Abstract
The potential of stem cell (SC) therapies for eye diseases is well-recognized. However, the results remain only encouraging as little is known about the mechanisms responsible for eye renewal, regeneration and/or repair. Therefore, it is critical to gain knowledge about the specific tissue environment (niches) where the stem/progenitor cells reside in eye. A new type of interstitial cell–telocyte (TC) (http://www.telocytes.com) was recently identified by electron microscopy (EM). TCs have very long (tens of micrometres) and thin (below 200 nm) prolongations named telopodes (Tp) that form heterocellular networks in which SCs are embedded. We found TCs by EM and electron tomography in sclera, limbus and uvea of the mouse eye. Furthermore, EM showed that SCs were present in the anterior layer of the iris and limbus. Adhaerens and gap junctions were found to connect TCs within a network in uvea and sclera. Nanocontacts (electron-dense structures) were observed between TCs and other cells: SCs, melanocytes, nerve endings and macrophages. These intercellular ‘feet’ bridged the intercellular clefts (about 10 nm wide). Moreover, exosomes (extracellular vesicles with a diameter up to 100 nm) were delivered by TCs to other cells of the iris stroma. The ultrastructural nanocontacts of TCs with SCs and the TCs paracrine influence via exosomes in the epithelial and stromal SC niches suggest an important participation of TCs in eye regeneration.
Keywords: telocytes, stem cells, limbus, iris, cornea, cell junctions, exosomes, eye regeneration
Introduction
The potential of stem cell (SC) therapies for eye diseases is well-recognized even the results remain only encouraging [1–7].
Recently, telocytes (TCs) have been described as a new type of interstitial cell by electron microscopy [8–10]. Telocytes have a small cell body and very long and thin cell prolongations (telopodes; Tp) with moniliform appearance, dichotomous branching and 3D-network distribution. Telocytes were found in close relationship with nerve endings, blood vessels and different types of resident cells, suggesting a role in the complex intercellular signalling throughout heterocellular junctions, shed vesicles and/or exosomes [11–14]. Particularly, TCs seem to be involved in the regenerative process because of their tandem with SCs in a variety of organs: heart [12, 15, 16], skeletal muscle [17], lung [18, 19], choroid plexus [20] or skin [21].
Adult tissue SCs are undifferentiated cells, capable of proliferation, self-renewal and differentiation into different tissue-specific progeny. Because of the functional definition, SCs are studied in vitro experiments and the microenvironmental interactions are not seen as integral part for their function [5]. Little is known about the cellular mechanisms responsible for eye renewal, regeneration and/or repair in situ but SCs and progenitor cells have been described in different areas of the eye [22–25]. Particularly, limbus seems to be rich in undifferentiated pluripotent cells which serve as an important source of new corneal epithelium [26–30] and a stem-cell niche has been described at this level [24].
We believe that it is critical to gain knowledge about the specific tissue environment where the stem/progenitor cells reside in eye. Therefore, we investigated the presence of TCs and SCs in mouse eye and their relationships using electron microscopy and electron tomography, as the appropriate diagnostic tools.
Material and methods
Eyes from four C57BL/6 mice (12 months old) were used for the ultrastructural study after the Institutional Ethical Committee approval. Small samples of about 1 mm3 were fixed by immersion in 4% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4. Samples were post-fixed in 1% OsO4 with 1.5% K4Fe(CN)6 (potassium ferrocyanide reduced osmium) in 0.1 M cacodylate buffer. Samples were further dehydrated in increased graded of ethanol followed by propylene oxide and embedded in Epon [31]. Semi-thin sections (1 μm thick) were stained with 1% toluidine blue and examined by light microscopy (Nikon Eclipse E600, Tokyo, Japan).
Transmission electron microscopy
Transmission electron microscopy (TEM) was performed on 60 nm thin sections stained with uranyl acetate and lead citrate using a Morgagni 268 electron microscope (FEI Company, Eindhoven, The Netherlands) at 80 kV. Digital electron micrographs were acquired with a MegaView III CCD and iTEM-SIS software (Olympus, Soft Imaging System GmbH, Münster, Germany). All measurements were performed with iTEM-SIS software, using 50 randomly selected structures/images.
Electron microscope tomography
Electron microscope tomography (ET) was performed on 250-nm thick sections of Epon-embedded tissue [32] using a Tecnai G2 Spirit BioTwin transmission electron microscope with a single-tilt specimen holder (FEI Company) at 100 kV. Electron tomographic data sets were recorded with a MegaView G2 CCD camera (Olympus) in ET mode. Projection images (1024 × 1024 pixels) were acquired at 1-degree angular increments from −65 to +65 degrees around an axis perpendicular to the optical axis of the microscope, at 36,000× magnification (pixel size 2.65 nm). After data alignment, the data sets were reconstructed into 3D volume (data collection, reconstruction and visualization) using Xplore3D Tomography Suite software (FEI Company). Amira 5.0.1 software (Visage Imaging GmbH, Berlin, Germany) was used for 3D imaging.
