Abstract
Chemokine-driven interactions of immune cells are essential for effective anti-tumor immunity. Human natural killer (NK) cells can be primed by the IL1-related pro-inflammatory cytokine IL-18 for unique helper activity, which promotes dendritic cell (DC) activation and DC-mediated induction of type-1 immune responses against cancer. Here we show that such IL-18-primed ‘helper’ NK cells produce high levels of the immature DC (iDC)-attracting chemokines CCL3 and CCL4 upon exposure to tumor cells or the additional inflammatory signals IFNα, IL-15, IL-12, or IL-2. These ‘helper’ NK cells potently attract iDCs in a CCR5-dependent mechanism and induce high DC production of CXCR3 and CCR5 ligands (CXCL9, CXCL10, and CCL5), facilitating the subsequent recruitment of type-1 effector CD8+ T (Teff) cells. Using cells isolated from the malignant ascites of patients with advanced ovarian cancer, we show that ‘helper’ NK cell-inducing factors can be used to enhance local production of Teff cell-recruiting chemokines. Our findings reveal the unique chemokine expression profile of ‘helper’ NK cells and highlight the potential for utilizing two-signal-activated NK cells to promote homing of type-1 immune effectors to the human tumor environment.
Keywords: natural killer cells, dendritic cells, chemokines, IL-18, ovarian cancer
Introduction
Natural killer (NK) cells are innate sentinel cells recognizing early signs of tissue stress, infection, or transformation (1, 2). NK cells integrate signals from activating and inhibitory receptors engaged by pathogen products and/or products released from affected cells, such as type I interferons (IFNα/β) (3, 4), and play a critical ‘helper’ role in initiating and directing dendritic cell (DC)-regulated immune responses (5–8). Constituting an early source of IFNγ and TNFα, NK cells are capable of promoting DC maturation and DC-mediated induction of type-1-polarized helper CD4+ T cell (Th1) and cytotoxic CD8+ T cell (CTL) responses (9–12).
Resting NK cells require activation for the acquisition of different effector functions, and specific NK cell functions can be preferentially driven by distinct cytokines, including IL-18 (1). IL-18 is an IL-1 family cytokine widely expressed by multiple barrier cell types, including epithelial cells in the gut and lung and keratinocytes in the skin, and by early-responding innate cells, such as monocytes and macrophages (13). Expression of the IL-18 pro-cytokine is further enhanced by toll-like receptor signaling, with production of the mature cytokine controlled by activated caspase-1 (14). Caspase-1 activity in turn depends on inflammasome activation, which is likewise downstream of pattern receptor recognition (14). Thus, IL-18 represents an early product of the developing response to tissue damage, infection, or transformation. We have previously shown that unlike IL-2, which promotes ‘killer’ NK cell differentiation characterized by enhanced cytotoxicity against tumor and DC targets, IL-18 uniquely primes human NK cells for preferential non-cytotoxic ‘helper’ activity upon subsequent stimulation with multiple distinct secondary factors, including tumor cells and type I interferons. We demonstrated that these IL-18-primed ‘helper’ NK cells are capable of inducing DC activation and potentiating DC-mediated induction of tumor-specific Th1 and CTL adaptive immune responses through an IFNγ- and TNFα-dependent mechanism (10, 11), including in patients with late-stage cancer (15). Here, we investigate whether IL-18 may also uniquely regulate human NK cell chemokine production to enhance interaction with DCs, and subsequently influence productive chemokine-driven interactions with effector T cells, particularly in the context of the human tumor environment.
While prior studies have described the ability of activated DCs to attract NK cells, in mechanisms involving CXCR3 and CXCR1 and their chemokine ligands (16–18), our current data indicate that human NK cells can initiate chemokine-driven NK-DC interaction in response to signals associated with infection or neoplastic cell transformation. We show that IL-18-primed NK cells can act as the inducers of local immune cell accumulation, promoting the CCR5-dependent attraction of immature DCs and driving subsequent DC production of the effector CD8+ T (Teff) cell-recruiting chemokines CXCL9, CXCL10, and CCL5, both in cells isolated from the blood of healthy donors as well as in tumor-associated cells isolated from the malignant ascites of advanced (stage III–IV) ovarian cancer (OvCa) patients.
Materials and Methods
Media, cell lines, and reagents
Serum-free CellGenix DC medium (CellGenix Technologie Transfer GmbH) was used for short-term culture of human NK cells and for DC generation. T cells, ovarian cancer ascites-derived cells, and K562 cells were cultured in Iscove’s Modified Dulbecco’s Medium (IMDM) containing 10% fetal bovine serum and 1% L-glutamine and penicillin/streptomycin (all from Gibco, Invitrogen). K562 cells were obtained from American Type Culture Collection, expanded and cryopreserved after receipt, and used for experiments from recently thawed stocks. The following factors were used throughout the study: IL-18 (MBL International); IL-2 (Chiron); IFNα (Intron A, IFN-α-2b; Schering-Plough); IL-12 (PeproTech); IL-15 (Sigma-Aldrich); IL-1β (Miltenyi Biotech); and granulocyte macrophage colony-stimulating factor (GM-CSF) and IL-4 (Schering-Plough).
NK cell and CD8+ T cell isolation
Peripheral blood from healthy donors was harvested by venipuncture under IRB-approved protocols. NK cells (CD56+CD3−) and naïve CD8+ T cells (CD8+CD45RA+CCR7highCD45RO−CD56−CD57−) were isolated by negative magnetic selection (>95% pure in both cases) using the EasySep system (StemCell Technologies), according to the manufacturer’s protocol. When indicated, CD3−CD56brightCD16− and CD3−CD56dimCD16+ NK cell subsets were flow-sorted using a MoFlo high-speed cell sorter (DakoCytomation), after labeling with appropriate antibodies.
Blood DC isolation
Human blood DCs, including all three major subsets [CD1c+ (BDCA-1+), CD141+ (BDCA-3+), and CD304+ (BDCA-4+)], were isolated from healthy donor peripheral blood by magnetic selection using the Blood Dendritic Cell Isolation Kit II (Miltenyi Biotec), according to the manufacturer’s protocol. Cells were >95% HLA-DR+ and >99% CD14−CD19−CD3−CD56−.
