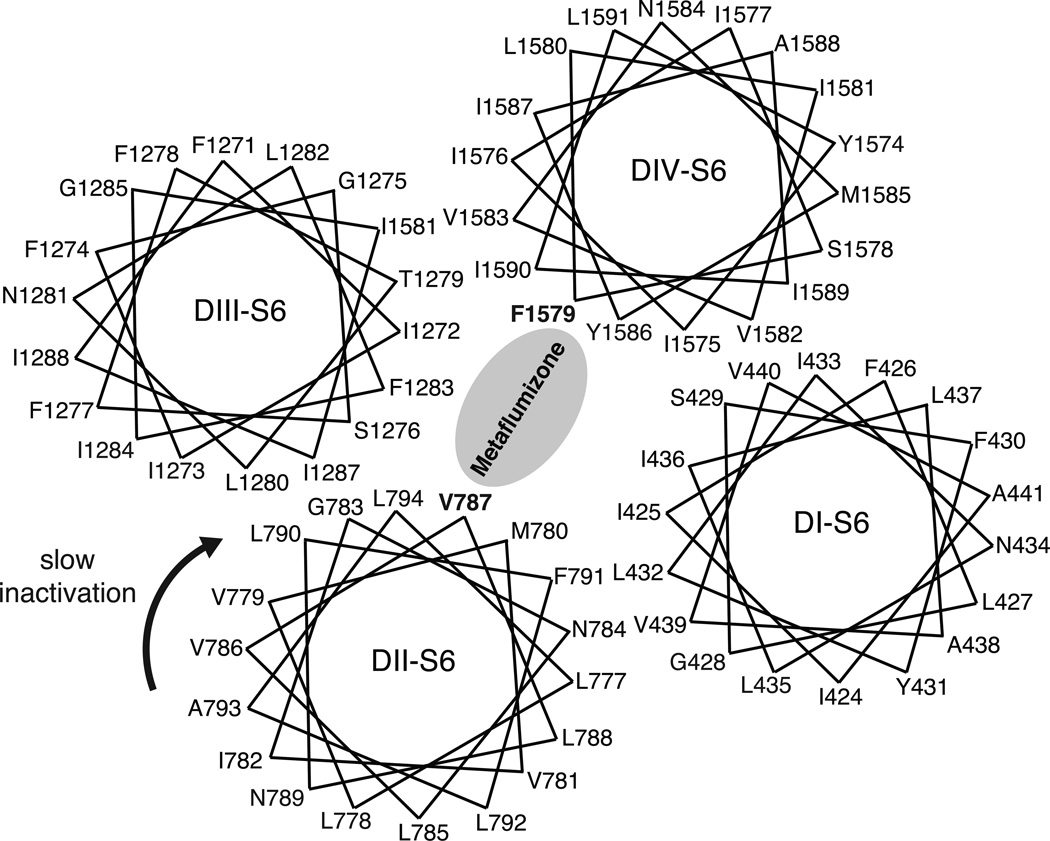
Numerous site-directed mutagenesis studies (reviewed in [23]) have identified amino acid residues in the S6 segments of homology domains DI, DIII, and DIV that contribute to sodium channel modification by SCI drugs. Figure 7 shows the location of 11 amino acid residues affecting SCI drug action on a postulated orientation of the four sodium channel S6 segments [23]. Phe1579 in DIV-S6 (boxed in Fig. 7; rat Nav1.4 residue numbering) is the single most important determinant of SCI drug binding and action [23]. Models of SCI drug binding to the LA receptor implicate Phe1579 as an element of the LA receptor that is intimately involved in drug binding within the ion pore [45,46,48,50]. Tyr1586, located on the same face of the DIV-S6 helix as Phe1579, is also an important determinant of anticonvulsant action but its direct role in drug binding is less well established [23]. Whereas the orientation of DIV-S6 shown in Fig. 7 is widely accepted, there is less consensus regarding the orientation of the other S6 helices in relation to the ion pore. Moreover, the distribution of residues affecting SCI drug action on multiple faces of three S6 domains ensures that many of these residues affect drug action indirectly, via effects on channel gating or conformational flexibility, rather than by forming part of the LA receptor.
Fig. 7.
Helical wheel representation of the amino acid sequences of the four S6 transmembrane domains arranged in relation to the central ion pore as proposed by Mike and Lukacs [23]. Residues shown by site-directed mutagenesis are shown in boldface; The Phe1579 residue thought to be a critical determinant of binding for all SCI drugs is boxed.
5.3. Effects of LA receptor mutations on SCI insecticide action
Evidence for competitive interactions between SCI insecticides and SCI drugs and the significance of sodium channel DIV-S6 residues as determinants of SCI drug binding or action led us to investigate the role of mutations at Phe1579 and Tyr1586 of rat Nav1.4 sodium channels in the action of SCI insecticides. Replacement of Phe1579 with alanine (F1579A) universally impairs the binding of SCI drugs to the LA receptor [23]. Nav1.4/F1579A sodium channels expressed in oocytes were significantly less sensitive to inhibition by RH3421, RH4841, DCJW and metaflumizone [21,52]. This effect of the F1579A mutation is fully consistent with its effect on the binding and action of SCI drugs to mammalian sodium channels and provides further evidence that the receptors for SCI insecticides and SCI drugs may overlap. Interestingly, the cognate alanine substitution (F1817A) in insect (Blattella germanica) BgNav sodium channels had no effect on the sensitivity of channels to indoxacarb or DCJW but enhanced channel sensitivity to metaflumizone [53]. Nevertheless, the F1817A mutation reduced use-dependent block of BgNav channels by lidocaine [54]. Taken together, these results suggest that the relationship between the receptors for SCI insecticides and SCI drugs may differ in mammalian and insect sodium channels.