Results
Ultrastructural analysis was performed on all three tunics of mouse eye (Fig. 1): fibrous (cornea and sclera), vascular pigmented (choroid, ciliary body and iris) and nervous (retina). Transmission electron microscopy showed the presence of interstitial cells with distinctive ultrastructural features defining TCs in lamina propria of the conjunctiva, in limbal area (Fig. 2A), sclera (Fig. 2B), beneath Bruch's membrane (Fig. 2C) and in the iris stroma (Figs 3A and B, 4A). TCs were not found in the ciliary processes, iris muscle, cornea or retina.
Fig. 1.
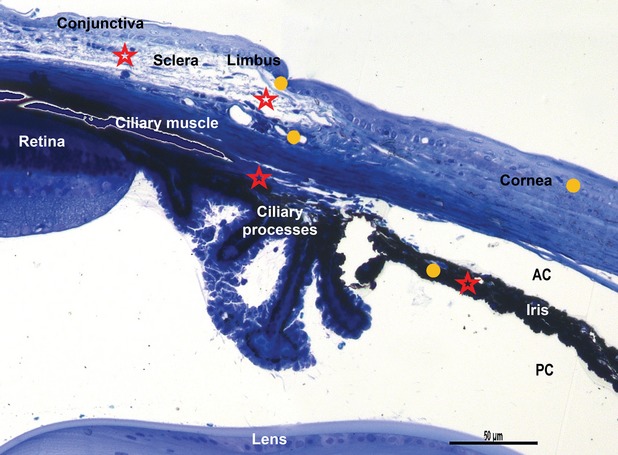
Light microscopy on semi-thin blue section of resin-embedded mouse eye. Stars indicated areas where telocytes are located: lamina propria of conjunctiva, limbal area, sclera, pars plana of the ciliary body, iris. Circles indicate the sites where stem cells have been found: cornea, limbus and iris. AC: anterior chamber; PC: posterior chamber. Toluidine blue staining, scale bar 50 μm, 20 × magnification.
Fig. 2.
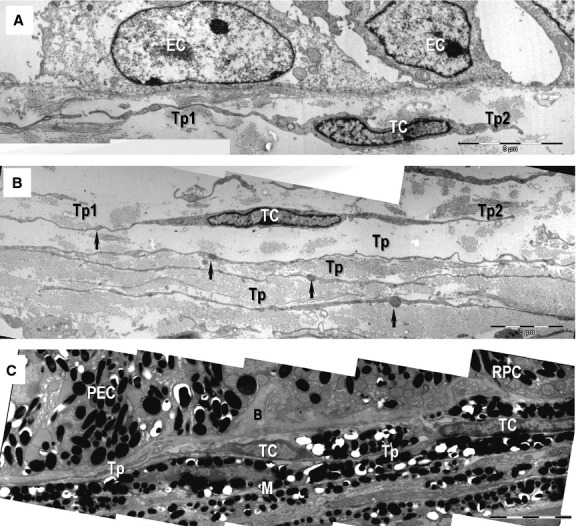
Transmission electron microscopy images show telocytes (TC) with telopodes (Tp) beneath the basement membrane of the conjunctival epithelium (A), sclera (B) and choroid (C). (A) Telocyte with two telopodes (Tp1, Tp2) beneath the corneal epithelium (EC) are visible. (B) Telocytes with overlapping telopodes (Tp) run in parallel layers in the sclera. The alternating thin segments (podomeres) and small dilations (podoms, arrows) generate the moniliform appearance of telopodes. (C) Telocytes extend telopodes (Tp) beneath Bruch's membrane (B) of the pigmentary cell of the retina (RPC) and ciliary body (PEC). Telopodes are more difficult to observe at lower magnification because of the electron-dense melanocytes (M); scale bars: A–C – 5 μm.
Fig. 3.
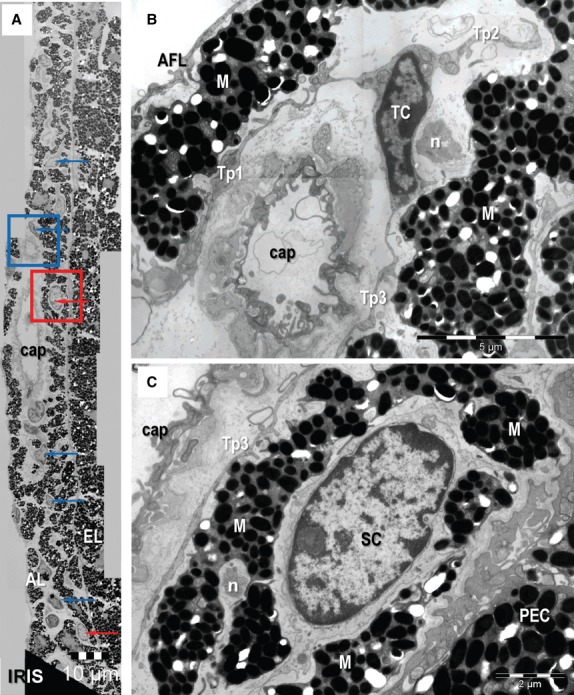
Transmission electron microscopy images of the mouse iris. (A) Telocytes (TC) (blue arrows) and putative stem cells (red arrows) are located between melanocytes in the anterior layer (AL). They are not present in the pigmented epithelial layer (EL) of the iris. Telocytes form a network in the stroma of the anterior marginal layer of the iris. (B) A higher magnification of the blue-square marked area in (A) shows a TC with three telopodes (Tp1-Tp3) extended among melanocytes (M), capillary (cap) and nerve endings (n). (C) Higher magnification of red square marked area in (A) shows a putative stem cell (SC) between M in the anterior layer of iris. PEC: pigmented epithelial cells of the iris. Scale bars: A – 10 μm; B – 5 μm; C – 2 μm.