Generation of DCs
Peripheral blood mononuclear cells (PBMCs) were isolated from the peripheral blood of healthy donors by density gradient separation using Lymphocyte Separation Medium (Cellgro Mediatech). Monocyte fractions were further isolated by CD14 positive magnetic selection (Miltenyi Biotech). Immature DCs were generated from monocytes cultured for 6 days in 24-well plates at 4×105 cells/well in GM-CSF and IL-4 (both 1000 IU/ml).
NK cell stimulation and DC co-culture
NK cells were isolated and plated in 48-well plates at 1×106 cells/ml. NK cells were stimulated with IL-18 (200 ng/ml) or IL-2 (250 IU/ml) together with IFNα (1000 IU/ml), IL-12 (5 ng/ml), IL-2 (250 IU/ml), IL-15 (100 ng/ml), or K562 cells (1×105 cells/well). Alternatively, NK cells were pre-treated with IL-18 or IL-2 for 24 h, washed thoroughly, and re-plated in the presence of IFNα, IL-12, IL-2, IL-15, or K562 cells as a secondary stimulus. Expression of chemokines was analyzed at 4 h by quantitative real-time PCR or at 24 h by specific ELISA. When indicated, NK cells were pre-treated for 30 min at 37°C with blocking antibodies to NKG2D (clone 1D11; 10 μg/ml; Biolegend), NKp30 (clone P3015; 10 μg/ml; Biolegend), or DNAM-1 (clone DX11; 10 μg/ml; Abcam) before co-culture with K562 cells. For NK cell activation of DCs, previously isolated and cryopreserved autologous NK cells were thawed and added to DC cultures at 1.5×105 cells/well to day 6 DC cultures in the presence of IL-18 (200 ng/ml) and IFNα (1000 IU/ml). When indicated, soluble decoy receptors to TNFα (sTNFR1; 1 μg/ml; R&D Systems) and IFNγ (sIFNγR1; 10 μg/ml; R&D Systems) were added to cultures at co-culture initiation. Supernatants were collected at 48 h for chemokine analysis. To assess the stability of DC chemokine production, NK-DC co-cultures were harvested and washed, and NK cells were removed by CD56 positive magnetic selection (StemCell Technologies). DCs were then re-plated in 96-well plates at 2×104 cells/well. To mimic interaction with CD40L-expressing CD4+ T cells, DCs were co-cultured with CD40L-transfected J558 cells (a gift from Dr. P. Lane, University of Birmingham, United Kingdom) at 5×104 cells/well, which in previous studies proved equivalent to activated CD4+ T cells and soluble CD40L (19). Supernatants were collected after 24 h and analyzed by specific ELISAs for CXCL9, CXCL10, CCL5, and CCL22 (PeproTech).
Generation of effector CD8+ T cells by in vitro sensitization
Naive CD8+ T cells were activated with staphylococcal enterotoxin B-pulsed DCs matured from day 6 immature DCs by 36 h treatment with TNFα (50 ng/ml), IL-1β (25 ng/ml), IFNγ (1000 IU/ml), poly-I:C (20 μg/ml), and IFNα (3000 IU/ml), as previously described (19). DCs matured in this manner have been extensively demonstrated to be efficient inducers of CD45RO+granzymeBhigh effector-type CD8+ T cells (Teff cells) expressing high levels of the peripheral homing chemokine receptors CXCR3 and CCR5 (19, 20). On days 5–6, expanded CD8+ T cells were analyzed to confirm CTL phenotype and expression of chemokine receptors, and were subsequently used for chemotaxis assays.
Chemotaxis
Chemotaxis assays were performed using 24-(Trans)well plates with 5 μm pore size polycarbonate membranes (Corning), as previously described (21). For DC chemotaxis, the lower chamber was filled with supernatants from 36 h cultures of NK cells treated with IL-18 (200 ng/ml) or IL-2 (250 IU/ml) together with IFNα (1000 IU/ml) in CellGenix medium, and the upper chamber was loaded with blood-isolated DCs or day 6 monocyte-derived immature DCs (2×105). When indicated, DCs were treated for 30 min with an anti-CCR5 blocking antibody (Clone 2D7, 20 μg/ml; BD Biosciences) before chemotaxis to block CCR5-dependent chemotaxis. Alternatively, DCs were treated for 30 min with recombinant CCL3, CXCL8, XCL1, CCL20, or CXCL12 (all at 200 ng/ml; all from PeproTech) before chemotaxis, previously shown to be effective for desensitizing specific chemokine receptor responsiveness (16, 21). For effector CD8+ T cell chemotaxis, the lower chamber was filled with supernatants from 42 h co-cultures of NK cells and DCs, and the upper chamber was loaded with effector CD8+ T cells (2×105) generated as described above. Cell numbers in the bottom chambers were assessed after 3 h by flow cytometry, and specific chemotaxis for each condition was calculated as the number of migrated cells subtracted by the number of migrated cells toward media-only controls.
Isolation of OvCa ascites cells
Human OvCa ascites were obtained intraoperatively from previously-untreated patients with advanced (stage III or IV) epithelial ovarian cancer undergoing primary surgical debulking for clinical staging. Written informed consent was obtained prior to any specimen collection, and the nature and possible consequences of the studies were explained. All specimens were provided under a protocol approved by the University of Pittsburgh Institutional Review Board (IRB0406147). Primary OvCa ascites cells were harvested by centrifugation. NK cell-enriched and NK cell-depleted fractions were generated from bulk OvCa ascites cells by CD56 positive magnetic selection (StemCell Technologies).
Flow cytometry
Cell surface and intracellular immunostaining analyses were performed using an Accuri C6 Flow Cytometer. NK cells and T cells were stained with the dye-conjugated anti-human mouse monoclonal antibodies CD56-PE-Cy5 (Beckman Coulter), CD3-PE (eBioscience), Granzyme B-PE (Invitrogen), and CD16-FITC, CD8-PE-Cy5, CD45RA-FITC, CD45RO-PE, and CD57-FITC (BD Biosciences). Chemokine receptors on DCs and T cells were stained with the dye-conjugated anti-human mouse monoclonal antibodies CCR1-PE and CCR7-FITC (R&D Systems) and CCR5-FITC, CCR6-PE, CXCR1-FITC, CXCR3-PE, and CXCR4-PE (BD Biosciences), and the dye-conjugated anti-human goat polyclonal antibody XCR1-PE (R&D Systems). The corresponding mouse antibody isotype controls IgG1-FITC, IgG2a-FITC, IgG2b-FITC, IgG1-PE, IgG2a-PE, IgG2b-PE, and IgG1-PE-Cy5 (BD Biosciences) and normal goat antibody control IgG-PE (R&D Systems) were used, as appropriate. Before staining, the cells were treated for 20 min at 4°C in PBS buffer containing 2% human serum, 0.5% BSA, 0.1% NaN3, and 1 μg/ml of mouse IgG (Sigma-Aldrich) to block non-specific binding. Cell permeabilization for intracellular staining was performed using 0.1% Triton X-100 (Sigma) in PBS for 15 min. Cells were stained for 40 min at 4°C followed by washing with PBS buffer containing 0.5% BSA and 0.1% NaN3, then fixed and stored in 4% paraformaldehyde until analysis.
