Abstract
Pharmacovigilance has not progressed well in India and the concept is still in its infancy. India rates below 1% in pharmacovigilance as against the world rate of 5%.
Objective
The aim of our study was to evaluate the knowledge, perception and practice of pharmacovigilance among registered community pharmacists in Hyderabad, India.
Methods
This was a prospective study to find out the knowledge, perception and practice of adverse drug reaction reporting among community pharmacists. It was conducted by a face to face questionnaire and the convenience factor of the pharmacist was taken into consideration.
Results
From the 650 questionnaire administered to community pharmacists, 347 (53.3%) were returned completely filled questionnaires. A number of 120 (34.6%) pharmacists could define the term ‘pharmacovigilance’ to an acceptable extent and 119 (34.3%) knew about the National Pharmacovigilance Programme in India. 96 (27.7%) had good knowledge, 36(10.4%) had fair knowledge and 215(61.9%) had poor knowledge about pharmacovigilance. We have found that 196 (56.5%) had good perception, 94(27.1%) had fair perception and 57(16.4%) had poor perception. Only 41(11.8%) pharmacists ever reported an ADR and the other never reported ADR. The majority of pharmacists 223(64.3%) felt that the AE is very simple and non-serious and hence did not report. Pharmacists have poor knowledge, good perception and negligibly low reporting rates.
Conclusions
Incorporation of ADR reporting concepts in education curriculum, training of pharmacists and voluntary participation of pharmacists in ADR reporting is very crucial in achieving the safety goals and safeguarding public health.
Keywords: Adverse Drug Reaction Reporting Systems, Pharmacists, Professional Practice, Attitude of Health Personnel, India
Introduction
As famous quotations state "Safety is not a gadget but a state of mind" and "Safety first is safety always". These quotations also apply to safety of drugs that we use in day to day life to treat different ailments and illnesses. The Thalidomide disaster in 1961 drew attention to the domain of adverse drug reaction monitoring and following further resolutions in 1966, 1967 and 1970. In 2005, the Berlin declaration on pharmacovigilance concluded that 'the systems for pharmacovigilance are not well organized and funded to serve patients and public optimally.1 Pharmacovigilance has gained significant importance with increased number of drug molecules entering the market and the increase in the number of drug recalls due to the involvement of high health risks. In several high risks profile incidents involving marketed pharmaceuticals have propelled the issues of patient safety and the adverse events to the regulatory attention.
Pharmacovigilance
According to World Health Organization (WHO) Pharmacovigilance defined as the "science and activities relating to the detection, assessment, understanding and prevention of the adverse effects (AE)", particularly long term and short term side effects of medicines or any other drug related problems.2
This plays a vital role in ensuring that the doctors together with the patients are provided with adequate safety information to make an educated decision when choosing a drug for treatment. The process of collection of such safety information about a drug normally begins in phase-I of the clinical trial before approval of the drug and continues after the approval. Additionally, several post-market safety studies are conducted, with many mandatory requirements by drug regulatory agencies around the world. Out of several methods of detecting ADRs, spontaneous reporting is the one that significantly contributed to the improved levels of pharmacovigilance in many countries.3 Pharmacovigilance is particularly concerned with adverse drug reactions, or ADRs, which are officially described as: "A response to a drug which is noxious and unintended and which occurs at doses that are normally used for the prophylaxis, diagnosis or therapy of disease or for modification of physiological function".4 ADRs are fourth to sixth leading cause of death among the hospitalized patients and occur in every 0.3 percent to 7 percent of hospital admissions.5
