Abstract
The extracellular matrix (ECM) plays diverse regulatory roles throughout development. Coordinate interactions between cells within a tissue and the ECM result in the dynamic remodeling of ECM structure. Both chemical signals and physical forces that result from such microenvironmental remodeling regulate cell behavior that sculpts tissue structure. Here, we review recent discoveries illustrating different ways in which ECM remodeling promotes dynamic cell behavior during tissue morphogenesis. We focus first on new insights that identify localized ECM signaling as a regulator of cell migration, shape, and adhesion during branching morphogenesis. We also review mechanisms by which the ECM and basement membrane can both sculpt and stabilize epithelial tissue structure, using as examples Drosophila egg chamber development and cleft formation in epithelial organs. Finally, we end with an overview of the dynamic mechanisms by which the ECM can regulate stem cell differentiation to contribute to proper tissue morphogenesis.
Keywords: fibronectin, basement membrane, cell dynamics, development, morphogenesis
Introduction
Numerous studies in diverse developmental systems have revealed that dynamic changes in cell motility, shape, and adhesion are major driving forces sculpting tissue-specific form and function. Over the last decade, it has become increasingly apparent that many of these processes can be modulated by chemical, physical, and topographical cues present in the cellular microenvironment [1-3]. Since the extracellular matrix (ECM) surrounding cells in vivo is a major component of this microenvironment, it comes as no surprise that the ECM is a critical regulator of developmental dynamics [4-6].
The ECM, composed of a fibrous mesh of glycoproteins and proteoglycans [7], is more than a static structure supporting tissue architecture. The binding of ECM proteins to cell surface integrins and other receptors promotes a variety of cellular responses including survival, proliferation, adhesion, and migration [1,2,8]. Furthermore, the ECM is dynamically remodeled during development and disease states, as cells constantly degrade and resynthesize the ECM to promote rapid changes in the microenvironment [5,6]. In this review, we describe particularly insightful recent examples highlighting ways in which ECM remodeling can regulate cell dynamics during tissue morphogenesis. We focus on specific concepts, including ECM effects on cell motility and adhesion, basement membrane-mediated sculpting of tissue shape, and ECM regulation of tissue differentiation, which provide clear examples of the reciprocity between ECM and cellular dynamics governing epithelial tissue morphogenesis. For recent comprehensive reviews on the role of ECM in development, please see references [5,6,9-12].
ECM promotes local changes in cell dynamics during tissue morphogenesis
An evolving theme in developmental biology is that signals from the ECM promote localized (rather than global) changes in cell behavior. For example, localized deposition of a specific matrix protein can trigger integrin signals that alter patterns of cell motility and adhesion. Recent work has delineated a fibronectin (FN)-mediated signaling cascade that promotes local cell dynamics during branching morphogenesis [13,14], a conserved developmental mechanism by which a primary epithelial bud or tube undergoes dynamic, coordinated cellular rearrangements to give rise to the complex branched epithelial architecture of many mammalian organs [15,16]. Cleft formation is a major mode of branching, which subdivides an epithelial bud into two new buds. Local FN deposition rapidly induces Btbd7 [BTB (POZ) domain containing 7] in a focal region at the base of progressing clefts, which in turn up-regulates the transcription factor Snail2 and down-regulates the adhesion molecule E-cadherin (Figure 1). These focal changes in cell signaling promote localized changes in cell behavior at the base of progressing clefts associated with altered cell shape, a more motile phenotype, and decreased cell adhesion leading to the formation of transient intercellular gaps [13] (Figure 1). Thus, cooperative interactions between FN and local cell dynamics appear to drive cleft progression.
Figure 1. Focal ECM deposition regulates dynamic cell behavior during branching morphogenesis.