Quantitative real-time PCR
Analysis of mRNA expression was performed using the StepOne Plus System (Applied Biosystems), as previously described (21), using inventoried primer/probe sets. Preliminary kinetic analysis (data not shown) determined optimal expression of NK cell-expressed IFNγ, TNFα, CCL3, CCL4, CXCL8, and XCL1 at 4 h following cytokine stimulation of both purified NK cells and bulk OvCa ascites cells, and optimal expression of CXCL9 and CXCL10 in bulk OvCa ascites cells at 24 h following IL-18/IFNα stimulation. The expression of each gene was normalized to HPRT1 and expressed as fold increase (2−ΔCT), where ΔCT = CT (target gene) − CT (HPRT1).
ELISA
Supernatants from 48 h co-cultures of NK cells and DCs were analyzed for CXCL9, CXCL10, CCL5, and CCL22 by indirect sandwich ELISA using specific matched primary and biotinylated-secondary antibody pairs (PeproTech), as previously described (21). When indicated, DCs were harvested, washed, and re-plated in the presence of CD40L-transfected J558 cells (for rationale, see above), and 24 h culture supernatants were analyzed for levels of CXCL9, CXCL10, CCL5, and CCL22.
Statistical analysis
Data was analyzed using unpaired and paired t tests (two-tailed) and one-way and two-way ANOVA, where appropriate. Significance was judged at an α of 0.05.
Results
Unique role of IL-18 in priming human NK cell attraction of DCs
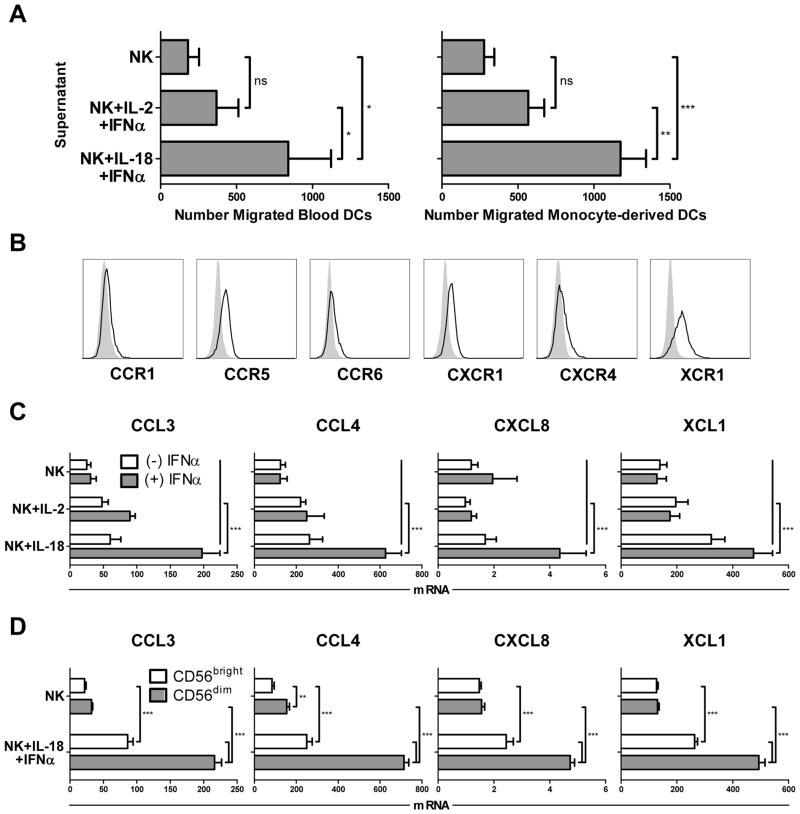
While activated DCs have been previously reported to attract and activate NK cells (16–18), we observed that the ‘helper’ pathway of NK cell activation, induced by IL-18 and secondary signals like tumor cells or IFNα (10, 22), strongly promoted NK cell attraction of autologous DCs, either directly isolated from peripheral blood or generated in vitro from monocyte precursors (Fig. 1A). In contrast, IL-2-induced ‘killer’ effector NK cells, characterized by enhanced cytotoxicity and ability to kill DCs (10), were not similarly effective in attracting DCs. Prompted by the essential role of the chemokine system in governing cellular interactions during immune responses (23, 24), we investigated the role of ‘helper’ NK cell-produced chemokines in their superior ability to attract DCs. Immature monocyte-derived DCs (iDCs) expressed similarly high levels of CCR5 and CXCR1 as blood-isolated DCs, as well as distinct but lower levels of CXCR4, with XCR1 expression limited to monocyte-derived iDCs (Fig. 1B and Supplementary Fig. S1). Analysis of the expression of the known ligands for these DC chemokine receptors in NK cells revealed that the NK cells treated with either IL-2 or IL-18 alone expressed only limited levels of CCL3, CCL4, and CXCL8 (Fig. 1C), ligands for the chemokine receptors CCR1/5 and CXCR1, respectively, which are known to mediate migration of iDCs in peripheral tissues (25). Similarly, NK cells treated with IL-2 or IL-18 alone showed minimal to modest enhancement, respectively, in the expression of XCL1, the ligand for the receptor XCR1 and a chemokine implicated in the attraction of DCs highly efficient in antigen cross-presentation (26, 27). In contrast, combined stimulation of NK cells with IL-18 and IFNα, a factor recently shown to be important for initiating spontaneous anti-tumor immune responses in vivo (28, 29) and a secondary signal known to co-activate cytokine secretion in human ‘helper’ NK cells (10, 11), induced a strong synergistic enhancement in the expression of CCL3, CCL4, CXCL8, and XCL1 (Fig. 1C). Such two-signal induction of DC-attracting chemokines parallels the mode of induction of the DC-activating cytokines IFNγ and TNFα in human NK cells (10, 11). Expression of the additional chemokines CCL20 and CXCL12, ligands for the receptors CCR6 and CXCR4, were not observed in NK cells under any mode of stimulation (data not shown).
