Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) has been widely used for the treatment of hematologic malignant and non-malignant hematologic diseases and other diseases. However, acute graft-versus-host disease (GVHD) is a life-threatening complication of allogeneic transplantation. Acute GVHD may occur in 30% of transplant recipients, which is a syndrome of erythematous skin eruption, cholestatic liver disease and intestinal dysfunction, resulting from the activation of donor T lymphocytes by host antigen-presenting cells, resulting in an immune-mediated inflammatory response. Recent scientific advances in the understanding of the pathogenesis involved in the development of acute GVHD and clinical investigation have provided more effective therapeutic strategies for acute GVHD. This review focuses on major scientific and clinical advances in the treatment of acute GVHD.
Keywords: Acute graft-versus-host disease, stem cell transplantation, hydrogen, treatment
Introduction
Acute Graft-versus-host disease (GVHD)
Pathophysiology of acute GVHD
Prevention of acute GVHD
Treatment of acute GVHD
First-line therapy
A recent phase II trial conducted by the blood and marrow transplant
Second-line therapy
Future directions
Introduction
Hematopoietic stem cell transplantation (HSCT) has been widely used for the treatment of hematologic malignant and non-malignant hematologic diseases and other diseases (Table 1). However, the widespread application of HSCT is restricted by the poor availability of suitable donors [1–4]. Acute graft-versus-host disease (GVHD) remains a major cause of post-transplant morbidity and mortality, even in patients who receive human leucocyte antigen (HLA) identical sibling grafts [5, 6]. Even through use of adequate post-transplantation immunosuppressive therapy, successfully engrafted recipients, free from active GVHD, immune response often show delayed immune reconstitution and remain susceptible to fatal infection [5–8]. Thus, acute GVHD continues to be a major limitation to successful HSCT. The objective of this review was to offer an overview of current management of acute GVHD regarding the pathophysiology, regimens in common clinical use, and regimens under investigation.
Table 1.
| Malignant disease |
| Acute myelogenous leukaemia |
| Acute lymphoblastic leukaemia |
| Chronic myelogenous leukaemia |
| Chronic lymphocytic leukaemia |
| Non-Hodgkin lymphoma |
| Hodgkin lymphoma |
| Multiple Myeloma |
| Myelodysplastic syndromes |
| Myeloproliferative syndromes |
| Waldenstrom macroglobulinemia |
| Hairy cell leukaemia |
| Amyloidosis |
| Testicular cancer |
| Paediatric tumorus |
| Neuroblastoma |
| Nonmalignant diseases |
| Acquired aplastic anaemia |
| Diamond-Blackfan syndrome |
| Dyskeratosis congenita/Hoyeraal-Hreidarsson syndrome |
| Fanconi anaemia |
| Shwachman-Diamond syndrome |
| Thalassemia |
| Sickle cell disease |
| Paroxysmal nocturnal hemoglobinuria |
| Severe combined immunodeficiency |
| Congenital leucocyte dysfunction |
| Osteopetrosis |
| Familial erythrophagocytic lymphohistiocytosis |
| Glanzmann disease |
| Hereditary storage diseases |
| Autoimmune lymphoproliferative syndrome (ALPS) |
| Ataxia-telangiectasia |
| Chediak-Higashi syndrome |
| Chronic granulomatous disease |
| Complete interferon-γ receptor 1 deficiency |
| DiGeorge syndrome |
| Familial hemophagocytic lymphohistiocytosis |
| Griscelli's syndrome |
| Hyper-IgM syndrome |
| Kostmann syndrome |
| Leucocyte adhesion deficiency |
| Wiskott-Aldrich syndrome |
| X-linked syndrome |
| Fucosidosis |
| Gaucher's disease |
| Mucopolysaccharidoses |
| Congenital erythropoietic porphyria (Günther's disease) |
| Essential thrombocytopenia |
| Histiocytoses |
| Idiopathic hypereosinophilic syndrome |
| Myelofibrosis |
| Polycythemia vera |
Acute Graft-versus-host disease
Acute GVHD remains a clinical challenge and a major source of morbidity and mortality following allogeneic HSCT. Traditionally, GVHD was divided into acute and chronic GVHD based on the timing of the onset of GVHD symptoms. Graft-versus-host disease occurs on or before the 100th day following transplantation was defined as acute GVHD, and the onset of chronic GVHD occurs after the 100th day. However, this temporal distinction is somewhat arbitrary, as patients may manifest classic signs of acute GVHD even after day 100, and chronic manifestations may occur before 100 days post-transplantation. Acute GVHD diagnosis should be confirmed by biopsy of an affected organ if possible; in addition, other non-GVHD complications involving the skin, liver and GI tract should be ruled out, such as cytomegalovirus enteritis or drug eruption from medications [9]. However, ultimate diagnosis of acute GVHD needs integration of all available clinical information, because the sensitivity of these biopsies is only approximately 60% [10]. Thus, the development of diagnostic tests for acute GVHD is needed [11]. Because long-term survival from acute GVHD is directly related to the severity of skin, liver and gut involvement, to facilitate the study and prognostication of acute GVHD, a clinical staging and grading system was developed. The severity score was clinically based and ranged between grades 0 and IV according to the Keystone 1994 consensus criteria, as defined by the involvement of each organ system (Table 2) [12]. The staging and grading system of acute GVHD has been updated by the Center for international Bone Marrow Transplant Registry (IBMTR) (IBMTR) criteria [13–15], in which acute GVHD can be diagnosed after 100 days post-transplantation, and patients manifest the only clinic signs including anorexia, nausea and vomiting with a positive upper gastrointestinal tract biopsy for acute GVHD are included under overall grade II acute GVHD.
Table 2.
Staging and grading of acute graft-versus-host [12]
| Stage | Skin | Liver | Gut |
|---|---|---|---|
| 1 | Rash <25% of body surface area | Bilirubin 2–3 mg/dl | Diarrhoea 500–1000 ml/day or persistent nausea |
| 2 | Rash 25–50% of body surface area | Bilirubin 3–6 mg/dl | Diarrhoea 1000–1500 ml/day |
| 3 | Rash >50% of body surface area | Bilirubin 6–15 mg/dl | Diarrhoea >1500 ml/day |
| 4 | Erythroderma with bullae formation | Bilirubin >15 mg/dl | Severe abdominal pain with or without ileus |
| Grade | |||
| I | Stage 1–2 | None | None |
| II | Stage 3 or | Stage 1 or | Stage 1 |
| III | Stage 2–3 or | Stage 2–4 | |
| IV | Stage 4 or | Stage 4 | |
Pathophysiology of acute GVHD
The pathophysiology of acute GVHD involves complex three stages as proposed by Ferrara and Reddy [16]. Stage I involves tissue damage and cellular activation induced by preconditioning (Fig. 1). The first phase occurs prior to transplantation of the graft, during which time the transplant conditioning regimen such as chemotherapy and irradiation, damages and activates host tissues leading to secretion of inflammatory cytokines [tumour necrosis factor-α (TNF-α), interleukin-1 (IL-1), IL-6 and IFN-γ], and danger signals such as adenosine-5′-triphosphate (ATP) and nicotine adenine dinucleotide, as well as extracellular matrix proteins such as biglycan that promote activation and maturation of antigen-presenting cells (APCs) [17–20].
Fig. 1.
