Abstract
Phospho-sulindac (PS) is a sulindac derivative with promising anticancer activity in lung cancer, but its limited metabolic stability presents a major challenge for systemic therapy. We reasoned that inhalation delivery of PS might overcome first-pass metabolism and produce high levels of intact drug in lung tumors. Here, we developed a system for aerosolization of PS and evaluated the antitumor efficacy of inhaled PS in an orthotopic model of human non-small cell lung cancer (A549 cells). We found that administration by inhalation delivered high levels of PS to the lungs and minimized its hydrolysis to less active metabolites. Consequently, inhaled PS (6.5mg/kg) was highly effective in inhibiting lung tumorigenesis (75%, p<0.01) and significantly improved the survival of mice bearing orthotopic A549 xenografts. Mechanistically, PS suppressed lung tumorigenesis by 1) inhibiting EGFR activation, leading to profound inhibition of Raf/MEK/ERK and PI3K/AKT/mTOR survival cascades; 2) inducing oxidative stress, which provokes the collapse of mitochondrial membrane potential and mitochondria-dependent cell death; and 3) inducing autophagic cell death. Our data establish that inhalation delivery of PS is an efficacious approach to the prevention of lung cancer, which merits further evaluation.
Keywords: Aerosol delivery, chemoprevention, lung cancer, non-steroidal anti-inflammatory drugs, phospho-sulindac
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, with non-small cell lung cancer (NSCLC) accounting for the majority of the cases (80%) (1). Despite advances in chemotherapy and the recent advert of molecularly-targeted drugs, patients diagnosed with advanced NSCLC only have a median survival time of 8 to 11 months (2). Given its poor prognosis, novel agents for lung cancer control are urgently needed.
NSAIDs are promising agents in the control of lung cancer. Epidemiological data suggest that NSAIDs can decrease the risk of lung cancer among smokers (3); although recent evidence suggests that individual NSAIDs may have contrasting effects on lung cancer risk and survival (4). Sulindac and its sulfone metabolite are efficacious in suppressing lung tumorigenesis in pre-clinical models (5, 6). Long-term use of NSAIDs, however, is associated with gastrointestinal and renal toxicities (7). Our group has developed phospho-sulindac (PS, Figure 1A), a sulindac derivative that is safe and effective against colon cancer (8–10). In our continuing effort to evaluate the anticancer activity of PS, the present study examined the activity of PS against human NSCLC in cell culture and in an orthotopic mouse model of NSCLC.
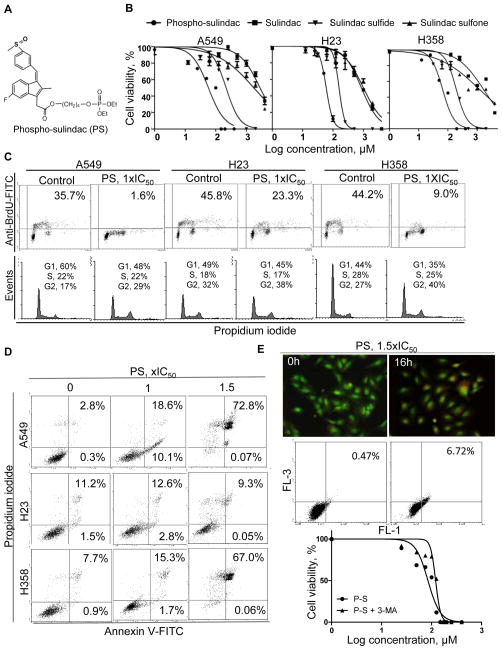
Figure 1. PS inhibits the growth of non-small-cell lung cancer (NSCLC) cells.
A, Structure of PS. B, 24-h Cell viability curves of PS in NSCLC cells. C, Upper: Anti-proliferative activity of PS (1xIC50, 24h) was determined by the BrdU incorporation assay. Lower: Effect of PS (1xIC50, 24h) on cell cycle progression was determined by staining of 70% ethanol-fixed cells with PI. D, Induction of apoptosis by PS (1x and 1.5xIC50, 24h) was determined by Annexin V/PI staining. Fluorescence intensity was measured by flow cytometry. E, Analysis of autophagy induction. Upper: Acridine orange staining of acidic vesicular organelles, which show bright orange to red fluorescence under blue excitation light (488nm). Middle: Flow cytometry analysis of red fluorescent complex after acridine orange-staining of A549 cells. Lower: Cell viability of A549 cells treated with PS in the presence/absence of a specific inhibitor of autophagosome formation, 3-methyl adenine, 5mM.
Inhalation administration represents an attractive strategy for the targeted prevention and treatment of lung cancer (11, 12). Aerosol delivery of drugs achieves high local drug levels at the lung epithelium and minimizes systemic exposure (13). A major challenge with effective delivery of PS to the target site(s) is its rapid inactivation by non-specific esterases (14). We reasoned that delivery of PS via inhalation will bypass the liver and gastrointestinal tract, major sites of esterase expression, and hence will require much lower doses to deliver intact PS to the lungs.
Aberrant activation of the epidermal growth factor receptor (EGFR) signaling cascades is a hallmark of NSCLC (15). The Raf/MEK/ERK pathway is a key downstream effector of the EGFR that is activated in the majority of NSCLCs (16). PI3K/AKT/mTOR is another downstream survival pathway that is constitutively activated in >50% of NSCLCs (17). The pharmacological inhibition of EGFR-dependent survival signaling may thus suppress tumor growth, manifested in the induction of apoptosis and/or autophagy (18).
Herein, we demonstrate that inhalation delivery of PS results in high levels of intact drug in the lungs and that inhaled PS is highly effective in inhibiting lung tumorigenesis in vivo. Mechanistically, PS inhibits EGFR-dependent survival cascades in NSCLC cells and induces oxidative stress to ultimately induce apoptosis/autophagy, leading to the potent inhibition of tumor growth. The findings of this study suggest that PS is a promising candidate for the development of an effective and safe drug for the control of NSCLC.
Materials and Methods
Reagents
Phospho-sulindac was a gift from Medicon Pharmaceuticals, Inc, Setauket, NY. All reagents used in the study are of analytical grade. All other chemicals, unless otherwise stated, were from Sigma-Aldrich (St. Louis, MO).
Cell culture
Human NSCLC cell lines A549, H23 and H358 were from American Type Culture collection (ATCC, Manassas, VA) which characterized these cell lines using cytogenetic analysis. These cell lines were grown as recommended by ATCC. A549-GFP cells were generated by transfection with GFP-lentiviral particles and selection with Geneticin. The highest 1% GFP-expressing cells sorted by flow cytometry were used for in vivo studies.