Figure 1.
Unique role of IL-18 in priming human NK cell attraction of DCs. (A) Chemotaxis of peripheral blood-isolated DCs (left) or day 6 monocyte-derived immature DCs (iDCs; right) toward culture supernatants from autologous NK cells stimulated for 36 h with IL-2 and IFNα or IL-18 and IFNα. Data shown represent the mean (± SD) number of specific migrated DCs from independent donors across independent experiments (3 donors for blood DCs; 5 donors for monocyte-derived DCs). (B) Surface expression (open histograms) of CCR1, CCR5, CCR6, CXCR1, CXCR4, and XCR1 on monocyte-derived iDCs. Gray filled histograms represent isotype controls. (C) NK cells were incubated for 4 h in the presence of IL-2, IL-18, and/or IFNα, and subsequently analyzed for expression of the chemokines CCL3, CCL4, CXCL8, and XCL1. Data are expressed as ratios between the expression of individual chemokine genes and HPRT1, and represent the mean (± SD) of 5 independent donors. (D) Sorted CD56bright and CD56dim NK cells were incubated for 4 h in the absence or presence of IL-18/IFNα, and subsequently analyzed for expression of CCL3, CCL4, CXCL8, and XCL1. Data are expressed as ratios between the expression of individual chemokine genes and HPRT1, and shown as the mean expression (± SD) of triplicate cultures. Data represent one of two independent experiments, which both yielded similar results. ***p<0.001, **p<0.01, *p<0.05, ns: p>0.05 compared to indicated groups.
Additional NK cell subset analysis revealed that the CD56dim population was particularly responsive to IL-18-driven chemokine induction (Fig. 1D), while the CD56bright NK cell subset responded to IL-18 to a significantly lesser degree. This is consistent with prior work implicating the CD56dim subset as the predominant target of IL-18 in driving NK cell acquisition of lymph node-homing CCR7 and the early secretion of DC-polarizing IFNγ (10), as well as recent findings implicating the CD56dim subset as a major producer of cytokines and chemokines, particularly at early activation time-points (30, 31).
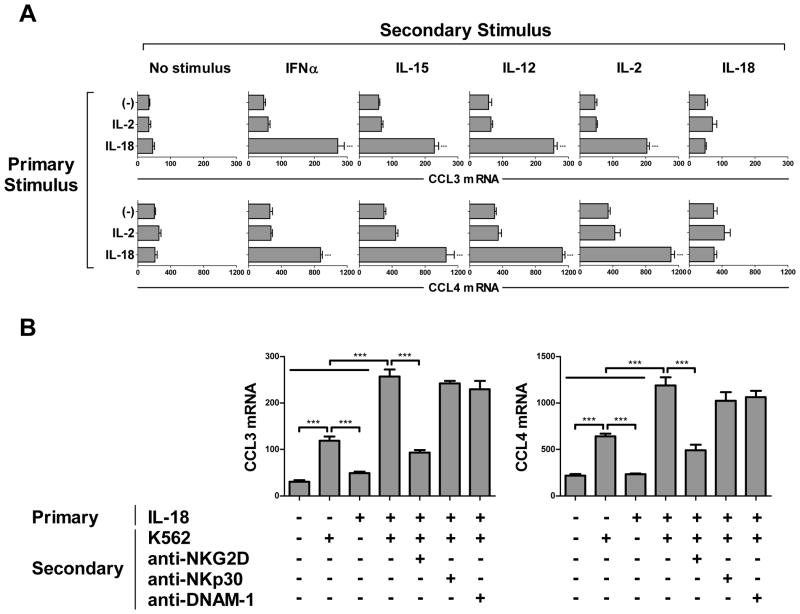
The ability to induce NK cell expression of DC-attracting chemokines was a specific feature of IL-18, since it could not be reproduced by IL-2 or IFNα (both known NK cell activating factors (2)) alone or in combination (Fig. 1C), nor by IL-1β, a member of the same family of cytokines as IL-18 (data not shown). The unique character of IL-18-induced ‘helper’ NK cells was further supported by the observation that only NK cells primed with IL-18 responded with enhanced expression of DC-attracting chemokines when exposed to such secondary stimuli as IFNα, IL-15, IL-12, and IL-2 (Fig. 2A). In contrast, providing these stimuli in reverse order (primary IL-2 followed by secondary stimulation with IL-18) was ineffective in inducing NK cell expression of DC-attracting chemokines, demonstrating that enhanced DC-recruiting function is a specific feature of IL-18-induced helper NK cells, rather than a general outcome of NK cell activation. IL-18 further primed NK cells for the expression of DC-attracting chemokines in response to multiple secondary pro-inflammatory cytokines (Fig. 2A) or in response to K562 tumor cells (Fig. 2B and Supplementary Fig. S2), with the latter effect involving NKG2D-mediated recognition of tumor targets. These results indicate the role of IL-18 in priming NK cells for the attraction of DCs following subsequent NK cell exposure to such diverse signals as inflammatory cytokines and tumor cell recognition.
Figure 2.
IL-18 synergizes with multiple secondary stimuli in inducing NK cell expression of DC-attracting chemokines. (A) NK cells were pre-treated for 24 h in the absence or presence of IL-2 or IL-18, washed, and re-plated in the absence or presence of IFNα, IL-15, IL-12, IL-2, or IL-18. The expression of CCL3 (top) and CCL4 (bottom) were analyzed after 4 h incubation with the secondary stimulus. Data are expressed as ratios between the expression of individual chemokine genes and HPRT1, and recorded as the mean expression (± SD) assayed in triplicate cultures. Data represent one of three independent experiments, which all yielded similar results. (B) NK cells were pre-treated for 24 h in the absence or presence of IL-18, washed, and re-plated in the absence or presence of K562 cells (5:1 NK:K562 ratio). When indicated, NK cells were pre-treated for 30 min with blocking antibodies to NKG2D, NKp30, or DNAM-1 before co-culture with K562 cells. The expression of CCL3 (left) and CCL4 (right) were analyzed after 4 h activation with the secondary stimulus. Data are expressed as ratios between the expression of individual chemokine genes and HPRT1, and recorded as the mean expression (± SD) assayed in triplicate cultures. Data represent one of three independent experiments, which all yielded similar results. ***p<0.001 compared to indicated groups or compared to all groups when not specifically indicated.