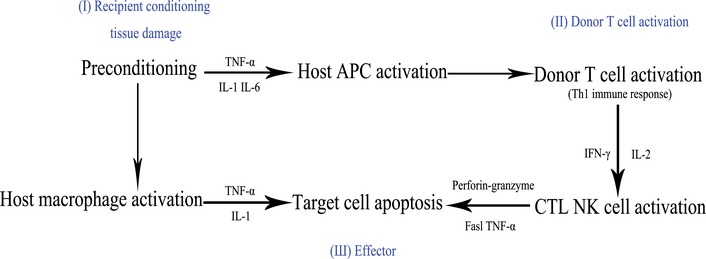
Pathophysiology of acute GVHD.
Cytokine cascade plays an important role in the occurrence and severity of acute GVHD, and the polymorphism of cytokine genes have been shown to affect the severity of acute GVHD [21]. Stage II involves the activation of donor lymphocytes (T cells). Resting donor T cells become activated in secondary lymphoid organs by both recipient and donor APCs as well as inflammatory cytokines, which expand and differentiate into effector cells [18, 22]. Activated T cells result in the production of IL-2 and IFN-γ (Th1) [23] or secreting IL-4, IL-5, IL-10, and IL-13 (Th2). Interleukin-2 plays a central role in controlling and amplifying the allogeneic immune response [23, 24], activating further T cell and natural killer cell responses, priming macrophages to release TNF-α and further inflammation damages the skin, liver, and gut. Both IL-2 and its receptor have been and are used as targets for the management of acute GVHD [25–27].
In the third stage, cellular and inflammatory factors are released, including TNF-α, IL-1, IL-6, IL-10, IL-12, which underlie the clinical manifestations of acute GVHD [28, 29]. A polymorphism in the TNF-α gene was demonstrated to increase incidence of severe acute GHVD [30], but polymorphisms in IL-10, which was considered as suppressing TNF-α, IL-1, and other inflammatory cytokines, was demonstrated with the ability to decrease incidence of acute GVHD [31].
Prevention of acute GVHD
The original prevention of acute GVHD developed since 1950s. In 1958, methotrexate (MTX) was used by Uphoff et al. because of its ability to delete proliferating donor lymphocytes through inhibition of dihydrofolate reductase and production of thymidylate and purines. In the1970s, cyclosporine was successfully used in the prevention of acute GVHD, which showed equivalency with MTX in prospective clinical studies by inhibiting T cell proliferation [32]. Since 1980s, calcineurin inhibitor cyclosporine and tacrolimus (TAC) combination with MTX have been successfully used in clinical trials, which laid the foundation of the following development of acute GVHD prophylaxis [32, 33]. In the 1990s, tacrolimus was used in a controlled clinical trial by Hiraoka et al. [34]. The incidence of grade II to IV acute GVHD within 100 days of transplantation was significantly lower among patients that received tacrolimus compared to patients that received cyclosporine. In two large randomized phase trials, TAC was used in combination with short-course MTX. Both trials showed reductions in overall acute GVHD incidence among patients receiving TAC/MTX. It has been demonstrated that TAC/MTX was superior to cyclosporine/MTX in the prevention of acute GVHD. Grade II-IV acute GVHD was significantly lower with TAC/MTX compared to cyclosporine/MTX both sibling donor (32% versus 44%; P = 0.01) and unrelated donor (56% versus 74%; P = 0.0002) transplant trials [35]. But a randomized trial comparing combination of cyclosporine and MTX to the combination of cyclosporine, prednisone, and MTX did not show any significant difference in acute GVHD incidence, relapse risk, and overall survival [8]. Nowadays, the combination of (cyclosporine or TAC) and MTX is the commonly used standard to prevent acute GVHD, which has been demonstrated better than single-agent MTX. [33, 36, 37] In recent clinical trials, post-transplant cyclophosphamide also promoted graft-host tolerance shows promise [38]. However, these agents have numerous side effects, including anorexia, nausea, vomiting and gastrointestinal tract reaction, gingival hyperplasia, renal toxicity, delayed cell count and immunological recovery, thrombotic microangiopathy, and posterior reversible encephalopathy syndrome, et al. [39–41] These side effects associated with methotrexate in particular has led investigators to examine the activity of alternative agents, such as tacrolimus combined with either mycophenolate mofetil (MMF) or sirolimus [42–46].
Mycophenolate mofetil inhibits proliferation of T lymphocytes via its metabolite mycophenolic acid and is a selective inhibitor of inosine monophosphate dehydrogenase, an enzyme critical to the de-novo synthesis of guanosine nucleotide, is now commonly used in combination with a calcineurin inhibitor for acute GVHD prophylaxis in preventing acute GVHD [47]. Mycophenolate mofetil and calcineurin inhibitor did not show better effect in acute GVHD prevention than MTX and calcineurin inhibitor, but the incidence and severity or oropharyngeal mucositis with the use of MMF was significantly decreased [42, 43, 48–52].
Approaches for the prevention of acute GVHD have utilized donor T cell depletion from the graft prior to infusion since 1980s [1, 53–55], by using physical techniques, density gradient centrifugation, anti-thymocyte globulin [56–59] or monoclonal antibody-based depletion methods, and CD34-cell-positive selection, et al. However, this approach is associated with a higher risk of graft rejection, impaired immune reconstitution, infectious complications, and relapse, and increased risk of primary disease relapse after HCT [60, 61]. Recent single-arm trials have shown 3-year disease-free survival approaching 60% with T cell-depleted peripheral blood stem cell transplantation [62, 63]. But T cell depletion did not improve overall survival, and the concept of partial marrow T cell depletion was evaluated by counterflow elutriation and T10B9 antibody plus complement in a multicenter randomized trial of 405 transplant recipients of HLA-matched unrelated donor grafts [64]. The incidence of acute GVHD grades II to IV was significantly lower in the partial T cell depletion group. However, partial marrow T cell depletion did not improve event-free and overall survival either. Besides, administration of the anti-CD52 antibody alemtuzumab (Campath 1H) was another approach to establish partial T cell depletion of the donor graft which was demonstrated could facilitate transplants from HLA-mismatched haploidentical donors without significant acute GVHD [65, 66]. This shows the promise of HSCT to patients without HLA-matched donors. But patients who received partial T cell depletion with administration of the anti-CD52 antibody were at increased risk for opportunistic infections, graft loss, and relapse. Therefore, investigators refined protocols for T cell depletion. In a phase II trial, Jakubowski et al. [63] reported ex vivo T cell depletion employing CD34 enrichment in 35 unrelated donor transplants. With no pharmacologic prophylaxis, acute GVHD grade II-III developed in 9% and chronic GVHD in 29% of patients. Fatal infections occurred in 5 of 35 (14%) patients. There was one late graft failure. The efficacy of this protocol was also confirmed by Devine et al. [62] in HLA-matched sibling donor transplantation. These results demonstrate that partial depletion of donor T cells provides protection against acute GVHD.
Agents attempt to block the cytokine pathways in the development of acute GVHD. In a recent phase 1/2 study, exciting new success has been reported in acute GVHD prevention using a well-tolerated oral CCR5 antagonist [67]. A strikingly low incidence of gastrointestinal and liver acute GVHD was observed in the study by using maraviroc which blocks T cell chemotaxis [67]. Interleukin-1 and TNF-α also play a central role in the development of acute GVHD, but drugs that target these cytokine/chemokine-receptor interactions (etanercept, infliximab) failed to improve incidence rates of acute GVHD [68, 69].