Replacement of Tyr1586 by alanine (Y1586A) in rat Nav1.4 sodium channels or the cognate mutation in other mammalian sodium channel isoforms reduces channel sensitivity to anticonvulsants and some other SCI drugs but has limited effects on the activity of local anesthetics [23]. We found that Nav1.4/Y1586A sodium channels expressed in oocytes were paradoxically more sensitive to inhibition by RH3421, RH4841, DCJW and metaflumizone [21,52]. Moreover, Nav1.4/Y1586A sodium channels acquired sensitivity to inhibition by indoxacarb that was not evident with wildtype Nav1.4 channels [52]. The magnitude of the increase in sensitivity of Nav1.4/Y1586A sodium channels was compound-specific: 58-fold for DCJW, 16-fold for RH3421, and approximately 10-fold for metaflumizone [21,52]. These results suggest that Tyr1586 does not form part of the SCI insecticide receptor. Instead, we postulate that the large aromatic side chain of the tyrosine residue impedes the binding of these insecticides. By this interpretation, alanine substitution at Tyr1586 relieves steric hindrance and increases the affinity of channels for these compounds. The failure of the cognate (Y1824A) mutation in insect (BgNav) sodium channels to enhance SCI insecticide sensitivity [53] further underscores the apparent divergence of the structure of the SCI insecticide receptor on mammalian and insect sodium channels.
6. Do Mutations at Val787 in DII-S6 Identify an Novel Determinant of Metaflumizone Binding?
6.1. Mutations at Val787 of Nav1.4 as probes of slow inactivation
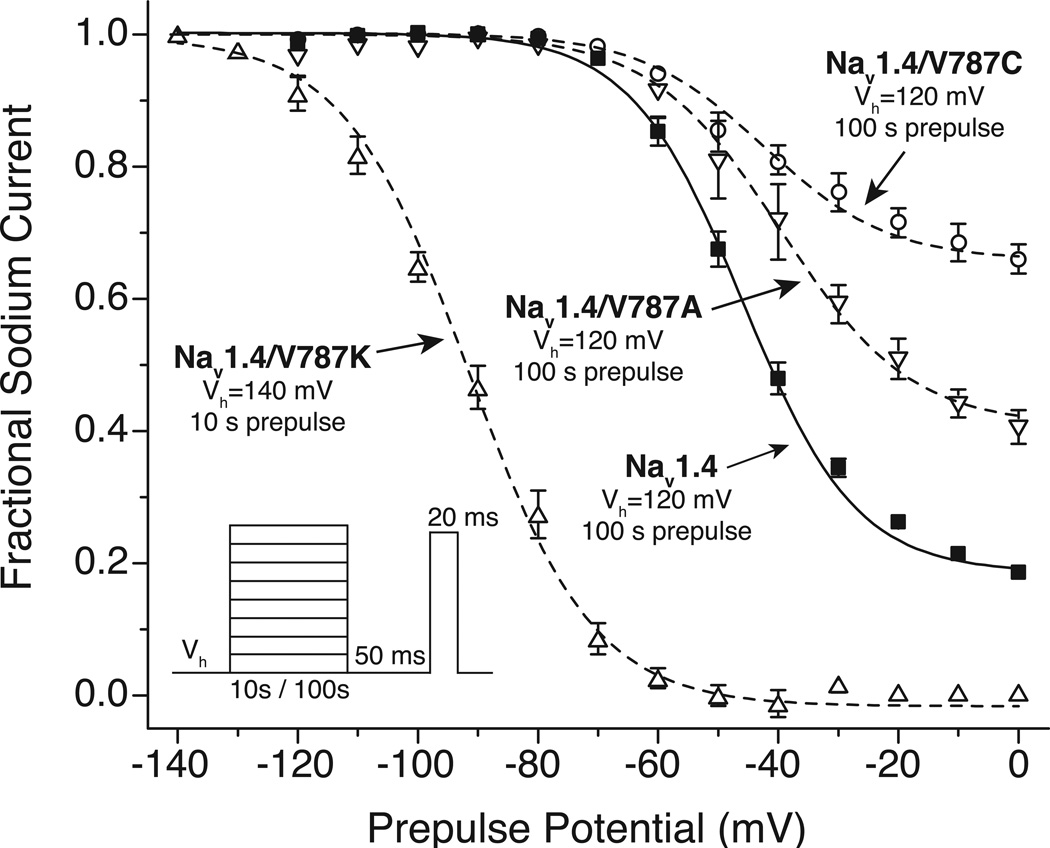
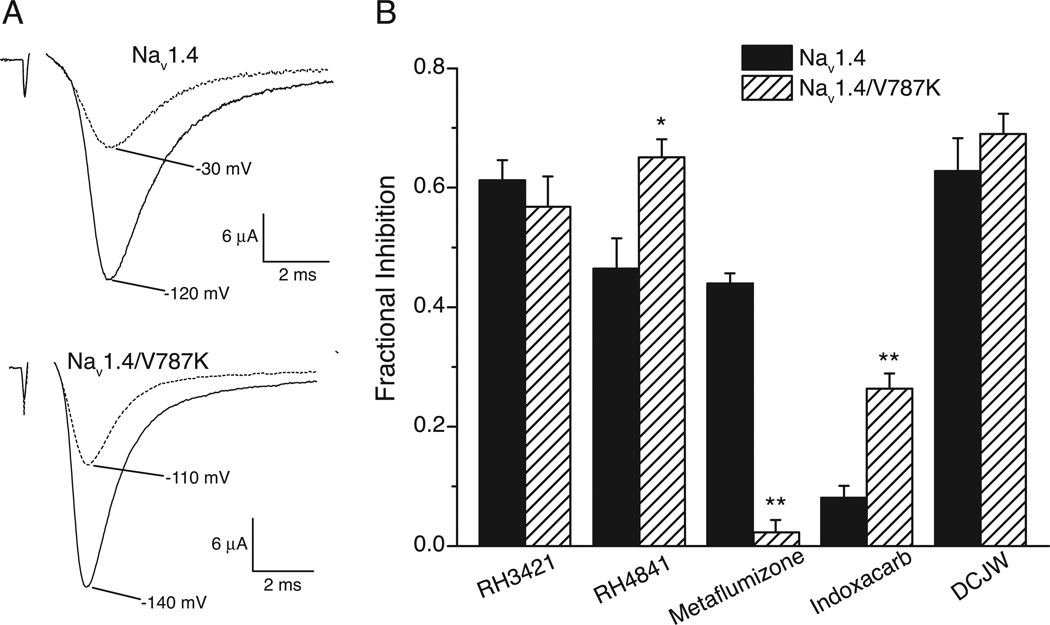
Mutations at Val787 of the rat Nav1.4 sodium channel isoform alter both the extent and voltage dependence of slow inactivation [55] and provide an opportunity to explore further the relationship between slow inactivation and sensitivity to inhibition by SCI insecticides. Figure 8 shows the voltage dependence of slow inactivation for wildtype Nav1.4 channels and for channels containing the V787K, V787C and V787A mutations [22]. The V787K mutation conferred complete slow inactivation at depolarized potentials and shifted the voltage dependence of slow inactivation by more that 45 mV in the direction of hyperpolarization. By contrast, the V787C and V787A mutations reduced the maximal extent of slow inactivation relative to wildtype channels without affecting its voltage dependence.