Key role of CCR5 in the recruitment of immature DCs by IL-18-primed NK cells
Desensitization of specific chemokine receptors on iDCs with a large panel of DC and NK cell-relevant chemokines (Fig. 3A; see Materials and Methods for discussion of the technique) revealed a highly selective role for CCR5, but not CCR6, XCR1, CXCR1, or CXCR4, in the recruitment of autologous iDCs by IL-18-primed NK cells. Specific antibody blockade of the CCR5 receptor (Fig. 3B) confirmed the key role of this receptor in helper NK cell-mediated iDC attraction.
Figure 3.
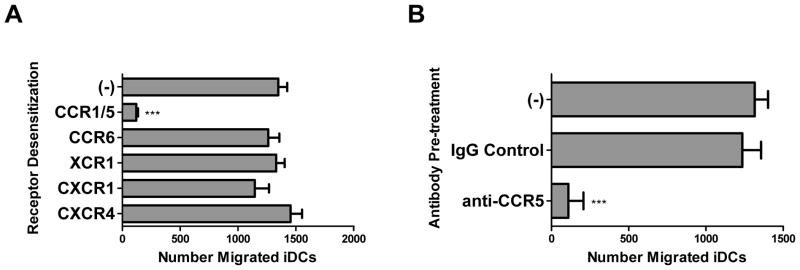
IL-18-primed NK cells attract immature DCs through a CCR5-dependent mechanism. (A–B) iDCs were pre-treated for 30 min with the chemokines CCL3, CCL20, XCL1, CXCL8, or CXCL12 (A) to block the DC chemokine receptors CCR1/5, CCR6, XCR1, CXCR1, or CXCR4, respectively, or treated with a blocking anti-CCR5 monoclonal antibody (B) before migration toward 36 h supernatants collected from IL-18/IFNα-stimulated autologous NK cells. Data is shown as mean (± SD) number of specific migrated iDCs in triplicate cultures. Data shown was obtained from one representative experiment of three performed, all yielding similar results. ***p<0.001 compared to all groups.
IL-18-primed NK cells collaborate with DCs in the recruitment of effector CD8+ T cells
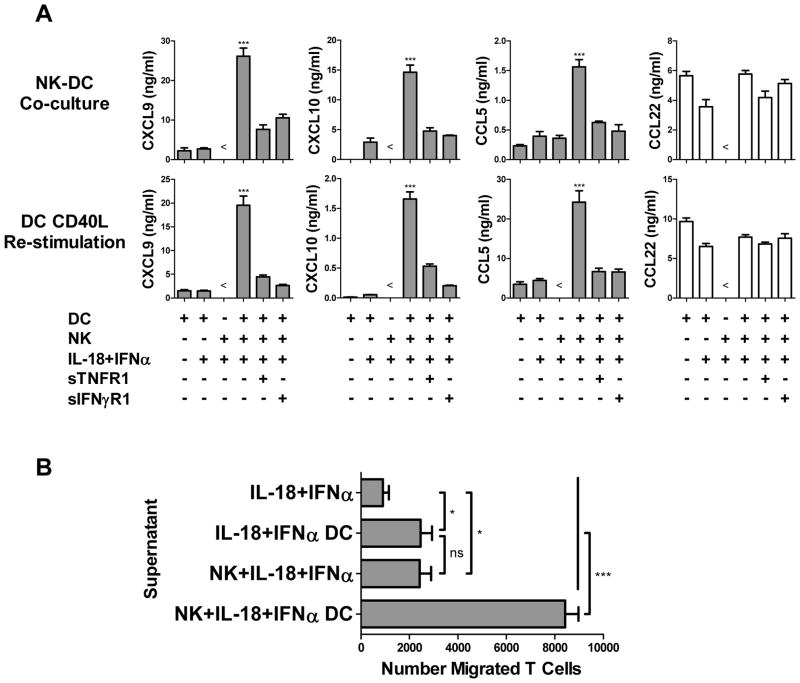
Co-culture of IL-18-primed NK cells with autologous iDCs resulted in highly elevated levels of CXCL9, CXCL10, and CCL5 (Fig. 4A, top), the chemokines that have been implicated in the attraction of type-1 Teff cell subsets central to efficient anti-tumor responses (32–34). This effect was not accompanied by an increase in the secretion of CCL22, a regulatory T cell-attracting chemokine (35). The induction of these Teff cell-recruiting chemokines in NK-DC co-cultures was dependent on TNFα and IFNγ, since the enhanced chemokine secretion was abrogated upon addition of soluble TNF and IFNγ decoy receptors to the co-cultures (Fig. 4A, top). Elevated production of Teff cell-recruiting chemokines by NK cell-activated DCs was maintained even after subsequent harvesting, washing, removal of NK cells, and re-stimulation of the DCs with CD40L (Fig. 4A, bottom), demonstrating the long-term impact of IL-18-primed NK cells on DC chemokine production.
Figure 4.
IL-18-primed NK cells induce DC production of Teff cell-recruiting chemokines, promoting Teff cell attraction. NK cells were added to autologous day 6 DCs (1:2 NK:DC ratio) in the presence of IL-18 and IFNα. After 48 h, co-culture supernatants were harvested for analysis and chemotaxis experiments, and DCs were harvested, washed, depleted of NK cells, and re-stimulated with CD40L for 24 h. (A) CXCL9, CXCL10, CCL5, and CCL22 levels in supernatants of untreated immature DCs (iDCs) or DCs exposed to IL-18/IFNα with or without autologous NK cells, in the additional presence or absence of soluble TNF (sTNFR1) or IFNγ (sIFNγR1) decoy receptors, after 48 h co-culture (top) or following harvesting, washing, NK cell depletion, and 24 h CD40L stimulation (bottom). (B) Migration of effector CD8+ T cells (see Materials and Methods for generation) toward supernatants collected from 48 h cultures of IL-18/IFNα alone, NK cells treated with IL-18/IFNα, or DCs exposed to IL-18/IFNα with or without autologous NK cells. Data recorded as mean (± SD) in triplicate cultures from one representative experiment of three performed, all yielding similar results. ***p<0.001, *p<0.05, ns: p>0.05 compared to indicated groups or compared to all groups when not specifically indicated. < indicates levels were below the limit of detection of the assay.