Other Agents are in the early stages of clinical development for acute GVHD prevention and give more promise [70]. Bortezomib, a proteasome inhibitor also show promise in acute GVHD prevention in a phase 1/2 study [71]. In experimental models of mismatched HSCT, T-regulatory cells (Tregs) suppressed lethal GVHD [72] and favoured post-transplantation immune reconstitution when co-infused with conventional T cells (Tcons) [73]. In humans, Tregs was also demonstrated preventing acute GVHD [74, 75] and promote immune reconstitution in HLA-haploidentical transplantation [74].
Treatment of acute GVHD
First-line therapy
The therapy of grade I acute GVHD should include topical therapy (topical steroid creams or topical tacrolimus) without the need for additional systemic immunosuppression. Suitable strengths of topical steroids are critically reviewed and detailed by Dignan et al. [76]. Adults should commence on 0.1% tacrolimus until resolution.
Glucocorticoids are the initial standard for treatment of grade II–IV acute GVHD, including methylprednisolone or prednisone at a dose of 1–2 mg/kg per day with subsequent gradual dose reduction once disease activity resolves [2, 77–79]. The optimal rate of tapering steroid doses after initial treatment has not been defined. Long–term prednisone therapy showed no advantage in a prospective randomized trial [80]. In general, steroids doses should be gradual reduced when acute GVHD manifestations start showing major improvement. Inappropriately rapid taper rates carry a risk of acute GVHD exacerbation or recurrence, whereas inappropriately slow taper rates increase the risk of steroid-related complications. Doses should be gradual reduced 0.2 mg/kg/day every 3–5 days, slower after prednisone doses are decreased to less than 20–30 mg/day [81]. The taper schedules provided in national, multicenter trials for acute GVHD, such as Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0302 or 0802, reflect current practice and are appropriate. The mechanism underline their effects may because of lympholytic effects and anti-inflammatory properties [82]. Higher doses of methylprednisolone (10 mg/kg/day) do not prevent evolution to grade III or IV acute GVHD or improve survival [77]. Mielcarek et al. reported that initial therapy of acute GVHD with low-dose prednisone (1.0 mg/kg) does not compromise patient outcome compared to standard dose prednisone (2 mg/kg/day) in a retrospective study in those with grade I/II disease. Definitive conclusions could not be drawn for those patients with grade III or IV disease because of the small numbers in this group [83]. Adverse effects of glucocorticoids include hypertension hyperglycemia and psychosis, immunosuppression, infections, hairy, myopathy, osteoporosis and avascular necrosis of bone, cataracts, and fat distribution, et al.
It has been demonstrated that as acute GVHD treatment response decreases, severity of the disease increases [84]. In a retrospective, 5-year survival in those patients response to steroid was significantly higher than those non-response to steroid (51% versus 32%). Similar result was also reported by Martin et al. [84, 85]. Unfortunately, only ∼60% of acute GVHD patients respond to systemic steroids and many of these responses are not durable [82, 86, 87]. The therapy effects of other agents in addition to prednisone on acute GVHD were studied for initial therapy [81, 88]. Agents evaluated in prospective studies have included Calcineurin inhibitors, MMF, pentostatin, etanercept,infliximab,Abs against IL-2R, horse ATG, anti-TNF drugs, however, most of these agents have proven futility [89–95].
A recent phase II trial conducted by the Blood and Marrow Transplant
Clinical Trials Network (BMT-CTN) has identified the combination of corticosteroids and MMF as a promising strategy [94]. In a phase III study, which has been recently closed for accrual and the data analysis is being awaited (http://www.clinicaltrials.gov identifier NCT01002742), this combination was compared against standard corticosteroid therapy alone in the therapy of acute GVHD.
Non-glucocorticoid systemic immune suppressive agents for the first-line therapy of acute GVHD may be an alternative approach with comparable efficacy and less morbidity related to glucocorticoids [95], which requires further exploration.
Second-line therapy
Acute GVHD is considered steroid-refractory when acute GVHD progresses within 3 days or is not improved after 5–7 days of initial treatment with 2 mg/kg dose methylprednisolone [82]. Sometimes, if acute GVHD is of a milder grade II, a longer observation interval of up to 2 weeks is acceptable [96]. Decisions to initiate secondary therapy should be made sooner for patients with more severe acute GVHD.
Very few prospective studies have evaluated the efficacy and safety of second-line therapy for acute GVHD, and interpretation of these studies is hampered by the lack of standardization. Agents that have been investigated over the last two decades in these trials include the following: low-dose MTX, MMF, extracorporeal photopheresis, IL-2R targeting, antibody therapy against CD3, CD7, CD25, CD52, CD147, IL-2R, IL-1, and TNF-α (i.e., basiliximab, daclizumab, denileukin, diftitox and alemtuzumab), horse ATG, etanercept, infliximab, and sirolimus, infusions of mesenchymal stem cells (MSCs) [27, 81, 97–116]. Intravenous immunoglobulin (IVIG) is effective, but with significant morbidity and mortality, mainly because of the infectious complications. Its cost and concern for impaired humoural recovery limit its widespread use [117–120]. More recently, numerous clinical trials using MSCs to treat acute GVHD have been reported [121–128]. Mesenchymal stem cells are suggested to suppress acute GVHD without impairing graft-versus-leukaemia effects and increasing systemic infections. However, there are many unsolved problems in the treatment of acute GVHD with MSCs (e.g., the source of MSCs, the single dose of MSCs, the total dose of MSCs and the interval of MSC administration). It is unclear whether MSCs preferentially suppress gut aGVHD or aGVHD in paediatric patients. Because few prospective comparative data on superior efficacy for any particular agent has been carried out, there are currently no criteria to identify patients who are likely to benefit from these second-line agents. The second-line regimen is chosen based on the effects of prior treatments, desired toxicity profile, considerations for drug interactions, logistical practicality, costs, and patient and physician preferences. Second-line treatments, especially those associated with the depression of T cells, are associated with increased infection and viral reactivation (including CMV, EBV, HHV-6, adenovirus, and polyoma [129, 130]. Many novel approaches are currently under investigation, but, to our knowledge to date, none of these approaches have achieved any improvements in overall survival in patients with steroid- refractory acute GVHD. Whether these approaches are truly representative of broader practices should be determined by retrospective studies on contemporary patients.
Future directions
The mechanisms of acute GVHD have been progressively elucidated over recent years. Many approaches have been developed and are being under investigation to prevent and treat acute GVHD using experimental models, including IL-21 blockade, Histone deacetylase inhibitors inducible costimulator, CSF-1, glycogen synthase kinase 3 inhibotion, Human CD8+ Regulatory T Cells [131–137].
In recent years, plasma biomarkers have been identified and validated as promising diagnostic tools for acute GVHD and prognostic tools by the development of proteomics technology. These biomarkers (Albumin, CRP, CXCL10, HGF, L-2Rα, IL-6, IL-8, IL-10, IL-12, IL-18, KRT18 REG3α, TNF-α, et al.) may represent novel therapeutic targets that could be inhibited by future acute GVHD-specific drugs which have been reviewed recently by Paczesny [138]. Because these drugs would target the appropriate effector T cells, they should increase efficacy and lower toxicity. These biomarkers may facilitate timely and selective therapeutic intervention, but should be more widely validated and incorporated into a new grading system for risk stratification of patients and better-customized treatment.
