Abstract
BACKGROUND
The use of neoadjuvant and adjuvant chemotherapy in soft tissue sarcomas is controversial. This is a report of long-term (≥5 years) follow-up in patients with high-grade, high-risk soft tissue sarcomas treated with neoadjuvant chemotherapy, preoperative radiotherapy (RT), and adjuvant chemotherapy.
METHODS
Patients with high-grade soft tissue sarcoma ≥8 cm in diameter of the extremities and body wall received 3 cycles of neoadjuvant chemotherapy (mesna, doxorubicin, ifosfamide, and dacarbazine) and preoperative RT (44 grays administered in split courses), and 3 cycles of postoperative chemotherapy (mesna, doxorubicin, ifosfamide, and dacarbazine).
RESULTS
Sixty-four of 66 patients were analyzed. After chemotherapy and RT, 61 patients had surgery; 58 had R0 resections (5 amputations), and 3 had R1 resections. Ninety-seven percent experienced grade 3 or higher toxicity, including 3 deaths. These toxicities were short term. With a median follow-up of 7.7 years in surviving patients, the 5-year rates of locoregional failure (including amputation), and distant metastasis were 22.2% (95% confidence interval [CI], 11.8–32.6) and 28.1% (95% CI, 17.0–39.2). The most common site of metastasis was lung. Estimated 5-year rates of disease-free survival, distant disease-free survival, and overall survival were 56.1% (95% CI, 43.9–68.3), 64.1% (95% CI, 52.3–75.8), and 71.2% (95% CI, 60.0–82.5), respectively.
CONCLUSIONS
Although the toxicity was significant, it was limited in its course and for the most part resolved by 1 year. The long-term outcome was better than might be expected in such high-risk tumors.
Keywords: sarcoma, neoadjuvant chemotherapy, radiation
Management approaches for newly diagnosed primary sarcoma include wide local resection combined with preoperative or postoperative radiotherapy or wide local excision alone for small superficial lesions.1–6 Management in this manner results in control of local tumor in 80% to 95% of patients, and the majority of patients benefit with good extremity function.7,8 Patients with high-grade tumors >5 cm are at increased risk for distant treatment failure and death from metastatic disease.8,9 The risk of distant metastatic disease increases with the size of the primary high-grade tumor. The risk is 34% in patients with lesions 5.1 to 10 cm and increases to 43% and 58% for 10.1- to 15-cm and 15.1- to 20-cm lesions, respectively8. A potential role for adjuvant chemotherapy in these high-risk tumors has been investigated. In 2006, the Radiation Therapy Oncology Group published the short-term results of a phase 2 trial evaluating the efficacy and toxicity of a modified mesna, doxorubicin, ifosfamide, and dacarbazine regimen interdigitated with radiotherapy in patients with high-risk, high-grade soft tissue sarcomas of the extremities and torso ≥8 cm in maximum diameter.10 The primary goal of this intervention was to decrease distant metastasis and improve survival in this high-risk group of patients. The initial results from this trial showed this regimen to be associated with a high rate of toxicity, but 1 that could nevertheless be delivered in a cancer cooperative group setting. The short-term outcomes were consistent with the earlier institutional pilot study.11 The early toxicity reported, 73% grade 4 leukopenia with 3 treatment-related deaths, compared unfavorably with that reported by DeLaney et al from the Massachusetts General Hospital using a similar regimen, but with a 25% lower dose of ifosfamide.12 It was considered most likely that this toxicity was related to the higher dose of ifosfamide used in RTOG 9514. Although the early toxicity and outcome of this regimen have been reported by Kraybill et al and DeLaney et al, the long-term toxicity and outcomes have not been reported.10,12 Whether the early very significant local and systemic toxicity is also associated with long-term toxicity and whether any gain in local and systemic tumor control is maintained are important for planning future trials and the potential use of a modified form of the regimen in those trials.
MATERIALS AND METHODS
RTOG 9514 was an Intergroup trial conducted by the Radiation Therapy Oncology Group and the Eastern Cooperative Oncology Group. Protocol eligibility requirements, treatment plans, study endpoints, and statistical methods have been detailed previously.10 Briefly, eligible patients had large (≥8 cm), high-grade (grade 2 or 3 in a 3-tier grading system) primary or locally recurrent soft tissue sarcoma of the extremities or torso clinically judged to be amenable to an R0 resection on completion of neoadjuvant chemotherapy and radiation therapy. For extremity lesions, a requirement for admission to the protocol was that patients be deemed candidates for a limb salvage procedure by the surgeon and the radiation oncologist. Resections were defined as R2 if the margins were macroscopically positive and with visible tumor left behind, R1 if all macroscopic disease was removed but with the margins microscopically positive, or R0 if margins were microscopically negative. Protocol treatment was 3 cycles of preoperative modified mesna, doxorubicin, ifosfamide, and dacarbazine chemotherapy with 44 grays (Gy) of radiation given in split courses of 22 Gy between the first and second cycles and between the second and third cycles, followed by surgery and 3 cycles of postoperative mesna, doxorubicin, ifosfamide, and dacarbazine. Rates of locoregional failure and distant metastases were estimated using the method of cumulative incidence.13 Amputation for any indication was considered locoregional failure because of the protocol’s aim of achieving limb preservation. Rates of overall, disease-free, and distant disease-free survival were estimated using the Kaplan-Meier method.14 All efficacy endpoints were measured from the date of registration in the study.
