Abstract
Tryptase(+) mast cells (MCs), abundant in the invasive front of tumours, contribute to tissue remodelling. Indeed, protease-activated receptor-2 (PAR-2) activation by MC-tryptase is considered an oncogenic event in colorectal cancer (CRC). Recently, we have suggested NHERF1 as a potential new marker in CRC. In this study, we aimed to determine the distribution of tryptase(+) MCs and PAR-2 and to examine the relationship between PAR-2 and NHERF1, investigating their reputed usefulness as tumour markers. We studied a cohort of 115 CRC specimens including primary cancer (C) and adjacent normal mucosa (NM) by immunohistochemical double staining, analyzing the protein expression of MC-tryptase, PAR-2 and cytoplasmic NHERF1. MC density was higher in NM than in C. Tumours with high TNM stage and poor grade showed the highest MC density. A higher PAR-2 immunoreactivity characterized tumours most infiltrated by MCs compared with samples with low MC density. Furthermore, PAR-2 overexpression was associated with advanced TNM stage, poor grade and lymphovascular invasion (LVI). A positive correlation existed between tryptase(+) MC density and PAR-2 expression. Cytoplasmic NHERF1 was higher in C than in NM and overexpressing tumours resulted associated with nodal and distant metastases, poor grade and LVI. PAR-2 correlated with cytoplasmic NHERF1 and the PAR-2(+)/cytoplasmic NHERF1(+) expression immunophenotype identified tumours associated with unfavourable prognosis and aggressive clinical parameters. Our data indicate that the high density of tryptase(+) MCs at invasive margins of tumours was associated with advanced stages of CRC and was strongly correlated with PAR-2 expression.
Keywords: Colorectal cancer, PAR-2, mast cell, tryptase, NHERF1, prognostic factor, invasiveness, aggressiveness
Introduction
Cancer-related inflammation is a key component of tumours and has been suggested as the seventh hallmark of cancer progression [1]. In fact, patients with inflammatory bowel disease, Crohn's disease and chronic ulcerative colitis have a much higher risk of CRC [2, 3]. MCs play a role as cancer promoters through various molecular and cellular mechanisms, including participation in immunosuppression, release of pro-angiogenic and mitogenic factors and remodelling of the tumour microenvironment [4–6]. MCs contribute to tissue remodelling through a rich number of proteases, including tryptase. Indeed, tryptase(+) MCs have been found to be abundant in the invasive front of tumours [7].
It is well-established that a subclass of proteases acts as signal molecules controlling cell functions through PARs, a subfamily of specific G protein–coupled receptors consisting of four members designated PAR1-4 [8]. In particular, PAR-2 is activated by multiple trypsin-like enzymes including trypsin and MC-tryptase, contributing to a range of normal and disease processes including cancer. Activation of PAR-2 is an oncogenic event in colon cancer [9] and it may facilitate the progression of CRC [10, 11]. MC-tryptase cleaves and activates PAR-2 on gut epithelial cells and myocytes [12, 13]. PAR-2 activity also stimulates proliferation of colon cancer cells and induces the expression of the prostaglandin-synthesizing enzyme COX2 and its metabolite prostaglandin-E2 [14]. Moreover, recent studies have shown that the PAR-2/trypsin system facilitates colorectal carcinogenesis by promoting cell proliferation, migration and invasion [15].
Signalling pathways are assembled into multi-protein signalling complexes by scaffolding proteins that permit the creation of new tumour-specific pathways to drive the subverted cellular functions exhibited by tumour cells [16]. The Na+/H+ exchanger regulating factor 1 (NHERF1, also named SLC9A3R1) is an adaptor protein that functions as a regulator of transmembrane receptors, transporters and other proteins localized at or near the plasma membrane [17] and is considered a new player in CRC progression [18]. Recently, we have suggested that nuclear NHERF1 could be a potential new marker, representing an early indicator of pre-morphological triggering of CRC carcinogenesis [19] and demonstrating an association with the hypoxic tumour microenvironment in nodal and liver metastases [20].
This study examined the distribution of tryptase(+) MCs and the expression of PAR-2 in primary tumours compared with adjacent uninvolved mucosa, in an attempt to understand their role in the progression of colonic cancer. Moreover, we examined protein expression of NHERF1 and its relationship with PAR-2, with the aim to investigate their supposed usefulness as tumour markers in CRC.
Materials and methods
Ethics statement
The study was approved by the Institutional Review Board of the National Cancer Research Centre ‘Giovanni Paolo II’, Bari, Italy. Before undergoing routine surgery, all patients were informed of the investigational nature of the study and provided their written informed consent, authorizing the Institute to utilize their removed biological tissue according to ethical standards.
Tissue specimens
A series of 115 colonic cases of pathologically confirmed primary adenocarcinoma Not Otherwise Specified (NOS) matched with adjacent cancer-uninvolved colonic mucosa was identified by a retrospective search of the surgical pathology files of the Pathology Department of our Institute.
Sections of 4-μm thickness were cut from formalin-fixed and paraffin-embedded histological blocks and for each sample, the cancer (C) and the paired NM present on the same slide were used for comparison. Histopathologic features of the colon carcinoma tissue specimens were confirmed by blinded review of the original pathology slides, stained with routine haematoxylin and eosin.
Disease was staged according to the tumour-node-metastasis (TNM) classification system of the American Joint Committee on Cancer [21]. The World Health Organization classification was used for pathologic grading [22]. Assessment of LVI was based on examination of sections stained with haematoxylin and eosin and was considered evident if at least one cohesive clump of tumour cells was clearly visible within peritumoural endothelial-lined spaces, both lymphatic channels and small blood vessels—closely associated with primary invasive carcinoma [23].
Information regarding patient characteristics, including age, gender, histological type, tumour site, depth of invasion, nodal status, distant metastases, differentiation grade, LVI, TNM stage and EGFR status, was collected from the Pathology Department of our Institute. The EGFR status was realized in accordance with the EGFR pharmDx™ scoring guidelines. Results were reported as positive when a complete or incomplete circumferential membrane staining was observed in at least 1% of the tumour cells. Staining was defined as immunostaining of cell membranes above background level and scored as follows: 1+=weak, 2+=moderate and 3+=strong. The absence of membrane staining or cytoplasmic staining was reported as negative. The percentage of stained cells was assessed as follows: 1–10, 10–50 and >50%. Slides were scored in a blind manner by two independent observers, who were blinded to the patients' characteristics. In the case of disagreement, the EGFR status was determined by consensus after simultaneous dual re-examination.
Immunohistochemistry and immunohistochemical double staining
Sections were immunohistochemically stained for NHERF1 using standard immunoperoxidase techniques, as previously described [24]. Briefly, sections were deparaffinized with xylene, rehydrated through a graded ethanol series and pre-treated with 0.01 M sodium citrate buffer at pH 6.0 in water bath. After endogenous peroxidase activity blocking with 0.3% H2O2 buffer solution, sections were incubated with a rabbit polyclonal EBP50 antibody for NHERF1 (clone PA1-090; Affinity Bioreagents, Golden, CO, USA; 1:150 dilution in PBS/BSA 1%) overnight at 4°C. Bound antibody was visualized with 3-amino-9-ethylcarbazole substrate-chromogen (DakoCytomation, Glostrup, Denmark) in the dark and counterstained with Mayer's haematoxylin. As a positive internal control, we used paraffin-embedded cells pellets from MCF-7 cell lines, expressing high levels of NHERF1. For negative controls, the primary antibody was omitted and replaced by PBS pH 7.6.