Fig. 4.
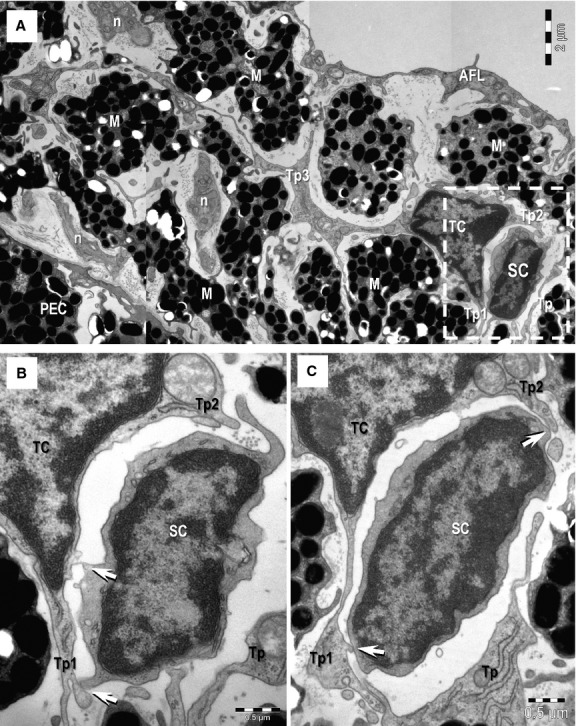
(A) Transmission electron microscopy image shows direct membrane-to-membrane contact (rectangular mark) between a telocyte (TC) and a putative stem cell (SC). M: melanocytes; n: nerves; AFL: anterior fibroblast layer. A TC with three telopodes (Tp1–Tp3) can be seen in the stroma of the iris. Tp3 extends between M and the dichotomous pattern of branching is noticeable. (B, C) Higher magnification of the TC-SC heterocellular connection (from rectangular marked area in A) – serial ultrathin sections. Tp2 and Tp3 enclose the putative SC. Small point contacts (arrows) connect the telocyte with the stem cell. Scale bars: A – 2 μm; B, C – 0.5 μm.
Telocytes showed an oval nucleus surrounded by a thin layer of cytoplasm (Figs 2A and B, 3B, 4A) and long cellular processes named Tp (Figs 2, 3B and 4A). Tp, very thin (below 100 nm) and long processes (up to 50 μm; 37.23 ± 9.72 μm), were the most prominent ultrastructural feature of TCs (Figs 8). The number of Tp per TC appears variable, usually two Tp in the sclera, choroid and ciliary body (Fig. 2) and three or more Tp in the iris stroma (Figs 3B and 4A). Tp showed characteristic uneven caliber and moniliform aspect (Fig. 2B) generated by alternating podoms (dilation of Tp, 294.47 ± 97.56 nm; Fig. 5) and podomers (slender segments; 95.67 ± 70.07 nm thickness; Figs 2 and 8). Podoms accommodate ‘Ca2+-uptake/release units’ formed by mitochondria, endoplasmic reticulum, caveolae (Fig. 5A and B).
Fig. 8.
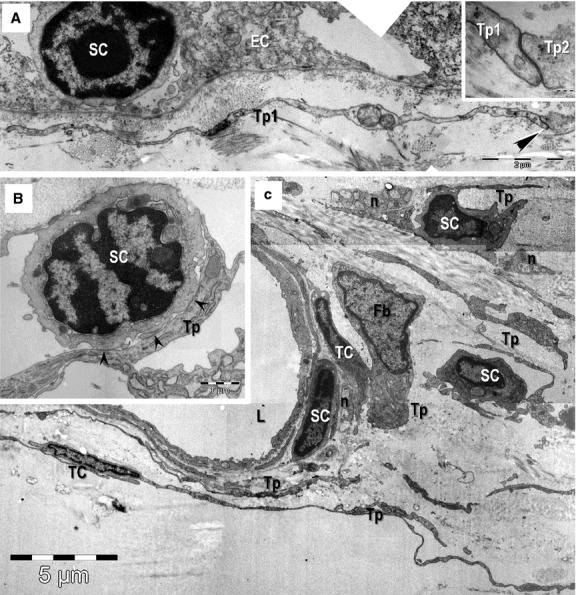
Transmission electron microscopy images of epithelial (A) and stromal (B, C) stem cell (SC) niches in the mouse eye. (A) Basal SC is sited on the basement membrane of limbus epithelium. A telopode (Tp1) runs parallel with the basement membrane and a gap junction (arrowheads) connect it with another one (Tp2; higher magnification in inset). (B, C) Stem cells in the stromal SC niches located in the corneoscleral junction. Direct contacts (arrowheads) between a Tp and the SC are visible in B. TC: telocytes; Tp: telopodes; Fb: fibroblast; n: nerve endings; L: lumen of an arteriole. Scale bars: A – 2 μm; inset – 0.1 μm; B – 1 μm; C – 5 μm.