The National Cancer Institute (NCI) Common Toxicity Criteria version 1.0 was used for chemotherapy toxicity, and the Radiation Therapy Oncology Group acute and late toxicity criteria were used to describe toxicity secondary to radiotherapy.15 The follow-up regimen was designed to assess in detail both early and late toxicity. The early regimen was designed to identify and record expected toxicities from the combined chemotherapy and radiation therapy regimen. After completion of therapy, patients were followed at a minimum of every 3 months for the first 2 years, every 6 months for years 2 through 5, and yearly thereafter. These postoperative evaluations included history and physical exam, blood work, and imaging. The imaging consisted of computerized tomography (CT) of the chest and either magnetic resonance imaging (MRI) or CT of the primary tumor site. Late grade 2 to 4 toxicity rates at 1, 2, 3, 4, and 5 years from the start of radiation therapy were estimated using the maximum grade reported for each toxicity type over 1-year intervals around the time point of interest (ie, ±6 months). This was done to avoid underestimation of the toxicity rates. Although careful follow-up for long-term complications was performed as described above, detailed evaluation of post-treatment function was not done.
RESULTS
Patient Population
Sixty-six patients from 31 institutions were enrolled between February 1997 and February 2000. Two patients were ineligible (1 with metastatic disease and 1 with ineligible histology), leaving 64 patients for analysis. The data that form the basis of this report represent all information received and processed at Radiation Therapy Oncology Group headquarters through July 25, 2007. Median follow-up for surviving patients was 7.7 years (range, 2.0–9.3 years), with all but 4 of the surviving patients having >5 years follow-up, compared with median 2.7 years for the initial report. Pretreatment characteristics have been detailed previously. Eighty percent were histologically grade 3 (in a 3-tier grading system). The median tumor size as measured by MRI, CT, or clinical finding was 15 cm (range, 8.2–55 cm).
Treatment Summary
Treatment delivery with respect to the protocol prescription has been summarized previously.10 Briefly, only 59% of patients received all 6 cycles of mesna, doxorubicin, ifosfamide, and dacarbazine chemotherapy, and 89% received a preoperative radiation dose per protocol (within 5%). Of 5 patients that received <95% of prescribed radiation, 3 had a local failure. However, 2 of these stopped radiation because of failure. One had an amputation, and 1 had progression. Local failures are not thought to be secondary to inadequate radiation dose. Open biopsy for diagnosis was used in 82%, core needle biopsy in 15%, and aspiration cytology in 4%. Sixty-one patients underwent resection in RTOG 9514, and 3 patients did not. Of these 3 patients, 2 had persistent and progressive primary tumors and were not candidates for R0 resections. The third patient’s primary was controlled; however, he had progressive distant disease and refused local resection. Fifty-eight (91%) patients had R0 resection (of which 5 were amputation), and the other 3 had R1 resections. Simple wound closure was used in 47% of patients, local muscle flaps in 13%, myocutaneous or muscle flaps in 11%, some other method in 13%, and a combination of reconstructive techniques was used in 16%.
Treatment Toxicity
The toxicity of this regimen, as previously reported, is considerable. Three (5%) patients died of treatment-related causes, 2 of which were secondary to acute myelogenous leukemia (AML), which occurred at 28 and 29 months. Ninety-seven percent experienced grade 3 or higher toxicities, including 3 grade 5 (death) toxicities. These toxicities were, for the most part, acute and transitory (Table 1). At 1 year, 25% (15 of 59) of patients had 1 or more grade 3 to 4 toxicities, but this rate was reduced to 7% (4 of 58) at 2 years, 4% (2 of 47) at 3 years, 3% (1 of 37) at 4 years, and 3% (1 of 34) at 5 years. At 1 year, 19% (11 of 59) had grade 1 to 2 toxicity, which was reduced to 6% (3 of 58) at 2 years, 2% (1 of 47) at 3 years, 3% (1 of 37) at 4 years, and 6% (2 of 34) at 5 years. The toxicities at 5 years were grade 2 fracture in 1 patient, grade 2 endocrine in 1 patient, and in a third patient grade 2 pain and grade 3 infection. Late grade 2 to 4 toxicities by type at 1, 2, 3, 4, and 5 years from the start of therapy are summarized in Table 1. This table demonstrates the severe acute toxicity and marked decrease in incidence and severity of treatment-associated toxicity later in follow-up.
Table 1.