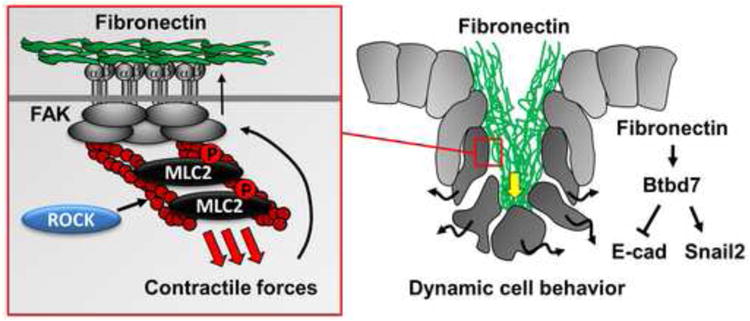
Fibronectin (FN) is focally assembled to promote cleft progression during epithelial morphogenesis. FN induces Btbd7 at the base of an initiated cleft, which in turn up-regulates Snail2 and down-regulates E-cadherin. This increases local cell dynamics at the cleft base (black wavy arrows) and opens up transient intercellular gaps (between dark gray cells) to advance the cleft (yellow arrow). The FN assembly requires intracellular Rho kinase (ROCK)-mediated actomyosin contraction and focal adhesion kinase (FAK) activation to unfold dimeric globular FN for fibril assembly (left panel).
Since Snail2 is a well-known promoter of epithelial-to-mesenchymal transition (EMT) [17], it is possible that branch formation involves FN-induced partial EMT at focal locations at the epithelial periphery. Indeed, EMT scatter factors such as Snail2 are transiently expressed at mammary gland branch sites in vitro, where they promote dynamic cell migration [18]. Furthermore, mammary terminal end buds migrate collectively as multilayered epithelia exhibiting reductions in apico-basal polarity and cell-cell adhesion [19,20], which are features of epithelial cells undergoing partial EMT. Although a role for ECM molecules such as FN in this process has not been investigated, dynamic signaling from the ECM to the nucleus may be a general mechanism to promote such EMT-related dynamic cell behavior during branching morphogenesis.
Recent work has also elucidated the upstream mechanisms by which FN is assembled into the basement membrane in branching organs. FN assembly is a cell-mediated process involving cytoskeletal forces applied to FN-bound integrin receptors to unfold dimeric FN molecules for fibril assembly [21-23]. Rho kinase (ROCK)-mediated actomyosin contraction and focal adhesion kinase (FAK) activation promote FN fibril assembly in branching organs [24,25]. Because FN accumulation itself also appears to induce activation of integrins and FAK in salivary epithelial cells [14,25], it is interesting to speculate that a dynamic FN-mediated feedforward regulatory loop could promote ongoing FN assembly to drive continued cleft propagation once this process is initiated.
Basement membrane sculpts and stabilizes tissue structure
As discussed above, localized ECM-regulated signaling causes motile cells to undergo dynamic changes in shape, protrusive activity, and adhesion during morphogenesis. Such plasticity provides a morphogenetic substrate for the forces that guide and stabilize changes in global tissue structure. Indeed, structural stabilization by the ECM itself, especially via the basement membrane, can contribute to sculpting of overall epithelial tissue structure by providing regional force anisotropies in the microenvironment to permit tissue expansion in only certain directions [26,27]. We will consider two recent examples that illustrate this role of the ECM during development: Drosophila egg chamber elongation and branching morphogenesis.
Egg elongation requires an ECM “molecular corset”
The Drosophila egg follicle consists of a cyst that develops into an oocyte surrounded by a simple follicular epithelium; as the oocyte matures, this initially rounded structure elongates along the anterior/posterior axis to produce an oval-shaped egg. Recent investigations into the mechanisms of this shape change have provided surprising insight into a new morphogenetic behavior.
Using live imaging, Haigo and Bilder recently demonstrated that as it elongates, the entire egg chamber rotates around its circumferential axis [28]. Interestingly, Drosophila mutants lacking either integrin βPS or collagen IV fail to rotate and elongate, suggesting that coordinate interactions between the follicular epithelium and basement membrane are required for this behavior. Individual cell motility is also required: Misshapen (Msn) kinase promotes cell motility in this system by decreasing integrin levels at the rear of migrating cells to facilitate tail retraction as the cells migrate [29]. What is the purpose of this novel morphogenetic behavior? Further analyses revealed that as the follicle rotates, it creates a planar polarized basement membrane around its anterior/posterior axis by rearranging randomly oriented fibers existing prior to these rotational motions (Figure 2). Moreover, round egg mutants that fail to elongate lack this polarized basement membrane, while experimental treatment of elongated chambers with collagenase results in a return to a symmetrical rounded morphology [28]. Taken together, these results suggest a model in which epithelial rotation is required to produce a planar polarized basement membrane around the circumferential axis of the egg chamber, which may in turn serve as a molecular corset that acts to physically restrict the direction of tissue expansion, thereby stabilizing an elongated tissue structure [28].
