Abstract
The use of generic drugs has increased significantly in recent years. Since generic drugs are available at a lower cost, they provide an opportunity for savings in drug expenditure. Thus, use of generic drugs is encouraged especially in developing countries. There are only a few studies concerning the perceptions and attitudes of the healthcare providers and patients towards generic drug use.
Methods
The present study was conducted by a face to face questionnaire in the Kadikoy district of Istanbul in April 2010. From randomly chosen respondents, 68 pharmacists, 56 prescribers and 101 patients consented to participate in the study.
Results
Thirty one and 32 % of the pharmacists and prescribers, respectively, expressed that they believed that the generics did not differ from the original drugs, whereas only 24% of the patients believed so. Forty percent of the pharmacists and 82% of the prescribers told that they were unsure about the bioequivalence of the generics. Ten percent of the patients claimed that they immediately accept generic substitution by the pharmacist, while 26% accepted it if it was substituted by the prescriber. Cost was the most important factor taken into consideration about generic substitution (92% for prescribers; 83% for patients and 82% for pharmacists).
Conclusions
Our findings demonstrated that healthcare providers as well as the drug consumers have insufficient knowledge about generic drugs. Therefore, they should be better educated with respect to generic substitution.
Keywords: Drugs, Generic; Health Knowledge, Attitudes, Practice; Pharmacists; Physicians; Turkey
Introduction
A generic drug is defined as "a pharmaceutical product which has the same characteristics as the reference medicinal product (innovators product) regarding the quality and composition of the active ingredients and pharmaceutical form, and also whose bioequivalence with the reference product has been demonstrated by appropriate bioavailability studies".1,2,3,4 Generic drugs are identical or within an acceptable bioequivalent range to the brand-name counterpart with respect to pharmacokinetic and pharmacodynamic properties. Many generic drugs as well as their brand-name counterparts are available in all countries. Since innovator drugs are no longer protected by patents; and since generic drug manufacturers do not have to spend extra money for drug discovery, pre-clinical and clinical trials, as well as for some other reasons, generics are generally cheaper than generics. In all countries, the use of generic drugs has increased significantly in recent years. Since generics are available at a lower cost, they provide an opportunity for savings in drug expenditure without reducing the quality.2,3,4,5,6,7,8,9,10,11
Both in developed and developing countries, health insurance agencies, health authorities and governments have suffered from pharmaceutical expenditures that has risen rapidly especially in the last two decades.2,3,4,5,10,11,12,13,14,15 Substitution of generics for brand-name might be an alternative way to reduce drug expenditure. Turkey has its own national pharmaceutical industry and policies supporting the growth of generic industry can increase the export potential of Turkey. Recently, higher discounts on innovator drugs and a revised reference pricing system for generics has been regulated by the government.16 However, this policy needs giving satisfactory information about generics to all of the respondents. Except for the trade name, colour, size, shape, taste and the cost, generics must have the same pharmacological characteristics as the alternative for the brand-name. However, some healthcare providers and patients may not have enough knowledge about generics especially about these features. For instance, patients and healthcare providers may have concerns about generics and sometimes this may be a major barrier to a wider use of these products.3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18 In Turkey, Ministry of Health examines generic drugs and approves them as bioequivalent to brand-name in terms of strength, quality, and safety.1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19 Patients, physicians, and pharmacist may also have some misinformation about generics which can cause hesitation about the use of these drugs and especially about their efficacies. In addition the rising healthcare cost is frequently challenged by some habits of healthcare providers and patients. There are many factors that can influence the choosing, prescribing, dispensing and taking medication behavior. Inaccurate or insufficient knowledge about generics can cause ineffective and unsafe treatment. This situation can also lead to a high cost, contrary to expectations.2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18
The use of generics has been discussed at various platforms in Turkey16,20, but well-designed survey studies especially with the contribution of related partners such as patients, community pharmacist and physicians are required to take a general picture. The aim of the present study was to determine the knowledge and attitudes of patients, their community pharmacists and physicians regarding the use of generic drugs.
Methods
The present study was conducted by a face to face questionnaire with pharmacists and patients in the Kadikoy district of Istanbul in April 2010. Also prescribers from the nearby hospitals (Haydarpasa Numune Education and Training Hospital, Marmara University Hospital) were asked to answer the questionnaire. From randomly asked respondents, a total number of 56 prescribers, 68 pharmacists and 101 patients consented to participate in the study.
The questionnaires consisted of structured and open questions which were prepared to determine socio-demographic characteristics of the pharmacists, prescribers and patients and their knowledge of generic drug use.
The questionnaire was examined in linguistic and interpretive terms by an investigator who works in the department of public health and it was validated. A pilot test was performed with 10 pharmacists, 10 prescribers and 15 patients twice with 4-week intervals to assess its reproducibility.
Legal permissions were taken from the local health authority-Provincial Health Directorate of Istanbul, Turkey [Istanbul Il Sağlık Müdürlüğü] (April 5th 2010; SG.26/2).
Statistical Analysis: The data were subjected to frequency analysis and Pearson's chi-square tests by SPSS software version 13.0 for Windows. Values were compared by independent sample t-test and p<0.05 were considered as significant.