Every serial section adjacent to that stained for NHERF1 was submitted to immunohistochemical double staining, a procedure that combined the two markers with the aim to analyse both density of MCs and the expression of PAR-2. Following the epitope antigen retrieval with 0.01 M sodium citrate buffer at pH 6.0 in water bath, protein blocking was performed with Tris-buffered saline pH 7.6/Bovine Serum Albumin (BSA) 1% and then the slides were exposed overnight to a mixture of antibodies directed against human MC-tryptase (clone FL-275, rabbit polyclonal; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and PAR-2 (clone SAM11, mouse monoclonal; Santa Cruz Biotechnology Inc.; for both 1:150 dilution in Tris-buffered saline/BSA 1%). After tissue rinsing in Tris-buffered saline 1× wash buffer, sections were incubated with MACH 2 Double Stain (Polymer Detection Kit 2, mouse-Horseradish Peroxidase + rabbit-Alkaline Phosphatase; Biocare Medical LLC, Concord, CA, USA) and bound antibodies were visualized with 3,3′-diamino-benzidine (DAB Chromogen Kit; Biocare Medical LLC) and Vulcan Fast Red chromogens (Vulcan Fast Red Chromogen Kit 2; Biocare Medical LLC). Finally, the slides were counterstained with Mayer's haematoxylin and mounted with an aqueous mounting medium (DakoCytomation). As a result, the staining with human MC-tryptase was visualized as a bright fuchsin-red colour, whereas the immunostaining of PAR-2 appeared as brown. In each assay, a section of CRC noted on routine histological examination with extensive infiltration by MCs and with the presence of muscularis mucosae served as positive control for MC-tryptase and PAR-2 respectively. As a negative control, the primary antibody was replaced with Tris-buffered saline/BSA 1%.
Immunohistochemical analysis for MCs was based on the appearance of tryptase(+) MCs in the tissue stroma, as previously reported [25]. To evaluate MC density, the stained sections were screened under ×50 microscopic fields to identify representative fields. Then, MCs were counted in six to eight non-overlapping ×200 microscopic fields, covering both peripheral and central regions (core) of each section. Cells showing an equivocal staining or lacking a nucleus were not counted. MC density in every histological section was expressed as a median and interval of variation, which was used as cut-off to classify cases in low (≤median) and high (>median) MC density.
Immunohistochemical analysis for PAR-2 and NHERF1 was based on subcellular localization of analysed markers. For PAR-2 immunoreactivity, membranous/cytoplasmic localization was considered, whereas for NHERF1, the cytoplasmic localization was examined. As previously described [20], protein expression analysis was determined by multiplying the number of stained cells (0–100) by the intensity staining (0, no immunoreactivity; 1, weak; 2, moderate; 3, strong) in three to five representative areas for each section at ×200 magnification. The total score obtained (0–300) was therefore converted to a percentage of stained positive cells/section. Moreover, the median value of positive cells was used as a cut-off to group cases into two categories of negative (≤median) and positive (>median) expression. All stained samples were scored in a blind manner by two independent investigators who had no prior knowledge of the clinicopathological data. When a section was uninformative—either lost or contained no tumour tissue—a case was judged as ‘not evaluable’ in the statistical analysis. Photomicrographs were acquired under bright-field illumination with a Leica DMLB optical microscope (Leica, Cambridge, UK), coupled with a Leica DCC camera (Leica).
Immunohistofluorescence
Immunofluorescent analysis was performed as previously described [20]. Briefly, after antigen retrieval, tissues were incubated overnight at 4°C in a humidified chamber with the rabbit policlonal antibody anti-NHERF1 (clone PA1-090, 1:150 dilution; Affinity Bioreagents) and with the mouse monoclonal anti-PAR-2 (clone SAM11, 1:150 dilution; Santa Cruz Biotechnology Inc.). Slides were then incubated at room temperature for 1 hr with the Alexa Fluor 488 and Alexa Fluor 568 immunoglobulin G secondary conjugated antibodies (1:2000 dilution; Molecular Probes Inc., Eugene, OR, USA).
Images were obtained on a BX40 microscope (Olympus, Tokyo, Japan) with a SenSys 1401E-Photometrics charge–coupled device camera. To verify protein co-localization, each acquired stack was merged by transforming the two channels corresponding to red (tetramethylrhodamine B isothiocyanate) and green (fluorescein isothiocyanate) into a single two-colour stack using the ‘RGB merge’ command of ImageJ software (National Institutes of Health Bethesda, MD, USA).
Statistical analysis
The non-parametric Mann–Whitney U-test was used to compare expression levels of tumour markers considered within C and NM compartments. Association analysis between tumour markers and clinicopathological data was analysed using a χ2 or Fisher's exact tests. Correlation between two continuous variables was assessed by the non-parametric Spearman's rank test. All tests were two sided with a 95% CI and a P < 0.05 was considered statistically significant. Data analysis was carried out using the statistical package SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Patients
This study considered 115 patients, consisting of 66 men (57.4%) and 49 women (42.6%), with a median age of 66 years (range 40–89 years). Location of cancer was colon and rectum in 73 (64%) and 41 (36%) patients respectively. Sixty-two (62.6%) patients presented lymph node metastases at the time of diagnosis and 47 (45.6%) had synchronous distant metastases. Seven tumours (6%) were classified as well-differentiated, 57 (50%) as moderate and 50 (43.9%) as poorly differentiated. Based on the TNM stage classification, there were 18 (15.7%) stage I patients, 19 (16.5%) stage II patients, 27 (23.5%) stage III patients and 51 (44.3%) stage IV patients.
The clinicopathological characteristics of the 115 tumours analysed are summarized in Table 1.
Table 1.
Clinicopathological data and tumour marker expressions in 115 colorectal cancers
| Characteristics, n (%) | Total (n = 115) |
|---|---|
| Age at diagnosis, median (range) | 66 (40–89) |
| Gender | |
| Male | 66 (57.4) |
| Female | 49 (42.6) |
| Histological type | |
| Adenocarcinoma (NOS) | 115 (100.0) |
| Tumour site | |
| Colon | 73 (63.5) |
| Rectum | 42 (36.5) |
| Depth of invasion | |
| pT1 | 4 (3.5) |
| pT2 | 18 (15.7) |
| pT3 | 69 (60.0) |
| pT4 | 24 (20.9) |
| Nodal status | |
| pN0 | 43 (37.4) |
| pN1 | 32 (27.8) |
| pN2 | 37 (32.2) |
| pN3 | 3 (2.6) |
| Distant metastases | |
| M0 | 68 (59.1) |
| M1 | 47 (40.9) |
| Differentiation grade | |
| Well | 7 (6.1) |
| Moderate | 57 (50.0) |
| Poor | 50 (43.9) |
| LVI | |
| Absent | 60 (68.2) |
| Present | 28 (31.8) |
| TNM stage | |
| I | 18 (15.7) |
| II | 19 (16.5) |
| III | 27 (23.5) |
| IV | 51 (44.3) |
| EGFR | |
| Negative | 49 (73.1) |
| Positive | 18 (26.9) |
| MC density | |
| Low (≤56) | 59 (51.3) |
| High (>56) | 56 (48.7) |
| PAR-2 expression | |
| Negative (≤26) | 61 (53.0) |
| Positive (>26) | 54 (47.0) |
| Cytoplasmic NHERF1 expression | |
| Negative (≤44%) | 58 (50.4) |
| Positive (>44%) | 57 (49.6) |
Adenocarcinoma (NOS): adenocarcinoma Not Otherwise Specified; LVI: lymphovascular invasion; EGFR: epidermal growth factor receptor; MC density: density of tryptase(+) Mast Cell; PAR-2: Proteinase-Activated Receptor 2; NHERF1: Na+/H+ exchanger regulating factor 1.