Figure 2. Directional cell migration orients ECM to drive tissue shape change during egg chamber morphogenesis.
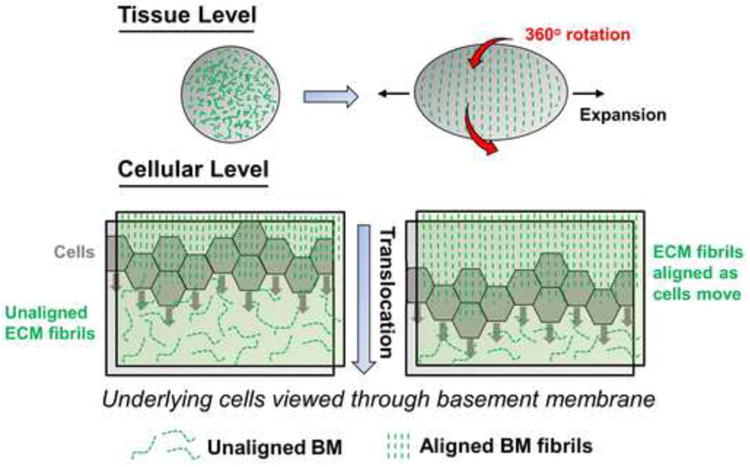
The Drosophila egg chamber is an initially rounded structure that elongates along the anterior/posterior axis to produce an oval-shaped egg. This requires the complete 360° rotation of the entire egg chamber (red arrows), which polarizes the alignment of basement membrane fibrils (green dashed lines) to act as a molecular corset restricting the direction of tissue expansion. At the cellular level, the mechanism of this ECM alignment involves the directional rotational migration (gray arrows) of individual follicular epithelial cells.
Two recent studies have identified similar rotational motions during mammary epithelial acinar development in an in vitro system, which cease upon acinar maturation [30,31]. In the first study, these rotations were correlated with the presence of planar polarized actin microfilaments at the basal surface aligned in the same direction as rotational migration. Furthermore, rotational motility was disrupted by inhibitors of actin and myosin II activity, implicating actomyosin contraction as a critical regulator of this process [30]. The second of these studies identified a direct relationship between rotational motion and the assembly of endogenous basement membrane; when structures failed to rotate, they could not assemble a laminin matrix, and conversely, when basement membrane was disrupted in mature non-rotating acinar structures, they began to rotate again to reassemble the matrix [31]. These studies suggest that rotational cell motility can provide an evolutionarily conserved mechanism for basement membrane reorganization necessary for morphogenesis.
Regional accumulation of ECM proteins stabilizes tissue structure during branching morphogenesis
Early studies of salivary gland development established that collagen accumulates preferentially at branch points, and removal of collagen not only prevents subsequent branching morphogenesis, but also triggers the regression of pre-existing clefts [32,33]. Similar observations in the mammary gland and lung have also identified heterogeneous ECM expression patterns, with thick accumulations of basement membrane around bud flanks, ductal structures, and in cleft regions. In contrast, ECM is thinner at end bud tips at which epithelial expansion occurs (Figure 3) [34,35]. Taken together, these findings suggest that local density of collagen and basement membrane can modulate expansion or stabilization, somewhat analogous to the molecular corset theory during Drosophila egg development.
Figure 3. Differential regional accumulation of ECM proteins stabilizes tissue structure during branching morphogenesis.

ECM is deposited heterogeneously in the basement membrane and stroma surrounding epithelial branched organs. This produces regional force anisotropies that either stabilize or guide the direction of epithelial tissue expansion. For example, thick accumulations of basement membrane (heavy green lines) are present around bud flanks and ducts, and in cleft regions. In contrast, ECM is thinner at end bud tips where epithelia expand (thin green lines).
More dynamically, however, ECM proteins can also actively translocate. For example, ECM appears to serve as an inward-directed wedge to promote cleft formation during branching. Pulse-chase experiments using salivary epithelial rudiments in three-dimensional ECM reveal that FN moves to the base of progressing clefts [36]. Moreover, live imaging with fluorescent FN during epiblast cell migration in Xenopus development shows that these cells appear to carry the ECM with them as they migrate [37,38]. Whether these cases of ECM translocation are mechanistically related to the production and alignment of ECM by rotational cell motility remains to be determined, but both processes show how ECM remodeling can regulate morphogenesis by physically stabilizing or promoting tissue shape change.