Fig. 5.
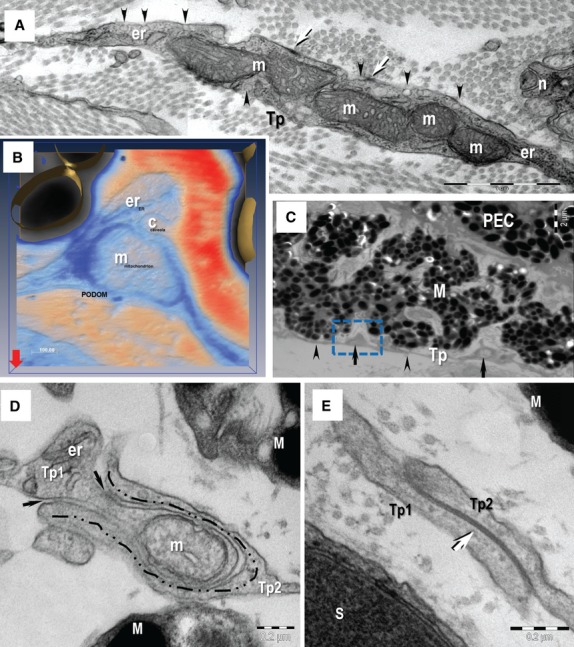
Transmission electron microscopy (TEM) image of the telopodes (Tp). (A) The Tp present small dilatation named podoms. The podoms accommodates mitochondria (m) and endoplasmic reticulum cisternae (er). Caveolae (arrowheads) and focal adhesion (arrows) are visible on the cellular membrane of telocyte at the podom level. n – nerve. (B) Electron tomography (3D isosurface reconstruction) of a podom illustrate the ‘Ca2+-uptake/release unit’ formed by mitochondrion (m), endoplasmic reticulum (er) and caveolae (c). (C) Rectangular mark indicates the podom on which electron tomography was performed on a thick section (200 nm). The Tp present alternating thin segments (podomeres, arrowheads) and small dilatation (podoms, arrows). (D, E) TEM images show different types of homocellular junctions connecting the telopodes (Tp1, Tp2): manubria adhaerentia (dashed line in B), puncta adhaerentia (black arrows in B) and gap junction (white arrow in C). M: melanocyte; S: Schwann cell; scale bars: A – 1 μm; B – 0.1 μm; C – 2 μm; D, E – 0.2 μm.
Telocytes via Tp were connected in an interstitial network in sclera and uvea by different types of homocellular junctions (Fig. 5D and E): manubria adhaerentia, puncta adhaerentia, gap junctions or combination of these. In addition, non-canonical, heterocellular junctions connected TCs with stromal SCs (Figs 4 and 8B), nerve endings (Fig. 6A), melanocytes (Fig. 6B), and macrophages (Fig. 7A and B). These atypical junctions were characterized by direct membrane-to-membrane point contacts or planar contacts (Figs 4, 6 and 8B). The intercellular cleft was 10.99 ± 3.76 nm wide. Usually, about 10 nm small electron-dense nanostructures (feet) were observed bridging adjoining cellular membranes (Fig. 6). We did not found any connections between TCs and smooth muscle cells of pupillary and ciliary muscles or, between TCs and pigmented epithelial cells.
Fig. 6.
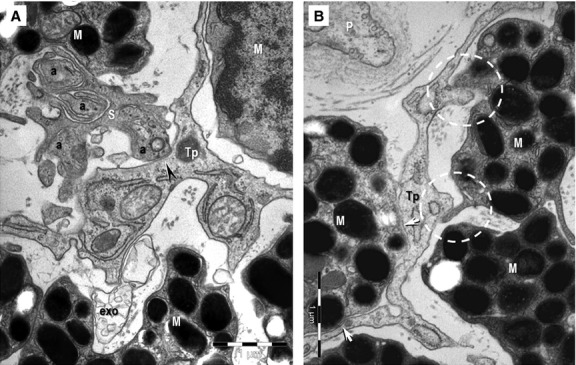
(A, B) Transmission electron microscopy images of the hetero-cellular connections formed by telocytes in the anterior layer of the iris. (A) Direct contact (arrowhead) can be seen between an axon (a) and a telopode (Tp). S: Schwann cell; exo: exosomes. (B) Point contacts (encircled) and planar contacts (arrows) are visible between a Tp and two melanocytes (M). P: pericyte.