Supernatants from NK cell-activated DCs were highly efficient at recruiting Teff cells (Fig. 4B). Importantly, although supernatants from two-signal-activated NK cells alone or IL-18/IFNα-activated DCs alone were capable of mild Teff cell attraction over baseline, the supernatants generated from NK-DC interaction had a greatly- and synergistically-enhanced capacity for Teff cell recruitment, demonstrating the key role of DCs in NK cell-initiated Teff cell attraction.
NK cell-mediated enhancement of Teff cell-recruiting chemokines can be induced in the human ovarian cancer environment
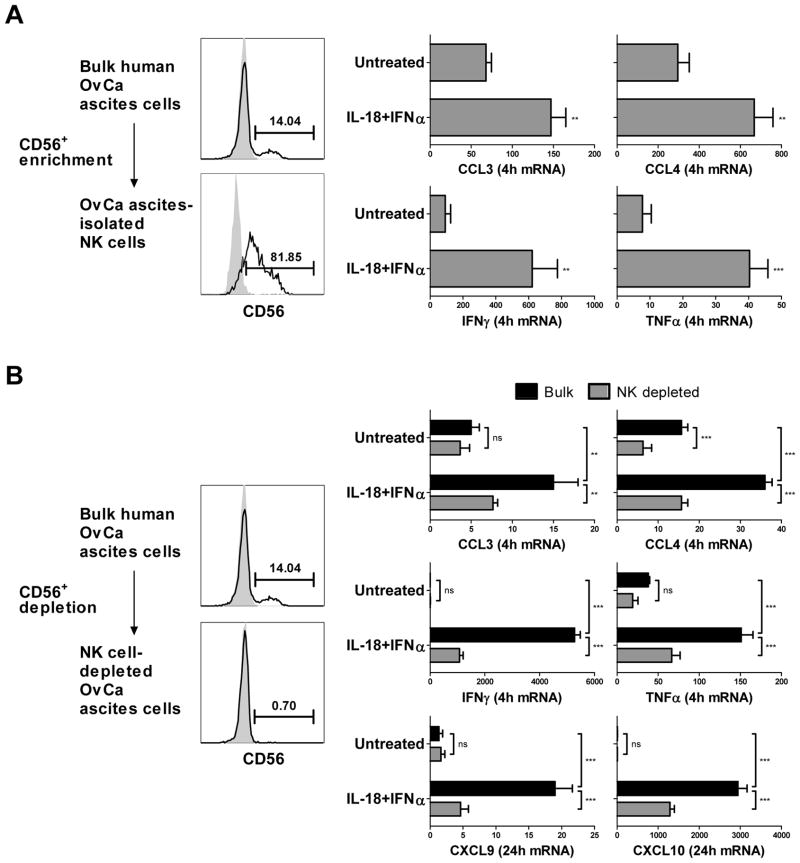
In order to assess the potential for utilizing NK ‘helper’ cell paradigms in the therapy of cancer patients, we evaluated the ability of IL-18 and NK cells to regulate chemokine production in the malignant ascites of patients with stage III–IV epithelial ovarian cancer. Similar to what has been previously reported (36), significant populations of CD3−CD56+ NK cells were found within the ascites cells (NK cells as percentage of tumor-associated lymphocytes: median=15.42%; range=2.1–30.5%; n=4). Although tumor-associated NK cells can demonstrate reduced functionality (37, 38), we observed that NK cells freshly isolated from malignant ovarian ascites could be effectively activated by IL-18/IFNα to express the NK helper-associated DC-attracting and DC-activating factors CCL3, CCL4, IFNγ, and TNFα (Fig. 5A).
Figure 5.
NK cell-driven production of Teff cell-recruiting chemokines in the human ovarian cancer environment. (A) CD56+ NK cells were enriched from human ovarian cancer (OvCa) ascites cells (left), cultured for 4 h in the absence or presence of IL-18/IFNα, and analyzed for expression of the chemokines CCL3 and CCL4 and the cytokines IFNγ and TNFα (right). (B) Bulk OvCa ascites cells or ascites cells depleted of CD56+ NK cells (left) were cultured for 4 or 24 h in the absence or presence of IL-18/IFNα, and analyzed for expression of the chemokines CCL3, CCL4, CXCL9, and CXCL10 and the cytokines IFNγ and TNFα (right). Flow cytometric analyses of NK cell enrichment and depletion are representative of one of four patients. Gene expression data are expressed as ratios between the expression of individual chemokine or cytokine genes and HPRT1, and represent the mean (± SD) across four independent experiments using ascites cells from four different patients. ***p<0.001, **p<0.01, ns: p>0.05.
Furthermore, IL-18/IFNα treatment of bulk ascites cells induced the production of high levels of the DC activators IFNγ and TNFα and the Teff cell-recruiting chemokines CXCL9 and CXCL10 (Fig. 5B), demonstrating the ability of IL-18/IFNα–activated NK cells to function even in the suppressive environment of OvCa. Depletion of NK cells from the bulk ascites cell population suppressed the production of these chemokines and cytokines mediated by IL-18/IFNα treatment, indicating that the activation of tumor-associated NK cells by the NK ‘helper’-driving stimuli plays the key role in the induction of DC-attracting, DC-activating, and Teff cell-recruiting factors within the human cancer environment.
Discussion
While IL-18-induced NK cells can promote antitumor immunity by elevating the production of Th1- and CTL-driving IL-12 by local DCs, our current data indicate their additional role in promoting DC attraction and the conditioning of tumor sites for the chemokine-driven infiltration of desirable effector-type T cells. We demonstrate that chemokine production is not a general consequence of NK cell activation, but is strictly regulated and selectively associated with a ‘helper’ NK cell phenotype driven by IL-18. Although IL-18 and IL-2 are both known to be potent NK cell activating factors, these data indicate that only IL-18 is efficient in enhancing NK cell expression of the DC-attracting chemokines CCL3, CCL4, CXCL8, and XCL1, resulting in the attraction of immature DCs. Such IL-18-driven enhancement in the expression of DC-attracting chemokines corresponds closely to the previously-reported regulation of IFNγ and TNFα, factors essential for NK cell-mediated activation of DCs, in IL-18-primed human NK cells (10). This suggests that NK cell recruitment of DCs and NK cell-mediated activation of DCs are closely related phenomena governed by similar mechanisms, supporting the role of NK cells as important modulators of DC-mediated immune responses.