Grade 2 to 4 Toxicities
| Toxicity | Acute (n=64),a Grade | Late (n=60),b Grade | 1 Year (n=59),c Grade | 2 Years (n=58),c Grade | 3 Years (n=47),c Grade | 4 Years (n=37),c Grade | 5 Years (n=34),c Grade | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | |
| Hematologic | 3 (4.7%) | 9(14.1%) | 50(78.1%) | 3 (5.0%) | 1 (1.7%) | 8 (13.3%) | 2 (3.4%) | 1 (1.7%) | 7 (11.9%) | 1 (1.7%) | 0 | 2 (3.4%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Infection | 9(14.1%) | 14(21.9%) | 3 (4.7%) | 4 (6.7%) | 3 (5.0%) | 1 (1.7%) | 4 (6.8%) | 3(5.1%) | 1 (1.7%) | 1 (1.7%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.9%) | 0 |
| Skin | 22 (34.4%) | 18(28.1%) | 3 (4.7%) | 2 (3.3%) | 2 (3.3%) | 0 | 1 (1.7%) | 2 (3.4%) | 0 | 1 (1.7%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mucous membrane | 9(14.1%) | 1 (1.6%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subcutaneous tissue | 0 | 0 | 1 (1.6%) | 1 (1.7%) | 1 (1.7%) | 1 (1.7%) | 1 (1.7%) | 1 (1.7%) | 1 (1.7%) | 0 | 1 (1.7%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hepatic | 4 (6.3%) | 0 | 0 | 1 (1.7%) | 0 | 0 | 1 (1.7%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 8(12.5%) | 3 (4.7%) | 0 | 1 (1.7%) | 0 | 0 | 0 | 0 | 0 | 1 (1.7%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nausea/vomiting | 29 (45.3%) | 5 (7.8%) | 2 (3.1%) | 2 (3.3%) | 0 | 0 | 2 (3.4%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other gastrointestinal | 5 (7.8%) | 3 (4.7%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neurological | 5 (7.8%) | 2 (3.1%) | 0 | 2 (3.3%) | 0 | 0 | 1 (1.7%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pain | 9(14.1%) | 1 (1.6%) | 1 (1.6%) | 1 (1.7%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.9%) | 0 | 0 |
| Genitourinary | 3 (4.7%) | 1 (1.6%) | 0 | 2 (3.3%) | 0 | 0 | 1 (1.7%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.7%) | 0 | 0 | 0 | 0 | 0 |
| Respiratory | 2 (3.1%) | 0 | 1 (1.6%) | 1 (1.7%) | 0 | 1 (1.7%) | 1 (1.7%) | 0 | 1 (1.7%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cardiac | 0 | 0 | 1 (1.6%) | 1 (1.7%) | 1 (1.7%) | 0 | 1 (1.7%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fever | 8(12.5%) | 1 (1.6%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Joint | 1 (1.6%) | 0 | 0 | 3 (5.0%) | 1 (1.7%) | 0 | 2 (3.4%) | 0 | 0 | 1 (1.7%) | 0 | 0 | 1 (2.1%) | 0 | 0 | 0 | 1 (2.7%) | 0 | 0 | 0 | 0 |
| Peripheral nerves | 1 (1.6%) | 0 | 0 | 1 (1.7%) | 2 (3.3%) | 0 | 1 (1.7%) | 1 (1.7%) | 0 | 0 | 1 (1.7%) | 0 | 0 | 1 (2.1%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vascular | 1 (1.6%) | 2 (3.1%) | 0 | 2 (3.3%) | 1 (1.7%) | 0 | 1 (1.7%) | 1 (1.7%) | 0 | 0 | 0 | 0 | 1 (2.1%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 20 (31.3%) | 6 (9.4%) | 3 (4.7%) | 5 (8.3%) | 0 | 2 (3.3%) | 5 (8.5%) | 0 | 0 | 0 | 0 | 1 (1.7%) | 0 | 0 | 1 (2.1%) | 0 | 0 | 0 | 2 (5.9%) | 0 | 0 |
The acute period is ≤6 months from the initiation of radiotherapy.
The late period is >6 months from initiation of radiotherapy.
The yearly rates are measured from the initiation of radiotherapy using intervals of ±6 months
Five of 53 extremity sarcomas underwent amputation, for a 9.4% amputation rate. We considered 2 of them to be treatment related, where the patients developed leukopenia-associated sepsis attributed to infection at the biopsy site. Two other patients were thought to have an inadequate clinical response to neoadjuvant treatment. One underwent a disarticulation, and the other underwent Van Ness rotationplasty with a bone graft. There was no viable tumor in either specimen. The fifth patient with a high-grade leiomyosarcoma of the axilla completed neoadjuvant chemotherapy and radiation therapy. On exploration of the axilla, the tumor was deemed too close to the neurovascular bundle, and he underwent forequarter resection. Pathology showed extensive necrosis with islands of viable tumor.