Fig. 8.
Voltage dependence of slow inactivation of unmodified and specifically mutated rat Nav1.4 sodium channels expressed in Xenopus oocytes. The holding potentials (Vh) and prepulse durations employed for each channel variant are indicated. The figure is redrawn from the data of von Stein and Soderlund [22].
6.2. Effects of mutations at Val787 of Nav1.4 on SCI insecticide sensitivity
Consistent with results obtained using Nav1.4 channels, SCI insecticides did not affect Nav1.4/V787A or Nav1.4/V787C channels at a hyperpolarized holding potential (−120 mV) [22]. However, indoxacarb, DCJW and RH4841 significantly inhibited Nav1.4/V787K channels when assayed at −140 mV whereas metaflumizone had no effect under these conditions. The sensitivity of Nav1.4/V787K channels to indoxacarb, DCJW and RH4841 at −140 mV was subsequently shown to result from incomplete removal of slow inactivation of Nav1.4/V787K channels at this membrane potential.
The unexpected resistance of Nav1.4/V787K channels to metaflumizone at −140 mV led us to assess the relative sensitivity of Nav1.4 and Nav1.4/V787K channels to SCI insecticides under conditions producing an approximately equivalent degree of slow inactivation of both channels [22]. Depolarization of the holding potential of Nav1.4 channels from −120 mV to −30 mV and of Nav1.4/787K channels from −140 mV to −110 mV reduced peak sodium currents by approximately 50% (Fig. 9A). Assays of SCI insecticides under these conditions (Fig. 9B) showed that the impact of the V787K mutation on channel inhibition was compound-specific: Nav1.4/V787K channels were resistant to metaflumizone but hypersensitive to RH4841 and indoxacarb and equally sensitive to either RH3421 or DCJW.
Fig. 9.
Inhibition of rat Nav1.4 and Nav1.4/V787K sodium channels expressed in Xenopus ooyctes. (A) Example current traces illustrating the approximately equivalent induction of slow inactivation of both variants. Top: traces recorded from Nav1.4 channels at holding potentials of either −120 mV or −30 mV. Bottom: traces recorded from Nav1.4/V787K channels at holding potentials of −140 mV or −110 mV. Currents from both channels held at depolarized potentials were recorded during 20-ms test pulses to −10 mV that was preceded by a 2-s repolarizing pulse to the holding potential (−120 or −140 mV depending on the variant examined). (B) Fractional inhibition of Nav1.4 and Nav1.4/V787K channels measured using the pulse protocols illustrated in Panel A 15 min after perfusion with SCI insecticides at 10 µM. The figure is re-drawn from the data of von Stein and Soderlund [22].
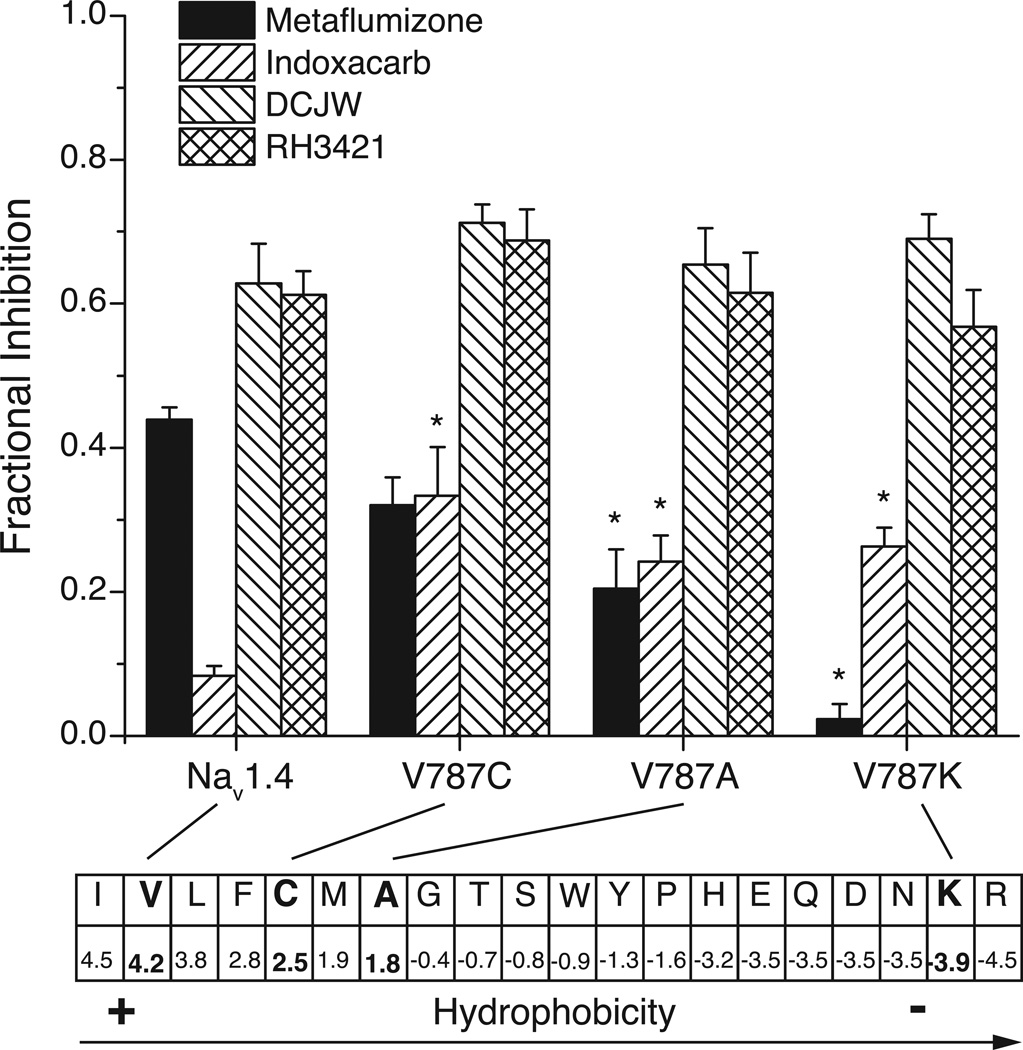
Fig. 10 summarizes the effects of all three mutations at Val787 on SCI insecticide sensitivity [22]. In these experiments Nav1.4, Nav1.4/V787C and Nav1.4/V787A channels were assayed following depolarization from −120 mV to −30 mV whereas Nav1.4/V787K channels were assayed following depolarization from −140 mV to −110 mV. These conditions produce approximately equivalent degrees of slow inactivation for Nav1.4 and Nav1.4/V787K channels but reduced slow inactivation for the Nav1.4/V787C and Nav1.4/V787A variants (see Fig. 8). If the availability of slow-inactivated channels were the only determinant of SCI inhibition in these experiments we would expect to see approximately equivalent inhibition of Nav1.4 and Nav1.4/V787K channels by all four insecticides based on the similar availability of slow-inactivated channels at the membrane holding potentials used (see Fig. 9). Similarly, we would expect reduced inhibition of Nav1.4/V787C and Nav1.4/V787A channels relative to Nav1.4 based on the reduced availability of slow-inactivated states with the mutated channels at a membrane holding potential of −30 mV (see Fig. 8).