Fig. 7.
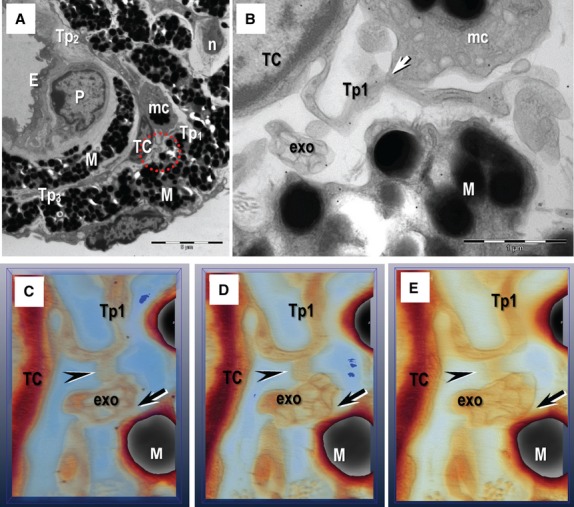
Electron tomography images on 200 nm thick section of resin-embedded iris. (A) General view shows a telocyte (TC) with three telopodes (Tp1–Tp3) in the anterior layer of iris. M: melanocyte; mc: macrophage; n: nerve; E: endothelial cell; P: pericyte. (B) Multi-vesicular structure containing exosomes (exo) is visible between a telocyte (telopode Tp1) and a M at higher magnification. Arrow indicates a point contact between telopode Tp1 and the mc. (C–E) Serial digital sections in the tomographic volume show that exo are connected with the Tp (arrowheads), as well as a M (arrows). Scale bars: A – 5 μm; B – 1 μm; C–E – 0.5 μm.
We often observed exosomes and shed vesicles (extracellular vesicles with a diameter below 100 nm) near TCs (Figs 6A and 7). Usually, an external membrane enclosed up to 10 exosomes near TCs (Figs 6 and 7C–E). Electron tomography showed that the exosomes were connected by point contacts with Tp and another type of cell, for example with melanocytes (Fig. 7) in the iris stroma.
Small cells (about 5 μm) with a high nucleo-cytoplasm ratio, containing few mitochondria, endoplasmic reticulum cisternae and numerous ribosomes in the scanty cytoplasm were found in the basal conjunctival epithelial layer at limbal level (Fig. 8A), in the stroma of the iris (Figs 3 and 4) and around blood vessels in the corneoscleral meshwork (Fig. 8B and C). Telocytes and SCs, alongside with nerve endings and blood vessels have been found as discrete clusters in these locations and have interpreted as stem-cell niches. Direct membrane-to-membrane contacts (nanocontacts or planar contacts) between TCs and SCs were often observed (Figs 4 and 8B).
Discussion
Telocytes, as a novel type of interstitial cells, were characterized in details by electron microscopy [8, 10, 12, 13, 33, 34]. We report here the presence of TCs and SCs in limbus, sclera and uvea of mouse eye. Earlier electron microscope studies [35, 36] overlooked the existence of TCs at the level of sclera and uvea. In fact, TCs and SCs exist in the mouse eye alongside with melanocytes, pigmented epithelial cells, myoepithelial cells, smooth muscles cells, pigment-laden macrophages, fibroblasts, Schwann cells with nerve endings and capillaries. Moreover, TCs are interconnected in an interstitial network and are connected by ‘stromal synapses’ [37] with SCs, melanocytes, nerve endings, or macrophages.
Noteworthy, we found that TCs have contacts with SCs in discrete sites that seem to be stem-cell niches in the iris stroma and corneoscleral meshwork. The tandem TC-SC has been found in stem-cell niches in various organs (e.g. epicardium, lungs, skeletal muscle, choroid plexus, skin) [10]. Stem-cell niches are highly organized interactive structural units which commonly occur at tissue intersections or transition zones and coordinate tissue repair and renewal [24, 38–40]. The functionality of a stem-cell niche relies on the physical contact and signalling interactions of SCs with neighbouring nurse cells as well as the paracrine and endocrine signals from local or distant sources, neural input and metabolic products of tissue [38]. Telocytes seem to have ‘strategic’ positioning in the eye tissue, in between blood capillaries and their specific target cells (SCs, melanocytes, macrophages, etc.) and are in close contact with nerve ending. Telocytes could be nurse cells integrating local (short-distance signals: direct contacts, exosomes, shed vesicles) and long-distance signals through the long TPs, because of their 3D network in the eye stroma. Extracellular vesicles, exosomes and shed vesicles, participate in intercellular communications and seem to play key role in horizontal transfer of important bioactive macromolecules (e.g. membrane receptors, proteins, mRNAs) among neighbouring cells [41–44] and stem-cell niche [45]. Telocytes can even act in immune system modulation [46] or being ‘cellular’ guides for immune cells that arrive via blood stream [37]. On the basis of our observations, we agree with Cantarero et al. [33] supporting that TCs could be part of the ‘mesenchymal cell niche’ together with nerves fibres and blood vessels.