Protein expression analysis of tryptase(+) MCs and PAR-2
We carried out an immunohistochemical double-staining assay to examine the distribution of mucosal MCs and to determine PAR-2 levels, obtaining bright fuchsine-red and brown-coloured precipitates at the antigen sites of cancer and corresponding normal mucosa compartments of the same colonic tumour respectively (Fig. 1A). Infiltration of MCs was already present in the uninvolved compartment of each sample, distributed in the lamina propria and submucosa (Fig. 1A, upper panel). Conversely, PAR-2 was lowly expressed or not detectable in epithelial cells of normal mucosa (Fig. 1A, upper panel).
Fig. 1.
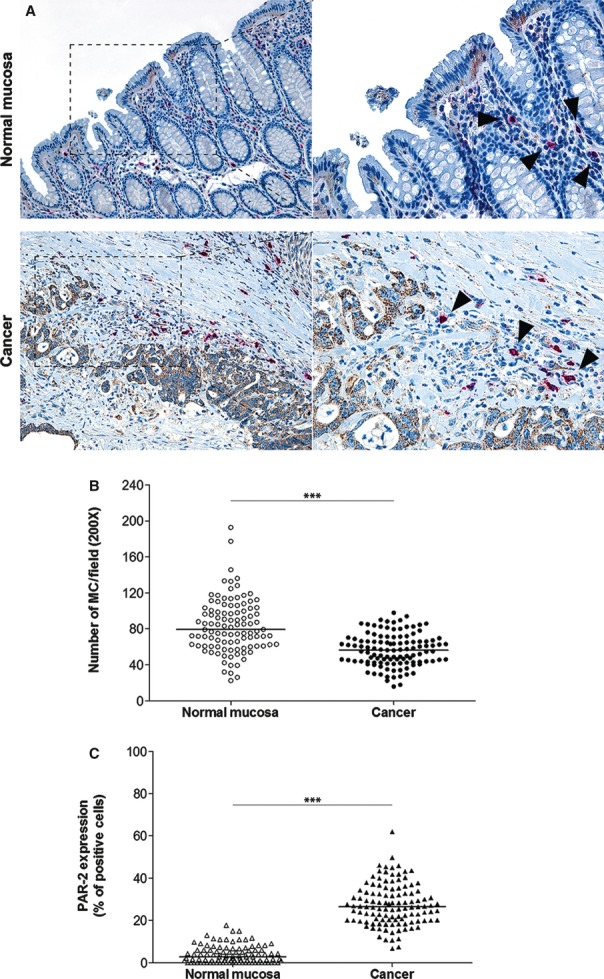
Expression analysis of tryptase-positive mast cells and PAR-2 in human colorectal cancer. (A) Representative images of tryptase(+) MCs (arrowheads) and PAR-2 immunoreactivity in primary tumours matched with adjacent cancer-uninvolved colonic mucosa by immunohistochemical double staining (original magnification on the left ×100, enlargement on the right ×200). (B and C) The MC density and the distribution of PAR-2(+) cells, respectively, on normal mucosa and the tumour compartment of the same colonic lesion. (Horizontal bold line in each box = median value; ***P < 0.0001).
In the tumour compartment, we observed MCs located mainly in the connective stroma, in the interface between growing cancer and healthy tissue and, frequently, in close association with small blood vessels within the tumour microenvironment. Interestingly, MCs were also present within the core of the invasive tumours and foci of microinvasion of the tumour mass (Fig. 1A, lower panel). In contrast, immunoreactivity for PAR-2 was detected both at the tumour centre and at the invasive front of the lesion. However, a higher intensity of staining could be detected along the invasion front and in most cases, along tumour borders adjacent to vascular structures (Fig. 1A, lower panel). In particular, PAR-2 was strongly expressed on cytoplasm or focally localized on membranous surface of epithelial tumour cells.
The distribution of MCs and PAR-2 immunoreactive epithelial cells in colonic tumours is summarized in Figure 1B and C respectively. The MC count significantly decreased passing from adjacent NM towards C tissue [median 79.15 (range 22.5–192.7) versus 56.3 (15.8–97.8), respectively; P < 0.0001, by Mann–Whitney test]. Moreover, a statistically higher median expression of PAR-2 was observed in C compared with NM [2.7 (0–17.7) versus 26.5 (6.7–62.0), respectively; P < 0.0001].
PAR-2 expression was evaluated both on the entire section and at the sites where MCs most intensively accumulated in serial sections (Fig. 2). Intriguingly, we noted a more distinct immunoreactivity (Fig. 2A) and a statistically higher median value of PAR-2 at the sites most infiltrated by MCs than in areas with low density of MCs [34.4 (7.7–78.4) versus 26.5 (6.7-62.0), respectively; P < 0.0001; Fig. 2B).
Fig. 2.
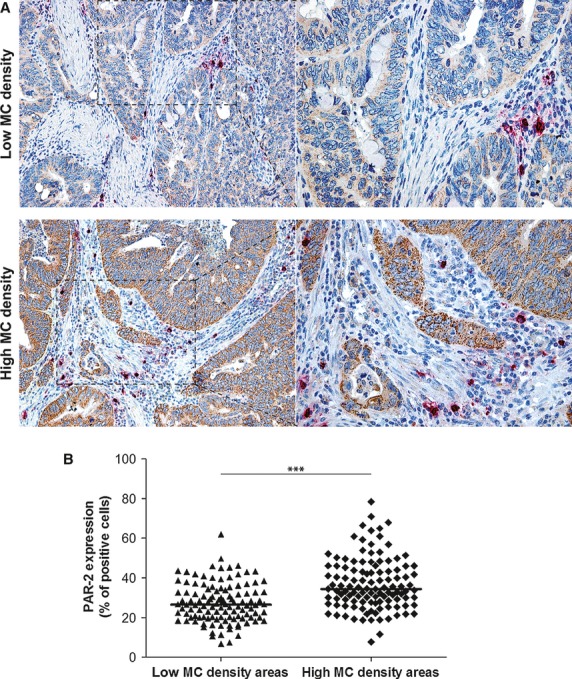
Comparison of PAR-2 expression in low and high mast cell density tumour areas. (A) Two representative images of PAR-2 immunoreactivity in colonic tumour areas with low and high MC counts by immunohistochemical double staining (original magnification on the left ×100, enlargement on the right ×200). (B) The distribution of PAR-2(+) cells, evaluated on the whole section with low MC density and at the sites where MCs most intensively accumulated in tumour lesion. (Horizontal bold line in each box = median value; ***P < 0.0001).