Fig. 10.
Fractional inhibition of native and specifically mutated rat Nav1.4 sodium channels expressed in Xenopus oocytes by SCI insecticides. The extent of inhibition was determined following perfusion with insecticide (10 µM) as described in the legend to Fig. 9. The Nav1.4/V787A and Nav1.4/V787C variants were assayed using the pulse protocol described in Fig. 9 for Nav1.4 channels. Asterisks indicate inhibition of mutated channels that is significantly different from that measured for the same compound assayed against native Nav1.4 channels. The extent of inhibition (top) is correlated with the relative hydrophobicity of amino acid residues (bottom). The amino acids at position 787 in the four channel variants employed are shown in boldface type. The figure is drawn from the data of von Stein and Soderlund [22].
As shown in Fig. 10, the relative sensitivity of native Nav1.4 channels and channels with mutations at Val787 to inhibition by SCI insecticides was not well-correlated with the relative availability of slow-inactivated states under these assay conditions. Mutations at Val787 had no effect on the sensitivity of Nav1.4 channels to RH3421 and DCJW, but all three mutations significantly increased sensitivity to indoxacarb relative to the parent Nav1.4 channel. By contrast, sensitivity to metaflumizone depended on the amino acid substituted for Val787. The reduction in sensitivity for the three mutated channels correlated with the magnitude of decrease in hydrophobicity of the amino acid sidechain relative to that of the parent valine. These results are consistent with the hypothesis that Val787 participates uniquely in the binding of metaflumizone, but not DCJW or indoxacarb, through hydrophobic interactions and that introduction of more hydrophilic residues disrupts this interaction. By contrast, our data suggest that a hydrophobic interaction with Val787 is an impediment to the optimal binding of indoxacarb that is relieved by hydrophilic substitution. However, our data do not rule out the possibility that one or both of these effects are mediated indirectly by the effects of mutations on the conformation of the binding region in slow-inactivated channels rather than by direct participated in binding.
Figure 11 provides a conceptual model of possible binding interactions for metaflumizone in the inner pore of the Nav1.4 sodium channel. Mutagenesis studies show that Phe1579 in DIV-S6, a component of the LA receptor, is an important determinant of the binding of metaflumizone and other SCI insecticides. We postulate that slow inactivation of Nav1.4 channels involves conformational changes that include the rotation of the DII-S6 helix, exposing Val787 to the inner pore where it contributes to the binding of metaflumizone. The state-dependent reorientation of Val787 toward the ion pore is also consistent with results of accessibility experiments using reactive sulfhydryl reagents. In Nav1.4/V787C channels the introduced cysteine residue is only available for covalent modification by methanethiosulfonate ethylammonium at membrane potentials that promote slow inactivation [55].
Fig. 11.
Helical wheel representation of the amino acid sequences of the four S6 transmembrane domains showing putative binding interactions of metaflumizone and the proposed conformational rotation of DII-S6 (arrow) in slow-inactivated channels required to expose Val787 to the channel pore.
There is not sufficient information at present to expand this model to include other SCI insecticides. Modification of sodium channels by chiral SCI insecticides is stereospecific [6,35], thereby implying the existence of a minimum of three points of contact between these insecticides and their receptor. Our data rule out Tyr1586 as a determinant of SCI insecticide binding and show that the putative importance of Va787 in binding is limited to metaflumizone. Thus, additional determinants of SCI binding in the region of the sodium channel LA receptor remain to be identified.
7. Conclusions and Implications
7.1. SCI insecticides share a common, novel mode of action on sodium channels
SCI insecticides, despite their structural diversity, are unified and grouped as a single class based on their common mode of action on both insect and mammalian voltage-gated sodium channels. All SCI insecticides interact preferentially with channels in the slow- inactivated state, forming long-lived, insecticide-modified complexes (Fig. 4). This sequestration of channels in the non-conducting slow-inactivated state leads to a reduction in sodium currents and, ultimately, block of nerve conduction. Neurons that fire frequently or otherwise exhibit partially depolarized membrane potentials are particularly sensitive to SCIs. There is a good correspondence between state-dependent channel modification by SCI insecticides, selective nerve block by SCI insecticides, and the pseudoparalysis that is the characteristic symptom of intoxication by SCI insecticides in intact insects. Some SCI insecticides exhibit high intrinsic neurotoxicity to mammals, but the causal links between state-dependent sodium channel modification and acute toxicity are less clear for mammals than for insects.