Survival and Pattern of Failure
At a median follow-up of 7.7 years (range, 2.0–9.3 years) for 42 surviving patients, 35 (54.7%) patients were alive without any disease failure (Fig. 1). Since the initial report, there have been 3 additional locoregional failures (for a total of 14), and 1 additional patient (1, lung) with distant metastases (for a total of 19). There have been 2 newly reported (1, posterior neck sarcoma; 1, pancreatic cancer) second primaries, for a total of 6 second primaries. Two of these second primaries were AML and considered a complication of chemotherapy. There have been 8 additional deaths from disease for a total of 22. The estimated 5-year locoregional failure and distant metastases rates are 22.2% (95% confidence interval [CI], 11.8–32.6) and 28.1% (95% CI, 17.0–39.2) (Fig. 1). If amputation is not considered a locoregional failure, the 5-year locoregional failure rate is 20.7%. Excluding amputations, local recurrences were managed with radiotherapy + surgery (n = 3), surgery (n = 1), chemotherapy (n = 2), and no treatment (n = 3). The most common site of distant failure was the lung (16 of 19). There have been only 4 locoregional failures after 2 years and none after 5 years. Only 2 patients have experienced distant metastases after 2 years, 1 of which was after 5 years. In total, 25 patients have experienced locoregional failure and/or distant metastases. Of 42 patients still at risk for both failures at 2 years, only 3 subsequently failed (1 locoregional; 1 distant; 1 both). Of 36 patients still at risk for both failures at 5 years, only 1 subsequently failed (1 distant).
Figure 1.
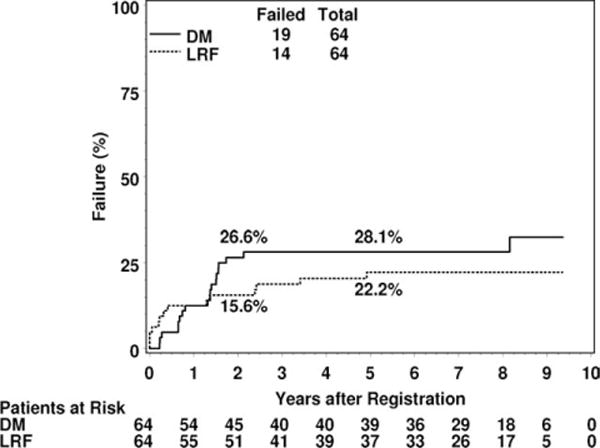
Cumulative incidence estimates of distant metastasis (DM) and locoregional failure (LRF) are shown.
The estimated 5-year rate of second primaries is 9.7% (95% CI, 2.2–17.1). Again, 2 of these were AML. The 5-year estimated rates of disease-free and distant disease-free survival are 56.1% (95% CI, 43.9–68.3) and 64.1% (95% CI, 52.3–75.8) (Fig. 2). The estimated 5-year survival rate is 71.2% (95% CI, 60.0–82.5). Cause of death was the study cancer in 15 patients, second primary in 1 patient, protocol treatment in 3 patients (this includes the 2 AML patients), and unknown in 3 patients.
Figure 2.
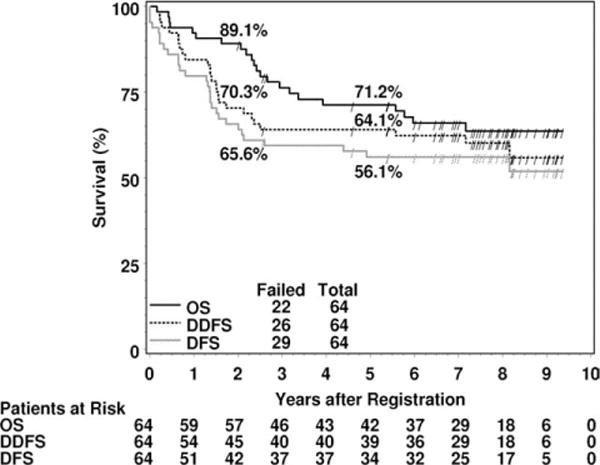
Kaplan-Meier estimates of overall survival (OS), distant disease-free survival (DDFS), and disease-free survival (DFS) are shown. ‘‘/’’ indicates a censored patient.
DISCUSSION
RTOG 9514 was opened in 1997 to assess what appeared at that time to be a promising regimen of adjuvant treatment for advanced primary soft tissue sarcomas of the extremities and torso developed at the Massachusetts General Hospital.11 As with the pilot, the study group was selected because they had extraordinary risk for developing distant metastasis and dying of metastatic disease.8 Since RTOG 9514 was opened and began to accrue patients, several other investigators have studied and reported their outcomes in the management of high-risk primary soft tissue sarcomas. These reports have not infrequently been contradictory in their results with regard to toxicity and outcomes. Excellent results have been reported with surgery alone in selected patients.5,16 Overall, factors identified as being important have included margins ≥1 cm, compartmental resections, and tumors amenable to wide resection despite being locally advanced. However, most groups have concluded that improved local control with limb salvage has more potential for success when wide excision is combined with either preoperative or postoperative radiation in patients with tumors >5 cm in diameter that are high grade17; furthermore, the use of radiation therapy may permit closer surgical margins18 while maintaining high rates of local control, which may be important when wider resection would compromise limb function.