In addition, our study suggests that there are two different types of stem-cell niches into the eye: epithelial niches (basal cells in cornea and conjunctiva) and stromal niches (iris, corneoscleral junction). If in the epithelial niche, the contact of SCs with basement membrane seems to be prerequisite in the stromal niches the existence of stromal supporting cells (telocytes!?) is required [38, 39]. Recent studies show that spindle cells subjacent to limbal basal epithelial SCs serve as niche supporting cells which maintain clonal growth of limbal epithelial progenitors [47] possible by direct adhesion [48]. Present data show that TCs are neighbouring both epithelial and stromal SCs, but show (a)typical junctions [12] only with the stromal SCs (Fig. 8). It is required smart reparative cells to restore or repair or renew eye tissues, but it is also needed an architectural structure that keeps on the unit. And here is where TCs could play a nursing key role. The TCs network could even be a scaffold for SC migration between different layers of the eye.
Recent results showed a particular immunofenotype [9, 14, 19, 49], distinct microRNA expression [11, 50], specific gene-expression profile [51] and peculiar electrophysiological proprieties [52] of TCs in various organs. It remains to explore if all these proprieties are shared by eye TCs. Telocytes secrete VEGF and express platelet-derived growth factor receptor (PDGFR-β), both in situ and in vitro 10 in skeletal muscle [14] and border zone of myocardial infarction [11]. These finding suggest that TCs are an important player in promoting vasculogenesis. Their involvement in the pathophysiology of neovascular eye diseases should be investigated as antagonist of VEGF, and PDGF changed clinical practices for neovascular eye diseases [53–55].
Conclusion
This ultrastructural study shows that TCs, coupled through adhaerens and gap junctions, form an interstitial network into the sclera and uvea creating a scaffold for SC migration between different layers of the eye. The tandem TC-SC present in eye stem-cell niches suggests that a heterocellular mixture could be more effective in the therapy of eye diseases.
Acknowledgments
This study was supported by, University of Zaragoza-Spain, project number UZ 210-150.UZ 2011-BIO-07; Diputación General de Aragón Group number B83 (MJLB) and CNCSIS– UEFISCSU, project number PNII–IDEI 350/2012 PN-II-ID-PCE-2011-3-0134.
Conflicts of interest
No authors have any financial/conflicting interests to disclose.
References
- 1.Kelley MJ, Rose AY, Keller KE, et al. Stem cells in the trabecular meshwork: present and future promises. Exp Eye Res. 2009;88:747–51. doi: 10.1016/j.exer.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Notara M, Alatza A, Gilfillan J, et al. In sickness and in health: corneal epithelial stem cell biology, pathology and therapy. Exp Eye Res. 2010;90:188–95. doi: 10.1016/j.exer.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Blenkinsop TA, Corneo B, Temple S, et al. Ophthalmologic stem cell transplantation therapies. Regen Med. 2012;7:32–9. doi: 10.2217/rme.12.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmeer CW, Wohl SG, Isenmann S. Cell-replacement therapy and neural repair in the retina. Cell Tissue Res. 2012;349:363–74. doi: 10.1007/s00441-012-1335-6. [DOI] [PubMed] [Google Scholar]
- 5.Wester ST, Goldberg J. In: Stem cells in ophthalmology, new advances in stem cell transplantation. Taner D, editor. InTech; 2012. ISBN: 978-953-51-0013-3. Available from: http://www.intechopen.com/books/new-advances-in-stem-cell-transplantation/stems-cells-in-ophtamology. [Google Scholar]
- 6.Ong JM, da Cruz L. A review and update on the current status of stem cell therapy and the retina. Br Med Bull. 2012;102:133–46. doi: 10.1093/bmb/lds013. [DOI] [PubMed] [Google Scholar]
- 7.Sasai Y, Eiraku M, Suga H. In vitro organogenesis in three dimensions: self-organizing stem cells. Development. 2012;139:4111–21. doi: 10.1242/dev.079590. [DOI] [PubMed] [Google Scholar]
- 8.Popescu LM, Faussone-Pellegrini MS. TELOCYTES – a case of serendipity: the winding way from interstitial cells of Cajal (ICC), via interstitial Cajal-like cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010;14:729–40. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faussone-Pellegrini MS, Popescu LM. Telocytes. BioMol Concepts. 2011;2:481–9. doi: 10.1515/BMC.2011.039. [DOI] [PubMed] [Google Scholar]
- 10.Popescu LM, Nicolescu MI. Telocytes and stem cells. In: Goldenberg RCdS, Campos deCarvalhoAC., editors. Resident stem cells and regenerative therapy. 1st ed. Academic Press/Elsevier; 2013. pp. 205–331. Chapter 11. ISBN 9780124160125. Available at: http://www.sciencedirect.com/science/article/pii/B9780124160125000116. [Google Scholar]