Cytokinetic analyses
Cell viability was determined by a modified MTT assay (8). Apoptosis and cell proliferation were assessed by Annexin V/Propidium iodide (PI) staining (Life Technologies, Grand Island, NY) and the bromodeoxyuridine (BrdU) incorporation method (BD Biosciences, San Jose, CA), respectively (19). Autophagy vacuolization was determined by acridine orange staining (20).
Analysis of ROS induction
Reactive oxygen species (ROS) levels were determined by staining with DCFDA (10μM) for 30min; mitochondrial superoxide anion levels were determined by staining with 5μM MitoSOX Red for 30min at 37°C, and their fluorescence intensity was measured by flow cytometry.
Mitochondria-depleted cells
Mitochondria-depleted (ρ0) derivatives of A549 cells were generated by incubation with 200ng/ml ethidium bromide, 50μg/ml uridine and 1μM sodium pyruvate for 8 weeks as previously described (21).
siRNA silencing of EGFR
A549 cells were transfected with 10nM EGFR- or control-siRNA (Applied Biosystems, Foster City, CA) using lipofectamine 2000 (Life Technologies, Grand Island, NY). The cells were treated with PS 72h post-transfection.
Aerosol drug delivery
The set up for aerosol drug delivery is shown in Suppl. Figure 1. Inhalation delivery conditions (50mg/ml PS in ethanol, 8-min exposure time) were optimized in pilot studies (Suppl. Figure 2). The estimated total amount of PS deposited in the lungs of mice in 8min was 15mg/kg/day, and the estimated dose of treatment was 6.5mg/kg/day.
Pharmacokinetic analyses
Mice were exposed to aerosolized PS (50mg/ml PS in ethanol, 8-min exposure) and euthanized at designated time points. Their plasma and lungs were collected and the levels of PS and its metabolites were determined by HPLC (22).
Efficacy study in an orthotopic lung cancer model
Seven-week old BALB/c nude mice (Harlan Inc., Indianapolis, IN) were pretreated for 5 days with aerosol generated from vehicle (ethanol) or 50mg/ml PS solution (n=15/group). On day 6, A549-GFP cells (1.5×106/mouse) were intrapulmonarily injected into the left lung parenchyma of the mice (23). Inhalation treatment (five times/week) was resumed 3 days later. After 8 weeks, the mice were euthanized, and blood and lung tissues were collected. Images of the lungs were taken on a fluorescence imaging system (Maestro, Wobum, MA). All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee, State University of New York at Stony Brook.
Analysis of in vivo oxidative stress
The effect of PS on the redox state of the mice bearing orthotopic lung tumors was assessed by measuring the levels of plasma 8-iso-prostaglandin F2α (8-iso-PGF2α) using an ELISA kit (Enzo Life Sciences, NY, USA).
Immunoblotting and antibodies
For immunoblotting, total protein lysates were diluted in loading buffer and subjected to SDS-PAGE, followed by electrotransfer to nitrocellulose membrane. Anti-EGFR and anti-phospho-EGFR antibodies were from Santa Cruz Biotechnology; all other antibodies were from Cell Signaling Technology.
Immunohistochemistry of orthotopic lung tumor tissues
Immunohistochemical staining of paraffin-embedded tumor sections from vehicle- and PS-treated mice were performed as previously described (9). Proliferating and apoptotic cells were stained using anti-PCNA and TUNEL, respectively.
Statistical Analysis
Differences between experimental groups were calculated using Student’s t-test. P values <0.05 were considered significant. Comparison of the survival rate between the control and treatment group was performed with the Kaplan-Meier method. Z>1.96 was considered significant.
Results
PS inhibits the growth of NSCLC cell lines in vitro
To study the effect of PS (Figure 1A) on cell growth, we determined its 24 h-IC50 values in NSCLC cell lines (Figure 1B & Suppl. Table 1). The IC50 values of PS varied little (75–94μM) among p53 wild-type (A549), p53-mutant (H23) and p53-null (H358) cells. Importantly, PS was considerably more potent in inhibiting NSCLC cells compared to conventional sulindac (IC50>1000μM), representing a potency enhancement of >12-fold. The IC50 values of sulindac metabolites sulindac sulfone (566–1895μM) and sulindac sulfide (159–222μM) in these cell lines were also much higher compared to those of PS. PS thus has a much more potent effect on lung cancer cells, although it cannot be ruled out that sulindac may exert its anticancer effect through alternative mechanisms, such as affecting the tumor microenvironment.
We further examined the cytokinetic effect of PS in vitro (Figure 1C–E). PS markedly reduced cell proliferation. At 1xIC50, PS inhibited the proliferation of A549, H23 and H358 cells by 94%, 49% and 80%, respectively. PS blocked cell cycle progression (G2/M arrest), leading to significant accumulation of cells in the G2/M phase in all three NSCLC cell lines. PS significantly induced apoptosis in NSCLC cells. At 1xIC50, apoptosis (Annexin V (+) cells) was induced by 7-fold in A549 cells and 2-fold in H358 cells, whereas at 1.5xIC50, apoptosis was induced by 26-fold in A549 cells and 9-fold in H358 cells. However, PS did not appreciably induce apoptosis in H23 cells. PS also induced autophagy, evidenced by the formation of acidic vesicular organelles, widely accepted as a hallmark for autophagy in mammalian cells (24) (Figure 1E & Suppl. Figure 3).
To better understand the role of autophagy in the growth inhibitory effect of PS, we compared the cytotoxicity of PS in A549 cells in the presence and absence of 3-MA, a specific inhibitor of autophagosome formation (25). Blockade of autophagy by 3-MA significantly reduced the cytotoxicity of PS as reflected in the 50% increase in its IC50 (Figure 1E). Thus, the induction of autophagy plays an important role in mediating the cytotoxic effect of PS. Our results indicate that PS inhibits the growth of NSCLC cells in vitro via a potent cytokinetic effect, which includes suppression of cell proliferation and induction of apoptotic and autophagic cell death.
Inhalation treatment effectively delivers intact PS to the lungs
Given that PS is much more potent than its metabolites in inhibiting NSCLC, we reasoned that the efficacy of PS could be improved by inhalation administration, which bypasses the major sites of drug metabolism and results in the direct delivery of PS to the lungs. We utilized a nose-only inhalation exposure system, which was optimized for the following parameters: particle size distribution, concentration of drug solution for atomization, and exposure time (Suppl. Figure 2).