There are 2 important randomized trials that are relevant. Yang and his coauthors from the NCI reported 141 patients with soft tissue sarcomas randomized between surgical resection alone and resection with postoperative radiation.19 This trial demonstrated improved local control in those patients receiving postoperative radiation. An accompanying quality of life study showed a decrease in joint motion and an increase in limb weakness and edema in patients receiving postoperative radiation. The National Cancer Institute of Canada randomized 190 patients to preoperative radiation (50 Gy in 25 fractions) versus postoperative radiation (66 Gy in 33 fractions).20 In several publications from 2002 to 2004, this group reported comparable 5-year results for local control (93% vs 92%); metastatic relapse-free survival (67% vs 69%), recurrence-free survival (58% vs 59%), and overall survival (78% vs 73%) (P = .64).21 However, preoperative radiation was clearly associated with a higher incidence of acute wound complications. An evaluation of radiation-associated morbidity in this series demonstrated that patients treated with postoperative radiation tended to have greater late fibrosis, joint stiffness, and edema that adversely affected patient function.22 These trials both support the use of radiation in combination with surgery as a means to achieve limb salvage and local control of extremity tumors. Neither trial suggested that radiation would enhance long-term survival or decrease distant metastasis. Both group’s cohorts of patients were at lower risk than those managed in RTOG 9514.
The locoregional failure rate and the amputation rate were higher in RTOG 9514 than might be expected. Since the initial report, there have been 3 additional locoregional failures (for a total of 14), with a locoregional failure rate of 22.2%. Five of 53 extremity sarcomas underwent amputation, for a 9.4% amputation rate. Although a primary objective of the trial was to assess its possible role in decreasing distant metastasis and in enhancing survival, an important secondary goal was to do so without compromising local control. What were some of the factors influencing the local recurrence rate and amputation rate? Patient selection may have had a role. Although patients were admitted to the trial only with the agreement of the surgical or orthopedic oncologist and the radiation oncologist, this was a cooperative group trial involving 31 institutions. For tumors >8 cm in maximum diameter, it is likely that committed investigators may differ in their experience and view of which of these patients would be candidates for this trial. Also, there were 2 patients judged at the time of surgery not to be candidates for limb salvage and found to have no viable tumor in the specimen after amputation. It is also likely that surgeons would differ in their experience and view of what is an acceptable margin after neoadjuvant therapy. It is reasonable to suggest that these 2 factors in the selection of patients for this protocol and the selection of the surgical procedure in the management of patients may have resulted in decreased locoregional control and an increased amputation rate. Another factor that almost certainly can effect outcome, both local control and distant control, is the response to neoadjuvant and adjuvant therapy. Central review of the resected specimens demonstrated that some tumors clearly responded and some did not. In 14 of 51 assessable patients (27%), there was no viable tumor identified.10 Three (6%) patients had >75% viable tumor. The number of patients assessed for viable tumor was inadequate to assess its importance statistically. Variation by patient in terms of response to chemotherapy and radiation is common and may have impacted outcome in this protocol. Furthermore, 2 of the amputations occurred because of wound infections at the tumor biopsy site in association with chemotherapy-induced leukopenia, a rare cause of amputation in most series.
The 5-year estimated rates of disease-free and distant disease-free survival, 56.1% and 64.1%, respectively, are better than might be expected for tumors of this size and grade (Fig. 2). The estimated 5-year survival rate was 71.2%. This compares favorably with historical controls for tumors of this size and grade.8,23 In a series of patients treated with a similar regimen but with a lower dose of ifosfamide from the Massachusetts General Hospital, the local control, disease-free, and distant disease-free survival were 92%, 75%, and 70%, respectively.12 The estimated 5-year survival rate was 87%. There was significant early toxicity in this trial as well, considered to be secondary to the mesna, doxorubicin, ifosfamide, and dacarbazine chemotherapy. However, it was not nearly as severe as that in RTOG 9514. This difference is thought to be secondary to the increased ifosfamide dose given in RTOG 9514. Although it is difficult to compare single institution results with those from cooperative groups, outcomes noted above are also clearly better in the Massachusetts General Hospital trial. This is possibly secondary to a greater percentage of tumors in RTOG 9514 being grade 3 tumors (80% vs 50% in the Massachusetts General Hospital trial) and the inclusion of a greater percentage of truncal lesions in RTOG 9514. Also, the selection process for the Massachusetts General Hospital trial is likely to be more consistent than in RTOG 9514. Another trial assessing preoperative chemotherapy concurrently with radiation was that reported by Edmonson et al from the Mayo Clinic.24 Thirty-nine patients received 2 cycles of aggressive chemotherapy consisting of ifosfamide, mitomycin, doxorubicin, and cisplatin. After 2 monthly cycles of this regimen, radiation to a total dose of 45 Gy was given concurrently with mitomycin, cisplatin, and doxorubicin. One month after completion of external beam radiation these patients were resected with an additional 10 to 20 Gy of intraoperative or postoperative radiation being given to the field after resection. These were all grade 3 or 4 tumors and were large tumors, with 44% being >10 cm in maximum diameter, although they were probably smaller overall than those reported in RTOG 9514 and the Massachusetts General Hospital study. The Kaplan-Meier estimate of 5-year survival was 80%. This was also a toxic regimen, with grade 3 or greater toxicity consisting of leukopenia (54%), thrombocytopenia (77%), and infection (10%), principally neutropenic fever. All 3 of these regimens consisting of neoadjuvant chemotherapy given with radiation have been associated with significant toxicity but have provided interesting results in terms of disease-free survival, distant disease-free survival, and overall survival. These outcomes are some-what better than might be expected in locally advanced high-grade soft tissue sarcomas. The estimated rate of distant metastasis for high-grade tumors of this size approaches or exceeds 50%.8,23