Gene transfer technologies are also promising tools to manipulate donor T cell immunity to enforce graft-versus-tumour/graft-versus-infection while preventing or controlling acute GVHD. For this purpose, several cell and gene transfer approaches have been investigated at the pre-clinical level and implemented in clinical trials [139, 140].
Our group recently has been suggested and demonstrated the therapeutic effects of hydrogen on acute GVHD in a mice model [141, 142]. H2 exert anti-oxidative and anti-inflammatory effects with few toxic side effects. Mutagenicity, genotoxicity and subchronic oral toxicity of hydrogen in a rat model was assessed by Saitoh et al. [143]. Significant changes basophil ratio of blood in female rats and decreased aspartate aminotransferase and alanine aminotransferase in male rats were observed which were not considered biologically significant. Similar clinical chemistry parameters were also observed by Nakao et al. in human beings [144]. Because few side effects of H2 have been reported, it is a promising and novel finding, which is easy to be used in clinic. However, acute GVHD remain difficult to prevent and treat. The most effective approach to treat acute GVHD is likely to be one that disrupts all three phases of the acute GVHD cascade synergistically. In the future, we would like to see targeted interventions to prevent and treat acute GVHD.
Acknowledgments
This study was supported by a grant from the National Natural Science Foundation of China (grant no. 81072241).
Conflicts of interest
The authors declare no competing interest.
References
- 1.Prentice HG, Blacklock HA, Janossy G, et al. Depletion of T lymphocytes in donor marrow prevents significant graft-versus-host disease in matched allogeneic leukaemic marrow transplant recipients. Lancet. 1984;1:472–6. doi: 10.1016/s0140-6736(84)92848-4. [DOI] [PubMed] [Google Scholar]
- 2.Thomas ED, Storb R, Clift RA, et al. Bone-marrow transplantation (second of two parts) N Engl J Med. 1975;292:895–902. doi: 10.1056/NEJM197504242921706. [DOI] [PubMed] [Google Scholar]
- 3.Deeg HJ, Storb R. Graft-versus-host disease: pathophysiological and clinical aspects. Annu Rev Med. 1984;35:11–24. doi: 10.1146/annurev.me.35.020184.000303. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324:667–74. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- 5.Gajewski J, Champlin R. Bone marrow transplantation from unrelated donors. Curr Opin Oncol. 1996;8:84–8. doi: 10.1097/00001622-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Goker H, Haznedaroglu IC, Chao NJ. Acute graft-vs-host disease: pathobiology and management. Exp Hematol. 2001;29:259–77. doi: 10.1016/s0301-472x(00)00677-9. [DOI] [PubMed] [Google Scholar]
- 7.Basara N, Kiehl MG, Fauser AA. New therapeutic modalities in the treatment of graft-versus-host disease. Crit Rev Oncol Hematol. 2001;38:129–38. doi: 10.1016/s1040-8428(00)00123-2. [DOI] [PubMed] [Google Scholar]
- 8.Chao NJ, Schmidt GM, Niland JC, et al. Cyclosporine, methotrexate, and prednisone compared with cyclosporine and prednisone for prophylaxis of acute graft-versus-host disease. N Engl J Med. 1993;329:1225–30. doi: 10.1056/NEJM199310213291703. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter PA, Macmillan ML. Management of acute graft-versus-host disease in children. Pediatr Clin North Am. 2010;57:273–95. doi: 10.1016/j.pcl.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weisdorf DJ, Hurd D, Carter S, et al. Prospective grading of graft-versus-host disease after unrelated donor marrow transplantation: a grading algorithm versus blinded expert panel review. Biol Blood Marrow Transplant. 2003;9:512–8. doi: 10.1016/s1083-8791(03)00162-9. [DOI] [PubMed] [Google Scholar]
- 11.Levine JE, Paczesny S, Sarantopoulos S. Clinical applications for biomarkers of acute and chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2012;18:S116–24. doi: 10.1016/j.bbmt.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 13.Cahn JY, Klein JP, Lee SJ, et al. Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: a joint Societe Francaise de Greffe de Moelle et Therapie Cellulaire (SFGM-TC), Dana Farber Cancer Institute (DFCI), and International Bone Marrow Transplant Registry (IBMTR) prospective study. Blood. 2005;106:1495–500. doi: 10.1182/blood-2004-11-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–64. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 15.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol. 2006;43:3–10. doi: 10.1053/j.seminhematol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95:2754–9. [PubMed] [Google Scholar]
- 18.Ferrara JL, Cooke KR, Teshima T. The pathophysiology of acute graft-versus-host disease. Int J Hematol. 2003;78:181–7. doi: 10.1007/BF02983793. [DOI] [PubMed] [Google Scholar]
- 19.Zeiser R, Marks R, Bertz H, et al. Immunopathogenesis of acute graft-versus-host disease: implications for novel preventive and therapeutic strategies. Ann Hematol. 2004;83:551–65. doi: 10.1007/s00277-004-0890-7. [DOI] [PubMed] [Google Scholar]
- 20.Zeiser R, Penack O, Holler E, et al. Danger signals activating innate immunity in graft-versus-host disease. J Mol Med. 2011;89:833–45. doi: 10.1007/s00109-011-0767-x. [DOI] [PubMed] [Google Scholar]
- 21.Dickinson AM, Middleton PG, Rocha V, et al. Genetic polymorphisms predicting the outcome of bone marrow transplants. Br J Haematol. 2004;127:479–90. doi: 10.1111/j.1365-2141.2004.05216.x. [DOI] [PubMed] [Google Scholar]
- 22.Bader P, Kreyenberg H, Hoelle W, et al. Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: possible role for pre-emptive immunotherapy? J Clin Oncol. 2004;22:1696–705. doi: 10.1200/JCO.2004.05.198. [DOI] [PubMed] [Google Scholar]
- 23.Mosmann TR, Cherwinski H, Bond MW, et al. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 24.Via CS, Finkelman FD. Critical role of interleukin-2 in the development of acute graft-versus-host disease. Int Immunol. 1993;5:565–72. doi: 10.1093/intimm/5.6.565. [DOI] [PubMed] [Google Scholar]
- 25.Anasetti C, Martin PJ, Hansen JA, et al. A phase I-II study evaluating the murine anti-IL-2 receptor antibody 2A3 for treatment of acute graft-versus-host disease. Transplantation. 1990;50:49–54. doi: 10.1097/00007890-199007000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Anasetti C, Hansen JA, Waldmann TA, et al. Treatment of acute graft-versus-host disease with humanized anti-Tac: an antibody that binds to the interleukin-2 receptor. Blood. 1994;84:1320–7. [PubMed] [Google Scholar]
- 27.Przepiorka D, Kernan NA, Ippoliti C, et al. Daclizumab, a humanized anti-interleukin-2 receptor alpha chain antibody, for treatment of acute graft-versus-host disease. Blood. 2000;95:83–9. [PubMed] [Google Scholar]
- 28.Hill GR, Crawford JM, Cooke KR, et al. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–13. [PubMed] [Google Scholar]