Results
A hundred and fifteen prescribers working in Haydarpasa Numune Education and Training Hospital and Marmara University Hospitals were visited in Kadikoy region in Istanbul. Fifty six prescribers answered the questionnaire (response rate 49%). Also 68 community pharmacist of the 121 community pharmacists in the Kadikoy region responded the questionnaire (response rate 56%). The socio-demographic characteristics of the pharmacists and prescribers are presented in Table 1.
Table 1.
Socio-demographical characteristics of the pharmacists and prescribers
| Prescribers | Pharmacists | |||
|---|---|---|---|---|
| N | % | N | % | |
| Age | ||||
| 25-35 | 39 | 69.6 | 9 | 13.2 |
| 36-45 | 10 | 17.9 | 18 | 26.5 |
| 46-55 | 6 | 10.7 | 13 | 19.1 |
| 55+ | 1 | 1.8 | 28 | 41.2 |
| Gender | ||||
| Male | 35 | 62.5 | 25 | 36.8 |
| Female | 21 | 37.5 | 43 | 63.2 |
| Experience (years) | ||||
| 0-5 | 19 | 33.9 | 5 | 7.4 |
| 6-10 | 17 | 30.4 | 6 | 8.8 |
| 11-20 | 11 | 19.6 | 17 | 25 |
| 20+ | 9 | 16.1 | 40 | 58.8 |
| TOTAL | 56 | 100 | 68 | 100 |
The 101 patients that participated in the study were also from the Kadikoy district, and they were asked to answer the face to face questionnaire right after they had their prescriptions dispensed and were leaving the pharmacy The socio-demographical characteristics of the patients are presented in Table 2.
Table 2.
Socio-demographical characteristics of the patients (n=101)
| N | % | |
|---|---|---|
| Age | ||
| 25-35 | 55 | 54.5 |
| 36-45 | 28 | 27.7 |
| 46-55 | 10 | 9.9 |
| 55+ | 8 | 7.9 |
| Gender | ||
| Male | 46 | 45.5 |
| Female | 55 | 54.5 |
| Education | ||
| Illiterate | 1 | 1.0 |
| Literate | 7 | 6.9 |
| Primary school | 1 | 1.0 |
| Secondary school | 1 | 1.0 |
| High school | 23 | 22.8 |
| University | 68 | 67.3 |
| Occupation | ||
| Blue collar employee | 23 | 22.8 |
| White collar employee | 21 | 20.8 |
| Self-employer | 10 | 9.9 |
| Retired | 7 | 6.9 |
| Housewife | 12 | 11.9 |
| Unemployed | 2 | 2.0 |
| Other | 26 | 25.7 |
| Monthly income (TRY) | ||
| 500 or less | 19 | 19.8 |
| 501-1000 | 31 | 30.7 |
| 1001-2000 | 41 | 40.6 |
| 2001-3000 | 8 | 7.9 |
| 3001-5000 | 1 | 1 |
| 5000 or higher | 1 | 1 |
| Social reimbursement | ||
| Yes | 93 | 92.1 |
| No | 8 | 7.9 |
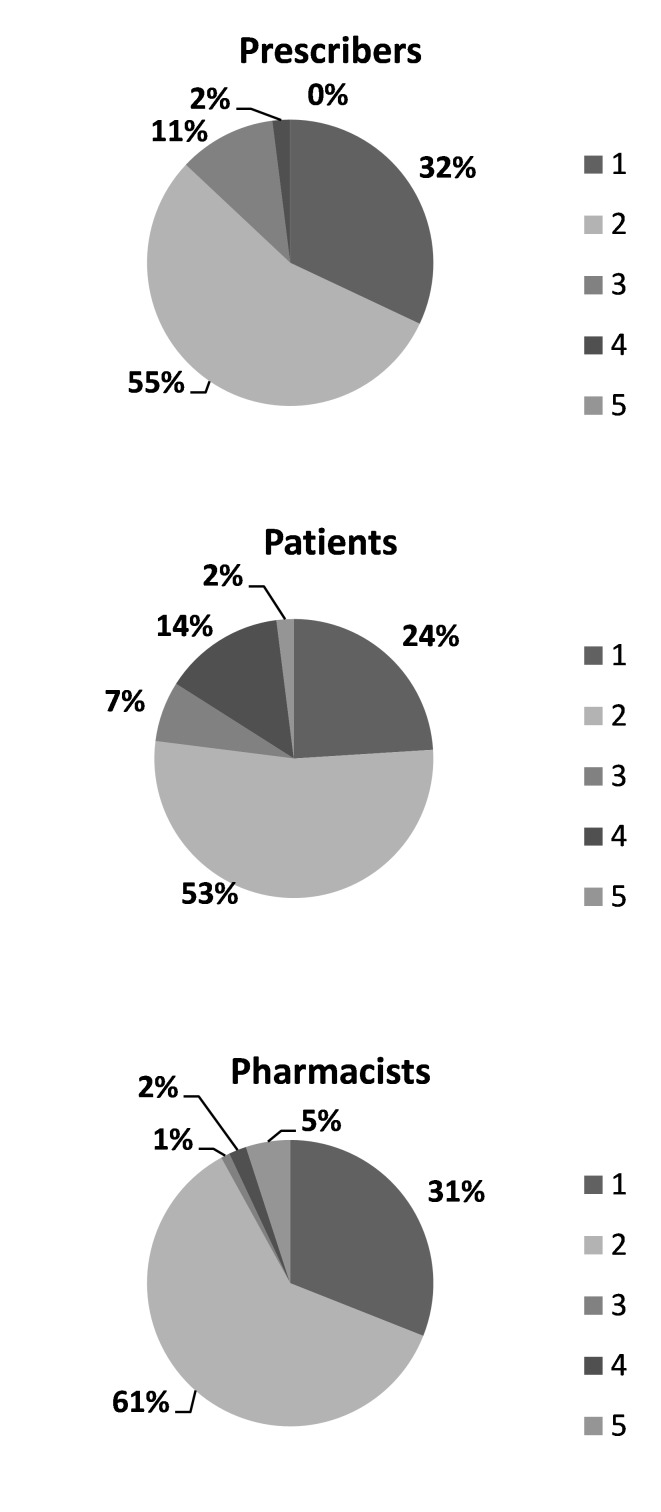
Thirty one and 32% of the pharmacists and prescribers respectively stated that they believed that the generic drugs did not differ from the original, whereas only 24% of the patients believed so (Figure 1). Forty percent of the pharmacists and 82% of the prescribers stated that they were unsure about the bioequivalence of the generic drugs (Figure 2). Three percent of the pharmacists and 9% of the prescribers claimed that they had never recommended generic drug. The prescribers and pharmacists knowledge and attitude towards generic substitution was not found to be related with their sex, age or professional experience.