ECM cues in tissue differentiation
Thus far, we have focused on ECM roles in tissue morphogenesis. Another important component of organogenesis, however, is the subsequent acquisition of cell type-specific function, particularly the regulation of stem cell differentiation. Although finding diverse ECM receptors on stem cells suggested a role for ECM [39], recent progress has firmly established crucial roles for ECM signaling in both maintenance of stem cells and regulation of their subsequent differentiation.
ECM regulation of the stem cell niche
The stem cell niche consists of unique supporting cell types and microenvironmental factors that provide instructional cues to maintain a stable stem cell population [40,41]. A general role of ECM within the niche is to promote stem cell interactions with various niche cells and the surrounding microenvironment (Figure 4). One example of such ECM interactions in the niche involves neural stem cell binding via integrin α6β1 to laminin in the vascular basement membrane (which comprises the niche) in the subventricular zone of the mouse brain [42]. Inhibition of this integrin receptor reduces numbers of neural stem cells. In the Drosophila testes, a distinct population of hub cells comprises the germline stem cell (GSC) niche, and the hub cells must bind to the ECM through integrin βPS to support GSC function [43].
Figure 4. ECM regulates stem cell retention within the niche and determines lineage commitment.
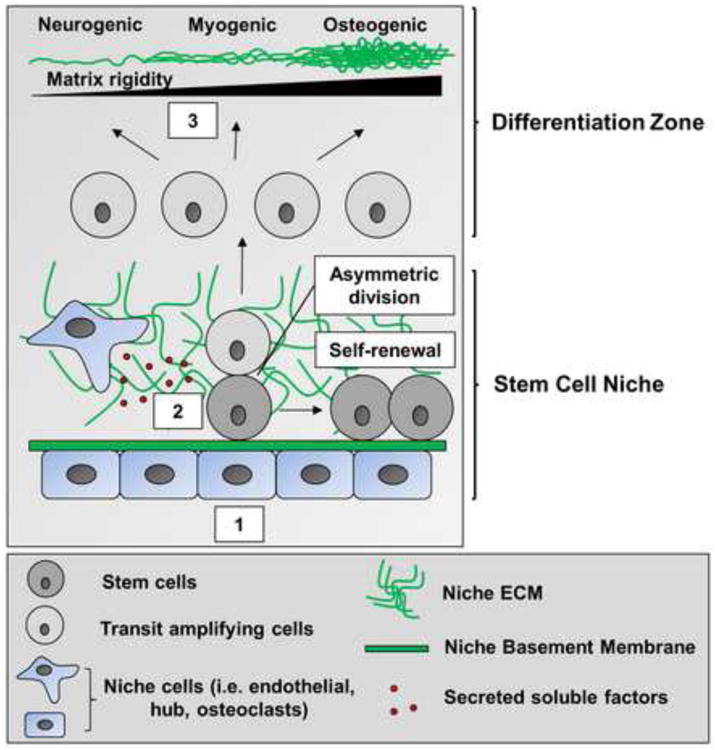
Binding to ECM and basement membrane components is required for both stem and niche cell localization within the niche (1). Niche-associated ECM and soluble factors secreted by cells present within the niche help regulate the decision between stem cell self-renewal and differentiation (2). ECM mechanical properties also determine mesenchymal stem cell lineage commitment along osteogenic, myogenic, and neurogenic differentiation pathways (3).
Besides promoting stem cell binding to a niche, specific ECM proteins can also directly influence stem cell behavior. For example, deposition of endogenous FN by mouse ES cells is required for their self-renewal [44]. In addition, muscle satellite cells (progenitor cells) transiently up-regulate FN upon skeletal muscle injury. In this system, FN binding to Syndecan 4 stimulates it to form a co-receptor with Frizzled 7 that supports downstream signaling via Wnt7a to increase satellite cell expansion within the niche [45]. Thus, stem cell binding to a specific ECM protein such as FN can repopulate the satellite cell niche as stem cells undergo differentiation for muscle regeneration. Other ECM proteins that either positively or negatively regulate stem cell number include osteopontin in bone marrow (the hematopoietic niche) [46], nephronectin in hair follicles [47], and biglycan in tendons [48].