- 29.Hill GR, Teshima T, Gerbitz A, et al. Differential roles of IL-1 and TNF-alpha on graft-versus-host disease and graft versus leukemia. J Clin Invest. 1999;104:459–67. doi: 10.1172/JCI6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullighan C, Heatley S, Doherty K, et al. Non-HLA immunogenetic polymorphisms and the risk of complications after allogeneic hemopoietic stem-cell transplantation. Transplantation. 2004;77:587–96. doi: 10.1097/01.tp.0000111769.45088.a2. [DOI] [PubMed] [Google Scholar]
- 31.Lin MT, Storer B, Martin PJ, et al. Relation of an interleukin-10 promoter polymorphism to graft-versus-host disease and survival after hematopoietic-cell transplantation. N Engl J Med. 2003;349:2201–10. doi: 10.1056/NEJMoa022060. [DOI] [PubMed] [Google Scholar]
- 32.Storb R, Deeg HJ, Fisher L, et al. Cyclosporine v methotrexate for graft-v-host disease prevention in patients given marrow grafts for leukemia: long-term follow-up of three controlled trials. Blood. 1988;71:293–8. [PubMed] [Google Scholar]
- 33.Storb R, Deeg HJ, Farewell V, et al. Marrow transplantation for severe aplastic anemia: methotrexate alone compared with a combination of methotrexate and cyclosporine for prevention of acute graft-versus-host disease. Blood. 1986;68:119–25. [PubMed] [Google Scholar]
- 34.Hiraoka A, Ohashi Y, Okamoto S, et al. Phase III study comparing tacrolimus (FK506) with cyclosporine for graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2001;28:181–5. doi: 10.1038/sj.bmt.1703097. [DOI] [PubMed] [Google Scholar]
- 35.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–8. [PubMed] [Google Scholar]
- 36.Storb R, Deeg HJ, Whitehead J, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314:729–35. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- 37.Nash RA, Pineiro LA, Storb R, et al. FK506 in combination with methotrexate for the prevention of graft-versus-host disease after marrow transplantation from matched unrelated donors. Blood. 1996;88:3634–41. [PubMed] [Google Scholar]
- 38.Luznik L, Fuchs EJ. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res. 2010;47:65–77. doi: 10.1007/s12026-009-8139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laskin BL, Goebel J, Davies SM, et al. Small vessels, big trouble in the kidneys and beyond: hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Blood. 2011;118:1452–62. doi: 10.1182/blood-2011-02-321315. [DOI] [PubMed] [Google Scholar]
- 40.Wong R, Beguelin GZ, de Lima M, et al. Tacrolimus-associated posterior reversible encephalopathy syndrome after allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2003;122:128–34. doi: 10.1046/j.1365-2141.2003.04447.x. [DOI] [PubMed] [Google Scholar]
- 41.Cutler C, Li S, Kim HT, et al. Mucositis after allogeneic hematopoietic stem cell transplantation: a cohort study of methotrexate- and non-methotrexate-containing graft-versus-host disease prophylaxis regimens. Biol Blood Marrow Transplant. 2005;11:383–8. doi: 10.1016/j.bbmt.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Perkins J, Field T, Kim J, et al. A randomized phase II trial comparing tacrolimus and mycophenolate mofetil to tacrolimus and methotrexate for acute graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 2010;16:937–47. doi: 10.1016/j.bbmt.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Mohty M, de Lavallade H, Faucher C, et al. Mycophenolate mofetil and cyclosporine for graft-versus-host disease prophylaxis following reduced intensity conditioning allogeneic stem cell transplantation. Bone Marrow Transplant. 2004;34:527–30. doi: 10.1038/sj.bmt.1704640. [DOI] [PubMed] [Google Scholar]
- 44.Nash RA, Johnston L, Parker P, et al. A phase I/II study of mycophenolate mofetil in combination with cyclosporine for prophylaxis of acute graft-versus-host disease after myeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005;11:495–505. doi: 10.1016/j.bbmt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez R, Parker P, Nademanee A, et al. Cyclosporine and mycophenolate mofetil prophylaxis with fludarabine and melphalan conditioning for unrelated donor transplantation: a prospective study of 22 patients with hematologic malignancies. Bone Marrow Transplant. 2004;33:1123–9. doi: 10.1038/sj.bmt.1704493. [DOI] [PubMed] [Google Scholar]
- 46.Sabry W, Le Blanc R, Labbe AC, et al. Graft-versus-host disease prophylaxis with tacrolimus and mycophenolate mofetil in HLA-matched nonmyeloablative transplant recipients is associated with very low incidence of GVHD and nonrelapse mortality. Biol Blood Marrow Transplant. 2009;15:919–29. doi: 10.1016/j.bbmt.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Storb R, Antin JH, Cutler C. Should methotrexate plus calcineurin inhibitors be considered standard of care for prophylaxis of acute graft-versus-host disease? Biol Blood Marrow Transplant. 2010;16:S18–27. doi: 10.1016/j.bbmt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bolwell B, Sobecks R, Pohlman B, et al. A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;34:621–5. doi: 10.1038/sj.bmt.1704647. [DOI] [PubMed] [Google Scholar]
- 49.Bornhauser M, Schuler U, Porksen G, et al. Mycophenolate mofetil and cyclosporine as graft-versus-host disease prophylaxis after allogeneic blood stem cell transplantation. Transplantation. 1999;67:499–504. doi: 10.1097/00007890-199902270-00001. [DOI] [PubMed] [Google Scholar]
- 50.Kasper C, Sayer HG, Mugge LO, et al. Combined standard graft-versus-host disease (GvHD) prophylaxis with mycophenolate mofetil (MMF) in allogeneic peripheral blood stem cell transplantation from unrelated donors. Bone Marrow Transplant. 2004;33:65–9. doi: 10.1038/sj.bmt.1704299. [DOI] [PubMed] [Google Scholar]
- 51.Vogelsang GB, Arai S. Mycophenolate mofetil for the prevention and treatment of graft-versus-host disease following stem cell transplantation: preliminary findings. Bone Marrow Transplant. 2001;27:1255–62. doi: 10.1038/sj.bmt.1703076. [DOI] [PubMed] [Google Scholar]
- 52.Niederwieser D, Maris M, Shizuru JA, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620–9. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 53.Ho VT, Soiffer RJ. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood. 2001;98:3192–204. doi: 10.1182/blood.v98.12.3192. [DOI] [PubMed] [Google Scholar]
- 54.Filipovich AH, Vallera DA, Youle RJ, et al. Ex-vivo treatment of donor bone marrow with anti-T-cell immunotoxins for prevention of graft-versus-host disease. Lancet. 1984;1:469–72. doi: 10.1016/s0140-6736(84)92847-2. [DOI] [PubMed] [Google Scholar]
- 55.Marmont AM, Horowitz MM, Gale RP, et al. T-cell depletion of HLA-identical transplants in leukemia. Blood. 1991;78:2120–30. [PubMed] [Google Scholar]
- 56.Finke J, Bethge WA, Schmoor C, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–64. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 57.Michallet MC, Preville X, Flacher M, et al. Functional antibodies to leukocyte adhesion molecules in antithymocyte globulins. Transplantation. 2003;75:657–62. doi: 10.1097/01.TP.0000053198.99206.E6. [DOI] [PubMed] [Google Scholar]