Figure 1.
The statements of the prescribers, pharmacists and patients for comparing reference and generic in terms of efficacy. Items:
1: Generics do not differ from the originals
2: Some generics differ from the originals
3: Generics for sure differ from the originals
4: No idea
5: Other
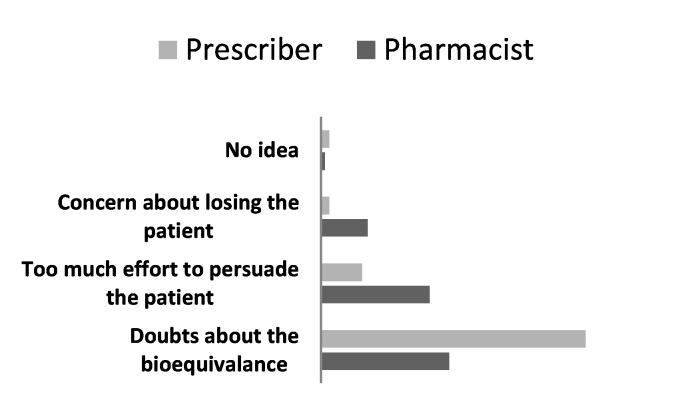
Figure 2.
The statements of the pharmacists and prescribers about the problems in switching a generic (%)
Prescribers and pharmacists opinions about the process of generic substitution are given in Table 3. More than half of the pharmacists stated that they may substitute a generic themselves, while almost half of the prescribers believed that a pharmacist should substitute after asking the prescriber and 88% complained about pharmacists' not consulting them about generic substitution. On the other hand, 55% expressed that generic substitution is one of the tasks of the pharmacists (Table 4).
Table 3.
Statements of pharmacist and prescribers about the process of generic substitution
| Prescribers | Pharmacists | |||
|---|---|---|---|---|
| N | % | N | % | |
| Pharmacist may substitute a generic on his own | 6 | 12 | 36 | 55 |
| Pharmacist should ask the prescriber for generic substitution of all drugs | 23 | 46 | 4 | 6 |
| Pharmacist may decide generic substitution for some of the drugs | 8 | 16 | 23 | 35 |
| I don’t agree generic substitution by the pharmacist | 13 | 26 | 2 | 3 |
| No idea | - | - | 1 | 1 |
| TOTAL | 50 | 100 | 66 | 100 |
Table 4.
Prescribers' opinions about the tasks of the pharmacists
| Agree/ strongly agree N (%) |
No idea N (%) |
Disagree/ strongly disagree N (%) |
Total N (%) |
|
|---|---|---|---|---|
| Ordering from the drug holder | 29 (54) | 7 (13) | 19 (35) | 55 (100) |
| Dispensing | 28 (51) | 4 (7) | 23 (42) | 55 (100) |
| Compounding | 48 (85) | 5 (9) | 3 (6) | 56 (100) |
| Deciding on a generic substitution | 30 (55) | 5 (9) | 20 (36) | 55 (100) |
| Informing the patient about instructions for use | 54 (96) | 1 (2) | 1 (2) | 56 (100) |
| Informing the patient about drug effects | 42 (75) | 0 (0) | 14 (25) | 56 (100) |
| Informing the patient about side effects | 41 (73) | 0 (0) | 15 (27) | 56 (100) |
| Informing the patient about herb, drug, food interactions | 41 (73) | 3 (5) | 12 (22) | 56 (100) |
| Warning the patients about contrindications | 45 (82) | 2 (4) | 8 (4) | 56 (100) |
| Filling out the social reimbursement forms | 23 (41) | 14 (25) | 19 (34) | 56 (100) |
| Monitoring the patients | 27 (49) | 13 (24) | 15 (27) | 56 (100) |
Pharmacists' rationale for not consulting a prescriber about generic substitution were as follows: Difficulty in getting in contact with the prescriber (47%), lack of time (7%), did not feel the need to consult the prescriber (27%), risk of being refused by the prescriber (10%) and others (9%).