The SCI insecticide family comprises several molecular scaffolds and includes no single toxophoric moiety. The conserved core partial structure identified by Takagi et al. [4] as a common feature of dihydropyrazole, oxadiazine and semicarbazone SCI insecticides (see Fig. 2) conveys the structural similarity among these groups of compounds but is not a toxophore in the formal sense and is not required for SCI insecticide-like activity. Thus the BZP class of SCI insecticides, which lacks the conserved core structure defined in Fig. 2, shares the pharmacological and insecticidal properties of the SCI insecticide class as a whole.
The mechanism by which SCI insecticides inhibit sodium channel function is a novel mode of insecticide action on sodium channels. SCI insecticides are distinguished from pyrethroids and DDT analogs, which also target the voltage-gated sodium channel, not only by their distinctly different effects on channel function but also by their discrete binding domains [56]. Thus, mutations in insect sodium channel genes that confer resistance to DDT and pyrethroids do not occur in sodium channel domains identified to date as part of the SCI insecticide receptor [57,58], and the SCI insecticides retain full insecticidal activity in insect populations that exhibit target site-mediated resistance to DDT and pyrethroids [6].
7.2. Do SCI insecticides bind to the sodium channel LA receptor?
Evidence summarized in this review shows that SCI insecticides interact competitively at the sodium channel with local anesthetics and other classes of SCI drugs. The majority of these studies involve mammalian sodium channels, either in native tissue or heterologous expression systems. Mutagenesis experiments using the rat Nav1.4 channel identify Phe1579 in DIV-S6, the single most important determinant of SCI drug binding to the LA receptor, as a critical determinant of the binding and action of SCI insecticides [21,52]. These results suggest that the receptors for SCI insecticides and SCI drugs both are located in the inner pore of the sodium channel and, at a minimum, overlap to the extent that joint occupancy of this domain by drug and insecticide molecules is precluded. However, our experiments involving mutations at Val787 in DII-S6 [22] show that determinants of SCI insecticide binding or action exist in regions of the inner pore that have not been previously identified as elements of the LA receptor. It is possible that further mutagenesis experiments to map the determinants of SCI insecticide binding, particularly in DII-S6, may help resolve the current ambiguity regarding the orientation of sodium channel S6 domain residues in relation to the ion pore. These studies could also provide new insight into structural rearrangements in the ion pore that accompany conversion to slow-inactivated channel states.
Available data, though limited, imply that the conclusions drawn above for SCI insecticide and drug action on mammalian sodium channels may not be applicable to insect sodium channels. The divergent results obtained with alanine mutations at DIV-S6 residues in cockroach sodium channels corresponding to the F1579A and Y1586A mutations in rat Nav1.4 channels [53] imply that the determinants of SCI insecticide binding and action may differ between insect and mammalian channels. This conclusion is surprising in light of the high degree of structural conservation in the S6 domains of evolutionarily divergent sodium channels and the difficulty that has been encountered in empirical efforts to identify SCI structures that retain high insecticidal activity without correspondingly high mammalian toxicity. Additional mutagenesis studies to map the determinants of SCI binding to cockroach and other insect sodium channels would provide important insight into the structural relationship between mammalian and insect receptors for SCI insecticides.
7.3. Pharmacological convergence of insecticide and drug discovery efforts on slow-inactivated sodium channels
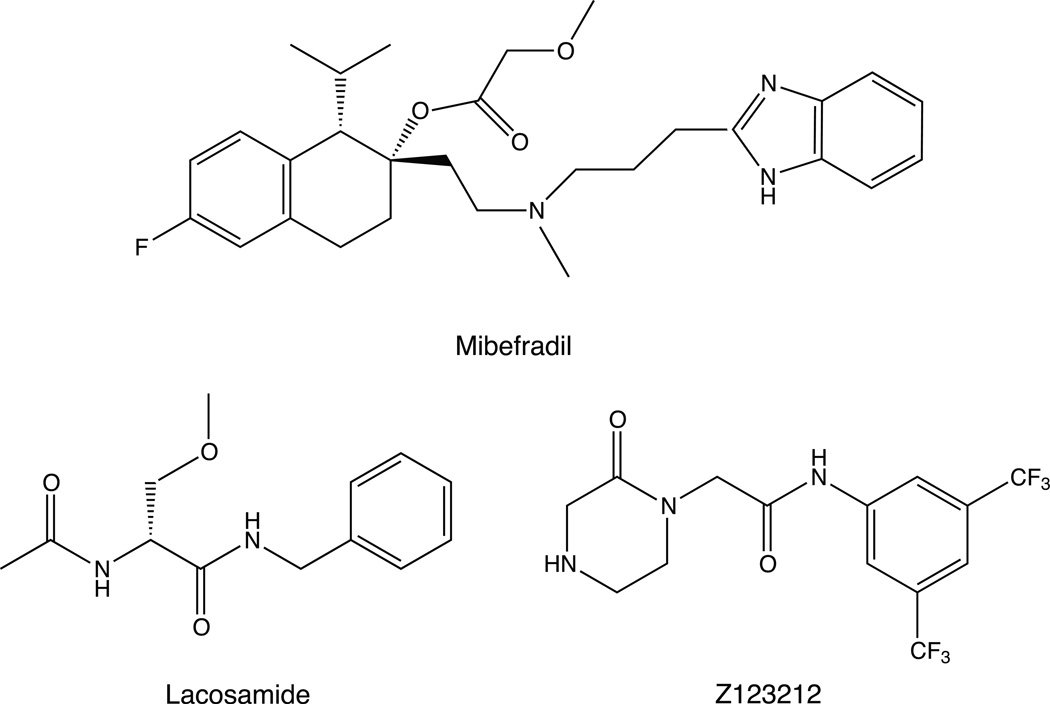
The majority of therapeutic SCI inhibitors (e.g., local anesthetics, anticonvulsants, antiarrhythmics) preferentially target sodium channels in the open or fast-inactivated state [59]. However, renewed interest in voltage-gated sodium channels as targets for new therapeutic agents to treat neuropathic pain and other disorders [60] has yielded novel drugs that, like SCI insecticides, preferentially target sodium channels in the slow-inactivated state. Figure 12 shows examples SCI drugs that selectively target slow-inactivated channels: mibefradil, an antihypertensive agent [61]; lacosamide, an anticonvulsant with anti-inflammatory and antinociceptive activity [62]; and Z123212, an experimental drug for neuropathic pain [63].