- 58.Genestier L, Fournel S, Flacher M, et al. Induction of Fas (Apo-1, CD95)-mediated apoptosis of activated lymphocytes by polyclonal antithymocyte globulins. Blood. 1998;91:2360–8. [PubMed] [Google Scholar]
- 59.Kumar A, Mhaskar AR, Reljic T, et al. Antithymocyte globulin for acute-graft-versus-host-disease prophylaxis in patients undergoing allogeneic hematopoietic cell transplantation: a systematic review. Leukemia. 2012;26:582–8. doi: 10.1038/leu.2011.349. [DOI] [PubMed] [Google Scholar]
- 60.Papadopoulos EB, Carabasi MH, Castro-Malaspina H, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood. 1998;91:1083–90. [PubMed] [Google Scholar]
- 61.Jakubowski AA, Small TN, Young JW, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110:4552–9. doi: 10.1182/blood-2007-06-093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Devine SM, Carter S, Soiffer RJ, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant. 2011;17:1343–51. doi: 10.1016/j.bbmt.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jakubowski AA, Small TN, Kernan NA, et al. T cell-depleted unrelated donor stem cell transplantation provides favorable disease-free survival for adults with hematologic malignancies. Biol Blood Marrow Transplant. 2011;17:1335–42. doi: 10.1016/j.bbmt.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wagner JE, Thompson JS, Carter SL, et al. Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): a multi-centre, randomised phase II-III trial. Lancet. 2005;366:733–41. doi: 10.1016/S0140-6736(05)66996-6. [DOI] [PubMed] [Google Scholar]
- 65.Rizzieri DA, Koh LP, Long GD, et al. Partially matched, nonmyeloablative allogeneic transplantation: clinical outcomes and immune reconstitution. J Clin Oncol. 2007;25:690–7. doi: 10.1200/JCO.2006.07.0953. [DOI] [PubMed] [Google Scholar]
- 66.Morris E, Thomson K, Craddock C, et al. Outcomes after alemtuzumab-containing reduced-intensity allogeneic transplantation regimen for relapsed and refractory non-Hodgkin lymphoma. Blood. 2004;104:3865–71. doi: 10.1182/blood-2004-03-1105. [DOI] [PubMed] [Google Scholar]
- 67.Reshef R, Luger SM, Hexner EO, et al. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med. 2012;367:135–45. doi: 10.1056/NEJMoa1201248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Antin JH, Weisdorf D, Neuberg D, et al. Interleukin-1 blockade does not prevent acute graft-versus-host disease: results of a randomized, double-blind, placebo-controlled trial of interleukin-1 receptor antagonist in allogeneic bone marrow transplantation. Blood. 2002;100:3479–82. doi: 10.1182/blood-2002-03-0985. [DOI] [PubMed] [Google Scholar]
- 69.Hamadani M, Hofmeister CC, Jansak B, et al. Addition of infliximab to standard acute graft-versus-host disease prophylaxis following allogeneic peripheral blood cell transplantation. Biol Blood Marrow Transplant. 2008;14:783–9. doi: 10.1016/j.bbmt.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolf D, von Lilienfeld-Toal M, Wolf AM, et al. Novel treatment concepts for graft-versus-host disease. Blood. 2012;119:16–25. doi: 10.1182/blood-2011-08-339465. [DOI] [PubMed] [Google Scholar]
- 71.Koreth J, Stevenson KE, Kim HT, et al. Bortezomib, tacrolimus, and methotrexate for prophylaxis of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation from HLA-mismatched unrelated donors. Blood. 2009;114:3956–9. doi: 10.1182/blood-2009-07-231092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoffmann P, Ermann J, Edinger M, et al. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–99. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nguyen VH, Shashidhar S, Chang DS, et al. The impact of regulatory T cells on T-cell immunity following hematopoietic cell transplantation. Blood. 2008;111:945–53. doi: 10.1182/blood-2007-07-103895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–8. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 75.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061–70. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dignan FL, Clark A, Amrolia P, et al. Diagnosis and management of acute graft-versus-host disease. Br J Haematol. 2012;158:30–45. doi: 10.1111/j.1365-2141.2012.09129.x. [DOI] [PubMed] [Google Scholar]
- 77.Bacigalupo A. Management of acute graft-versus-host disease. Br J Haematol. 2007;137:87–98. doi: 10.1111/j.1365-2141.2007.06533.x. [DOI] [PubMed] [Google Scholar]
- 78.Reddy P, Ferrara JL. Immunobiology of acute graft-versus-host disease. Blood Rev. 2003;17:187–94. doi: 10.1016/s0268-960x(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 79.Thomas E, Storb R, Clift RA, et al. Bone-marrow transplantation (first of two parts) N Engl J Med. 1975;292:832–43. doi: 10.1056/NEJM197504172921605. [DOI] [PubMed] [Google Scholar]
- 80.Hings IM, Filipovich AH, Miller WJ, et al. Prednisone therapy for acute graft-versus-host disease: short- versus long-term treatment. A prospective randomized trial. Transplantation. 1993;56:577–80. doi: 10.1097/00007890-199309000-00016. [DOI] [PubMed] [Google Scholar]
- 81.Martin PJ, Rizzo JD, Wingard JR, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18:1150–63. doi: 10.1016/j.bbmt.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deeg HJ. How I treat refractory acute GVHD. Blood. 2007;109:4119–26. doi: 10.1182/blood-2006-12-041889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mielcarek M, Storer BE, Boeckh M, et al. Initial therapy of acute graft-versus-host disease with low-dose prednisone does not compromise patient outcomes. Blood. 2009;113:2888–94. doi: 10.1182/blood-2008-07-168401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martin PJ, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood. 1990;76:1464–72. [PubMed] [Google Scholar]
- 85.Weisdorf D, Haake R, Blazar B, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood. 1990;75:1024–30. [PubMed] [Google Scholar]
- 86.Hings IM, Severson R, Filipovich AH, et al. Treatment of moderate and severe acute GVHD after allogeneic bone marrow transplantation. Transplantation. 1994;58:437–42. doi: 10.1097/00007890-199408270-00008. [DOI] [PubMed] [Google Scholar]
- 87.MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–94. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 88.MacMillan ML, DeFor TE, Weisdorf DJ. The best endpoint for acute GVHD treatment trials. Blood. 2010;115:5412–7. doi: 10.1182/blood-2009-12-258442. [DOI] [PubMed] [Google Scholar]
- 89.Levine JE, Paczesny S, Mineishi S, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood. 2008;111:2470–5. doi: 10.1182/blood-2007-09-112987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Couriel DR, Saliba R, de Lima M, et al. A phase III study of infliximab and corticosteroids for the initial treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:1555–62. doi: 10.1016/j.bbmt.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cahn JY, Bordigoni P, Tiberghien P, et al. Treatment of acute graft-versus-host disease with methylprednisolone and cyclosporine with or without an anti-interleukin-2 receptor monoclonal antibody. A multicenter phase III study. Transplantation. 1995;60:939–42. [PubMed] [Google Scholar]