Protein expression analysis of NHERF1
In normal mucosa, NHERF1 expression was detected at the apical pole of the well-polarized duct, with a characteristic distribution along apical cell membranes of both enterocytes and goblet cells (Fig. 3A, upper panel). In the cells of all tumour samples, a variable cytoplasmic NHERF1 expression was detected. Positive cells had no preferential distribution. In fact, they could be observed either along the invasion front or within the growing tumour mass (Fig. 3A, lower panel).
Fig. 3.
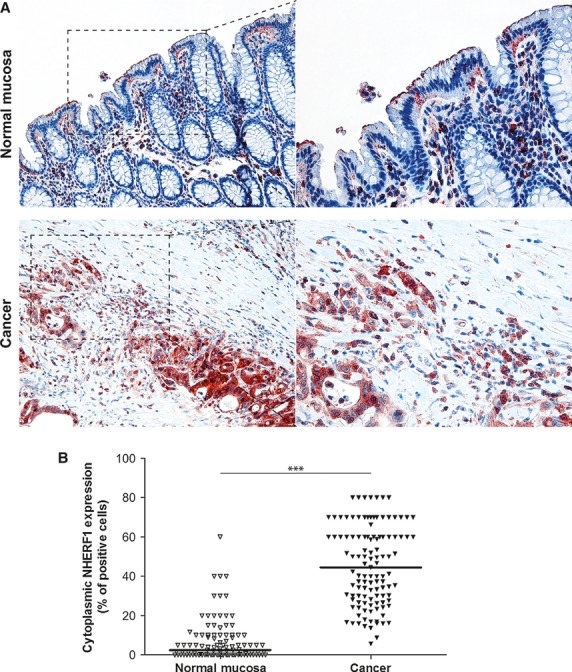
Expression analysis of cytoplasmic NHERF1 in human colorectal cancer. (A) Representative images of cytoplasmic NHERF1 immunoreactivity in the primary tumour matched with adjacent cancer-uninvolved colonic mucosa by immunohistochemistry (Original magnification on the left ×100, enlargement on the right ×200). (B) The distribution of cytoplasmic NHERF1(+) cells on normal mucosa and the tumour compartment of the same colonic lesion. (Horizontal bold line = median value; ***P < 0.0001).
A higher density of epithelial cells positive for cytoplasmic NHERF1 was observed in C compared with NM [44.5 (5.7–80.0) versus 2.5 (0–60.0) respectively] and the intergroup comparison showed that the median expression of NHERF1 was statistically higher in the C compartment than in NM (P < 0.0001; Fig. 3B).
Association of clinicopathological data with MC density, PAR-2 and NHERF1 expressions
Significant associations between tumour markers and clinicopathological characteristics are summarized in Table 2. In particular, a high density of tryptase(+) MCs was shown in 48.7% (n = 56/115) of tumours, exhibiting a statistical association with male patients (P = 0.004) and higher TNM stage (P = 0.025); a statistical trend was observed between the high density of tryptase(+) MCs and tumours with a poor differentiation grade (P = 0.051).
Table 2.
Association between tumour marker expressions and clinicopathological data in 115 colorectal cancers
| Characteristics, n (%) | MC density | PAR-2 expression | Cytoplasmic NHERF1 expression | |||
|---|---|---|---|---|---|---|
| Low | High | Negative | Positive | Negative | Positive | |
| Gender | ||||||
| Male | 26 (39.4) | 40 (60.6) | 31 (47.0) | 35 (53.0) | 32 (48.5) | 34 (51.5) |
| Female | 33 (67.3) | 16 (32.7) | 30 (61.2) | 19 (38.8) | 26 (53.1) | 23 (46.9) |
| P value | 0.004 | NS | NS | |||
| TNM stage | ||||||
| I + II | 17 (45.9) | 20 (54.1) | 23 (62.2) | 14 (37.8) | 28 (75.7) | 9 (24.3) |
| III | 20 (74.1) | 7 (25.9) | 21 (77.8) | 6 (22.2) | 23 (85.2) | 4 (14.8) |
| IV | 22 (43.1) | 29 (56.9) | 17 (33.3) | 34 (66.7) | 7 (13.7) | 44 (86.3) |
| P value | 0.025 | 0.000 | 0.000 | |||
| Differentiation grade | ||||||
| High | 6 (85.7) | 1 (14.3) | 5 (71.4) | 2 (28.6) | 5 (71.4) | 2 (28.6) |
| Moderate | 31 (54.4) | 26 (45.6) | 37 (64.9) | 20 (35.1) | 39 (68.4) | 18 (31.6) |
| Poor | 22 (44.0) | 28 (56.0) | 19 (38.0) | 31 (62.0) | 14 (28.0) | 36 (72.0) |
| P value | 0.051 | 0.005 | 0.000 | |||
| LVI | ||||||
| Negative | 34 (56.7) | 26 (43.3) | 42 (70.0) | 18 (30.0) | 44 (73.3) | 16 (26.7) |
| Positive | 10 (35.7) | 18 (64.3) | 11 (39.3) | 17 (60.7) | 8 (28.6) | 20 (71.4) |
| P value | NS | 0.010 | 0.000 | |||
| EGFR | ||||||
| Negative | 23 (46.9) | 26 (53.1) | 25 (51.0) | 24 (49.0) | 28 (57.1) | 21 (42.9) |
| Positive | 12 (66.7) | 6 (33.3) | 10 (55.6) | 8 (44.4) | 5 (27.8) | 13 (72.2) |
| P value | NS | NS | 0.053 | |||
MC density: density of Tryptase-positive Mast Cell; PAR-2: Proteinase-Activated Receptor 2; NHERF1: Na+/H+ exchanger regulating factor 1; NS: not significant; LVI: lymphovascular invasion.
Significant associations (P < 0.05) are indicated in bold.
Positive expression of PAR-2 was observed in 47% (n = 54/115) of tumours and these overexpressed cases showed a significant association with high nodal status, the presence of distant metastases (P = 0.007 and P = 0.002, respectively, data not shown), higher TNM stage (P = 0.000), poor differentiation grade (P = 0.005) and the presence of LVI (P = 0.010).
Tumours overexpressing cytoplasmic NHERF1 (49.6%) showed a significant association with pT3-pT4 depth of invasion, high nodal status, the presence of distant metastases (P = 0.000 for all, data not shown), higher TNM stage (P = 0.000), poor differentiation grade (P = 0.000) and the presence of LVI (P = 0.000). Finally, tumours overexpressing cytoplasmic NHERF1 showed a significant trend with positive EGFR status (P = 0.053).
Correlation among MC density, PAR-2 and NHERF1 expressions
A direct association existed between high MC density and PAR-2 overexpression. In fact, the Spearman's rank test revealed a positive correlation between tryptase(+) MCs and PAR-2 both in NM (r = 0.299, P = 0.002; Table 3B) and C compartments (r = 0.193, P = 0.039; Table 3A). A direct association existed between PAR-2 overexpression and increasing levels of NHERF1. The Spearman's rank test showed a significant positive correlation between PAR-2 and cytoplasmic NHERF1 both in NM (r = 0.319, P = 0.001; Table 3B) and C compartments (r = 0.362, P < 0.000; Fig. 4A). This association was also observed in immunofluorescence analysis, showing a co-overexpression of the two proteins in C samples. Tumour cells disseminated in the stroma and, frequently, very close to blood vessels within the tumour microenvironment, overexpressed PAR-2 and NHERF1, showing a wide co-localization of the two proteins in the cytoplasm compartment (Fig. 4B).