Fig. 12.
Structures of mibefradil, lacosamide and Z123212.
What properties differentiate toxic (i.e., insecticidal) and therapeutic SCI agents that target slow-inactivated channels? There are no direct comparative studies of toxic and therapeutic SCIs that might identify the pharmacological properties associated with either toxic or therapeutic effects. However, we speculate that association and dissociation kinetics for the binding of SCI agents to their receptor domains may play a large role in distinguishing therapeutic and toxic effects. SCI insecticides exhibit extremely slow association kinetics, requiring equilibration in vitro for up to 15 minutes to achieve steady-state channel modification (see Fig. 3). Moreover, the binding of SCI insecticides to slow-inactivated channels is very persistent and, in some cases, irreversible by washing unless a hyperpolarized membrane potential is restored. By contrast steady-state effects of mibefradil are achieved within 1–2 min of perfusion and can be reversed by washing [61]. We suggest that neurotoxicity among SCI agents may be due in part to persistent compromise of nerve function due to long-lasting sequestration of sodium channels in nonconducting slow-inactivated states.
Further efforts to define the properties that differentiate toxic and therapeutic SCIs may benefit the further development of the SCI insecticide class in two ways. First, knowledge of these properties has the potential to improve the safety of SCI insecticides while informing the further development of safe and effective SCI drugs. Second, lead compounds abandoned by the pharmaceutical industry due to adverse toxicity could identify new structural scaffolds for the discovery of novel SCI insecticides.
Research Highlights.
Sodium channel inhibitor (SCI) insecticides silence endogenous neural activity
SCI insecticides interact selectively with slow-inactivated sodium channels
The SCI insecticide receptor includes elements of the local anesthetic receptor
Metaflumizone exhibits unique actions that are not shared with other SCI insecticides
Acknowledgments
We thank the following current or former colleagues for their contributions to the research reviewed in this article: Pamela Adams, Jin Sung Choi, Bingjun He, Scott Kopatz, and Jianguo Tan. Research from this laboratory was supported by research (R01-ES08962, R01-ES014591) and training (T32-ES07052) grants from the National Institute of Environmental Health Sciences, National Institutes of Health and a grant (2001-35302-10880) from the United States Department of Agriculture. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of any of the sponsors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mulder R, Wellinga K, van Daalen JJ. A new class of insecticides. Naturwissenschaften. 1975;62:531–532. doi: 10.1007/BF00609070. [DOI] [PubMed] [Google Scholar]
- 2.McCann SF, Annis GD, Shapiro R, Piotrowski DW, Lahm GP, Long JK, Lee KC, Hughes MM, Myers BJ, Griswold SM, Reeves BW, March RW, Sharpe PL, Lowder P, Barnette WE, Wing KD. The discovery of indoxacarb: oxadiazines as a new class of pyrazoline-type insecticide. Pest Management Science. 2001;57:153–164. doi: 10.1002/1526-4998(200102)57:2<153::AID-PS288>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 3.Meier GA, Silverman R, Ray PS, Cullen TG, Ali SF, Marek FL, Webster CA. Insecticidal dihydropyrazoles with reduced lipophilicity. In: Baker DR, Fenyes JG, Steffens JJ, editors. Synthesis and Chemistry of Agrochemicals III. Washington, DC: American Chemical Society; 1992. pp. 313–326. [Google Scholar]
- 4.Takagi K, Hamaguchi H, Nishimatsu T, Konno T. Discovery of metaflumizonea novel semicarbazone insecticide. Veterinary Parasitology. 2007;150:177–181. doi: 10.1016/j.vetpar.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 5.Silver KS, Soderlund DM. Action of pyrazoline-type insecticides at neuronal target sites. Pesticide Biochemistry and Physiology. 2005;81:136–143. [Google Scholar]
- 6.Wing KD, Andaloro JT, McCann SF, Salgado VL. Indoxacarb and sodium channel blocker insecticides: chemistry, physiology, and biology in insects. In: Gilbert L, Iatrou K, Gill S, editors. Comprehensive Molecular Insect Science. New York: Elsevier; 2005. pp. 31–53. [Google Scholar]
- 7.Silver KS, Song W, Nomura Y, Salgado VL, Dong K. Mechanism of action of sodium channel blocker insecticides (SCBIs) on insect sodium channels. Pesticide Biochemistry and Physiology. 2010;97:87–92. doi: 10.1016/j.pestbp.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grosscurt AC, Hes RV, Wellinga K. 1-Phenylcarbamoyl-2-pyrazolines, a new class of insecticides. 3. Synthesis and insecticidal properties of 3,4-diphenyl-1-phenylcaramoyl-2-pyrazolines. Journal of Agricultural and Food Chemistry. 1979;27:406–409. [Google Scholar]
- 9.van Hes R, Wellinga K, Grosscurt AC. 1-Phenylcarbamoyl-2-pyrazolines: a new class of insecticides. 2. Synthesis and insecticidal properties of 3,5-diphenyl-1-phenylcarbamoyl-2-pyrazolines. Journal of Agricultural and Food Chemistry. 1978;26:915–918. [Google Scholar]
- 10.Wellinga K, Grosscurt AC, van Hes R. 1-Phenylcarbamoyl-2-pyrazolines: a new class of insecticides. 1. Synthesis and insecticidal properties of 3-phenylcarbamoyl-2-pyrazolines. Journal of Agricultural and Food Chemistry. 1977;25:987–992. [Google Scholar]
- 11.Scheele B. Untersuchung des Photoabbaus von PH 60-42 auf MaisblätternGlasplatten und Bodendünnschichtplatten. Chemosphere. 1980;9:483–494. [Google Scholar]
- 12.Führ F, Mittelstaedt W, Wieneke J. Bilanzversuche mit 14C-markiertem 1-(4-Chlorophenylcarbamoyl)-3(4-chlorophenyl)-4-phenyl-2-pyrazolin (PH 60-42) nach Boden- und Spritzapplikation in Freilandlysimetern. Chemosphere. 1980;9:469–482. [Google Scholar]