The optimized system (50mg/ml PS, 8-min exposure) generated promising pharmacokinetic profiles in terms of the delivery of intact PS to the lungs (Suppl. Figure 2D, Suppl. Table 2). The highest concentration of PS was 22.2nmol/g lung tissue right after inhalation treatment (Tmax=0h), which decreased towards later time points and was undetectable after 24h. The AUC0–24h was 7.7nmol/g lung tissue*h. Of note, intact PS persisted in the lungs even 2h after inhalation treatment.
Inhaled PS inhibits lung cancer growth in vivo
We next evaluated the efficacy of inhaled PS in vivo using an orthotopic lung cancer model in nude mice. In this model, the mice developed lung tumors 2–3 weeks after intrapulmonary implantation of A549 cells (26). Compared to control, the PS-treated group showed 75% (p<0.001) reduction in tumor volume assessed by GFP luminosity at sacrifice (Figure 2A). Lung weight (including cancerous and non-cancerous tissues) was also determined to analyze the relative tumor load in the control and treatment groups. The lung weight from another group of mice not implanted with cancer cells or subjected to vehicle or PS treatment served as the normal control. The lung weight of the vehicle-treated group was 2.6-fold higher than that of normal mice (Suppl. Figure 4A). PS inhalation treatment maintained lung weight near the normal levels. PS inhalation treatment also improved the overall survival of mice with orthotopic lung tumors. By week 8, 33% (5 out of 15) of the mice in the control group died from the disease while only 6% (1 out of 15) in the treatment group died (Suppl. Figure 4B&C).
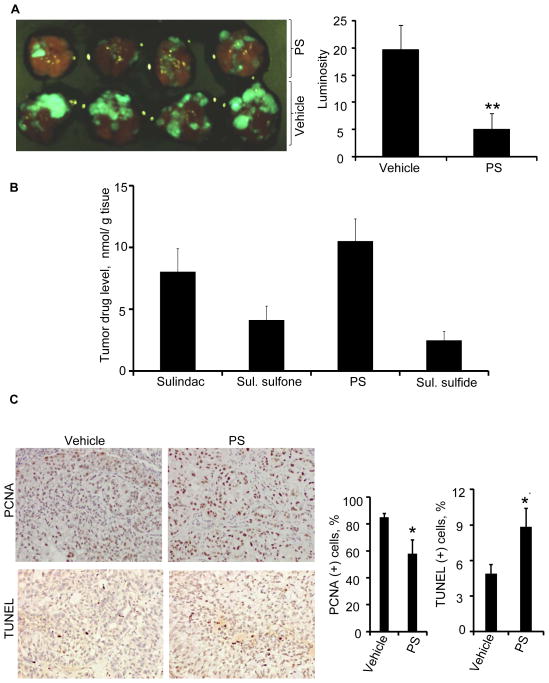
Figure 2. PS inhibits lung tumorigenesis in a mouse orthotopic model of human NSCLC.
GFP-transfected A549 cells (1.5×106) were implanted into the left lung parenchyma of athymic nude mice by intrapulmonary injection. Three days post tumor cell implantation the mice were treated with vehicle or aerosolized PS for 8 weeks (5 days/week). A, PS significantly (75% inhibition, p<0.001) inhibited lung tumorigenesis as indicated by the much lower levels of GFP luminosity in the treatment group compared to the controls. B, Tumor drug levels were determined by HPLC. C, Immunohistochemical staining of tumor sections for cell proliferation (PCNA) and apoptosis (TUNEL), respectively. *p<0.05, **p<0.01.
The potent inhibition of tumorigenesis was likely a result of the delivery of high levels of intact PS to the lung tumors. Figure 2B displays the relative levels of PS and its metabolites in the tumor tissues. PS was the dominant form identified (>10nmol/g), followed by sulindac, sulindac sulfone and sulindac sulfide. It merits emphasis that oral gavage of PS (even at much higher doses) could not deliver comparable amounts of intact PS to the lungs (Suppl. Figure 5). Consequently, oral PS treatment only moderately inhibited (30%, p<0.05) the growth of A549 xenografts in nude mice (Suppl. Figure 6). We have also evaluated the delivery of PS to the lungs via i.p. and i.v. administration. In agreement with our expectation, none of these alternative routes delivered significant amounts of intact PS to the lungs, which were less than 12% of that achieved with inhalation (Suppl. Figure 6C). PS exerted a strong cytokinetic effect on the orthotopic lung tumors. Immunohistochemical analyses (Figure 2C) revealed that PS significantly inhibited cell proliferation (p=0.018) and induced apoptosis (p=0.024) in the tumor cells in vivo.
PS suppresses EGFR activation
Antibody microarray analysis (Kinexus Bioinformatics Corporation) of tumor lysates from vehicle- and PS-treated mice revealed significant changes in EGFR signaling pathways. Aberrant activation of EGFR signaling has important implications for the pathogenesis of NSCLC (15, 27). We first explored in A549 cells in vitro the effect of PS on the level of EGFR phosphorylation and the expression of ADAM10 and ADAM17, known to induce EGFR activation through ligand cleavage (28, 29), (Figure 3A). PS significantly reduced EGFR phosphorylation (Y1068) starting 1h post treatment and the effect became more pronounced towards the later time points. Quantitative analysis of p-EGFR expression by flow cytometry also revealed a significant reduction after 16-h treatment with PS. In vitro assay showed that PS did not modulate the kinase activity of EGFR (data not shown) (EGFR KinEASE FP Fluorescein Green Assay, Upstate Cell Signaling Solutions). PS moderately decreased the level of ADAM17, starting 8h after treatment. PS, however, did not modulate the levels of ADAM10 or Notch1.
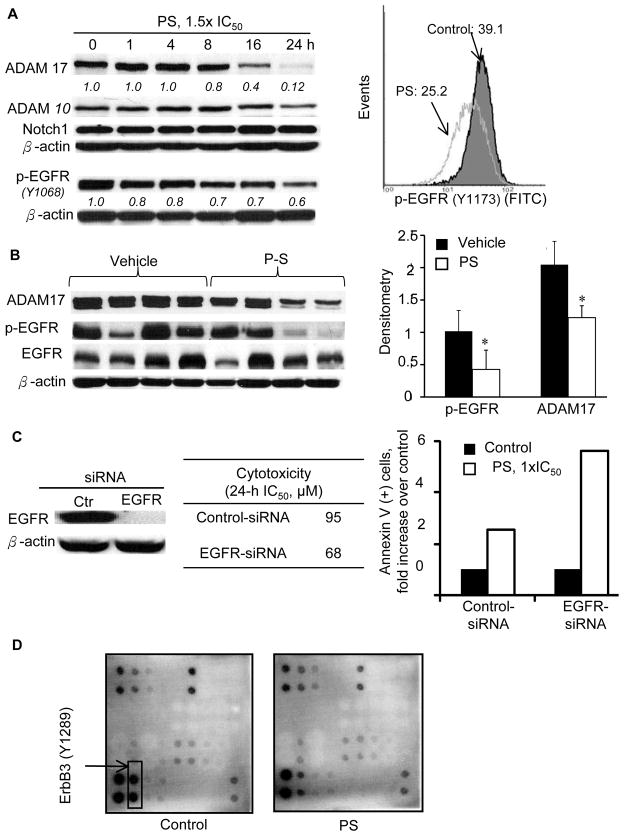
Figure 3. PS inhibits EGFR phosphorylation in vitro and in vivo.