Pharmacovigilance in India: The general range of incidence of Adverse Drug Reactions (ADR) in India was anywhere between 10-20% and in fewer cases even increased to 70% and above. Also in the current scenario, clinical trials are conducted in a very stringent environment with fixed criteria and thorough monitoring. With such a mind wobbling numbers and discreteness in data, the need for an effective vigilance program has aroused. Pharmacovigilance has not progressed well in India and the concept is still in its infancy. India rates below 1% in pharmacovigilance as against the world rate of 5%. In India, the Drugs Control Department within the Ministry of Health & Family Welfare initiated the establishment of a nationwide network to build a comprehensive Pharmacovigilance data system in 2004. The National Pharmacovigilance Programme (NPP) of India is sponsored by the WHO and is funded by the World Bank. NPP in India is divided in a three tier structure into 2 zonal centers, 5 regional centers and 24 peripheral centers. There are two zonal centers which collate information from all over the country and send it to the Uppsala Monitoring Centre in Sweden.6
The role of the community pharmacist: The pharmacists' contribution will remain an important element in effective pharmacovigilance. Pharmacists have a central role in drug safety by contributing to the prevention, identification, documentation and reporting of ADRs. All healthcare providers have key roles to play in maintaining a balance between medicines’ benefits and risks. National drug monitoring programs throughout the world differ in their sources of participation in the reporting of ADRs by healthcare professionals. In contrast to Canada or the US, where the majority of the reports come from pharmacists, some countries, such as France, Ireland, Malaysia, New Zealand, the Nordic countries, and in the UK, have the largest contribution of ADR reports coming from physicians.7 In many developed countries like the Netherlands, community pharmacists play a significant role in ADR reporting.
Factors for underreporting: Underreporting of ADRs is a common phenomenon in spontaneous post-marketing surveillance programs. Underreporting may delay signal detection and cause underestimation of the size of a problem. Correcting the underreporting scenario is difficult as the extent is unknown and variable. Involvement of community pharmacists in ADR reporting is lowest. This may be due to sub-optimal level of knowledge about the drugs, lack of confidence and inapt professional approach. Our community pharmacists restrict themselves to mere dispensing of marketed preparation.8
In a huge populated country like India, access to drugs is very easy. Most of the people buy the medicines from local community pharmacies without consulting a physician for many illnesses as it is easy, less time consuming and economic. Hyderabad, the capital of Andhra Pradesh is one of the biggest cities in India with many pharmacy colleges and universities. There are thousands of community pharmacies which operate on private norms or as a part of corporate chains.
Methods
The study was a cross-sectional questionnaire-based study involving community pharmacists working in parts of Hyderabad. This was conducted by a face to face questionnaire administered to randomly selected, registered community pharmacists during the pride of Aug. 2011 to April 2012. The questionnaire was adapted from a similar study investigating the attitudes and practice of ADR reporting. It was designed to capture the following information contained five sections comprising of demographic data, questions on knowledge, perception, practice and reasons for underreporting. In knowledge part 9 questions, perception part having 8 questions, practice part have a yes/no question and the question to know the best ADR reference aid was also included. In the knowledge score of the community pharmacists, out of 9 questions, a score of 6 and above was considered as good, score of 3-5 was considered as fair and score of less than 3 was considered as poor. The answer 'yes' was scored 1, the answer 'no' was scored zero.
Data analysis
The filled questionnaires were analyzed as per the study objectives. The various parameters such as 'sex distribution', 'age distribution', ‘professional status’, 'educational qualifications', 'worksite', 'duration of service' and the 'knowledge, attitude and practice scores' were analyzed. The data obtained were entered in Microsoft excel spread sheet and were analyzed. SPSS version 12.0.1 was used to conduct the descriptive statistics.
Results
There are 650 community pharmacists were offered to participate in the study, around 347 pharmacist completely filled questionnaire and were selected for analysis. The response rate was around 53%. Most of the pharmacists completed and returned the questionnaire. However only 53.3 % (347) properly filled and could be analyzed. The demographics of the respondents, distribution by professional cadre and experience are shown in Table 1.
Table 1.