- 92.Lee SJ, Zahrieh D, Agura E, et al. Effect of up-front daclizumab when combined with steroids for the treatment of acute graft-versus-host disease: results of a randomized trial. Blood. 2004;104:1559–64. doi: 10.1182/blood-2004-03-0854. [DOI] [PubMed] [Google Scholar]
- 93.Cragg L, Blazar BR, Defor T, et al. A randomized trial comparing prednisone with antithymocyte globulin/prednisone as an initial systemic therapy for moderately severe acute graft-versus-host disease. Biol Blood Marrow Transplant. 2000;6:441–7. doi: 10.1016/s1083-8791(00)70036-x. [DOI] [PubMed] [Google Scholar]
- 94.Alousi AM, Weisdorf DJ, Logan BR, et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114:511–7. doi: 10.1182/blood-2009-03-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pidala J, Kim J, Anasetti C. Sirolimus as primary treatment of acute graft-versus-host disease following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15:881–5. doi: 10.1016/j.bbmt.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pavletic SZ, Fowler DH. Are we making progress in GVHD prophylaxis and treatment? Hematology Am Soc Hematol Educ Program. 2012;2012:251–64. doi: 10.1182/asheducation-2012.1.251. [DOI] [PubMed] [Google Scholar]
- 97.de Lavallade H, Mohty M, Faucher C, et al. Low-dose methotrexate as salvage therapy for refractory graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation. Haematologica. 2006;91:1438–40. [PubMed] [Google Scholar]
- 98.Kim JG, Sohn SK, Kim DH, et al. Different efficacy of mycophenolate mofetil as salvage treatment for acute and chronic GVHD after allogeneic stem cell transplant. Eur J Haematol. 2004;73:56–61. doi: 10.1111/j.1600-0609.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- 99.Krejci M, Doubek M, Buchler T, et al. Mycophenolate mofetil for the treatment of acute and chronic steroid-refractory graft-versus-host disease. Ann Hematol. 2005;84:681–5. doi: 10.1007/s00277-005-1070-0. [DOI] [PubMed] [Google Scholar]
- 100.Furlong T, Martin P, Flowers ME, et al. Therapy with mycophenolate mofetil for refractory acute and chronic GVHD. Bone Marrow Transplant. 2009;44:739–48. doi: 10.1038/bmt.2009.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pidala J, Kim J, Perkins J, et al. Mycophenolate mofetil for the management of steroid-refractory acute graft vs host disease. Bone Marrow Transplant. 2010;45:919–24. doi: 10.1038/bmt.2009.252. [DOI] [PubMed] [Google Scholar]
- 102.Messina C, Locatelli F, Lanino E, et al. Extracorporeal photochemotherapy for paediatric patients with graft-versus-host disease after haematopoietic stem cell transplantation. Br J Haematol. 2003;122:118–27. doi: 10.1046/j.1365-2141.2003.04401.x. [DOI] [PubMed] [Google Scholar]
- 103.Perfetti P, Carlier P, Strada P, et al. Extracorporeal photopheresis for the treatment of steroid refractory acute GVHD. Bone Marrow Transplant. 2008;42:609–17. doi: 10.1038/bmt.2008.221. [DOI] [PubMed] [Google Scholar]
- 104.Schmidt-Hieber M, Fietz T, Knauf W, et al. Efficacy of the interleukin-2 receptor antagonist basiliximab in steroid-refractory acute graft-versus-host disease. Br J Haematol. 2005;130:568–74. doi: 10.1111/j.1365-2141.2005.05631.x. [DOI] [PubMed] [Google Scholar]
- 105.Willenbacher W, Basara N, Blau IW, et al. Treatment of steroid refractory acute and chronic graft-versus-host disease with daclizumab. Br J Haematol. 2001;112:820–3. doi: 10.1046/j.1365-2141.2001.02582.x. [DOI] [PubMed] [Google Scholar]
- 106.Cuthbert RJ, Phillips GL, Barnett MJ, et al. Anti-interleukin-2 receptor monoclonal antibody (BT 563) in the treatment of severe acute GVHD refractory to systemic corticosteroid therapy. Bone Marrow Transplant. 1992;10:451–5. [PubMed] [Google Scholar]
- 107.Ho VT, Zahrieh D, Hochberg E, et al. Safety and efficacy of denileukin diftitox in patients with steroid-refractory acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:1224–6. doi: 10.1182/blood-2004-01-0028. [DOI] [PubMed] [Google Scholar]
- 108.Shaughnessy PJ, Bachier C, Grimley M, et al. Denileukin diftitox for the treatment of steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:188–93. doi: 10.1016/j.bbmt.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 109.Macmillan ML, Couriel D, Weisdorf DJ, et al. A phase 2/3 multicenter randomized clinical trial of ABX-CBL versus ATG as secondary therapy for steroid-resistant acute graft-versus-host disease. Blood. 2007;109:2657–62. doi: 10.1182/blood-2006-08-013995. [DOI] [PubMed] [Google Scholar]
- 110.Van Lint MT, Milone G, Leotta S, et al. Treatment of acute graft-versus-host disease with prednisolone: significant survival advantage for day +5 responders and no advantage for nonresponders receiving anti-thymocyte globulin. Blood. 2006;107:4177–81. doi: 10.1182/blood-2005-12-4851. [DOI] [PubMed] [Google Scholar]
- 111.Busca A, Locatelli F, Marmont F, et al. Recombinant human soluble tumor necrosis factor receptor fusion protein as treatment for steroid refractory graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2007;82:45–52. doi: 10.1002/ajh.20752. [DOI] [PubMed] [Google Scholar]
- 112.Wolff D, Roessler V, Steiner B, et al. Treatment of steroid-resistant acute graft-versus-host disease with daclizumab and etanercept. Bone Marrow Transplant. 2005;35:1003–10. doi: 10.1038/sj.bmt.1704929. [DOI] [PubMed] [Google Scholar]
- 113.Hoda D, Pidala J, Salgado-Vila N, et al. Sirolimus for treatment of steroid-refractory acute graft-versus-host disease. Bone Marrow Transplant. 2010;45:1347–51. doi: 10.1038/bmt.2009.343. [DOI] [PubMed] [Google Scholar]
- 114.Gomez-Almaguer D, Ruiz-Arguelles GJ, del Carmen Tarin-Arzaga L, et al. Alemtuzumab for the treatment of steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2008;14:10–5. doi: 10.1016/j.bbmt.2007.08.052. [DOI] [PubMed] [Google Scholar]
- 115.Schub N, Gunther A, Schrauder A, et al. Therapy of steroid-refractory acute GVHD with CD52 antibody alemtuzumab is effective. Bone Marrow Transplant. 2011;46:143–7. doi: 10.1038/bmt.2010.68. [DOI] [PubMed] [Google Scholar]
- 116.Couriel D, Saliba R, Hicks K, et al. Tumor necrosis factor-alpha blockade for the treatment of acute GVHD. Blood. 2004;104:649–54. doi: 10.1182/blood-2003-12-4241. [DOI] [PubMed] [Google Scholar]
- 117.Ruutu T, Niederwieser D, Gratwohl A, et al. A survey of the prophylaxis and treatment of acute GVHD in Europe: a report of the European Group for Blood and Marrow, Transplantation (EBMT). Chronic Leukaemia Working Party of the EBMT. Bone Marrow Transplant. 1997;19:759–64. doi: 10.1038/sj.bmt.1700745. [DOI] [PubMed] [Google Scholar]
- 118.Roy J, McGlave PB, Filipovich AH, et al. Acute graft-versus-host disease following unrelated donor marrow transplantation: failure of conventional therapy. Bone Marrow Transplant. 1992;10:77–82. [PubMed] [Google Scholar]
- 119.Sullivan KM, Kopecky KJ, Jocom J, et al. Immunomodulatory and antimicrobial efficacy of intravenous immunoglobulin in bone marrow transplantation. N Engl J Med. 1990;323:705–12. doi: 10.1056/NEJM199009133231103. [DOI] [PubMed] [Google Scholar]