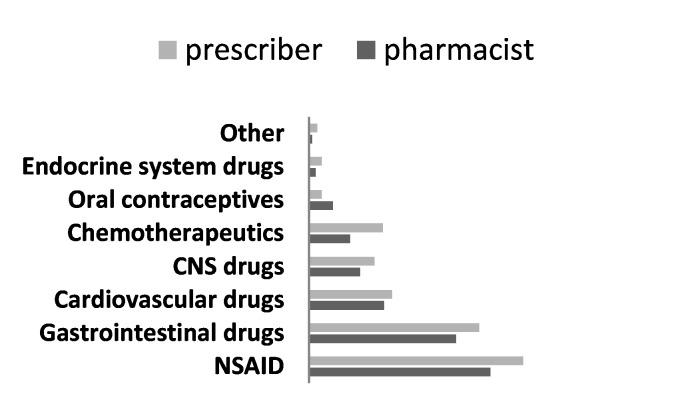
According to the Pharmacists' report, frequency of prescribing as an active ingredient was as given in Figure 3.
Figure 3.
Pharmacists' claim about the incidence of prescriptions by active ingredient (international non-proprietary name) of the drug (n=68)
Pharmacists and prescribers also stated that their approach for generic substitution differed in regard with the pharmacological group (Figure 4).
Figure 4.
Pharmacists (n=65) and prescribers (n=51) approval about generic substitution in some drug groups
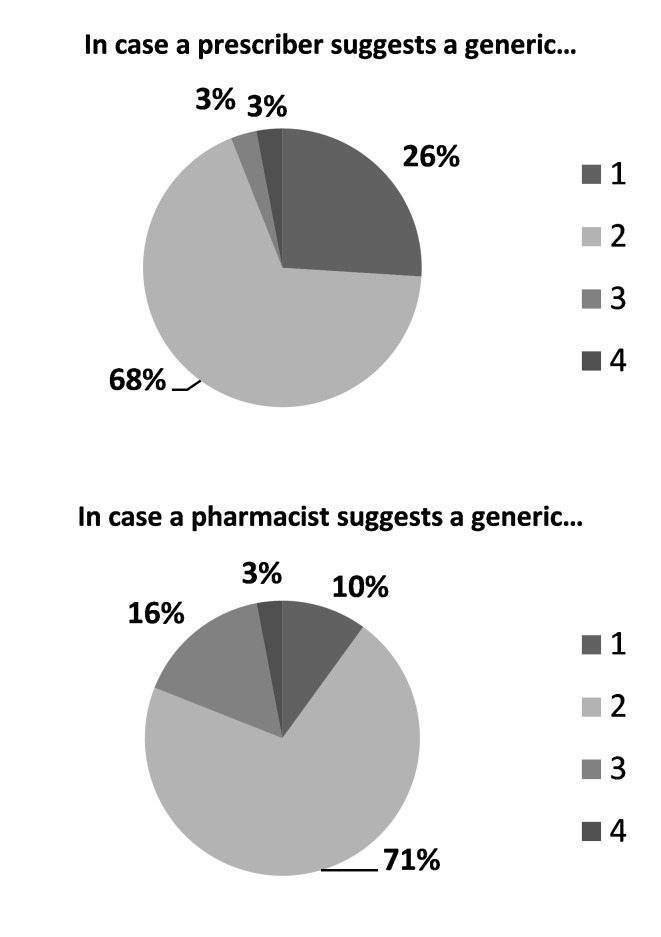
Nineteen percent of the patients told that generic substitution should be initiated by the first prescription, while 12% thinks it is relevant for a prescription refill. On the other hand, pharmacists’ opinions were as follows: Generic substitution may be performed in new prescriptions 30%, prescription refills 13%, in any prescription 33%, other 12%, no idea 3%. Ten percent of the patients claimed that they immediately accept generic substitution by the pharmacist, while 26% accepted it, if it was substituted by the prescriber (Figure 5).
Figure 5.
The statements of the patients about accepting a generic. Items:
1: I directly accept
2: I accept after persuation
3: I strictly don't accept
4: No idea
Their acceptance of generic drugs was not related to their sex, age or income, but it was significantly correlated with their degree of education. There was a negative relationship between the number of patients accepting the generic substitution and their education levels. The more educated the patients are, the less they accept generic substitution.