Demographic Data
| Categories | Total (%) |
|---|---|
| Gender | |
| Female | 114 (32.9%) |
| Male | 233 (67.1%) |
| Age | |
| 20-30 | 112 (32.2%) |
| 31-40 | 149 (43.0%) |
| 41-50 | 57 (16.4%) |
| 51-60 | 19 (5.5%) |
| Above 60 | 10 (2.9%) |
| Marital status | |
| Married | 239 (68.9%) |
| Unmarried | 108 (31.1%) |
| Pharmacy ownership | |
| Owned | 139 (40.1%) |
| Partnership | 23 (6.6%) |
| Employed | 185 (53.3%) |
| Qualification | |
| D.Pharm | 270 (77.8%) |
| B.Pharm | 71 (20.5%) |
| M.Pharm | 6 (1.7%) |
| Training status | |
| Trained | 48 (13.8%) |
| Untrained | 299 (86.2%) |
| Experience | |
| Less than 1 year | 164 (30%) |
| 1- 5 years | 102 (19%) |
| 6 - 10 years | 68 (12%) |
| Above 10 years | 72 (13%) |
Knowledge of Pharmacists on pharmacovigilance: In the knowledge score of the community pharmacists, out of 9 questions, each questions have one point, a score of 6 and above was considered as good, score of 3-5 was considered as fair and score of less than 3 was considered as poor. Out of the total respondents, 96 (27.7%) had good knowledge, 36 (10.4%) had fair knowledge and 215 (61.9%) had poor knowledge about pharmacovigilance. The knowledge of community pharmacists on pharmacovigilance was illustrated in Table 2. The educational qualification shows the significant difference.
Table 2.
Knowledge of community pharmacists in Pharmacovigilance
| Questions | Yes (%) | No (%) |
|---|---|---|
| 1. Can you define the term 'Pharmacovigilance' | 120(34.6) | 227(65.4) |
| 2. Are you aware of National Pharmacovigilance Program in India | 119(34.3) | 228(65.7) |
| 3. Do you know when to report and how to report an ADR | 113(32.6) | 234(67.4) |
| 4. Do you know where to obtain ADR form from | 114(32.9) | 233(67.1) |
| 5. Did you ever report an ADR and know where to report | 107(30.8) | 240(69.2) |
| 6. Do you report herbals, vaccines, blood products, biologicals | 48(13.8) | 299(86.2) |
| 7. Do you know that community Pharmacist is one of the responsible health care professionals to report ADRs | 223 (64.3) | 124 (35.7) |
| 8. Do you know the resources to be used when needed | 96(27.7) | 251(72.3) |
| 9. Do you know the reportability criteria for a valid report | 91(26.2) | 256(73.8) |
Perception of Pharmacist pertaining to pharmacovigilance: Out of the total respondents, 324(93.4%) stated that ADR reporting is an essential role of a community pharmacist. A majority of about 310 (89.3%) pharmacists felt that the pharmacovigilance aspects need to be included in their curriculum. 284 (81.8%) of the pharmacists agreed that their pharmacovigilance knowledge needs to be updated at regular intervals. Around 263 (75.8%) felt that the community pharmacist is usually the first point of contact for ADR reporting by the general public. Out of 347 pharmacists, 161(46.4%) were willing to practice pharmacovigilance if trained and 240 (69.2%) feel that ADR reporting will ultimately benefit the patient. When asked about whether ADR reporting must be made compulsory, 211 (60.8%) answered 'yes', 24(6.9%) answered 'no' and others 112 (32.3%) chooses not to respond. It was found that 196 (56.5%) felt ADR reporting needs to be involuntary to improve patient safety and to promote rational drug use and 151 (43.5%) perceived it only as a professional obligation as illustrated in Figure 1.
Figure 1.