These 3 phase 2 studies were developed to improve the long-term outcomes in patients with extraordinarily high-risk tumors, to decrease distant metastasis and improve long-term survival. These 3 studies appear to have better than expected long-term results in regard to disease-free survival and distant disease-free survival. Nedea et al updated the Massachusetts General Hospital experience. Their report comparing neoadjuvant mesna, doxorubicin, ifosfamide, and dacarbazine-treated patients with historical matched high-risk controls treated with radiation and surgery suggested improved outcomes with neoadjuvant mesna, doxorubicin, ifosfamide, and dacarbazine.12,25 A careful review of the long-term complications and recurrences, both local and distant, is important, as continuing complications occurring in patients treated with this regimen could preclude consideration of its potential use in future protocols or in combination with other therapeutic interventions. The most common late toxicity, defined as occurring at least 6 months after the initiation of radiation, was leukopenia, with 8 patients still manifesting grade 4 toxicity. However, this was for the most part resolved after the first year. The incidence of significant toxicity was markedly decreased after 1 and almost completely after 2 years.
In a careful and thoughtful review of the potential role of adjuvant and neoadjuvant chemotherapy with doxorubicin- and doxorubicin/ifosfamide-based regimens, Bramwell presented her view that it is premature to routinely manage high-risk soft tissue sarcomas with adjuvant or neoadjuvant chemotherapy.26 She based this view on the toxicity reported from regimens being used such as in RTOG 9514. She also emphasized that trials in breast cancer and osteosarcoma assessing a potential advantage with neoadjuvant chemotherapy versus adjuvant chemotherapy failed to demonstrate a benefit in giving chemotherapy before other therapies.27,28 Conceptually, there might be an advantage to starting chemotherapy before other therapies to treat microscopic metastatic disease. Published data in these tumors do not support this view. Also, there was a relatively small benefit identified in a rigorously done meta-analysis (SMAC meta-analysis) reported in 1997.29 In the SMAC meta-analysis, the original data sets were accessed, reviewed, and reanalyzed. A more recent meta-analysis studying the role of adjuvant chemotherapy used only the published results and a different statistical methodology. Trials were included that had a minimum of 2 years follow-up.30 This trial includes the addition of ifosfamide to the doxorubicin-based regimens. This trial found statistical significance for survival, with a 46% risk of death in patients not receiving chemotherapy versus 40% in patients receiving chemotherapy. This does not resolve the issue of toxicity. On the basis of its extensive studies, the European Organization for Research and Treatment of Cancer has concluded that adjuvant and/or neoadjuvant chemotherapy should not be used outside a clinical trial setting.31,32 The literature concerning the use of adjuvant or neoadjuvant doxorubicin and/or doxorubicin/ifosfamide is interesting, but is inconclusive with regard to toxicity and efficacy issues.
There have been reports of the use of adjuvant drug therapies that are directed toward specific histopathologic types of sarcomas. Eilber et al, combining sarcoma databases from Memorial Sloan-Kettering Cancer Center and University of California at Los Angeles, reported that adjuvant ifosfamide was associated with improved disease-specific survival for high-grade extremity liposarcomas.33 In a separate article, Eilber et al reported that adjuvant ifosfamide was associated with improved survival in synovial sarcomas.34 Targeted therapies for soft tissue sarcomas are being investigated. Imatinib, very successful in gastrointestinal stromal tumors, is being investigated as potential therapy in other soft tissue sarcomas such as dermatofibrosarcoma protuberans, chordoma, and aggressive fibromatosis.35 Insulinlike growth factor is being investigated as targeted therapy for pediatric sarcomas and adult sarcomas.36 The specific histone deacetylase inhibitor PCI-24,781 in combination with chemotherapy is being investigated in sarcoma animal models.37
In summary, RTOG 9514 assessed a regimen of very aggressive chemotherapy in combination with preoperative radiation as adjuvant therapy for uncommonly high-risk extremity and torso soft tissue sarcomas. Although probably the highest-risk primary soft tissue sarcoma cohort assessed in the cooperative group setting, the long-term outcomes were better than might be expected for this group of high-risk tumors. Although the early toxicity was severe, this abated for the most part after 6 months and did not continue to any significant degree beyond 1 year. Potential reasons for this toxicity have been outlined. This regimen should not be used outside a clinical trial setting, but in a modified form and possibly in combination with other therapies might still be considered for study. Future adjuvant therapy regimens in this group of tumors need to be less toxic and more efficacious. Efforts such as those of the RTOG 0630 seeking to safely decrease radiation fields in the management of extremity soft tissue sarcomas may well decrease the local toxicity of management of these tumors and facilitate combination with systemic therapy. Identifying specific tumor subtypes more responsive to specific chemotherapy regimens and regimens combined with targeted therapies may improve efficacy without increasing toxicity. Continued clinical and translational research directed toward these fascinating tumors is required to improve outcomes and decrease toxicity.