A, Left: Effect of PS (1.5xIC50) on the expression of ADAM10, ADAM17, notch1, and p-EGFR. Densitometry results are shown as numbers (italics) below the immunoblots. Right: Quantitative analysis of p-EGFR (Y1173) level after PS (1xIC50, 20h) treatment using a FITC-conjugated p-EGFR antibody and flow cytometry. B, Left: Immunoblots of orthotopic A549 tumor tissues. Right: Densitometry of the immunoblots normalized to the loading control. *p<0.05. C, EGFR-knockdown in A549 cells abrogated the cytotoxic effect of PS. Left: Immunoblotting confirmed knockdown of EGFR. Middle: Effect of EGFR knockdown on the cytotoxicity of PS in A549 cells. Right: Effect of EGFR-knockdown on apoptosis induction by PS (1xIC50, 24h) in A549 cells. D, A549 cells were treated with vehicle or PS, 1.5xIC50 for 8h, at which time cell lysates were collected and the supernatant (16,000xg, 10min) was subjected to immunoblotting using the RayBio Human EGFR Phosphorylation Antibody Array.
PS also suppressed EGFR activation in vivo. We compared the levels of ADAM17 in lung xenografts between the vehicle- and PS-treated groups (Figure 3B). Immunoblots showed that PS significantly reduced the level of ADAM17 by 40%, which presumably led to a significant reduction of EGFR phosphorylation (58%) and activation. Immunohistochemical studies also showed a significant reduction in the expression of p-EGFR (p<0.038) in the PS-treated group compared to the control (Suppl. Figure 7A).
Interestingly, the levels of p-EGFR were much lower in responders (small tumors) than those in non-responders (large tumors). A significant correlation was found between tumor load (GFP luminosity) and p-EGFR level (WB, densitometry) (Suppl. Figure 7B). siRNA knockdown of EGFR sensitized A549 cells to PS (Figure 3C). IC50 value (24-h) of PS in EGFR-knockdown A549 cells (68μM) was much lower compared to that in mock-siRNA-transfected cells (95μM). Consistent with this observation, treatment with equimolar PS (95μM) resulted in a >2-fold higher apoptosis induction in EGFR-knockdown cells versus mock-transfected cells. We observed increased basal level of ROS in EGFR-knockdown A549 cells, which was intensified upon PS treatment (30) (data not shown). This combined effect on ROS levels likely enhanced cell death further leading to decreased IC50 values.
To have a clearer understanding of the effect of PS on the activation of other HER family members in addition to EGFR, we performed an antibody array assay (RayBiotech. Inc. Norcross, GA) on lysates from vehicle- and PS-treated A549 cells. In agreement with previous studies (31), much higher levels of ErbB3 were observed relative to the other HER family members assayed. As shown in Figure 3D, PS significantly decreased the expression of p-ErbB3 (Y1289).
PS inhibits the Raf/MEK/ERK and PI3K/AKT signaling cascades
In A549 cells, inhibition of EGFR phosphorylation by PS resulted in sequential inactivation of downstream MAPKs manifested in the decreased expression of p-c-Raf, p-MEK and p-ERK (Figure 4A, Suppl. Figure 8). Of note, p-c-Raf was reduced by >80% relative to control 1h post PS treatment and was nearly abrogated towards later time points. A similar dramatic inhibition was observed for p-MEK and p-ERK, but starting at later time points (4h for p-MEK and 8h for p-ERK), reflecting a propagating signal.
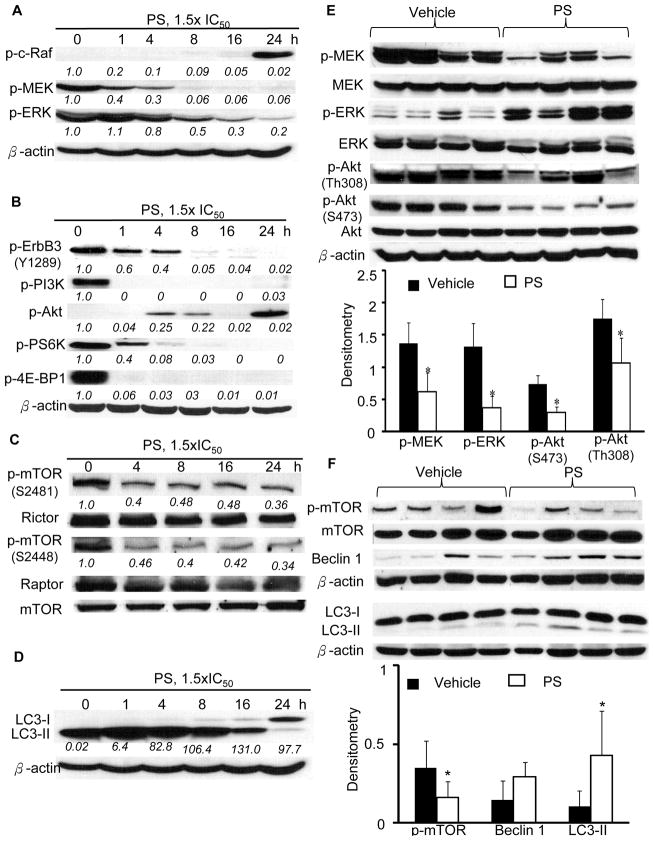
Figure 4. PS inhibits Raf/MEK/ERK and PI3K/Akt/mTOR signaling and induces autophagy in vitro and in vivo.
The inhibitory effect of PS on: A, p-Raf/p-MEK/p-ERK; B, p-ErbB3 and p-PI3K/p-Akt/p-PS6K/p-4E-PB-1; and C, p-mTOR (S2481 and S2448)/Rictor/Raptor expression in A549 cells (1.5xIC50) in vitro. Densitometry results are shown as numbers (italics) below the immunoblots. D, PS increased conversion of LC3-I to LC3-II as indicated by the increased LC3-II/LC3-I ratios (italics). E, Inhalation PS treatment significantly reduced phosphorylation of signaling components of Raf/MEK/ERK and PI3K/Akt pathways in orthotopic A549 tumors. F, Inhalation PS treatment significantly induced autophagy in the orthotopic tumors as indicated by the increased LC3-II/LC3-I ratios and reduced expression of p-mTOR. *p<0.05.