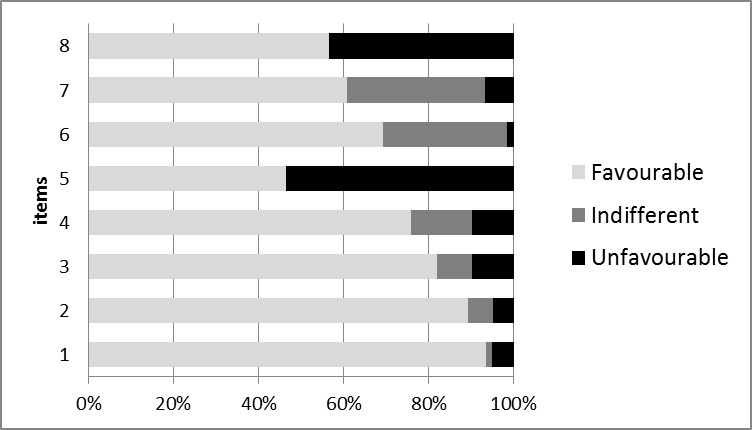
Figure 1. Perception of community pharmacists towards Pharmacovigilance. Items:
1. ADR reporting is an essential role of a community pharmacist
2. Pharmacovigilance needs to be included in curriculum
3. Pharmacovigilance knowledge to be regularly updated
4. Community Pharmacist usually first point of contact to report ADR
5. Will practice Pharmacovigilance if trained
6. ADR reporting & monitoring will benefit patient
7. ADR reporting must be made compulsory
8. ADR reporting needs to be involuntary/ professional obligation
Reporting of ADR among community pharmacists: Among the community pharmacists interviewed only 41 (11.8%) pharmacists showed interests of ADR reporting. However, majority of these 41 pharmacists reported only sporadically and did not depict consistency of reporting. Among these 41 pharmacists, only 14 (4.0%) reported ADRs in the last one month while 25 actually received ADR reports from the patients. Though efforts were made to obtain the number of reports sent in a one month time frame, pharmacists only answered on an average basis (0-6). It was found that 306 (88.2%) pharmacists did not show any interests in reporting. As this category was the majority, efforts were made to understand the reasons behind the underreporting and lack of interest in reporting.
About 14 (34.1%) of pharmacists preferred to send the ADR forms to CDSCO, 15 (36.5%) reported to National Pharmacovigilance Centers and 12 (29.3%) reported via other means. According to the results, 23 (56.1%) community pharmacists would prefer to fill it online, 7(17.1%) send them by physical mail, 1 (2.4%) inform by telephone, 6 (14.6%) hand over them directly and 4 (9.8%) opted for alternative means. The other means included filling the CDSCO, ADR form or their internal ADR forms and handing over to company sales and medical representatives. So majority of the pharmacists would prefer to report ADRs online as shown in Table 3. Out of the results obtained, 177 (51%) mentioned internet/ websites and 213 (61.4%) relied on drug information sheets/ package inserts which formed the major choice of drug references for ADRs as shown in Table 3.
Table 3.
Best / Preferred reference Aid
| Best / Preferred reference Aid | No (%) |
|---|---|
| Internet / Website | 177(51%) |
| Reference text books | 51(14.7%) |
| Medical journals / periodicals | 45(13.0%) |
| Medical / Sales representatives of Pharm. Companies | 82(23.6%) |
| Drug Information sheets/ leaflets | 213(61.4%) |
| Drug Information Centers | 9(2.6%) |
| Information bulletins by Ministry of Health | 13(3.7%) |
| Others | 79(22.8%) |
We found that 223 (64.3%) felt that the AE is very simple and non-serious and hence was not reported. And 144 (41.5%) felt that the AE is assumed to be already known, 113 (32.6%) felt it would affect business, waste of time and may cause differences with the physician. These formed the majority of the underreporting reasons. We noted that 106 (30.5%) felt it as an extra responsibility, 100 (28.8%) did not know how to do it and if pharmacists can do it, 98 (28.2%) were unsure about the causal relationship between the AE and the drug, 98 (28.2%) felt that the reporting procedure was too complex and time consuming. Proper training and introduction of pharmacovigilance concepts in their academic or on job curriculums would help to overcome this knowledge gap.
The other reasons included 84 (24.2%) ADR reporting is not needed in most of the cases, 77 (22.2%) mentioned that patients resisted them to report, 7 (22.2%) did not understand the importance and impact of reporting and 24 (6.9%) preferred not to report as they never got a response after submission. Among the reasons highlighted, all were equally important and interlinked and was found to have a significant impact on underreporting of ADRs.