Acknowledgments
We thank Thomas Pajak, PhD, Statistician at Radiation Therapy Oncology Group, and the data management personnel at Radiation Therapy Oncology Group, without whom this work could not have been accomplished.
CONFLICT OF INTEREST DISCLOSURES
Supported by RTOG U10 CA21661, CCOP U10 CA37422, and Stat U10 CA32115 grants from the NCI. The contents are the sole responsibility of the authors and do not necessarily represent the official views of the NCI. Dr. Ettinger has acted as a consultant for AstraZeneca, Bristol-Myers Squibb, Celgene, Eli Lilly & Co., Eisai, Genentech, GlaxoSmithKline, Imclone, Merck, Novartis, Pfizer, and Sanofi-Aventis. Dr. Letson has acted as a consultant for Stryker. Dr. Eisenberg has served on the Novartis advisory board.
References
- 1.Lawrence WJR, Donegan WL, Natarajan N, et al. A pattern of care survey of the American College of Surgeons. Ann Surg. 1987;205:349–359. doi: 10.1097/00000658-198704000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindberg RD, Martin RG, Romsdahl MM, et al. Conservative surgery and postoperative radiotherapy in 300 adults with soft-tissue sarcomas. Cancer. 1981;47:2392–2397. doi: 10.1002/1097-0142(19810515)47:10<2391::aid-cncr2820471012>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of limb-sparing surgery plus radiation therapy compared with amputation and the role of adjuvant chemotherapy. Ann Surg. 1982;196:305–315. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859–868. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 5.Rydholm A, Gustafson P, Rooser B, et al. Limb-sparing surgery without radiotherapy based on anatomic location of soft tissue sarcoma. J Clin Oncol. 1991;10:1757–1765. doi: 10.1200/JCO.1991.9.10.1757. [DOI] [PubMed] [Google Scholar]
- 6.Baldini EH, Goldberg J, Jenner C, et al. Long-term outcomes after function-sparing surgery without radiotherapy for soft tissue sarcoma of the extremities and trunk. J Clin Oncol. 1999;17:3252–3259. doi: 10.1200/JCO.1999.17.10.3252. [DOI] [PubMed] [Google Scholar]
- 7.Stinson SF, DeLaney TF, Greenberg J, et al. Acute and long-term effects on limb function of combined modality limb sparing therapy for extremity soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 1991;221:1493–1499. doi: 10.1016/0360-3016(91)90324-w. [DOI] [PubMed] [Google Scholar]
- 8.Delaney TF, Rosenberg D, Harmon DC, et al. Soft tissue sarcomas. In: Price P, Sikora K, editors. Treatment of Cancer. 4. London, UK: Arnold; 2002. pp. 867–907. [Google Scholar]
- 9.Potter DA, Glenn J, Kinsella T, et al. Patterns of recurrence in patients with high-grade soft-tissue sarcomas. J Clin Oncol. 1985;3:353–366. doi: 10.1200/JCO.1985.3.3.353. [DOI] [PubMed] [Google Scholar]
- 10.Kraybill WG, Harris J, Spiro IJ, et al. Phase II study of neoadjuvant chemotherapy and radiation therapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. J Clin Oncol. 2006;24:619–625. doi: 10.1200/JCO.2005.02.5577. [DOI] [PubMed] [Google Scholar]
- 11.Spiro IJ, Suit H, Gebhardt M, et al. Neoadjuvant chemotherapy and radiotherapy for large soft tissue sarcomas [abstract] Proc Am Soc Clin Oncol. 1996;15:524. Abstract 168. [Google Scholar]
- 12.DeLaney TF, Spiro IJ, Suit HD, et al. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int J Radiat Biol Phys. 2003;56:1117–1127. doi: 10.1016/s0360-3016(03)00186-x. [DOI] [PubMed] [Google Scholar]
- 13.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: Wiley; 1980. pp. 163–178. [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 15.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) [editorial] Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 16.Karakousis CP, Proimakis C, Walsh DL. Primary soft tissue sarcoma of the extremities in adults. Br J Surg. 1995;82:1208–1212. doi: 10.1002/bjs.1800820919. [DOI] [PubMed] [Google Scholar]
- 17.Fabrizio PL, Stafford SL, Pritchard DJ. Extremity soft-tissue sarcomas selectively treated with surgery alone. Int J Radiat Oncol Biol Phys. 2000;48:227–232. doi: 10.1016/s0360-3016(00)00601-5. [DOI] [PubMed] [Google Scholar]
- 18.Kim B, Chen YL, Goldberg SI, et al. An effective preoperative three-dimensional radiotherapy target volume for extremity soft tissue sarcoma and the effect of margin width on local control. Int J Radiat Biol Phys. 2010;77:843–850. doi: 10.1016/j.ijrobp.2009.06.086. [DOI] [PubMed] [Google Scholar]