Table 3.
Correlation analysis among Mast Cell density, PAR-2 and NHERF1 expressions in primary cancer (A) and paired normal colonic mucosa (B)
| Variables | MC density | PAR-2 | Cytoplasmic NHERF1 |
|---|---|---|---|
| (A) | |||
| MC density | |||
| Rho | 1 | 0.1931 | 0.167 |
| P value | – | 0.039 | 0.074 |
| PAR-2 | |||
| Rho | 0.1931 | 1 | 0.3621 |
| P value | 0.039 | – | <0.000 |
| Cytoplasmic NHERF1 | |||
| Rho | 0.167 | 0.3621 | 1 |
| P value | 0.074 | <0.000 | – |
| (B) | |||
| MC density | |||
| Rho | 1 | 0.2989 | 0.3485 |
| P value | – | 0.002 | 0.000 |
| PAR-2 | |||
| Rho | 0.2989 | 1 | 0.3191 |
| P value | 0.002 | – | 0.001 |
| Cytoplasmic NHERF1 | |||
| Rho | 0.3485 | 0.3191 | 1 |
| P value | 0.000 | 0.001 | – |
MC density: density of Tryptase-positive Mast Cell; PAR-2: Proteinase-Activated Receptor 2; NHERF1: Na+/H+ exchanger regulating factor 1.
Significant correlations (P < 0.05) are indicated in bold.
Fig. 4.
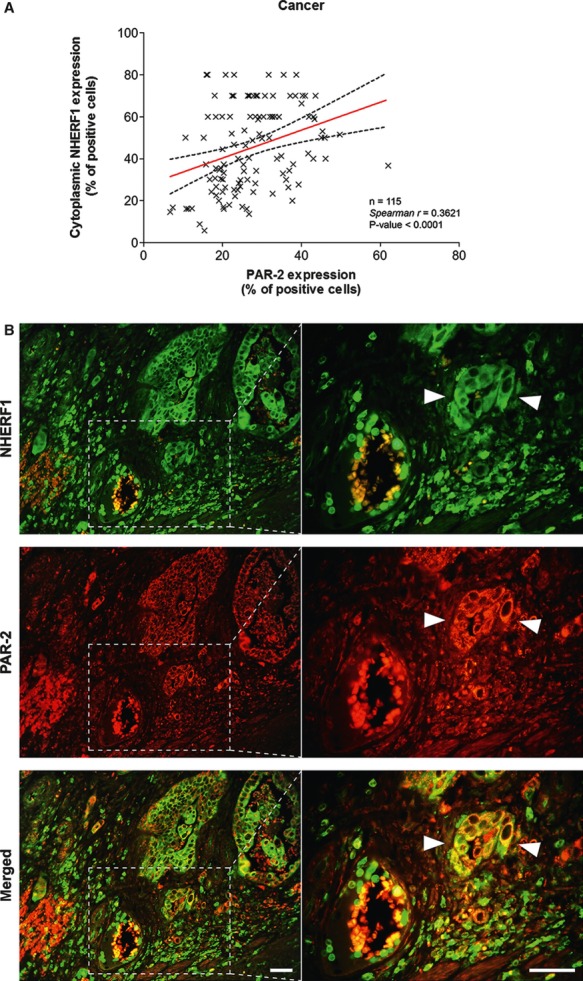
Analysis of PAR-2 and cytoplasmic NHERF1 in human colorectal cancer. As shown in (A), the correlation between protein expression of PAR-2 and cytoplasmic NHERF1 was evaluated by Spearman's rank correlation coefficient analysis, and a positive significant correlation was established. (B) A representative tissue sample stained with PAR-2 and EBP-50 antibodies and detected with Alexa Fluor 568 (red) and Alexa Fluor 488 (green) secondary antibodies, respectively, prior to fluorescence microscopy analysis. Overlaps between red and green signals (merged) point to co-localizations (in yellow). Arrowheads indicate invasive cells disseminated into the stroma close to a blood vessel with a high global expression of two proteins, where PAR-2 co-localized with NHERF1 on cytoplasmic and membranous compartments (scale bar = 20 μm).
Given the fundamental association between these two tumour markers, we examined the potential prognostic role of the PAR-2/NHERF1 immunophenotypes. With respect to the PAR-2(−)/cytoplasmic NHERF1(−) expression phenotype, the PAR-2(+)/cytoplasmic NHERF1(+) phenotype was associated with the highest depth of invasion (P = 0.004), positive nodal status (P < 0.000), the presence of distant metastases (P < 0.000), poor differentiation grade (P < 0.000) and the presence of LVI (P = 0.000; Table 4).
Table 4.
Association between PAR-2/NHERF1 expression immunophenotypes and clinicopathological data
| Characteristics, n (%) | PAR-2(+)/cytoplasmic NHERF1(+) | PAR-2(−)/cytoplasmic NHERF1(−) | P value |
|---|---|---|---|
| Depth of invasion | |||
| pT1 | 0 (0.0) | 3 (100.0) | 0.004 |
| pT2 | 2 (14.3) | 12 (85.7) | |
| pT3 | 28 (57.1) | 21 (42.9) | |
| pT4 | 7 (58.3) | 5 (41.7) | |
| Nodal status | |||
| pN0 | 7 (21.9) | 25 (78.1) | <0.000 |
| pN1 | 10 (41.7) | 14 (58.3) | |
| pN2 | 17 (89.5) | 2 (10.5) | |
| pN3 | 3 (100.0) | 0 (0.0) | |
| Distant metastases | |||
| M0 | 8 (20.0) | 32 (80.0) | <0.000 |
| M1 | 26 (92.9) | 2 (7.1) | |
| Differentiation grade | |||
| Well | 1 (20.0) | 4 (80.0) | <0.000 |
| Moderate | 13 (28.9) | 32 (71.1) | |
| Poor | 22 (75.9) | 7 (24.1) | |
| LVI | |||
| Negative | 10 (21.7) | 36 (78.3) | 0.000 |
| Positive | 13 (76.5) | 4 (23.5) | |
PAR-2: Proteinase-Activated Receptor 2; NHERF1: Na+/H+ exchanger regulating factor 1; LVI: lymphovascular invasion.
Significant associations with P < 0.05 are indicated in bold.
Furthermore, a significant correlation was highlighted between high MC density and increasing levels of cytoplasmic NHERF1 in the NM compartment (r = 0.349, P = 0.000; Table 3B).
Discussion
Although a variety of malignant tumours are accompanied by an increased infiltration of MCs, conflicting data about the relationship between MC density and prognosis in CRC have been reported. In fact, although some authors have found a direct correlation between high MC counts and improved prognosis [26, 27], the majority of studies have shown that MCs are directly associated with an unfavourable prognosis and tumour aggressiveness [28–31]. In this study, we investigated the distribution of MCs and their relationship with PAR-2(+) epithelial cells in primary CRC. Using the immunohistochemical double staining technique, we analysed the density of tryptase(+) MCs and the expression of PAR-2 in tumours and adjacent uninvolved colonic mucosa.