- 120.Khoury H, Kashyap A, Adkins DR, et al. Treatment of steroid-resistant acute graft-versus-host disease with anti-thymocyte globulin. Bone Marrow Transplant. 2001;27:1059–64. doi: 10.1038/sj.bmt.1703032. [DOI] [PubMed] [Google Scholar]
- 121.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–41. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 122.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–86. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 123.Fang B, Song YP, Liao LM, et al. Treatment of severe therapy-resistant acute graft-versus-host disease with human adipose tissue-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;38:389–90. doi: 10.1038/sj.bmt.1705457. [DOI] [PubMed] [Google Scholar]
- 124.Ringden O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–7. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 125.Fang B, Song Y, Liao L, et al. Favorable response to human adipose tissue-derived mesenchymal stem cells in steroid-refractory acute graft-versus-host disease. Transplant Proc. 2007;39:3358–62. doi: 10.1016/j.transproceed.2007.08.103. [DOI] [PubMed] [Google Scholar]
- 126.Muller I, Kordowich S, Holzwarth C, et al. Application of multipotent mesenchymal stromal cells in pediatric patients following allogeneic stem cell transplantation. Blood Cells Mol Dis. 2008;40:25–32. doi: 10.1016/j.bcmd.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 127.von Bonin M, Stolzel F, Goedecke A, et al. Treatment of refractory acute GVHD with third-party MSC expanded in platelet lysate-containing medium. Bone Marrow Transplant. 2009;43:245–51. doi: 10.1038/bmt.2008.316. [DOI] [PubMed] [Google Scholar]
- 128.Kebriaei P, Isola L, Bahceci E, et al. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:804–11. doi: 10.1016/j.bbmt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 129.Chakrabarti S, Mackinnon S, Chopra R, et al. High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of Campath-1H in delaying immune reconstitution. Blood. 2002;99:4357–63. doi: 10.1182/blood.v99.12.4357. [DOI] [PubMed] [Google Scholar]
- 130.Chakrabarti S, Mautner V, Osman H, et al. Adenovirus infections following allogeneic stem cell transplantation: incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood. 2002;100:1619–27. doi: 10.1182/blood-2002-02-0377. [DOI] [PubMed] [Google Scholar]
- 131.Bucher C, Koch L, Vogtenhuber C, et al. IL-21 blockade reduces graft-versus-host disease mortality by supporting inducible T regulatory cell generation. Blood. 2009;114:5375–84. doi: 10.1182/blood-2009-05-221135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hanash AM, Kappel LW, Yim NL, et al. Abrogation of donor T-cell IL-21 signaling leads to tissue-specific modulation of immunity and separation of GVHD from GVL. Blood. 2011;118:446–55. doi: 10.1182/blood-2010-07-294785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang L, Tao R, Hancock WW. Using histone deacetylase inhibitors to enhance Foxp3(+) regulatory T-cell function and induce allograft tolerance. Immunol Cell Biol. 2009;87:195–202. doi: 10.1038/icb.2008.106. [DOI] [PubMed] [Google Scholar]
- 134.Taylor PA, Panoskaltsis-Mortari A, Freeman GJ, et al. Targeting of inducible costimulator (ICOS) expressed on alloreactive T cells down-regulates graft-versus-host disease (GVHD) and facilitates engraftment of allogeneic bone marrow (BM) Blood. 2005;105:3372–80. doi: 10.1182/blood-2004-10-3869. [DOI] [PubMed] [Google Scholar]
- 135.Kwan WH, Hashimoto D, Paz-Artal E, et al. Antigen-presenting cell-derived complement modulates graft-versus-host disease. J Clin Invest. 2012;122:2234–8. doi: 10.1172/JCI61019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hashimoto D, Chow A, Greter M, et al. Pretransplant CSF-1 therapy expands recipient macrophages and ameliorates GVHD after allogeneic hematopoietic cell transplantation. J Exp Med. 2011;208:1069–82. doi: 10.1084/jem.20101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Klamer G, Shen S, Song E, et al. GSK3 inhibition prevents lethal GVHD in mice. Exp Hematol. 2013;41:39–55. doi: 10.1016/j.exphem.2012.09.005. e10. [DOI] [PubMed] [Google Scholar]
- 138.Paczesny S. Discovery and validation of graft-versus-host disease biomarkers. Blood. 2013;121:585–94. doi: 10.1182/blood-2012-08-355990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ciceri F, Bonini C, Stanghellini MT, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol. 2009;10:489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 140.Ciceri F, Bonini C, Marktel S, et al. Antitumor effects of HSV-TK-engineered donor lymphocytes after allogeneic stem-cell transplantation. Blood. 2007;109:4698–707. doi: 10.1182/blood-2006-05-023416. [DOI] [PubMed] [Google Scholar]
- 141.Qian L, Mei K, Shen J, et al. Administration of hydrogen-rich saline protects mice from lethal acute graft-versus-host disease (aGVHD) Transplantation. 2013;95:658–62. doi: 10.1097/TP.0b013e31827e6b23. [DOI] [PubMed] [Google Scholar]
- 142.Qian L, Shen J. Hydrogen therapy may be an effective and specific novel treatment for Acute Graft-versus-host disease(GVHD) J Cell Mol Med. 2013 doi: 10.1111/jcmm.12081. doi: 10.1111/jcmm.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Saitoh Y, Harata Y, Mizuhashi F, et al. Biological safety of neutral-pH hydrogen-enriched electrolyzed water upon mutagenicity, genotoxicity and subchronic oral toxicity. Toxicol Ind Health. 2010;26:203–16. doi: 10.1177/0748233710362989. [DOI] [PubMed] [Google Scholar]
- 144.Nakao A, Toyoda Y, Sharma P, et al. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome-an open label pilot study. J Clin Biochem Nutr. 2010;46:140–9. doi: 10.3164/jcbn.09-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hamilton BK, Copelan EA. Concise review: the role of hematopoietic stem cell transplantation in the treatment of acute myeloid leukemia. Stem Cells. 2012;30:1581–6. doi: 10.1002/stem.1140. [DOI] [PubMed] [Google Scholar]
- 146.Jones CV, Copelan EA. Treatment of acute myeloid leukemia with hematopoietic stem cell transplantation. Future Oncol. 2009;5:559–68. doi: 10.2217/fon.09.20. [DOI] [PubMed] [Google Scholar]
- 147.Hamadani M, Awan FT, Copelan EA. Hematopoietic stem cell transplantation in adults with acute myeloid leukemia. Biol Blood Marrow Transplant. 2008;14:556–67. doi: 10.1016/j.bbmt.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 148.Pant S, Copelan EA. Hematopoietic stem cell transplantation in multiple myeloma. Biol Blood Marrow Transplant. 2007;13:877–85. doi: 10.1016/j.bbmt.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 149.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–26. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 150.Gratwohl A, Niederwieser D. History of hematopoietic stem cell transplantation: evolution and perspectives. Curr Probl Dermatol. 2012;43:81–90. doi: 10.1159/000335266. [DOI] [PubMed] [Google Scholar]