- 11.Manole CG, Cişmaşiu V, Gherghiceanu M, et al. Experimental acute myocardial infarction: telocytes involvement in neo-angiogenesis. J Cell Mol Med. 2011;15:2284–96. doi: 10.1111/j.1582-4934.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gherghiceanu M, Popescu LM. Cardiac telocytes - their junctions and functional implications. Cell Tissue Res. 2012;348:265–79. doi: 10.1007/s00441-012-1333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicolescu MI, Popescu LM. Telocytes in the interstitium of human exocrine pancreas: ultrastructural evidence. Pancreas. 2012;41:949–56. doi: 10.1097/MPA.0b013e31823fbded. [DOI] [PubMed] [Google Scholar]
- 14.Suciu LC, Popescu BO, Kostin S, et al. Platelet-derived growth factor receptor-β-positive telocytes in skeletal muscle interstitium. J Cell Mol Med. 2012;16:701–7. doi: 10.1111/j.1582-4934.2011.01505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gherghiceanu M, Popescu LM. Cardiomyocyte precursors and telocytes in epicardial stem cell niche: electron microscope images. J Cell Mol Med. 2010;14:871–7. doi: 10.1111/j.1582-4934.2010.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gherghiceanu M, Popescu LM. Heterocellular communication in the heart: electron tomography of telocyte-myocyte junctions. J Cell Mol Med. 2011;15:1005–11. doi: 10.1111/j.1582-4934.2011.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Popescu LM, Manole E, Serboiu CS, et al. Identification of telocytes in skeletal muscle interstitium: implication for muscle regeneration. J Cell Mol Med. 2011a;15:1379–92. doi: 10.1111/j.1582-4934.2011.01330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popescu LM, Gherghiceanu M, Suciu LC, et al. Telocytes and putative stem cells in the lungs: electron microscopy, electron tomography and laser scanning microscopy. Cell Tissue Res. 2011b;345:391–403. doi: 10.1007/s00441-011-1229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng Y, Bai C, Wang X. Telocyte morphologies and potential roles in diseases. J Cell Physiol. 2012;227:2311–7. doi: 10.1002/jcp.23022. [DOI] [PubMed] [Google Scholar]
- 20.Popescu BO, Gherghiceanu M, Kostin S, et al. Telocytes in meninges and choroid plexus. Neurosci Lett. 2012;516:265–9. doi: 10.1016/j.neulet.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Ceafalan L, Gherghiceanu M, Popescu LM, et al. Telocytes in human skin; are they involved in skin regeneration? J Cell Mol Med. 2012;16:1405–20. doi: 10.1111/j.1582-4934.2012.01580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatia B, Singhal S, Jayaram H, et al. Adult retinal stem cells revisited. Open Ophthalmol J. 2010;4:30–8. doi: 10.2174/1874364101004010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du Y, Roh DS, Mann MM, et al. Multipotent stem cells from trabecular meshwork become phagocytic TM cells. Invest Ophthalmol Vis Sci. 2012;53:1566–75. doi: 10.1167/iovs.11-9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ordonez P, Di Girolamo N. Limbal epithelial stem cells: role of the niche microenvironment. Stem Cells. 2012;30:100–7. doi: 10.1002/stem.794. [DOI] [PubMed] [Google Scholar]
- 25.Wohl SF, Christian W, Schmeer CW, et al. Neurogenic potential of stem/progenitor-like cells in the adult mammalian eye. Prog Retin Eye Res. 2012;31:213–42. doi: 10.1016/j.preteyeres.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Schlötzer-Schrehardt U, Kruse FE. Identification and characterization of limbal stem cells. Exp Eye Res. 2005;81:247–64. doi: 10.1016/j.exer.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Shortt AJ, Secker GA, Munro PM, et al. Characterization of the limbal epithelial stem cell niche: novel imaging techniques permit in vivo observation and targeted biopsy of limbal epithelial stem cells. Stem Cells. 2007;25:1402–9. doi: 10.1634/stemcells.2006-0580. [DOI] [PubMed] [Google Scholar]
- 28.Secker GA, Daniels JT, StemBook . Limbal epithelial stem cells of the cornea. The Stem Cell Research Community, StemBook; 2009. doi: doi/ 10.3824/stembook.1.48.1. [PubMed] [Google Scholar]
- 29.Pinnamaneni N, Funderburgh JL. Concise review: stem cells in the corneal stroma. Stem Cells. 2012;30:1059–63. doi: 10.1002/stem.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meller D, Thomasen H, Steuhl KP. Ocular surface reconstruction in limbal stem cell deficiency: transplantation of cultivated limbal epithelium. Ophthalmologe. 2012;109:863–8. doi: 10.1007/s00347-011-2510-y. [DOI] [PubMed] [Google Scholar]