In this study, we showed a large distribution of MCs in the lamina propria and submucosa, representing an early and persistent infiltrating immune cell type. In line with findings from Xia et al. [32], we confirmed that the MC count in normal mucosa was higher than that in primary tumours. Interestingly, we also demonstrated a significant MC increase in male patients compared with female patients in agreement with Wu et al. [33]. This strong association seems to be related to the oestrogen/oestrogen receptor/tumour necrosis factor-α axis; in fact, MCs express oestrogen receptors and treatment with the specific ligand has been demonstrated to prevent the release of TNF-alpha, a major MC-derived autocrine growth factor [34].
In our study, MCs infiltrated stroma more abundantly at invasive margins than in the internal tumour field, probably to activate cell processes involved in cancer progression. Furthermore, we showed that tumours with distant metastases and with a poor differentiation grade had a higher density of tryptase(+) MCs. Tryptase(+) MCs have been demonstrated to play a role in tissue remodelling during wound healing and cancer, degrading selectively matrix proteins, synthesizing collagen [35] and stimulating fibroblast proliferation [36] and myofibroblast differentiation [25]. However, it has been suggested that in the context of developing tumours, the ability of MCs to remodel tissues is subverted, disrupting the surrounding extracellular matrix and increasing tumour spread [37]. In normal colonic mucosa, natural inhibitors of tryptase and normal tissue homeostasis maintain a proteolytic balance, even if during cancer progression, this balance is gradually disturbed and definitively broken in the invasive CRC by overexpression of several proteases [38]. On the basis of our results, it seems that MCs might functionally contribute to neoplastic aggressiveness by secreting tryptase, which increases tumour invasion and metastasis [39, 40].
Recent studies have altered the traditional view of the role of proteases in tumour evolution, showing that these enzymes serve as signal molecules controlling cell functions through specific membrane receptors [41]. In this context, it has been suggested that PAR-2 may play an active role in the setting of cancer growth and tumour progression (Fig. 5) [42–47]. Furthermore, most colon cancer cell lines express PAR-2 and its activation has shown to play an important role in the progression of colon cancer [10]. In our study, we demonstrated an increased expression of PAR-2 in cancer with respect to adjacent normal mucosa and PAR-2 overexpression was associated with advanced disease parameters, as measured by TNM stage, poor histological differentiation and LVI. Furthermore, we described a positive linear correlation between MC density and PAR-2 expression, hypothesizing that tryptase may act directly on PAR-2 by stimulating cancer progression. In fact, analogous results have been reported for trypsin, a serine proteinase similar to tryptase, which regulates cellular proliferation in gastric carcinoma [48] and promotes colon cancer proliferation by inducing PAR-2/G protein signalling pathways (Fig. 5) [10]. Thus, our data could support the view that PAR-2 expression is related to the progression of CRC and its tryptase-induced activation could have a role in tumour invasion.
Fig. 5.
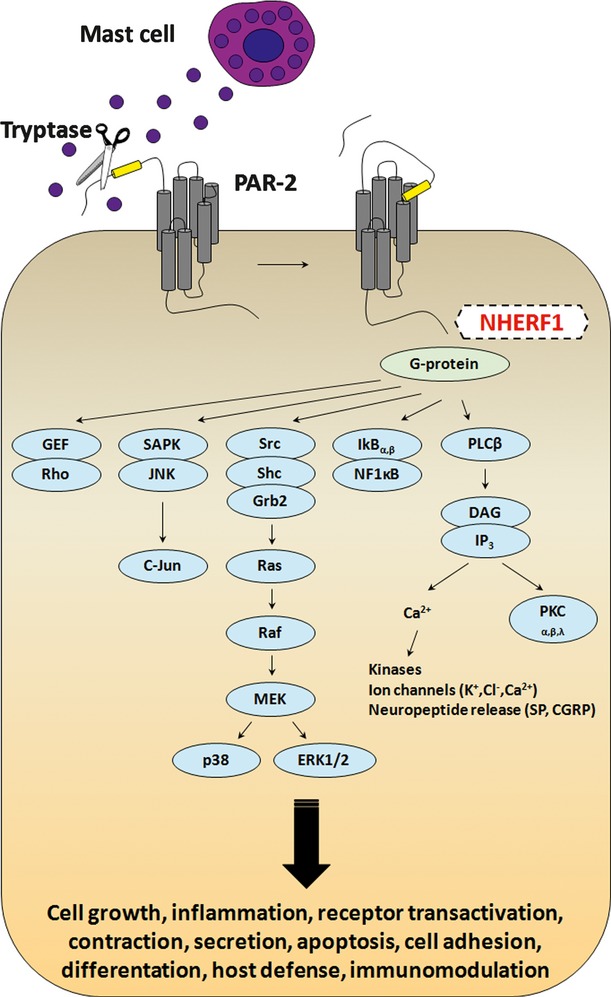
Diagram showing the major G protein–mediated signalling pathways coupled to PAR-2. Tryptase secreted in the tumour microenvironment by infiltrating mast cells cleaves the N-terminal domain of PAR-2, releasing a new N-terminal tail, which acts as a tethered ligand that binds the receptor itself. The association with G proteins initiates signal transduction, resulting in stimulation of phosphoinositide breakdown, cytosolic calcium mobilization and promoting several cell responses.
Mounting evidence highlights the role of cytoplasmic adapter proteins that contribute critically to the signalling, trafficking and function of many G protein–coupled receptors [49]. In our previous studies, it was established that NHERF1, an adaptor protein with two non-identical, type 1 tandem PDZ domains and a carboxyl-terminal ezrin-binding domain, is a potential candidate of clinical relevance for human breast cancer [16, 24, 50, 51]. Recently, we have also demonstrated that nuclear NHERF1, present in the early stages of colorectal carcinogenesis and related with poor prognosis, might contribute to the onset of the colonic malignant phenotype [19]. Furthermore, we have suggested nuclear NHERF1 as a potential new biomarker of advanced CRC, demonstrating an overexpression of nuclear NHERF1 in no longer polarized epithelial cells, converted to a mesenchymal phenotype in hypoxic colonic areas [20]. Therefore, given that the role of nuclear NHERF1 has been previously analysed in-depth and considering that reports investigating the distribution of cytoplasmic NHERF1 in CRC have not been published to date, in this study, we aimed to analyse the role of the NHERF1 protein localized in the cytoplasm. We demonstrated that overexpressing tumours resulted associated with unfavourable prognosis and aggressive clinical parameters. Moreover, we showed both a positive linear correlation between cytoplasmic NHERF1 and PAR-2 and a significant co-expression of the two proteins mostly in the margin of the tumour mass.
Even if PAR-2 expression was associated with clinicopathological characteristics of adverse prognosis, in some studies, PAR-2 has not represented an independent prognostic factor [52, 53]. Other markers interacting with the PAR-2 signalling pathway may be the reason of this trend, which influences survival outcome. Thus, study of PAR-2 expression in CRC in combination with other proteins could be helpful in evaluating the relationship of PAR-2 with patient survival [52]. We therefore examined the potential prognostic role of PAR-2/NHERF1 immunophenotypes on the basis of the significant correlation between these two tumour markers. Interestingly, the PAR-2(+)/cytoplasmic NHERF1(+) expression immunophenotype was able to predict poor prognosis of CRC patients, being associated with the presence of nodal and distant metastasis, poor differentiation grade and LVI.