- 13.Jacobson RM. A new class of insecticidal dihydropyrazoles. In: Crombie L, editor. Recent Advances in the Chemistry of Insect Control II. Cambridge: Royal Society of Chemistry; 1990. pp. 206–212. [Google Scholar]
- 14.Hasan R, Nishimura K, Okada M, Akamatsu M, Inoue M, Ueno T. Stereochemical basis for the insecticidal activity of carbamoylated and acylated pyrazolines. Pesticide Science. 1996;46:105–112. [Google Scholar]
- 15.Lyga J, Lyga I, Silverman R, Ali SF, Cullen TF, Cohen DH, Simmons KA. Discovery of the indolebenzhydrylpiperazines and benhydrolpiperidines: a new class of insecticides. In: Baker DR, Fenyes JG, Selby TP, Stevenson TM, editors. Synthesis and Chemistry of Agrochemicals VI. Washington, DC: American Chemical Society; 2002. pp. 199–210. [Google Scholar]
- 16.Wierenga JM, Warkentin DL, Staetz CA, Pitts DL, Dybas JA. Insecticidal activity of N-arylalkylbenzhydrolpiperidines. Pest Management Science. 2002;58:1266–1272. doi: 10.1002/ps.598. [DOI] [PubMed] [Google Scholar]
- 17.Bloomquist JR, Payne GT, Kinne L, Lyga J, Leong D, Nicholson RA. Toxicity and mode of action of benzhydrolpiperidines and related compounds in insects. Pesticide Biochemistry and Physiology. 2002;73:18–26. [Google Scholar]
- 18.Leong D, Bloomquist JR, Bempong J, Dybas JA, Kinne LP, Lyga JW, Marek FL, Nicholson RA. Insecticidal arylalkylbenzhydrolpiperidines: novel inhibitors of voltage-sensitive sodium and calcium channels in mammalian brain. Pest Management Science. 2001;57:889–895. doi: 10.1002/ps.352. [DOI] [PubMed] [Google Scholar]
- 19.Wing KD, Sacher M, Kagaya Y, Tsurubuchi Y, Mulderig L, Connair M, Schee M. Bioactivation and mode of action of the oxadiazine indoxacarb in insects. Crop Protection. 2000;19:537–545. [Google Scholar]
- 20.Hempel K, Hess FG, Bögi C, Fabian E, Hellwig J, Fegert I. Toxicological properties of metaflumizone. Veterinary Parasitology. 2007;150:190–195. doi: 10.1016/j.vetpar.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 21.von Stein RT, Soderlund DM. Role of the local anesthetic receptor in the state-dependent inhibition of voltage-gated sodium channels by the insecticide metaflumizone. Molecular Pharmacology. 2012;81:366–374. doi: 10.1124/mol.111.075283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Stein RT, Soderlund DM. Compound-specific effects of muations at Val787 in DII-S6 of Nav1.4 sodium channels on the action of sodium channel inhibitor insecticides. Neurotoxicology. 2012;33:1381–1389. doi: 10.1016/j.neuro.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mike A, Lukacs P. The enigmatic drug binding site for sodium channel inhibitors. Current Molecular Pharmacology. 2010;3:129–144. doi: 10.2174/1874467211003030129. [DOI] [PubMed] [Google Scholar]
- 24.Salgado VL. Mode of action of insecticidal dihydropyrazoles: selective block of impulse generation in sensory nerves. Pesticide Science. 1990;28:389–411. [Google Scholar]
- 25.Wing KD, Schnee ME, Sacher M, Connair M. A novel oxadiazine insecticide is bioactivated in Lepidopteran larvae. Archives of Insect Biochemistry and Physiology. 1998;37:91–103. [Google Scholar]
- 26.Salgado VL, Hayashi JH. Metaflumizone is a novel sodium channel blocker nsecticide. Veterinary Parasitology. 2007;150:182–189. doi: 10.1016/j.vetpar.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 27.Lapied B, Grolleau F, Sattelle DB. Indoxacarb an oxadiazine insecticide, blocks insect neuronal sodium channels. British Journal of Pharmacology. 2001;132:587–595. doi: 10.1038/sj.bjp.0703853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salgado VL. Slow voltage-dependent block of sodium channels in crayfish nerve by dihydropyrazole insecticides. Molecular Pharmacology. 1992;41:120–126. [PubMed] [Google Scholar]
- 29.Zhao X, Ikeda T, Salgado VL, Yeh JZ, Narahashi T. Block of two subtypes of sodium channels in cockroach neurons by indoxacarb insecticides. Neurotoxicology. 2005;26:455–465. doi: 10.1016/j.neuro.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Tsurubuchi Y, Kono Y. Modulation of sodium channels by the oxadiazine insecticide indoxacarb and its N-decarbomethoxylated metabolite in rat dorsal root ganglion neurons. Pest Management Science. 2003;59:999–1006. doi: 10.1002/ps.652. [DOI] [PubMed] [Google Scholar]
- 31.Zhao X, Ikeda T, Yeh JZ, Narahashi T. Voltage-dependent block of sodium channels in mammalian neurons by the oxadiazine insecticide indoxacarb and its metabolite DCJW. Neurotoxicology. 2003;24:83–96. doi: 10.1016/s0161-813x(02)00112-2. [DOI] [PubMed] [Google Scholar]
- 32.Catterall WA. From ionic currents to molecular mechanisms: structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 33.Goldin AL. Resurgence of sodium channel research. Annual Review of Physiology. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- 34.Soderlund DM. Sodium channels. In: Gilbert L, Iatrou K, Gill S, editors. Comprehensive Molecular Insect Science. New York: Elsevier; 2005. pp. 1–24. [Google Scholar]
- 35.Silver K, Soderlund DM. State-dependent block of rat Nav1.4 sodium channels expressed in Xenopus oocytes by pyrazoline-type insecticides. Neurotoxicology. 2005;26:397–406. doi: 10.1016/j.neuro.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their role in electrogenesis within dorsal root ganglion neurons. Journal of Physiology. 2007;579:1–14. doi: 10.1113/jphysiol.2006.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silver KS, Soderlund DM. Differential sensitivity of rat voltage-sensitive sodium channel isoforms to pyrazoline-type insecticides. Toxicology and Applied Pharmacology. 2006;214:209–217. doi: 10.1016/j.taap.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 38.Deecher DC. Soderlund DM RH 3421, an insecticidal dihydropyrazole inhibits sodium channel-dependent sodium uptake into mouse brain preparations. Pesticide Biochemistry and Physiology. 1991;39:130–137. [Google Scholar]