Discussion
There are 650 community pharmacists who were offered to participate in the study around 347 pharmacists completely filled the questionnaire and were selected for analysis. The response rate was around 53%. In the knowledge score of the community pharmacists, out of 9 questions, a score of 6 and above was considered as good, score of 3-5 was considered as fair and score of less than 3 was considered as poor. The answer 'yes' was scored 1, the answer ‘no’ was scored zero. Out of the total respondents, 96 (27.7%) had good knowledge, 36 (10.4%) had fair knowledge and 215 (61.9%) had poor knowledge about pharmacovigilance. The pharmacists with poor knowledge were the majority which was similar to a studies.9,10,11,12,13
The knowledge and perception of pharmacists pertaining to pharmacovigilance had major impact on the practice of pharmacovigilance. So if pharmacists are trained, there would be a positive drive towards increase in reporting and thereby would help in maintaining the safety profiles of drugs. Out of eight perception questions, favorable responses of 5-8 were considered good; 1-4 were considered fair and -8 to 0 were considered poor. It was found that 196 (56.5%) of respondent pharmacists had good perception, 94 (27.1%) had fair perception and 57 (16.4%) had poor perception about pharmacovigilance. These results were similar to other studies.9,10,11,12,13
Practice of pharmacovigilance was found that community pharmacists majorly relied on drug information sheets/ package inserts which only record limited information and the next lot depended on internet and websites to update their ADR knowledge. This is a clear avenue where awareness needs to be spread amongst pharmacists to use genuine websites for drug related information. It also throws light on the responsibility of pharmaceutical companies to update the drug leaflets. When required keeping in mind the safety of public this was similar to other studies.,10,11,12,13
Underreporting of ADRs is a common phenomenon in spontaneous post-marketing surveillance programs. Underreporting may delay signal detection and cause underestimation of the size of a problem. Correcting the underreporting scenario is difficult as the extent is unknown and variable. Barriers to improved monitoring and reporting of ADRs have been analyzed in various studies and can be summarized as: fear of personal and organizational liability, lack of resources for surveillance and reporting, labor-intensive, complex, and time-consuming reporting processes, ambiguity in causal relationship between drug and adverse event, minimal feedback provided to reporters no incentives, rewards, or motivation to report, lack of knowledge and confidence to distinguish between significant ADRs and minor ones, Surveillance and reporting functions without guidance.14,15,16
Conclusions
Pharmacists have very little basic knowledge about pharmacovigilance. The concepts of pharmacovigilance should be incorporated in the curriculum of diploma as they form major portion of practicing pharmacists in community pharmacies. Periodic trainings should be held by pharmacy authorities to update reporting knowledge like ADR reporting form availability, reporting centres, modes and benefits of reporting etc., and must be made mandatory to all community pharmacists. The authorities must create awareness among all the community pharmacists about National pharmacovigilance Program (NPP) in India. More peripheral pharmacovigilance centers should be set up to increase the convenience of reporting. Eg.: In cities like Hyderabad. The pharmacists must be encouraged and constantly motivated ADR reporting becomes a voluntary responsibility. Community pharmacists should achieve and maintain a positive attitude towards pharmacovigilance as this is an essential role of a pharmacist. Incentives and other encouraging perks should be given to reporting pharmacists to keep them motivated and focused.
Footnotes
Competing interests: None declared.
Contributor Information
Arul Prakasam, Department of Pharmacy Practice, JKK Munirajah Medical Research Foundation, College of Pharmacy, B. Komarapalyam, Namakkal. Tamil Nadu (India)..
Anitha Nidamanuri, Department of Pharmacy Practice, JKK Munirajah Medical Research Foundation, College of Pharmacy, B. Komarapalyam, Namakkal. Tamil Nadu (India)..
Senthil Kumar, JKK Munirajak Medical Research Foundation, College of Pharmacy, B. Komarapalyam, Namakkal. Tamil Nadu (India)..