- 31.Gherghiceanu M, Barad L, Novak A, et al. Cardiomyocytes derived from human embryonic and induced pluripotent stem cells: comparative ultrastructure. J Cell Mol Med. 2011;15:2539–51. doi: 10.1111/j.1582-4934.2011.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hinescu ME, Gherghiceanu M, Suciu L, et al. Telocytes in pleura: two- and three-dimensional imaging by transmission electron microscopy. Cell Tissue Res. 2011;343:389–97. doi: 10.1007/s00441-010-1095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cantarero I, Luesma MJ, Junquera C. The primary cilium of telocytes in the vasculature: electron microscope imaging. J Cell Mol Med. 2011;15:2594–600. doi: 10.1111/j.1582-4934.2011.01312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carmona IC, Bartolomé MJ, Escribano CJ. Identification of telocytes in the lamina propria of rat duodenum: transmission electron microscopy. J Cell Mol Med. 2011;15:26–30. doi: 10.1111/j.1582-4934.2010.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freddo TF. Ultrastructure of the iris. Microsc Res Tech. 1996;33:369–89. doi: 10.1002/(SICI)1097-0029(19960401)33:5<369::AID-JEMT1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 36.Imesch PD, Bindley CD, Khademian Z, et al. Melanocytes and iris color. Electron microscopic findings. Arch Ophthalmol. 1996;114:443–7. doi: 10.1001/archopht.1996.01100130439015. [DOI] [PubMed] [Google Scholar]
- 37.Popescu LM, Gherghiceanu M, Cretoiu D, et al. The connective connection: interstitial cells of Cajal (ICC) and ICC-like cells establish synapses with immunoreactive cells. Electron microscope study in situ. J Cell Mol Med. 2005;9:714–30. doi: 10.1111/j.1582-4934.2005.tb00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–9. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 39.Voog J, Jones DL. Stem cells and the niche: a dynamic duo. Cell Stem Cell. 2010;6:103–15. doi: 10.1016/j.stem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker MR, Patel KK, Stappenbeck TS. The stem niche. J Pathol. 2009;217:169–80. doi: 10.1002/path.2474. [DOI] [PubMed] [Google Scholar]
- 41.Camussi G, Deregibus MC, Bruno S, et al. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–48. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 42.Bang C, Thum T. Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol. 2012;44:2060–4. doi: 10.1016/j.biocel.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Ludwig AK, Giebel B. Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol. 2012;44:11–5. doi: 10.1016/j.biocel.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Pant S, Hilton H, Burczynski ME. The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol. 2012;83(11):1484–94. doi: 10.1016/j.bcp.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quesenberry PJ, Aliotta JM. The paradoxical dynamism of marrow stem cells: considerations of stem cells, niches, and microvesicles. Stem Cell Rev. 2008;4:137–47. doi: 10.1007/s12015-008-9036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stoorvogel W, Kleijmeer MJ, Geuze HJ, et al. The biogenesis and functions of exosomes. Traffic. 2002;3:321–30. doi: 10.1034/j.1600-0854.2002.30502.x. [DOI] [PubMed] [Google Scholar]
- 47.Xie HT, Chen SY, Li GG, et al. Isolation and expansion of human limbal stromal niche cells. Invest Ophthalmol Vis Sci. 2012;53:279–86. doi: 10.1167/iovs.11-8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Higa K, Kato N, Yoshida S, et al. Aquaporin 1-positive stromal niche-like cells directly interact with N-cadherin-positive clusters in the basal limbal epithelium. Stem Cell Res. 2013;10:147–55. doi: 10.1016/j.scr.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Hatta K, Huang ML, Weisel RD, et al. Culture of rat endometrial telocytes. J Cell Mol Med. 2012;16:1392–6. doi: 10.1111/j.1582-4934.2012.01583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cişmaşiu VB, Radu E, Popescu LM. miR-193 expression differentiates telocytes from other stromal cells. J Cell Mol Med. 2011;15:1071–4. doi: 10.1111/j.1582-4934.2011.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng Y, Zhang M, Qian M, et al. Genetic comparison of mouse lung telocytes with mesenchymal stem cells and fibroblasts. J Cell Mol Med. 2013;17:567–77. doi: 10.1111/jcmm.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cretoiu SM, Cretoiu D, Marin A, et al. Telocytes: ultrastructural, immunohistochemical and electrophysiological characteristics in human myometrium. Reproduction. 2013;145:357–70. doi: 10.1530/REP-12-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andreoli CM, Miller JW. Anti-vascular endothelial growth factor therapy for ocular neovascular disease. Curr Opin Ophthalmol. 2007;18:502–8. doi: 10.1097/ICU.0b013e3282f0ca54. [DOI] [PubMed] [Google Scholar]
- 54.Horsley MB, Kahook MY. Anti-VEGF therapy for glaucoma. Curr Opin Ophthalmol. 2010;21:112–7. doi: 10.1097/ICU.0b013e3283360aad. [DOI] [PubMed] [Google Scholar]
- 55.Campochiaro PA. Ocular neovascularization. J Mol Med. 2013;91:311–21. doi: 10.1007/s00109-013-0993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