- 19.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 20.O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet. 2002;359:2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 21.O’Sullivan B, Davis A, Turcotte R, et al. Five-year results of a randomized phase III trial of pre-operative vs post-operative radiotherapy in extremity soft tissue sarcoma. J Clin Oncol. 2004(22):9007. [Google Scholar]
- 22.Davis A, O’Sullivan, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75:48–53. doi: 10.1016/j.radonc.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Coindre JM, Terrier P, Bui NB, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma: a study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol. 1996;14:869–877. doi: 10.1200/JCO.1996.14.3.869. [DOI] [PubMed] [Google Scholar]
- 24.Edmonson JH, Petersen IA, Shives TC, et al. Chemotherapy, irradiation, and surgery for function-preserving therapy of primary extremity soft tissue sarcomas. Cancer. 2002;94:786–792. doi: 10.1002/cncr.10259. [DOI] [PubMed] [Google Scholar]
- 25.Nedea EA, Spiro IJ, Suit HD, et al. Long-term follow-up of patients treated with neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas [abstract] Int J Radiat Oncol Biol Phys. 2006;66(3 suppl):S118. doi: 10.1016/s0360-3016(03)00186-x. [DOI] [PubMed] [Google Scholar]
- 26.Bramwell VHC. Controversies in surgical oncology: routine anthracycline-based adjuvant chemotherapy for stage III extremities soft tissue sarcoma. Ann Surg Oncol. 2007;14:1254–1256. doi: 10.1245/s10434-006-9168-8. [DOI] [PubMed] [Google Scholar]
- 27.Bear HD, Anderson S, Smith RE, et al. A randomized trial comparing preoperative (preop) doxorubicin/cyclophosphamide (AC) to preop AC followed by preop docetaxel (T) and to preop AC followed by postoperative (postop) T in patients (pts) with operable carcinoma of the breast: results of NSABP B-27 [abstract] Breast Cancer Res Treat. 2005;88:S16. Abstract 26. [Google Scholar]
- 28.Goorin AM, Schwartzentruber DJ, Devidas M, et al. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol. 2003;21:1574–1580. doi: 10.1200/JCO.2003.08.165. [DOI] [PubMed] [Google Scholar]
- 29.Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet. 1997;350:1647–1654. [PubMed] [Google Scholar]
- 30.Pervaiz N, Cotterjohn N, Farrokhyar F, et al. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113:573–581. doi: 10.1002/cncr.23592. [DOI] [PubMed] [Google Scholar]
- 31.Italiano A, Penel N, Robin YM, et al. Neo/adjuvant chemotherapy does not improve outcome in resected primary synovial sarcoma: a study of the French Sarcoma Group. Ann Oncol. 2009;20:425–430. doi: 10.1093/annonc/mdn678. [DOI] [PubMed] [Google Scholar]
- 32.Le Cesne A, Van Glabbeke MV, Woll PJ, et al. The end of adjuvant chemotherapy (adCT) era with doxorubicin-based regimen in resected high-grade soft tissue sarcoma (STS): pooled analysis of the two STBSG-EORTC phase III clinical trials [abstract] J Clin Oncol. 2008;26(suppl):10525. Abstract. [Google Scholar]
- 33.Eilber FC, Eilber FR, Eckardt J, et al. The impact of chemotherapy on the survival of patients with high-grade primary extremity liposarcoma. Ann Surg. 2004;240:686–697. doi: 10.1097/01.sla.0000141710.74073.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eilber FC, Brennan MF, Eilber FR, et al. Chemotherapy is associated with improved survival in adult patients with primary extremity synovial sarcoma. Ann Surg. 2007;246:105–113. doi: 10.1097/01.sla.0000262787.88639.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duffaud F, Le Cesne A. Imatinib in the treatment of solid tumors. Target Oncol. 2009;4:45–56. doi: 10.1007/s11523-008-0101-x. [DOI] [PubMed] [Google Scholar]
- 36.Kolb EA, Gorlick R. Development of IGF-IR inhibitors in pediatric sarcomas. Curr Oncol Rep. 2009;11:307–313. doi: 10.1007/s11912-009-0043-1. [DOI] [PubMed] [Google Scholar]
- 37.Lopez G, Liu J, Ren W, et al. Combining PCI-24781, a novel histone deacetylase inhibitor, with chemotherapy for the treatment of soft tissue sarcoma. Clin Cancer Res. 2009;15:3472–3483. doi: 10.1158/1078-0432.CCR-08-2714. [DOI] [PubMed] [Google Scholar]