Almost half (45%) of the patients told that generics are surely cheaper than the originals, while 19% believed there was no difference in price. Cost was the most important factor taken into consideration about generic substitution (92% for prescribers; 83% for patients and 82% for pharmacists) (Table 5).
Table 5.
What issues do you take into consideration while preferring the generics?
| Pharmacist | Prescriber | Patient | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Agree/ strongly agree N (%) |
Unsure/ No idea N (%) |
Disagree/ strongly disagree N (%) |
Agree/ strongly agree N (%) |
Unsure/ No idea N (%) |
Disagree/ strongly disagree N (%) |
Agree/ strongly agree N (%) |
Unsure/ No idea N (%) |
Disagree/ strongly disagree N (%) |
|
| Manifacturer | 46 (82.1) | 5 (8,9) | 5 (8.9) | 28 (70) | 4 (10) | 8 (20) | 57 (56.4) | 7 (7) | 37 (36.6) |
| Place of production | 26 (49.1) | 10 (18.9) | 17 (32) | 20 (51) | 3 (8) | 16 (41) | 39 (38.6) | 12 (11.9) | 50 (49.5) |
| Cost | 45 (81.8) | 4 (7.3) | 6 (10.9) | 36 (92) | - (0) | 3 (8) | 84 (83.2) | 3 (3) | 14 (13.8) |
| Efficacy | 53 (96.4) | - | 2 (3.6) | 37 (92) | 1 (3) | 2 (5) | 92 (91.1) | 1 (1) | 8 (7.9) |
| Side effects | 41 (82.0) | 5 (10.0) | 3 (8.0) | 36 (90) | - (0) | 4 (10) | 90 (89.1) | 1 (1) | 10 (9.9) |
| Physical properties (taste, odour, colour, pharmaceutical form etc) | 30 (61.3) | 4 (8.2) | 15 (30.6) | 27 (68) | 4 (10) | 9 (22) | 62 (61.4) | 9 (8.9) | 30 (29.7) |
| Date of launching in the market | 27 (51.9) | 8 (15.4) | 17 (32.7) | 19 (50) | 7 (18) | 12 (32) | 58 (57.4) | 11 (10.9) | 32 (31.7) |
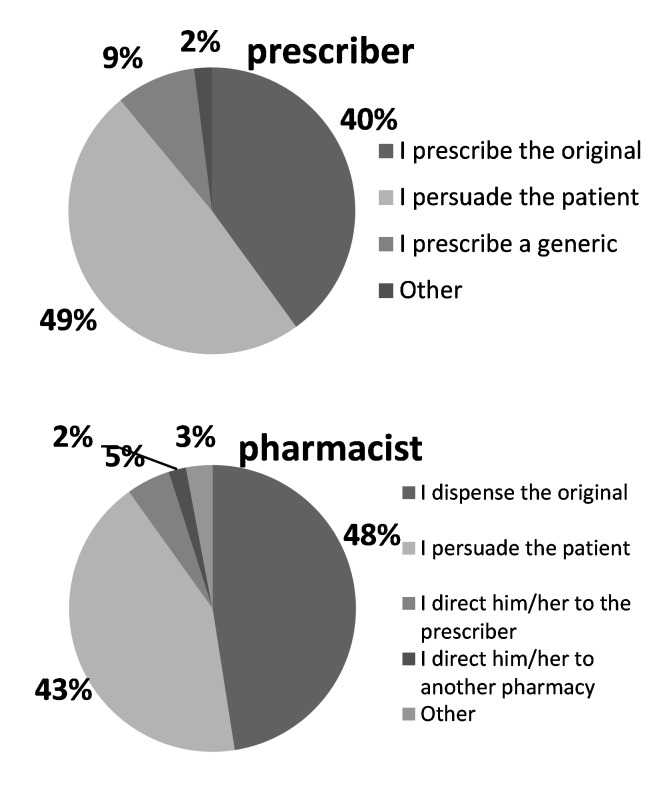
Thirty six percent stated that if the prescribed drug was not available in the pharmacy, they would prefer to go to another store. If the patient refuses to take the generic, twenty six percent told that they can be persuaded it by the pharmacist, while 17% told that they would ask the prescriber before accepting pharmacists substitution. Twenty percent told that they would ask for the original and wait for it to be ordered from the drug holder, if necessary. Pharmacists and prescribers approach towards the patient who does not accept generic drugs is shown in Figure 6.
Figure 6.
The prescribers and pharmacists approach in case a patient refuses a generic.
According to the pharmacists, the role of the prescribers and pharmacists for promoting generic substitution is essential (97% and 91% respectively), while it was 94% and 77% according to the prescribers.
Discussion
The use of generics has increased significantly in the last two decades. Since generics are available at a lower cost, they provide an opportunity for savings in health care expenditure. Therefore, use of generic drugs is encouraged especially in developing countries.