ErbB3 (HER3) is the principal HER family member, which binds PI3K and drives Akt signaling (32). Immunoblotting of the major signaling molecules of the PI3K/Akt pathway revealed substantially decreased expression of p-PI3K, p-Akt, p-mTOR, p-S6K, and p-4E-BP1 in A549 cells (Figure 4B&C). Of note, as early as 1h post PS treatment, the levels of most of the above signaling molecules were reduced by >80% compared to control. As a consequence, PS exerted a potent inhibitory effect on cell survival and growth in vitro.
It is well established that mTOR negatively regulates autophagy (33). During autophagy, LC3-I, the cytosolic soluble form of microtubule-associated protein 1A/1B-light chain 3 (LC3), is conjugated to phosphatidylethanolamine to form LC3-phosphatidylethanolamine conjugate (LC3-II) during autophagosome formation (34). Immunoblotting and densitometry analyses showed that PS significantly induced the conversion of LC3-I to LC3-II, an effect sustained throughout the timeframe investigated (Figure 4D).
Analyses of tumor samples confirmed our observations in vitro (Figure 4E&F). Immunoblotting of tumor lysates showed that PS significantly (p<0.05) down-regulated the phosphorylation of EGFR and its downstream kinases such as MEK (55%), ERK (72%), AKT (S473, 60%; Th308, 39%) and mTOR (55%). In addition, tumors of the treatment group had significantly higher LC3-II/LC3-I ratios. Taken together, PS strongly attenuated the Raf/MEK/ERK and PI3K/AKT/mTOR signaling cascades, which may explain the potent growth inhibition of PS in the orthotopic A549 xenografts.
PS induces oxidative stress and mitochondria-dependent cell death
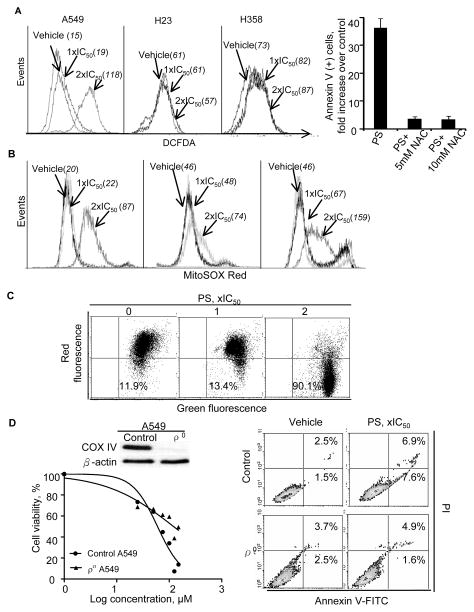
Induction of oxidative stress from the generation of reactive oxygen species (ROS) is a key mediator of cell death of phospho-NSAIDs (8, 9, 35). Using DCFDA, a general ROS probe, we showed that PS increased ROS levels in A549 cells by 1.2-fold at 1xIC50, and by >7-fold at 2xIC50. In contrast to the dramatic induction of ROS in A549 cells, PS only increased ROS levels in H358 cells by <20% at 2xIC50; and it failed to induce ROS in H23 cells (Figure 5A). The importance of ROS in the anticancer activity of PS was supported by the observation that pre-treatment of A549 cells with N-acetylcysteine almost completely abrogated PS’ capability to induce apoptosis (Figure 5A, Suppl. Figure 9A). Measurement of F2-isoprostanes is one of the most reliable indicators of oxidative stress in vivo (36). We found that plasma levels of 8-iso-PGF2α were more than 2-fold higher in the PS-treated group than in the control (Suppl. Figure 9B).
Figure 5. PS induces oxidative stress in vitro and in vivo.
NSCLC cells were treated with PS at the indicated concentrations for 1h. A, Left: PS induced ROS in A549 and H358 cells, but not in H23 cells as determined by DCFDA staining. Right: The effect of N-acetylcysteine (NAC, 10mM for 4h) pretreatment on apoptosis induction by PS. B, PS induced mitochondrial ROS stress in all three NSCLC cell lines as determined by MitoSOX Red staining. C, PS caused the collapse of mitochondrial membrane potential (Δψm), as indicated by the increased JC-1 fluorescence relative to the control. D, Mitochondria-depleted A549 cells (ρ0) showed resistance to PS. Left, upper: Immunoblotting of control and ρ0 A549 cell lysates for the specific marker mitochondrial protein cytochrome c oxidase subunit IV (COXIV). Left, lower: ρ0 and control A549 cells were treated with PS for 24h and cell viability was determined by trypan blue exclusion. Depletion of mitochondria substantially increased the resistance of A549 cells to the cytotoxic effect of PS. Right: ρ0 A549 cells were much more resistant to PS-induced apoptotic cell death, as measured by Annexin V/PI staining and flow cytometry.
Mitochondria are a major source of ROS (37). We measured the mitochondrial superoxide (O2·−) levels using the selective probe MitoSOX Red (19). Interestingly, PS (2xIC50) increased mitochondrial O2·− by 1.6- to 4-fold in A549, H23 and H358 cells (Figure 5B) while having minimal effect on total cellular ROS in the latter two. These data suggest that oxidative stress induction by PS in these cell lines is likely mitochondria-specific.
Generation of ROS stress led to collapse of mitochondrial membrane potential (Δψm) (Figure 5C, Suppl. Figure 10A), evidenced by the increased JC-1 fluorescence after PS treatment. Dissipation of Δψm resulted in activation of the intrinsic apoptotic pathway, demonstrated by the activation of caspase-9 and capase-3, the down-regulation of Bcl-2, and the cleavage of PARP (Suppl. Figure 10B). There was no cleavage of pro-caspase-8 (data not shown). Hence, PS activated the caspase cascades, culminating in apoptotic cell death.
To verify the involvement of the mitochondria in PS-induced death in NSCLC cells, we generated mitochondria-less (ρ0) A549 cells, confirmed by the absence of mitochondrial protein cytochrome c oxidase subunit IV (COXIV) (Figure 5D, Suppl. Figure 10C). Compared to their parental cells, the ρo cells were significantly more resistant to PS-induced apoptosis (3.6-fold increase over control in parental vs. no induction in ρo cells). Accordingly, the ρ0 cells were significantly more resistant to the cytotoxicity of PS, indicated by a 2-fold increase in its 24-h IC50 (124μM vs. 59.8μM for parental cells). Our results substantiate the role of mitochondria in the cancer cell killing effect of PS.