Interestingly, the unique ‘priming’ effects of IL-18 in promoting ‘helper’ NK cell-driven chemokine interactions with DCs requires secondary stimulation with other pro-inflammatory factors (see Fig. 2A) or recognition of target cells (see Fig. 2B and Supplementary Fig. S2). These secondary signals, described in this study and others (10, 39), can include direct interaction with tumor cells or type I interferons likely to be elaborated early in viral infection. This two-signal requirement for IL-18-primed NK cell function is likely to represent a critical checkpoint in NK cell activation, preventing inappropriate development of potent downstream immune responses, including those initiated through NK cell interaction with DCs. Although IL-18 has been reported to favor protective anti-tumor immunity (40, 41), IL-18 has also been recently implicated in tumor-associated immunosuppression through its promotion of a Kit+ subset of regulatory NK cells overexpressing PD-L1 (42, 43). Therefore, it is possible that in some situations, IL-18-primed NK cells may mediate different functions depending on the availability and/or character of associated secondary signals. Indeed, the activity of IL-18 has been shown to be highly context-dependent, and demonstrates the capacity to co-induce either the type-1 cytokine IFNγ or the type-2 cytokine IL-13 when combined with different secondary signals (44). The differential impact of distinct secondary signals specifically on IL-18-primed NK cell activity is the subject of our current ongoing investigations.
The quantity and quality of immune cell infiltration into the tumor environment, including the critical balance between effector and regulatory T cells, have been increasingly recognized as vital components of both spontaneous and therapy-induced anti-tumor immune control (45, 46). Importantly, the chemokines CXCL9, CXCL10, and CCL5 have been implicated in the attraction of type-1 Teff cell subsets central to effective anti-tumor responses (32–34), providing key targets for therapeutic ‘conditioning’ of the tumor chemokine environment for efficient anti-tumor effector cell entry. Although this study demonstrates that IL-18-primed NK cells can directly express CCR5 ligands, their role in generating a chemokine environment conducive to type-1 Teff cell recruitment is most likely to occur through their activity on DCs, given the apparent strong synergy between IL-18-driven NK cells and DCs in promoting Teff cell attraction that was significantly more efficient than NK cells or DCs alone (see Fig. 4B).
Notably, this study demonstrates that IL-18-primed human NK cells, including from directly within the human cancer environment, can enhance type-1 immune responses by selectively inducing high DC expression of Teff cell-recruiting chemokines, including CXCL9, CXCL10, and CCL5, without inducing the Treg cell-attracting chemokine CCL22. However, intratumoral NK cells have also been shown to be capable of secreting CCL22 and mediating the recruitment of CD4+CD25+FoxP3+ regulatory T cells, a process which can be driven by NK cell activation with IL-2 (47). This highlights the importance of carefully defining the relevant NK cell stimulatory factors for the therapeutic augmentation of intratumoral immune control.
In addition to their expression by Teff cells, resting NK cells have also been shown to express the chemokine receptors CCR5 and CXCR3, and can respond to their respective ligands produced by the interaction between IL-18-primed NK cells and DCs (48). Since DCs can be an important source of IL-18 during developing immune responses (14), and mature DCs can play a significant role in activating resting NK cells (49), this presents the possibility of a reciprocal, chemokine-driven feed-forward interaction between NK cells and DCs, in which NK cell-activated DCs can subsequently attract and activate additional resting NK cells, further promoting an amplifying cycle of immune activation. Indeed, reciprocal positive feedback has been demonstrated between NK cells and myeloid cells, including DCs (49), and spatial innate cell clustering has been shown to be important to developing protective immune responses in vivo (50). Our current data suggests that the suppressive nature of the human tumor environment may not, at least, represent an absolute, irreversible barrier to NK cell activation toward DC-stimulating helper function, and may be amendable to therapeutic modulation, for instance through the local application of IL-18 and IFNα. Thus, the ‘helper’ interaction between IL-18-primed NK cells and DCs may represent a powerful feed-forward loop amplifying endogenous immune responses, and may present an attractive target for cancer therapy in which modest initiation of the helper response may result in a much larger induction of effector activity.
In summary, these data identify the unique chemokine expression of ‘helper’ versus ‘killer’ pathways of NK cell differentiation, and demonstrates that human NK cells can serve important helper functions in facilitating the chemokine-driven attraction and activation of DCs and the accumulation of effector cells in the tumor environment. This study further demonstrates that NK cells in cancer patients, including NK cells infiltrating the tumor environment itself, are competent to undergo helper differentiation, and thus may serve as therapeutic targets for the modulation of the human tumor chemokine environment to facilitate type-1 immune responses against cancer.
Supplementary Material
Acknowledgments
Financial support: This work was supported by the following NIH grants: P01 CA101944, P01 CA132714, F30 CA165410, T32 CA082084, and TL1 RR024155.
The authors thank Dr. Ravikumar Muthuswamy for providing extensive training in many of the techniques used in this study, and Dr. Julie Urban and Dr. Eva Wieckowski for critical discussion of the manuscript.
Footnotes
Conflicts of interest: The authors declare that no conflicts of interest exist.