References
- 1.International Society of Drug Bulletins, Berlin Declaration on Pharmacovigilance, January, (2005), Available from http://www.isdbweb.org/documents/uploads/Declaration/Berlin_Declaration_Berlin%20Engl.pdf (Accessed on December. 2011).
- 2.World Health Organization. Safety of Medicines. A guide to detecting and reporting adverse drug reactions. Why health professionals need to take action. Geneva: World Health Organization; 2002. [Google Scholar]
- 3.www.fda.gov/consumer/updates/drugterms041108.html (Accessed on December. 2011)
- 4.Vallano A, Cereza G, Pedròs C, Agustí A, Danés I, Aguilera C, Arnau JM. Obstacles and solutions for spontaneous reporting of adverse drug reactions in the hospital. Br J Clin Pharmacol. 2005;60:653–658. doi: 10.1111/j.1365-2125.2005.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.www.Pharmacovigilance.co.in (Accessed on December. 2011)
- 6.The Learning Centre Continuing pharmacy education; fall 1999. Canada: University of British Columbia; 1999. Pharmacists are number one. [Google Scholar]
- 7.Bäckström M, Mjörndal T, Dahlqvist R, Nordkvist-Olsson T. Attitudes of reporting adverse drug reactions in Northern Sweden. Eur J Clin Pharmacol. 2000;56:729–732. doi: 10.1007/s002280000202. [DOI] [PubMed] [Google Scholar]
- 8.Amrita P, Roomi MT. Scenario of Pharmacovigilance and ADR Reporting among Pharmacists in Delhi. Indian Journal of Pharmacy Practice. 2011;4:29–38. [Google Scholar]
- 9.Oreagba IA, Ogunleye OJ, Olayemi SO. The knowledge, perceptions and practice of Pharmacovigilance amongst community pharmacists in Lagos state, south west Nigeria. Pharmacoepidemiol Drug Saf. 2011;20:30–35. doi: 10.1002/pds.2021. [DOI] [PubMed] [Google Scholar]
- 10.Green CF, Mottram DR, Raval D, Proudlove C, Randall C. Community pharmacists' attitude to adverse drug reaction reporting. Int J Pharm Pract. 1999;7:92–99. [Google Scholar]
- 11.Fadare JO, Enwere OO, Afolabi AO, Chedi BAZ, Musa A. Knowledge, Attitude and Practice of Adverse Drug Reaction Reporting among Healthcare Workers in a Tertiary Centre in Northern Nigeria. Trop J Pharm Res. 2011;10:235–242. [Google Scholar]
- 12.Madhan R, Parthasarathi G. Attitudes and perceptions of medical practitioners-Adverse drug reactions reporting. Asian Journal of pharmaceutical and Clinical Research. 2009;2:184–189. [Google Scholar]
- 13.Sweis D, Wong IC. A survey on factors that could affect adverse drug reaction reporting according to hospital pharmacists in Great Britain. Drug Saf. 2000;23:165–172. doi: 10.2165/00002018-200023020-00006. [DOI] [PubMed] [Google Scholar]
- 14.Gupta P, Udupa A. Adverse Drug Reaction Reporting and Pharmacovigilance: Knowledge, Attitudes and Perceptions amongst Resident Doctors. J Pharm Sci Res. 2011;3:1064–1069. [Google Scholar]
- 15.Eland IA, Belton KJ, van Grootheest AC, Meiners AP, Rawlins MD, Stricker BH. Attitudinal survey of voluntary reporting of adverse drug reactions. Br J Clin Pharmacol. 1999;48:623–627. doi: 10.1046/j.1365-2125.1999.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavaza P, Brown CM, Lawson KA, Rascati KL, Wilson JP, Steinhardt M. Influence of attitudes on pharmacists' intention to report serious adverse drug events to the Food and Drug Administration. Br J Clin Pharmacol. 2011;72:143–152. doi: 10.1111/j.1365-2125.2011.03944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