Discussion
Our data demonstrate that inhalation delivery of PS is efficacious in inhibiting lung tumorigenesis. This anticancer effect is a result of the following: 1) inhalation delivery substantially increased the exposure of the lungs to the active, intact PS, while limiting its systemic distribution to other healthy organs; 2) inhaled PS was able to down-regulate its molecular targets, namely, EGFR and its downstream kinases that mediate cell survival in tumor cells; and 3) PS induced oxidative stress, which led to apoptotic cell death, thus inhibiting tumour cell growth. Such a degree of efficacy could not be achieved by oral administration of PS.
The in vitro anticancer potency of PS is >12-fold stronger than sulindac, its parent NSAID. However, the carboxylic ester moiety in PS is highly liable to carboxylesterase (CES)-mediated hydrolysis in vivo, resulting in significant attenuation of its antitumor activity (38). CES is highly expressed in the liver and intestine (39). In our experience, PS (150–300mg/kg) given via the oral route undergoes extensive pre-systemic hydrolysis, resulting in minute distribution of the intact drug (<5% of total metabolites) to target organs (22). Rodent lungs, on the other hand, have carboxylesterase activity 5-fold lower than that in the liver and intestine (40). Here, we have demonstrated that aerosol administration, despite its much lower dose (6.5mg/kg body wt), successfully enhanced the delivery of intact PS to the lungs of mice (43% of PS plus metabolites). Accordingly, inhaled PS was remarkably effective in inhibiting the growth of orthotopically transplanted A549 tumors (75% reduction, p<0.001) in nude mice. Oral administration, however, only delivered minimal levels of intact PS to A549 tumors and resulted in moderate growth inhibition of the tumors.
EGFR, a molecular target of PS, is frequently overexpressed in NSCLCs and plays a crucial role in tumorigenesis (41). Phosphorylation of EGFR is accompanied by engagement of downstream signalling pathways, which promote cell survival, growth and resistance to apoptosis (41). Although PS did not modulate EGFR kinase activity in vitro (data not shown), it significantly suppressed EGFR phosphorylation in A549 cells in vitro and in orthotopic xenografts, thereby exerting a powerful inhibitory effect on the activation of the downstream RAF/MEK/ERK signalling.
NSCLC cells harboring the KRAS mutated gene, such as A549, are prone to develop resistance to EGFR tyrosinase kinase inhibitors (TKIs) (42, 43). However, these KRAS mutant, EGFR TKI-resistant cells are sensitive to PI3K inhibitors (43). One of the most potent activators of PI3K, ErbB3, is highly expressed in human lung adenocarcinomas and mediates resistance to TKIs (44). Our data showed that PS strongly inhibits the phosphorylation of ErbB3, resulting in attenuated PI3K/Akt signalling in A549 cells in vitro and in vivo. The concomitant inhibition of EGFR- and ErbB3-mediated survival pathways was consequential, as indicated by the increased expression of pro-apoptotic proteins (caspase-9, Bad) and the suppression of anti-apoptotic proteins (e.g. Bcl-2) (45). The dual inhibition of Raf/MEK/ERK and PI3K/Akt likely explains the substantial inhibition of the growth of A549 xenografts by PS.
Our data also establish the induction of oxidative stress as an important mechanism of cell death caused by PS. Increased ROS, an effect more pronounced in mitochondria, is an early event in PS-induced cell death in NSCLCs. PS also induced oxidative stress in mice bearing orthotopic lung tumors. In agreement with previous studies (46), a rapid ROS induction is a pivotal event in phospho-NSAIDs’ anticancer effect. PS may also modulate ROS indirectly through its inhibitory effect on Raf/MEK/ERK signalling. Oncogenic Kras was shown to up-regulate an Nrf-2-dependent cellular antioxidant program via Raf/MEK/ERK cascade in human lung cancer (47). Hence, the inhibition of Raf/MEK/ERK by PS may contribute to an enhanced oxidative state in A549 cells.
Inhibition of the survival cascades and induction of oxidative stress by PS in NSCLC culminates in apoptotic and autophagic cell death. PS-induced apoptotic cell death showed a marked dependence on mitochondria (intrinsic apoptosis pathway). We demonstrated that mitochondria-deficient A549 cells are more resistant to the pro-apoptotic effect of PS. The centrality of mitochondria in the induction of apoptosis by PS is also evident from the enhanced mitochondrial ROS levels, dissipation of mitochondria transmembrane potential, and activation of caspase-9 (but not caspase-8) observed upon PS treatment.
PS also induced autophagic cell death in NSCLC cell lines, evidenced by the formation of acidic vesicular organelles and the increased conversion of LC3-I to LC3-II. In cancer therapy, apoptosis almost invariably leads to cell death. In contrast, autophagy may be a pro-survival or cell-death mechanism depending on the stage of tumorigenesis and the extent of autophagy induced (48, 49). Here, PS-induced autophagy is involved in cell death, since chemical inhibition of autophagy confers a cyto-protective effect. The ability of PS to induce both apoptotic and autophagic cell death in NSCLC cells has important therapeutic implications. Defects in the apoptotic machinery in NSCLCs are a significant cause of resistance to chemotherapy (50). PS may thus have a therapeutic advantage in treating NSCLCs with apoptotic defects.
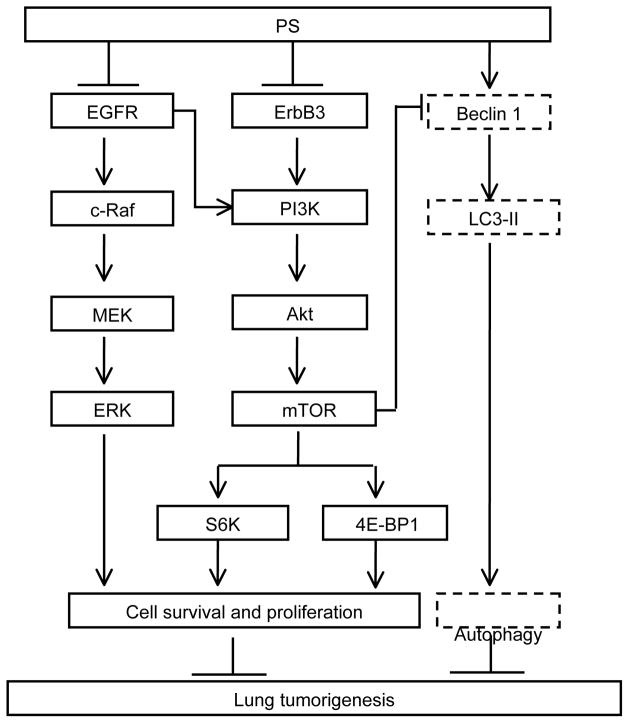
In conclusion, inhaled PS is an effective anticancer agent in preclinical models of NSCLC. This anticancer effect is mediated through the inhibition of EGFR survival pathways, in concert with the induction of ROS, culminating in apoptotic and autophagic death of NSCLC cells (Figure 6). Our data establish PS as a promising drug candidate for NSCLCs which merits further evaluation.