References
- 1.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 2.Shi FD, Ljunggren HG, La Cava A, Van Kaer L. Organ-specific features of natural killer cells. Nat Rev Immunol. 2011;11:658–71. doi: 10.1038/nri3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCartney S, Vermi W, Gilfillan S, Cella M, Murphy TL, Schreiber RD, et al. Distinct and complementary functions of MDA5 and TLR3 in poly(I:C)-mediated activation of mouse NK cells. J Exp Med. 2009;206:2967–76. doi: 10.1084/jem.20091181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hart OM, Athie-Morales V, O’Connor GM, Gardiner CM. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol. 2005;175:1636–42. doi: 10.4049/jimmunol.175.3.1636. [DOI] [PubMed] [Google Scholar]
- 5.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–51. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–33. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–41. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mocikat R, Braumuller H, Gumy A, Egeter O, Ziegler H, Reusch U, et al. Natural killer cells activated by MHC class I(low) targets prime dendritic cells to induce protective CD8 T cell responses. Immunity. 2003;19:561–9. doi: 10.1016/s1074-7613(03)00264-4. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–11. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 10.Mailliard RB, Alber SM, Shen H, Watkins SC, Kirkwood JM, Herberman RB, et al. IL-18-induced CD83+CCR7+ NK helper cells. J Exp Med. 2005;202:941–53. doi: 10.1084/jem.20050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mailliard RB, Son YI, Redlinger R, Coates PT, Giermasz A, Morel PA, et al. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J Immunol. 2003;171:2366–73. doi: 10.4049/jimmunol.171.5.2366. [DOI] [PubMed] [Google Scholar]
- 12.Vitale M, Della Chiesa M, Carlomagno S, Pende D, Arico M, Moretta L, et al. NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. Blood. 2005;106:566–71. doi: 10.1182/blood-2004-10-4035. [DOI] [PubMed] [Google Scholar]
- 13.Smith DE. The biological paths of IL-1 family members IL-18 and IL-33. Journal of leukocyte biology. 2011;89:383–92. doi: 10.1189/jlb.0810470. [DOI] [PubMed] [Google Scholar]
- 14.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 15.Wong JL, Mailliard RB, Moschos SJ, Edington H, Lotze MT, Kirkwood JM, et al. Helper activity of natural killer cells during the dendritic cell-mediated induction of melanoma-specific cytotoxic T cells. J Immunother. 2011;34:270–8. doi: 10.1097/CJI.0b013e31820b370b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–5. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 17.Gustafsson K, Ingelsten M, Bergqvist L, Nystrom J, Andersson B, Karlsson-Parra A. Recruitment and activation of natural killer cells in vitro by a human dendritic cell vaccine. Cancer Res. 2008;68:5965–71. doi: 10.1158/0008-5472.CAN-07-6494. [DOI] [PubMed] [Google Scholar]
- 18.Vujanovic L, Ballard W, Thorne SH, Vujanovic NL, Butterfield LH. Adenovirus-engineered human dendritic cells induce natural killer cell chemotaxis via CXCL8/IL-8 and CXCL10/IP-10. Oncoimmunology. 2012;1:448–57. doi: 10.4161/onci.19788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, et al. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–7. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 20.Watchmaker PB, Berk E, Muthuswamy R, Mailliard RB, Urban JA, Kirkwood JM, et al. Independent regulation of chemokine responsiveness and cytolytic function versus CD8+ T cell expansion by dendritic cells. J Immunol. 2010;184:591–7. doi: 10.4049/jimmunol.0902062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muthuswamy R, Urban J, Lee JJ, Reinhart TA, Bartlett D, Kalinski P. Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res. 2008;68:5972–8. doi: 10.1158/0008-5472.CAN-07-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agaugue S, Marcenaro E, Ferranti B, Moretta L, Moretta A. Human natural killer cells exposed to IL-2, IL-12, IL-18, or IL-4 differently modulate priming of naive T cells by monocyte-derived dendritic cells. Blood. 2008;112:1776–83. doi: 10.1182/blood-2008-02-135871. [DOI] [PubMed] [Google Scholar]
- 23.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–5. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 24.Hugues S, Scholer A, Boissonnas A, Nussbaum A, Combadiere C, Amigorena S, et al. Dynamic imaging of chemokine-dependent CD8+ T cell help for CD8+ T cell responses. Nat Immunol. 2007;8:921–30. doi: 10.1038/ni1495. [DOI] [PubMed] [Google Scholar]
- 25.Randolph GJ, Ochando J, Partida-Sanchez S. Migration of dendritic cell subsets and their precursors. Annu Rev Immunol. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- 26.Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273–81. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre CA, Ventre E, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1283–92. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005–16. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Maria A, Bozzano F, Cantoni C, Moretta L. Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation. Proc Natl Acad Sci U S A. 2011;108:728–32. doi: 10.1073/pnas.1012356108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115:2167–76. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luster AD, Leder P. IP-10, a -C-X-C- chemokine, elicits a potent thymus-dependent antitumor response in vivo. J Exp Med. 1993;178:1057–65. doi: 10.1084/jem.178.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:105–13. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 34.Musha H, Ohtani H, Mizoi T, Kinouchi M, Nakayama T, Shiiba K, et al. Selective infiltration of CCR5(+)CXCR3(+) T lymphocytes in human colorectal carcinoma. International journal of cancer Journal international du cancer. 2005;116:949–56. doi: 10.1002/ijc.21135. [DOI] [PubMed] [Google Scholar]
- 35.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 36.Ioannides CG, Platsoucas CD, Rashed S, Wharton JT, Edwards CL, Freedman RS. Tumor cytolysis by lymphocytes infiltrating ovarian malignant ascites. Cancer Res. 1991;51:4257–65. [PubMed] [Google Scholar]
- 37.Carlsten M, Norell H, Bryceson YT, Poschke I, Schedvins K, Ljunggren HG, et al. Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells. J Immunol. 2009;183:4921–30. doi: 10.4049/jimmunol.0901226. [DOI] [PubMed] [Google Scholar]
- 38.Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71:5412–22. doi: 10.1158/0008-5472.CAN-10-4179. [DOI] [PubMed] [Google Scholar]
- 39.Chaix J, Tessmer MS, Hoebe K, Fuseri N, Ryffel B, Dalod M, et al. Cutting edge: Priming of NK cells by IL-18. J Immunol. 2008;181:1627–31. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. 2010;207:1625–36. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207:1045–56. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terme M, Ullrich E, Aymeric L, Meinhardt K, Desbois M, Delahaye N, et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res. 2011;71:5393–9. doi: 10.1158/0008-5472.CAN-11-0993. [DOI] [PubMed] [Google Scholar]
- 43.Terme M, Ullrich E, Aymeric L, Meinhardt K, Coudert JD, Desbois M, et al. Cancer-induced immunosuppression: IL-18-elicited immunoablative NK cells. Cancer Res. 2012;72:2757–67. doi: 10.1158/0008-5472.CAN-11-3379. [DOI] [PubMed] [Google Scholar]
- 44.Hoshino T, Wiltrout RH, Young HA. IL-18 is a potent coinducer of IL-13 in NK and T cells: a new potential role for IL-18 in modulating the immune response. J Immunol. 1999;162:5070–7. [PubMed] [Google Scholar]
- 45.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 46.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mailloux AW, Young MR. NK-dependent increases in CCL22 secretion selectively recruits regulatory T cells to the tumor microenvironment. J Immunol. 2009;182:2753–65. doi: 10.4049/jimmunol.0801124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walzer T, Vivier E. G-protein-coupled receptors in control of natural killer cell migration. Trends in immunology. 2011;32:486–92. doi: 10.1016/j.it.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “l’union fait la force”. Blood. 2005;106:2252–8. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 50.Kang SJ, Liang HE, Reizis B, Locksley RM. Regulation of hierarchical clustering and activation of innate immune cells by dendritic cells. Immunity. 2008;29:819–33. doi: 10.1016/j.immuni.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.