In conclusion, our findings show that the high density of tryptase(+) MCs correlates with the advanced stages of CRC. Furthermore, the strong association between tryptase(+) MCs and PAR-2 expression seems to suggest that the activation of tryptase-induced PAR-2 contributes to tumour progression and invasiveness. Furthermore, examination of the PAR-2(+)/cytoplasmic NHERF1(+) expression immunophenotype appears able to predict the prognosis of CRC and may be useful for selecting subgroups of patients who should be treated with more aggressive therapies because of the higher risk of tumour invasion and metastasis.
Finally, we believe that further studies are needed to confirm usefulness of PAR2(+)/cytoplasmic NHERF1(+) expression immunophenotype for selecting subgroups of patients who should be treated with more aggressive therapies.
Acknowledgments
The authors thank Vincenza Rubini, Rossana Daprile and Alessia L. Marzano for their expert technical assistance. The authors also thank Caroline Oakley for language revision.
Conflict of interest
No authors have conflicts of interest to declare.
References
- 1.Colotta F, Allavena P, Sica A, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–81. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 2.Ferrone C, Dranoff G. Dual roles for immunity in gastrointestinal cancers. J Clin Oncol. 2010;28:4045–51. doi: 10.1200/JCO.2010.27.9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pages F, Galon J, Dieu-Nosjean MC, et al. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 4.Huang B, Lei Z, Zhang GM, et al. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood. 2008;112:1269–79. doi: 10.1182/blood-2008-03-147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Z, Zhang B, Li D, et al. Mast cells mobilize myeloid-derived suppressor cells and Treg cells in tumor microenvironment via IL-17 pathway in murine hepatocarcinoma model. PLoS ONE. 2010;5:e8922. doi: 10.1371/journal.pone.0008922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ch'ng S, Wallis RA, Yuan L, et al. Mast cells and cutaneous malignancies. Mod Pathol. 2006;19:149–59. doi: 10.1038/modpathol.3800474. [DOI] [PubMed] [Google Scholar]
- 7.Maltby S, Khazaie K, McNagny KM. Mast cells in tumor growth: angiogenesis, tissue remodelling and immune-modulation. Biochim Biophys Acta. 2009;1796:19–26. doi: 10.1016/j.bbcan.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macfarlane SR, Seatter MJ, Kanke T, et al. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–82. [PubMed] [Google Scholar]
- 9.MacNaughton WK. Epithelial effects of proteinase-activated receptors in the gastrointestinal tract. Mem Inst Oswaldo Cruz. 2005;100(Suppl. 1):211–5. doi: 10.1590/s0074-02762005000900036. [DOI] [PubMed] [Google Scholar]
- 10.Darmoul D, Marie JC, Devaud H, et al. Initiation of human colon cancer cell proliferation by trypsin acting at protease-activated receptor-2. Br J Cancer. 2001;85:772–9. doi: 10.1054/bjoc.2001.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vergnolle N. Clinical relevance of proteinase activated receptors (pars) in the gut. Gut. 2005;54:867–74. doi: 10.1136/gut.2004.048876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schechter NM, Brass LF, Lavker RM, et al. Reaction of mast cell proteases tryptase and chymase with protease activated receptors (PARs) on keratinocytes and fibroblasts. J Cell Physiol. 1998;176:365–73. doi: 10.1002/(SICI)1097-4652(199808)176:2<365::AID-JCP15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Corvera CU, Dery O, McConalogue K, et al. Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. J Clin Invest. 1997;100:1383–93. doi: 10.1172/JCI119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshii M, Jikuhara A, Mori S, et al. Mast cell tryptase stimulates DLD-1 carcinoma through prostaglandin- and MAP kinase-dependent manners. J Pharmacol Sci. 2005;98:450–8. doi: 10.1254/jphs.fpj05002x. [DOI] [PubMed] [Google Scholar]
- 15.Soreide K, Janssen EA, Korner H, et al. Trypsin in colorectal cancer: molecular biological mechanisms of proliferation, invasion, and metastasis. J Pathol. 2006;209:147–56. doi: 10.1002/path.1999. [DOI] [PubMed] [Google Scholar]
- 16.Bellizzi A, Malfettone A, Cardone RA, et al. NHERF1/EBP50 in breast cancer: clinical perspectives. Breast Care. 2010;5:86–90. doi: 10.1159/000298962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voltz JW, Weinman EJ, Shenolikar S. Expanding the role of NHERF, a PDZ-domain containing protein adapter, to growth regulation. Oncogene. 2001;20:6309–14. doi: 10.1038/sj.onc.1204774. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi Y, Molina JR, Hamilton SR, et al. NHERF1/EBP50 is a new marker in colorectal cancer. Neoplasia. 2010;12:1013–22. doi: 10.1593/neo.10780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mangia A, Saponaro C, Malfettone A, et al. Involvement of nuclear NHERF1 in colorectal cancer progression. Oncol Rep. 2012;28:889–94. doi: 10.3892/or.2012.1895. [DOI] [PubMed] [Google Scholar]
- 20.Malfettone A, Silvestris N, Paradiso A, et al. Overexpression of nuclear NHERF1 in advanced colorectal cancer: association with hypoxic microenvironment and tumor invasive phenotype. Exp Mol Pathol. 2012;92:296–303. doi: 10.1016/j.yexmp.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Scott-Conner CE, Christie DW. Cancer staging using the American Joint Committee on Cancer TNM System. J Am Coll Surg. 1995;181:182–8. [PubMed] [Google Scholar]
- 22.Hu H, Krasinskas A, Willis J. Perspectives on current tumor-node-metastasis (TNM) staging of cancers of the colon and rectum. Semin Oncol. 2011;38:500–10. doi: 10.1053/j.seminoncol.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Malfettone A, Saponaro C, Paradiso A, et al. Peritumoral vascular invasion and NHERF1 expression define an immunophenotype of grade 2 invasive breast cancer associated with poor prognosis. BMC Cancer. 2012;12:106. doi: 10.1186/1471-2407-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mangia A, Chiriatti A, Bellizzi A, et al. Biological role of NHERF1 protein expression in breast cancer. Histopathology. 2009;55:600–8. doi: 10.1111/j.1365-2559.2009.03424.x. [DOI] [PubMed] [Google Scholar]