Figure 6. Proposed mechanism for the anticancer effect of PS in A549 human NSCLC.
PS inhibits lung tumorigenesis by 1) inhibiting phosphorylation activation of EGFR, leading to attenuated phosphorylation of the downstream signaling molecules (c-Raf, MEK and ERK); 2) inhibiting phosphorylation of ErbB3 which presumably contributes to attenuated downstream signaling via PI3K, Akt, mTOR, S6K and 4E-BP1. PS also induces autophagic cell death as evidenced by the formation of acidic vesicular organelles and the enhanced conversion of LC3-I to LC3-II. Solid-line box, inhibition; Dash-line box, induction.
Supplementary Material
Acknowledgments
We thank Dr. K. Vrankova for the synthesis of phospho-sulindac for this work.
Grant Support
This work was supported by NIH R01CA139454 (B. Rigas) and DOD W81XWH11-1-0799 (B. Rigas).
Abbreviations
- PS
phospho-sulindac
- NSCLC
non-small cell lung cancer
- NSAID
non-steroidal anti-inflammatory drug
- EGFR
epidermal growth factor receptor
- ROS
reactive oxygen species
- BrdU
bromodeoxyuridine
- 8-iso-PGF2α
8-iso-prostaglandin F2α
- CES
carboxylesterases
Footnotes
Conflicts of interest: The authors have nothing to disclose except for Basil Rigas, who has an equity position in Medicon Pharmaceuticals, Inc. and Nengtai Ouyang who is an employee for the same.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Thatcher N, Chang A, Parikh P, Rodrigues PJ, Ciuleanu T, von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–37. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 3.Harris RE, Beebe-Donk J, Schuller HM. Chemoprevention of lung cancer by non-steroidal anti-inflammatory drugs among cigarette smokers. Oncol Rep. 2002;9:693–5. [PubMed] [Google Scholar]
- 4.Brasky TM, Baik CS, Slatore CG, Alvarado M, White E. Prediagnostic nonsteroidal anti-inflammatory drug use and lung cancer survival in the VITAL study. J Thorac Oncol. 2012;7:1503–12. doi: 10.1097/JTO.0b013e3182641bdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duperron C, Castonguay A. Chemopreventive efficacies of aspirin and sulindac against lung tumorigenesis in A/J mice. Carcinogenesis. 1997;18:1001–6. doi: 10.1093/carcin/18.5.1001. [DOI] [PubMed] [Google Scholar]
- 6.Castonguay A, Rioux N. Inhibition of lung tumourigenesis by sulindac: comparison of two experimental protocols. Carcinogenesis. 1997;18:491–6. doi: 10.1093/carcin/18.3.491. [DOI] [PubMed] [Google Scholar]
- 7.Henry D, Lim LL, Garcia Rodriguez LA, Perez GS, Carson JL, Griffin M, et al. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. BMJ. 1996;312:1563–6. doi: 10.1136/bmj.312.7046.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackenzie GG, Sun Y, Huang L, Xie G, Ouyang N, Gupta RC, et al. Phospho-sulindac (OXT-328), a novel sulindac derivative, is safe and effective in colon cancer prevention in mice. Gastroenterology. 2010;139:1320–32. doi: 10.1053/j.gastro.2010.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackenzie GG, Ouyang N, Xie G, Vrankova K, Huang L, Sun Y, et al. Phospho-sulindac (OXT-328) combined with difluoromethylornithine prevents colon cancer in mice. Cancer Prev Res (Phila) 2011;4:1052–60. doi: 10.1158/1940-6207.CAPR-11-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang L, Zhu C, Sun Y, Xie G, Mackenzie GG, Qiao G, Komninou D, et al. Phospho-sulindac (OXT-922) inhibits the growth of human colon cancer cell lines: a redox/polyamine-dependent effect. Carcinogenesis. 2010;31:1982–90. doi: 10.1093/carcin/bgq149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahl AR, Grossi IM, Houchens DP, Scovell LJ, Placke ME, Imondi AR, et al. Inhaled isotretinoin (13-cis retinoic acid) is an effective lung cancer chemopreventive agent in A/J mice at low doses: a pilot study. Clin Cancer Res. 2000;6:3015–24. [PubMed] [Google Scholar]
- 12.Wattenberg LW, Wiedmann TS, Estensen RD, Zimmerman CL, Steele VE, Kelloff GJ. Chemoprevention of pulmonary carcinogenesis by aerosolized budesonide in female A/J mice. Cancer Res. 1997;57:5489–92. [PubMed] [Google Scholar]
- 13.Tatsumura T, Koyama S, Tsujimoto M, Kitagawa M, Kagamimori S. Further Study of Nebulization Chemotherapy, a New Chemotherapeutic Method in the Treatment of Lung Carcinomas - Fundamental and Clinical. Brit J Cancer. 1993;68:1146–9. doi: 10.1038/bjc.1993.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie G, Sun Y, Nie T, Mackenzie GG, Huang L, Kopelovich L, et al. Phospho-ibuprofen (MDC-917) is a novel agent against colon cancer: efficacy, metabolism, and pharmacokinetics in mouse models. J Pharmacol Exp Ther. 2011;337:876–86. doi: 10.1124/jpet.111.180224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.She QB, Solit DB, Ye Q, O’Reilly KE, Lobo J, Rosen N. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell. 2005;8:287–97. doi: 10.1016/j.ccr.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 17.Balsara BR, Pei J, Mitsuuchi Y, et al. Frequent activation of AKT in non-small cell lung carcinomas and preneoplastic bronchial lesions. Carcinogenesis. 2004;25:2053–9. doi: 10.1093/carcin/bgh226. [DOI] [PubMed] [Google Scholar]
- 18.Wu WK, Coffelt SB, Cho CH, Wang XJ, Lee CW, Chan FK, et al. The autophagic paradox in cancer therapy. Oncogene. 31:939–53. doi: 10.1038/onc.2011.295. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Rowehl LM, Huang L, Mackenzie GG, Vrankova K, Komninou D, et al. Phospho-ibuprofen (MDC-917) suppresses breast cancer growth: an effect controlled by the thioredoxin system. Breast Cancer Res. 2012;14:R20. doi: 10.1186/bcr3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhoopathi P, Chetty C, Gujrati M, Dinh DH, Rao JS, Lakka S. Cathepsin B facilitates autophagy-mediated apoptosis in SPARC overexpressed primitive neuroectodermal tumor cells. Cell Death Differ. 2010;17:1529–39. doi: 10.1038/cdd.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naito A, Carcel-Trullols J, Xie CH, Evans TT, Mizumachi T, Higuchi M. Induction of acquired resistance to antiestrogen by reversible mitochondrial DNA depletion in breast cancer cell line. Int J Cancer. 