Since physicians and pharmacists have a key role in prescribing and dispensing generics, respectively, the effectiveness of policies targeting prescribing and dispensing behavior is disputable. Although generic prescribing by physicians, in principle, is promoted in most countries, the extent to which this is put to practice varies significantly as do monitoring and prescribing audit by health insurance companies.21
There are only a few studies concerning the perceptions and attitudes of the health workers and patients towards generic drug use. Thus, in this study, we aimed to evaluate the knowledge and attitude of pharmacists, prescribers and patients about generics. The main limitation of this study was the low response rate. However, the present study gives a sectional view to generics and generic substitution in Turkey. In a previous study in Istanbul regarding the dispensing practice of the community pharmacists, prescribed drug was replaced by an equivalent after informing the patient (5%) or without informing the patient (6%). The innovators product was dispensed in 81% of the pharmacies.22
According to the present study one third of the pharmacists and prescribers stated that they believed that the generics did not differ from the original, whereas only one fifth of the patients believed so. Moreover, almost half of the pharmacists and 82% prescribers told that they were unsure about the bioequivalence of the generics. Our results also demonstrated that the prescribers and pharmacists knowledge and attitude towards generic substitution was not related with their sex, age or professional experience.
Kobayashi et al, (2011) conducted a study with 1253 pharmacists, and observed that the majority was in favor of dispensing generics, but they stated that they would carefully decide if it is appropriate.23 On the other hand, they indicated four obstacles preventing them from performing generic substitution: 1) the generic drug may not be in stock or no generic equivalent is available yet in the market, 2) only a very small cost savings resulting in patients' objections, 3) physicians' objections and 4) presence of skepticism in the quality of generic medicines and inadequate drug information from generic manufacturers.23
In a Japanese study of 1215 respondents, patients stated the main reasons for accepting generic substitutions were recommendations by physicians (49%) and by pharmacists (33%).18 Relatively less than the Japanese study, 10% of the Turkish patients claimed that they immediately accept generic substitution given by the pharmacist, while 26% accepted if it was substituted by the prescriber. Patients, who refused the generics, commented that their doubts about generic substitution were due to their refrainment about being an instrument to the pharmacists' or prescribers' commercial concerns. Surprisingly, cost seemed to be a relatively less important factor for them in switching to a generic. Almost half of the patients thought that the generics are cheaper than the originals and 24% believed generics do not differ from the originals in terms of drug effects. The efficacy was the most important factor determining the outcome in our study. In Germany 37% of the patients believed generics were less effective for they were cheaper than the branded drugs.24 In Norway it was observed that half of the patients used a generic at least once a lifetime and patients using multiple drugs preferred generics more. On the other hand, 1/3 of the patients reported negative experience by generic substitution. The experiences of the patients were not related to age, gender, or number of medications or information about generics from either the pharmacy or the physician. About 41% of the patients told they would not switch if they had no personal economic incentives.8
Also in the US, patients agreed that generics are less expensive and a better value than brand-name drugs, and are just as safe. However, although 56% reported that Americans should use more generics, only 37.6% preferred to take generics themselves. Similarly we found that 38% would prefer generics, whereas 70% told generics were as good as the branded ones. Almost two-thirds (67%) of respondents reported that they were comfortable asking their physician to substitute a generic for a branded medication, and 61 percent reported that they were comfortable asking their pharmacist. Almost 60% agreed with the statement, "I don’t mind when my pharmacist switches my prescription to a generic medication" while 31% disagreed.25
Consistent with a previous study26, we have also observed that level of education effects attitude towards generics. The more educated the patients are, the less they accept generic substitution. In another study conducted in US, disease condition was shown to be another factor. In patients' opinions, generic substitution of cardiovascular drugs were found to be 3 times more risky than substitution of antitussives.27
In Malaysia, half of the pharmacists agreed that all products that are approved as generic equivalents can be considered therapeutically equivalent with the innovator medicines. About 76% of respondents indicated that generic substitution of medicines with a narrow therapeutic index is inappropriate. About one fifth of respondents thought that generic medicines are of inferior quality compared to the innovator medicines. However, most of the pharmacists (62%) disagreed that generics produce more side-effects than the innovator brand.17
In our study majority (73%) of the pharmacists stated that they need to consult the prescriber for generic substitution especially for certain pharmacological groups, and 88 % of prescribers believed that pharmacists should consult them before dispensing a generic. Also one third of the pharmacists stated that "pharmacists can substitute for certain drug groups"; whereas 73% of Malaysian pharmacists believed so.17 Consistent in two studies, the leading rationale for not consulting the physician was difficulty in getting in contact.