- 25.Mangia A, Malfettone A, Rossi R, et al. Tissue remodelling in breast cancer: human mast cell tryptase as an initiator of myofibroblast differentiation. Histopathology. 2011;58:1096–106. doi: 10.1111/j.1365-2559.2011.03842.x. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen HJ, Hansen U, Christensen IJ, et al. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J Pathol. 1999;189:487–95. doi: 10.1002/(SICI)1096-9896(199912)189:4<487::AID-PATH484>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 27.Tan SY, Fan Y, Luo HS, et al. Prognostic significance of cell infiltrations of immunosurveillance in colorectal cancer. World J Gastroenterol. 2005;11:1210–4. doi: 10.3748/wjg.v11.i8.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Syrjanen KJ, Hjelt LH. Tumor-host relationships in colorectal carcinoma. Dis Colon Rectum. 1978;21:29–36. doi: 10.1007/BF02586543. [DOI] [PubMed] [Google Scholar]
- 29.Fisher ER, Paik SM, Rockette H, et al. Prognostic significance of eosinophils and mast cells in rectal cancer: findings from the National Surgical Adjuvant Breast and Bowel Project (protocol R-01) Hum Pathol. 1989;20:159–63. doi: 10.1016/0046-8177(89)90180-9. [DOI] [PubMed] [Google Scholar]
- 30.Acikalin MF, Oner U, Topcu I, et al. Tumour angiogenesis and mast cell density in the prognostic assessment of colorectal carcinomas. Dig Liver Dis. 2005;37:162–9. doi: 10.1016/j.dld.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 31.Gulubova M, Vlaykova T. Prognostic significance of mast cell number and microvascular density for the survival of patients with primary colorectal cancer. J Gastroenterol Hepatol. 2009;24:1265–75. doi: 10.1111/j.1440-1746.2007.05009.x. [DOI] [PubMed] [Google Scholar]
- 32.Xia Q, Wu XJ, Zhou Q, et al. No relationship between the distribution of mast cells and the survival of stage IIIB colon cancer patients. J Transl Med. 2011;9:88. doi: 10.1186/1479-5876-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu X, Zou Y, He X, et al. Tumor-infiltrating mast cells in colorectal cancer as a poor prognostic factor. Int J Surg Pathol. 2013;21:111–20. doi: 10.1177/1066896912448836. [DOI] [PubMed] [Google Scholar]
- 34.Kim MS, Chae HJ, Shin TY, et al. Estrogen regulates cytokine release in human mast cells. Immunopharmacol Immunotoxicol. 2001;23:495–504. doi: 10.1081/iph-100108596. [DOI] [PubMed] [Google Scholar]
- 35.Cairns JA, Walls AF. Mast cell tryptase stimulates the synthesis of type I collagen in human lung fibroblasts. J Clin Invest. 1997;99:1313–21. doi: 10.1172/JCI119290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gailit J, Marchese MJ, Kew RR, et al. The differentiation and function of myofibroblasts is regulated by mast cell mediators. J Invest Dermatol. 2001;117:1113–9. doi: 10.1046/j.1523-1747.2001.15211.x. [DOI] [PubMed] [Google Scholar]
- 37.Coussens LM, Werb Z. Matrix metalloproteinases and the development of cancer. Chem Biol. 1996;3:895–904. doi: 10.1016/s1074-5521(96)90178-7. [DOI] [PubMed] [Google Scholar]
- 38.Noel A, Jost M, Maquoi E. Matrix metalloproteinases at cancer tumor-host interface. Semin Cell Dev Biol. 2008;19:52–60. doi: 10.1016/j.semcdb.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 39.Yano H, Kinuta M, Tateishi H, et al. Mast cell infiltration around gastric cancer cells correlates with tumor angiogenesis and metastasis. Gastric Cancer. 1999;2:26–32. doi: 10.1007/s101200050017. [DOI] [PubMed] [Google Scholar]
- 40.Elpek GO, Gelen T, Aksoy NH, et al. The prognostic relevance of angiogenesis and mast cells in squamous cell carcinoma of the oesophagus. J Clin Pathol. 2001;54:940–4. doi: 10.1136/jcp.54.12.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–8. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- 42.Massi D, Naldini A, Ardinghi C, et al. Expression of protease-activated receptors 1 and 2 in melanocytic nevi and malignant melanoma. Hum Pathol. 2005;36:676–85. doi: 10.1016/j.humpath.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 43.Darmoul D, Gratio V, Devaud H, et al. Protease-activated receptor 2 in colon cancer: trypsin-induced MAPK phosphorylation and cell proliferation are mediated by epidermal growth factor receptor transactivation. J Biol Chem. 2004;279:20927–34. doi: 10.1074/jbc.M401430200. [DOI] [PubMed] [Google Scholar]
- 44.Nishibori M. Physiology and pathophysiology of proteinase-activated receptors (PARs): preface. J Pharmacol Sci. 2005;97:7. doi: 10.1254/jphs.fmj04005x1. [DOI] [PubMed] [Google Scholar]
- 45.Yada K, Shibata K, Matsumoto T, et al. Protease-activated receptor-2 regulates cell proliferation and enhances cyclooxygenase-2 mRNA expression in human pancreatic cancer cells. J Surg Oncol. 2005;89:79–85. doi: 10.1002/jso.20197. [DOI] [PubMed] [Google Scholar]
- 46.Caruso R, Pallone F, Fina D, et al. Protease-activated receptor-2 activation in gastric cancer cells promotes epidermal growth factor receptor trans-activation and proliferation. Am J Pathol. 2006;169:268–78. doi: 10.2353/ajpath.2006.050841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanchez-Hernandez PE, Ramirez-Duenas MG, Albarran-Somoza B, et al. Protease-activated receptor-2 (PAR-2) in cervical cancer proliferation. Gynecol Oncol. 2008;108:19–26. doi: 10.1016/j.ygyno.2007.08.083. [DOI] [PubMed] [Google Scholar]
- 48.Miyata S, Koshikawa N, Yasumitsu H, et al. Trypsin stimulates integrin alpha(5)beta(1)-dependent adhesion to fibronectin and proliferation of human gastric carcinoma cells through activation of proteinase-activated receptor-2. J Biol Chem. 2000;275:4592–8. doi: 10.1074/jbc.275.7.4592. [DOI] [PubMed] [Google Scholar]
- 49.Ardura JA, Friedman PA. Regulation of G protein-coupled receptor function by Na+/H+ exchange regulatory factors. Pharmacol Rev. 2011;63:882–900. doi: 10.1124/pr.110.004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bellizzi A, Mangia A, Malfettone A, et al. Na+/H+ exchanger regulatory factor 1 expression levels in blood and tissue predict breast tumour clinical behaviour. Histopathology. 2011;58:1086–95. doi: 10.1111/j.1365-2559.2011.03844.x. [DOI] [PubMed] [Google Scholar]
- 51.Mangia A, Malfettone A, Saponaro C, et al. Human epidermal growth factor receptor 2, Na+/H+ exchanger regulatory factor 1, and breast cancer susceptibility gene-1 as new biomarkers for familial breast cancers. Hum Pathol. 2011;42:1589–95. doi: 10.1016/j.humpath.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 52.Chang JH, Park JM, Kim SW, et al. Expression of protease activated receptor-2 in human colorectal cancer and its association with tumor progression. Dis Colon Rectum. 2010;53:1202–8. doi: 10.1007/DCR.0b013e3181d536f6. [DOI] [PubMed] [Google Scholar]
- 53.Li Z, Bian LJ, Li Y, et al. Expression of protease-activated receptor-2 (PAR-2) in patients with nasopharyngeal carcinoma: correlation with clinicopathological features and prognosis. Pathol Res Pract. 2009;205:542–50. doi: 10.1016/j.prp.2009.01.015. [DOI] [PubMed] [Google Scholar]