2008;122:1506–11. doi: 10.1002/ijc.23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie G, Nie T, Mackenzie GG, Sun Y, Huang L, Ouyang N, et al. The metabolism and pharmacokinetics of phospho-sulindac (OXT-328) and the effect of difluoromethylornithine. Br J Pharmacol. 2012;165:2152–66. doi: 10.1111/j.1476-5381.2011.01705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doki Y, Murakami K, Yamaura T, Sugiyama S, Misaki T, Saiki I. Mediastinal lymph node metastasis model by orthotopic intrapulmonary implantation of Lewis lung carcinoma cells in mice. Br J Cancer. 1999;79:1121–6. doi: 10.1038/sj.bjc.6690178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–75. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang JH, Chang YC, Maurizi MR. 4-O-carboxymethyl ascochlorin causes ER stress and induced autophagy in human hepatocellular carcinoma cells. J Biol Chem. 2012;287:15661–71. doi: 10.1074/jbc.M112.358473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fulzele SV, Chatterjee A, Shaik MS, Jackson T, Singh M. Inhalation delivery and anti-tumor activity of celecoxib in human orthotopic non-small cell lung cancer xenograft model. Pharm Res. 2006;23:2094–106. doi: 10.1007/s11095-006-9074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blasco RB, Francoz S, Santamaria D, Cañamero M, Dubus P, Charron J, et al. c-Raf, but not B-Raf, is essential for development of K-Ras oncogene-driven non-small cell lung carcinoma. Cancer Cell. 2011;19:652–63. doi: 10.1016/j.ccr.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahin U, Blobel CP. Ectodomain shedding of the EGF-receptor ligand epigen is mediated by ADAM17. FEBS Lett. 2007;581:41–4. doi: 10.1016/j.febslet.2006.11.074. [DOI] [PubMed] [Google Scholar]
- 29.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–4. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 30.Rigas B, Sun Y. Induction of oxidative stress as a mechanism of action of chemopreventive agents against cancer. Br J Cancer. 2008;98:1157–60. doi: 10.1038/sj.bjc.6604225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sithanandam G, Smith GT, Masuda A, Takahashi T, Anderson LM, Fornwald LW. Cell cycle activation in lung adenocarcinoma cells by the ErbB3/phosphatidylinositol 3-kinase/Akt pathway. Carcinogenesis. 2003;24:1581–92. doi: 10.1093/carcin/bgg125. [DOI] [PubMed] [Google Scholar]
- 32.Soltoff SP, Carraway KL, 3rd, Prigent SA, Gullick WG, Cantley LC. ErbB3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factor. Mol Cell Biol. 1994;14:3550–8. doi: 10.1128/mcb.14.6.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Din FV, Valanciute A, Houde VP, Zibrova D, Green KA, Sakamoto K, et al. Aspirin Inhibits mTOR Signaling, Activates AMP-Activated Protein Kinase, and Induces Autophagy in Colorectal Cancer Cells. Gastroenterology. 2012;142:1504–15. e3. doi: 10.1053/j.gastro.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol. 2004;36:2503–18. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang L, Mackenzie GG, Sun Y, Ouyang N, Xie G, Vrankova K, et al. Chemotherapeutic properties of phospho-nonsteroidal anti-inflammatory drugs, a new class of anticancer compounds. Cancer Res. 2011;71:7617–27. doi: 10.1158/0008-5472.CAN-11-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montuschi P, Barnes PJ, Roberts LJ., 2nd Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18:1791–800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 37.Marchi S, Giorgi C, Suski JM, Agnoletto C, Bononi A, Bonora M, et al. Mitochondria-ros crosstalk in the control of cell death and aging. J Signal Transduct. 2012;2012:329635. doi: 10.1155/2012/329635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong CC, Cheng KW, Xie G, Zhou D, Zhu CH, Constantinides PP, et al. Carboxylesterases 1 and 2 hydrolyze phospho-nonsteroidal anti-inflammatory drugs: relevance to their pharmacological activity. J Pharmacol Exp Ther. 2012;340:422–32. doi: 10.1124/jpet.111.188508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hosokawa M. Structure and catalytic properties of carboxylesterase isozymes involved in metabolic activation of prodrugs. Molecules. 2008;13:412–31. doi: 10.3390/molecules13020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morton CL, Wierdl M, Oliver L, Ma MK, Danks MK, Stewart CF, et al. Activation of CPT-11 in mice: identification and analysis of a highly effective plasma esterase. Cancer Res. 2000;60:4206–10. [PubMed] [Google Scholar]
- 41.Scagliotti GV, Selvaggi G, Novello S, Hirsch FR. The biology of epidermal growth factor receptor in lung cancer. Clin Cancer Res. 2004;10:4227s–32s. doi: 10.1158/1078-0432.CCR-040007. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 43.Gong HC, Wang S, Mayer G, Chen G, Leesman G, Singh S, et al. Signatures of drug sensitivity in nonsmall cell lung cancer. Int J Proteomics. 2011:215496. doi: 10.1155/2011/215496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sergina NV, Rausch M, Wang D, Blair J, Hann B, Kevan M, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–41. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu Z, Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life. 2006;58:621–31. doi: 10.1080/15216540600957438. [DOI] [PubMed] [Google Scholar]
- 46.Sun Y, Huang L, Mackenzie GG, Rigas B. Oxidative stress mediates through apoptosis the anticancer effect of phospho-nonsteroidal anti-inflammatory drugs: implications for the role of oxidative stress in the action of anticancer agents. J Pharmacol Exp Ther. 2011;338:775–83. doi: 10.1124/jpet.111.183533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–9. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levine B. Cell biology: autophagy and cancer. Nature. 2007;446:745–7. doi: 10.1038/446745a. [DOI] [PubMed] [Google Scholar]
- 50.Shivapurkar N, Reddy J, Chaudhary PM, Gazdar AF. Apoptosis and lung cancer: a review. J Cell Biochem. 2003;88:885–98. doi: 10.1002/jcb.10440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.