In the study from Saudi Arabia with 772 physicians 96% reported that they knew enough about the therapeutic value of generics. The majority reported that they were aware about the price differences, and this knowledge helped them to switch to generic prescription medication. Most physicians supported generic substitution, but they indicated that there are certain clinical situations where they preferred to use branded drugs. Physicians did not report a significant difference in pressure from patients to prescribe either generic or brand drugs. Most physicians had a positive attitude towards the government's role in assuring the quality of local drug products and in enforcing physicians to prescribe generics.28
In Jamaica, almost half of the physicians mostly prescribed generic brands willingly because lower cost was a significant factor. There were doubts about whether bioequivalence of a generic was equitable to therapeutic equivalence to innovator drug. Additionally, 33% of the physicians were able to identify at least one case in the past year, of clinical problems with generic substitutes that they perceived would not have occurred with the innovators product.29
In the review of Kirking et al, (2001), it was concluded that both community and hospital pharmacists generally support generic drug use.30 However, they continue to have concerns about generic substitution of medications in certain classes, such as those with a narrow therapeutic index. Pharmacists also have concerns about pressure from managed care to mandate generic substitution. The perceived influence of patient preference is less significant. After the decision is made to dispense a generic product, the selection of a supplier is driven less by the price than by the confidence pharmacists have in the supplier, while product quality is given as a key determinant of the specific product chosen.30
Conclusions
Findings of this study reveal the doubts about the generics due to the insufficient information. Therefore, healthcare providers as well as the public should be better educated about generic drugs. Health authorities and reimbursement institutes should cooperate with pharmacists and prescribers' medical associations for educational programs and promoting the more rational use of generic drugs.
Acknowledgments
The present study was the graduation project of Hacı Mustafa Gannemoğlu, Aslıhan Yetim and Haşim Güneş. The authors are grateful to Prof. Sevim Rollas, the Dean of Marmara University School of Pharmacy for her valuable support. Also the authors would like to thank Binnur Erdem and Çiğdem Engin from the Local Health Authority for their contributions in the procedure of getting legal permissions.
Footnotes
Competing interests: None.
Contributor Information
Hale Z. Toklu, Department of Pharmacology, School of Pharmacy, Marmara University. Istanbul (Turkey).
Gül A. Dülger, Department of Pharmacology, School of Pharmacy, Marmara University. Istanbul (Turkey).
Seyhan Hıdıroğlu, Department of Public Health, School of Medicine, Marmara University. Istanbul (Turkey)..
Ahmet Akici, Department of Pharmacology and Clinical Pharmacology, School of Medicine, Marmara University. Istanbul (Turkey)..
Aslıhan Yetim, School of Pharmacy, Marmara University. Istanbul (Turkey)..
H. Mustafa Gannemoğlu, School of Pharmacy, Marmara University. Istanbul (Turkey)..
Haşim Güneş, School of Pharmacy, Marmara University. Istanbul (Turkey)..
References
- 1.Ministry of Health of Turkey, General Directorate of Pharmaceuticals and Pharmacy Regulation on the Registration of Medicinal Products for Human Use. Official Gazette #25705 of 19.01.2005 and last amended published by Official Gazette # 27208 of 22.April.2009
- 2.Weekes L, Ramzan I. Generic medicines--how confident should we be? Pac Health Dialog. 2010;16:109–111. [PubMed] [Google Scholar]
- 3.Godman B, Shrank W, Andersen M, Berg C, Bishop I, Burkhardt T, Garuoliene K, Herholz H, Joppi R, Kalaba M, Laius O, McGinn D, Samaluk V, Sermet C, Schwabe U, Teixeira I, Tilson L, Tulunay FC, Vlahović-Palčevski V, Wendykowska K, Wettermark B, Zara C, Gustafsson LL. Comparing policies to enhance prescribing efficiency in Europe through increasing generic utilization: changes seen and global implications. Expert Rev Pharmacoecon Outcomes Res. 2010;10:707–722. doi: 10.1586/erp.10.72. [DOI] [PubMed] [Google Scholar]
- 4.Hassali MA, Shafie AA, Jamshed S, Ibrahim MI, Awaisu A. Consumers' views on generic medicines: a review of the literature. Int J Pharm Pract. 2009;17:79–88. [PubMed] [Google Scholar]
- 5.García Rada A. Mandatory generic prescribing is expected to save Spain €2bn a year. BMJ. 2011;343:d4803 [Google Scholar]
- 6.Tsiantou V, Zavras D, Kousoulakou H, Geitona M, Kyriopoulos J. Generic medicines: Greek physicians' perceptions and prescribing practices. J Clin Pharm Ther. 2009;34:547–554. doi: 10.1111/j.1365-2710.2009.01037.x. [DOI] [PubMed] [Google Scholar]
- 7.Kersnik J, Peklar J. Attitudes of Slovene general practitioners towards generic drug prescribing and comparison with international studies. J Clin Pharm Ther. 2006;31:577–583. doi: 10.1111/j.1365-2710.2006.00776.x. [DOI] [PubMed] [Google Scholar]