- 39.Payne GT, Deecher DC, Soderlund DM. Structure-activity relationships for the action of dihydropyrazole insecticides on mouse brain sodium channels. Pesticide Biochemistry and Physiology. 1998;60:177–185. [Google Scholar]
- 40.Catterall WA. Inhibition of voltage-sensitive sodium channels in neuroblastoma cells by antiarrhythmic drugs. Molecular Pharmacology. 1981;20:356–362. [PubMed] [Google Scholar]
- 41.Creveling CR, McNeal ET, Daly JW, Brown GB. Batrachotoxin-induced depolarization and [3H]batrachotoxinin-A 20a-benzoate binding in a vesicular preparation from Guinea pig cerebral cortex. Molecular Pharmacology. 1983;23:350–358. [PubMed] [Google Scholar]
- 42.Deecher DC, Payne GT, Soderlund DM. Inhibition of [3H]batrachotoxinin A 20-a-benzoate binding to mouse brain sodium channels by the dihydropyrazole insecticide RH 3421. Pesticide Biochemistry and Physiology. 1991;41:265–273. [Google Scholar]
- 43.Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biology. 2003;4:207.1–207.7. doi: 10.1186/gb-2003-4-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lipkind GM, Fozzard HA. KcsA crystal structure as framework for a molecular model of the Na+ channel pore. Biochemistry. 2000;39:8161–8170. doi: 10.1021/bi000486w. [DOI] [PubMed] [Google Scholar]
- 45.Lipkind GM, Fozzard HA. Molecular modeling of local anesthetic drug binding by voltage-gated sodium channels. Molecular Pharmacology. 2005;68:1611–1622. doi: 10.1124/mol.105.014803. [DOI] [PubMed] [Google Scholar]
- 46.Lipkind GM, Fozzard HA. Molecular model of anticonvulsant drug binding to the voltage-gated sodium channel inner pore. Molecular Pharmacology. 2010;78:6331–638. doi: 10.1124/mol.110.064683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Reilly AO, Khambay BPS, Williamson MS, Field LM, Wallace BA, Davies TGE. Modelling insecticide binding sites at the voltage-gated sodium channel. Biochemical Journal. 2006;396:255–263. doi: 10.1042/BJ20051925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheib H, McLay I, Guex N, Clare JJ, Blaney FE, Dale TJ, Tate SN, Robertson GM. Modeling the pore structure of voltage-gated sodium channels in closed, open and fast-inactivated conformation reveals details of site 1 toxin and local anesthetic binding. Journal of Molecular Modeling. 2006;12:813–822. doi: 10.1007/s00894-005-0066-y. [DOI] [PubMed] [Google Scholar]
- 49.Tikhonov DB, Zhorov BS. Modeling P-loops domain of sodium channel: homology with potassium channels and interactions with ligands. Biophysical Journal. 2005;88:184–197. doi: 10.1529/biophysj.104.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tikhonov DB, Zhorov BS. Sodium channels: ionic model of slow inactivation and state-dependent drug binding. Biophysical Journal. 2007;93:1557–1570. doi: 10.1529/biophysj.106.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–359. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silver KS, Soderlund DM. Point mutations at the local anesthetic receptor site modulate the state-dependent block of rat Nav1.4 sodium channels by pyrazoline-type insecticides. Neurotoxicology. 2007;28:655–663. doi: 10.1016/j.neuro.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Silver KS, Nomura Y, Salgado VL, Dong K. Role of the sixth transmembrane segment of domain IV of the cockroach sodium channel in the action of sodium channel blocker insecticides. Neurotoxicology. 2009;30:613–621. doi: 10.1016/j.neuro.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song W, Silver KS, Du Y, Liu Z, Dong K. Analysis of the action of lidocaine on insect sodium channels. Insect Biochemistry and Molecular Biology. 2011;41:36–41. doi: 10.1016/j.ibmb.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Reilly JP, Wang S-Y, Wang GK. Residue-specific effects on slow inactivation at V787 in D2-S6 of Nav1.4 sodium channels. Biophysical Journal. 2001;81:2100–2111. doi: 10.1016/S0006-3495(01)75858-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soderlund DM. Molecular mechanisms of pyrethroid insecticide neurotoxicity: recent advances. Archives of Toxicology. 2011 doi: 10.1007/s00204-011-0726-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong K. Insect sodium channels and insecticide resistance. Invertebrate Neuroscience. 2007;7:17–30. doi: 10.1007/s10158-006-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soderlund DM. Pyrethroids, knockdown resistance and sodium channels. Pest Management Science. 2008;64:610–616. doi: 10.1002/ps.1574. [DOI] [PubMed] [Google Scholar]
- 59.Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- 60.Thiele JW, Cummins TR. Recent developments regarding voltage-gated sodium channel blockers for the treatment of inherited and acquired neuropathic pain disorders. Frontiers in Pharmacology. 2011;2:68. doi: 10.3389/fphar.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McNulty MM, Hanck DA. State-dependent mibefradil block of Na+ channels. Molecular Pharmacology. 2004;66:1652–1661. doi: 10.1124/mol.66.6.1652. [DOI] [PubMed] [Google Scholar]
- 62.Errington AC, Stöhr T, Heers C, Lees G. The investigational anticonvulsant lacosamide seletively enhancs slow inactivation of voltage-gated sodium channels. Molecular Pharmacology. 2008;73 doi: 10.1124/mol.107.039867. [DOI] [PubMed] [Google Scholar]
- 63.Hildebrand ME, Smith PL, Bladen C, Eduljee C, Xie JY, Chen L, Fee-Maki M, Doering DJ, Mezeyova J, Zhu Y, Belardetti F, Pajouhesh H, Parker D, Arneric SP, Parmar M, Porreca F, Tringham E, Zamponi GW, Snutch TP. A novel slow-inactivation-specific ion channel modulator attenuates neuropathic pain. Pain. 2011;152:833–843. doi: 10.1016/j.pain.2010.12.035. [DOI] [PubMed] [Google Scholar]