- 8.Kjoenniksen I, Lindbaek M, Granas AG. Patients' attitudes towards and experiences of generic drug substitution in Norway. Pharm World Sci. 2006;28:284–289. doi: 10.1007/s11096-006-9043-5. [DOI] [PubMed] [Google Scholar]
- 9.Suh DC. Trends of generic substitution in community pharmacies. Pharm World Sci. 1999;21:260–265. doi: 10.1023/a:1008781619011. [DOI] [PubMed] [Google Scholar]
- 10.Spiegel W, Mlczoch-Czerny MT, Jens R, Dowrick C. Quality circles for pharmacotherapy to modify general practitioners' prescribing behaviour for generic drugs. J Eval Clin Pract. 2012;18:828–834. doi: 10.1111/j.1365-2753.2011.01684.x. [DOI] [PubMed] [Google Scholar]
- 11.Decollogny A, Eggli Y, Halfon P, Lufkin TM. Determinants of generic drug substitution in Switzerland. BMC Health Serv Res. 2011;11:17. doi: 10.1186/1472-6963-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babar ZU, Grover P, Stewart J, Hogg M, Short L, Seo HG, Rew A. Evaluating pharmacists' views, knowledge, and perception regarding generic medicines in New Zealand. Res Social Adm Pharm. 2011;7:294–305. doi: 10.1016/j.sapharm.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Granlund D. Are private physicians more likely to veto generic substitution of prescribed pharmaceuticals? Soc Sci Med. 2009;69:1643–1650. doi: 10.1016/j.socscimed.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Andersson K, Sonesson C, Petzold M, Carlsten A, Lönnroth K. What are the obstacles to generic substitution? An assessment of the behaviour of prescribers, patients and pharmacies during the first year of generic substitution in Sweden. Pharmacoepidemiol Drug Saf. 2005;14:341–348. doi: 10.1002/pds.1055. [DOI] [PubMed] [Google Scholar]
- 15.Heikkilä R, Mäntyselkä P, Hartikainen-Herranen K, Ahonen R. Customers' and physicians' opinions of and experiences with generic substitution during the first year in Finland. Health Policy. 2007;82:366–374. doi: 10.1016/j.healthpol.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Country Report Turkey. Pharmaceutical Market Europe 2011; 55-61 Available from: http://www.imshealth.com/ims/Global/Content/Insights/Featured%20Topics/Emerging%20Markets/ICG_Turkey_Article1.pdf (Accessed: 31.July.2012)
- 17.Chong CP, Hassali MA, Bahari MB, Shafie AA. Exploring community pharmacists' views on generic medicines: a nationwide study from Malaysia. Int J Clin Pharm. 2011;33:124–131. doi: 10.1007/s11096-010-9470-1. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi E, Karigome H, Sakurada T, Satoh N, Ueda S. Patients' attitudes towards generic drug substitution in Japan. Health Policy. 2011;99:60–65. doi: 10.1016/j.healthpol.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Ministry of Health of Turkey, General Directorate of Pharmaceuticals and Pharmacy Regulation on the Evaluation of Pharmaceutical Products for Bioavailability and Bioequivalence. Official Gazette #21942 of 27May1994
- 20.Daşdemir S, Mercan S, Balta E Özcan V. Eşdeğer İlaç Türk Eczacıları Birliği Eşdeğer İlaçlar Raporu, 2009 Available in:http://www.teb.org.tr/images/upld2/rapor/Mai20090515141252ESDEGERILACRAPORU.pdf (Accessed: 31.July.2012)
- 21.Kanavos P. Generic policies: rhetoric vs reality. Euro Observer. The Health Policy Bulletin. 2008;10:1–6. [Google Scholar]
- 22.Toklu HZ, Akici A, Oktay S, Cali S, Sezen SF, Keyer Uysal M. The pharmacy practice of community pharmacists in Turkey. http://www.marmarapharmaceuticaljournal.com/pdf/pdf_MPJ_242.pdf Marmara Pharm J. 2010;14:53–60. [Google Scholar]
- 23.Kobayashi E, Satoh N, Uedain S. Community pharmacists’ perspectives on generic substitution in Japan. Journal of Public Health. 2011;19:249–256. [Google Scholar]
- 24.Himmel W, Simmenroth-Nayda A, Neibling W, Ledig T, Jansen RD, Kochen MM, Gleiter CH, Hummers-Pradier E. What do primary care patients think about generic drugs? Int J Clin Pharmacol Ther. 2005;43:472–479. doi: 10.5414/cpp43472. [DOI] [PubMed] [Google Scholar]
- 25.Shrank WH, Cox ER, Fischer MA, Mehta J, Choudhry NK. Patients’ perceptions of generic medications. Health Aff (Millwood) 2009;28:546–556. doi: 10.1377/hlthaff.28.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lebanova H, Manolov D, Getov I. Patients' attitude about generics –Bulgarian perspective. Marmara Pharm J. 2012;16:36–40. [Google Scholar]
- 27.Ganther JM, Kreling DH. Consumer perceptions of risk and required cost savings for generic prescription drugs. J Am Pharm Assoc. 2000;40:378–383. doi: 10.1016/s1086-5802(16)31086-5. [DOI] [PubMed] [Google Scholar]
- 28.Alghasham AA. Generic drug prescribing in central Saudi Arabia: perceptions and attitudes of physicians. Ann Saudi Med. 2009;29:24–29. doi: 10.4103/0256-4947.51819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gossell-Williams M. Generic Substitutions: A 2005 Survey of the Acceptance and Perceptions of Physicians in Jamaica. West Indian Med J. 2007;56:458–463. [PubMed] [Google Scholar]
- 30.Kirking DM, Gaither CA, Ascione FJ, Welage LS. Pharmacists' Individual and Organizational Views on Generic Medications. J Am Pharm Assoc. 2001;41:723–728. [Google Scholar]