The ECM can also directly influence stem cell function by modulating signaling by growth factors, many of which are required for stem cell maintenance and/or differentiation [49]. For example, the ECM protein anosmin is required for avian cranial neural crest formation [50]; stem cell-like neural crest cells migrate and differentiate to diverse tissues. Local anosmin deposition was recently shown to enhance FGF8 signaling in these cells while at the same time dampening BMP5 and Wnt3a, thereby positively regulating cranial neural crest formation. These results illustrate how a single ECM protein can coordinate multiple growth factor activities to affect the cell biology of a stem cell-like population.
Mechanical regulation of stem cell differentiation
Besides promoting stem cell retention and maintenance within the niche, ECM physical properties can have a profound effect on stem cell differentiation. A recent, recurring theme is that ECM mechanical signals influence stem cell lineage commitment. When mesenchymal stem cells (MSCs) are cultured on gels of varying stiffness, their fate depends on the mechanical compliance of their underlying substrate; specifically, MSCs are directed to neuronal, muscle, or osteogenic lineages by cell culture on surfaces that approximate the mechanical stiffness of the respective in vivo tissue [51]. Adding yet another level of complexity, other studies suggest that ECM can also indirectly affect stem cell differentiation by modulating cell shape. When micropatterned ECM substrates were used to control the amount of cell spreading in stem cell cultures, round cells became adipocytes while flattened cells underwent osteogenesis [52]. A common theme appears to be the level of Rho-stimulated actomyosin contractility in these cells, which is regulated by the mechanical properties of the underlying substrate; that is, both stiff matrices and micropatterns that support cell spreading trigger increased actomyosin contraction, while more compliant matrices and micropatterns that only allow cell rounding trigger a reduction [51,52]. Thus, ECM topography, as well as biomechanical rigidity, is clearly capable of influencing stem cell fate decisions. Recent progress in this area has further revealed that MSCs will first undergo durotaxis, migrating to a region of “preferred” substrate rigidity. When MSCs are cultured on hydrogels containing physiological gradients of matrix stiffness, some cells migrate to the stiffest regions and commit to an osteogenic lineage, while others prefer more compliant regions and undergo alternate lineage commitment [53]. Although all of these studies involved 2D culture conditions, these findings can be reproduced in a 3D environment. Just as on a 2D substrate [51], cells in 3D express neurogenic, myogenic, and osteogenic markers as a function of increasing substrate rigidity [54].
Conclusions
Recent research has established that dynamic ECM-modulated signaling plays important roles in the remodeling and morphogenesis of epithelial tissues. The dynamic mechanisms of cell-ECM interactions and the ways in which they regulate complex developmental processes such as epithelial morphogenesis constitute an exciting, rapidly expanding area of research. In this review, we have considered a number of emerging biological principles by which cell interactions with the ECM and basement membrane coordinately shape mammalian epithelial tissues; these include the highly localized regulation of cell dynamics by ECM-mediated signaling, ECM-induced forces that guide alterations in tissue structure, and the regulation of tissue differentiation by chemical and physical properties of the ECM. A major future challenge will be to integrate these multiple roles of cell-ECM interactions with our knowledge about the many critical soluble regulators of morphogenesis to provide a more complete systems biology understanding of tissue development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hynes RO, Yamada KM, editors. Extracellular matrix biology. Cold Spring Harbor Laboratory Press; 2011. [Google Scholar]
- 3.Mecham RP, editor. The extracellular matrix: an overview. Springer; 2011. [Google Scholar]
- 4.Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255–264. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- 5.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341:126–140. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hynes RO, Naba A. Overview of the matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol. 2011;4:a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geiger B, Yamada KM. Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan D, Lin X. Shaping morphogen gradients by proteoglycans. Cold Spring Harb Perspect Biol. 2009;1:a002493. doi: 10.1101/cshperspect.a002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Onodera T, Sakai T, Hsu JC, Matsumoto K, Chiorini JA, Yamada KM. Btbd7 regulates epithelial cell dynamics and branching morphogenesis. Science. 2010;329:562–565. doi: 10.1126/science.1191880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423:876–881. doi: 10.1038/nature01712. [The above two papers delineate a fibronectin-mediated pathway from the cell surface to the nucleus that promotes dynamic epithelial cell behavior via the up-regulation of the EMT transcription factor Snai2 and the down-regulation of the cell surface adhesion molecule E-cadherin. The authors demonstrate the importance of this pathway for cleft formation during branching morphogenesis of multiple epithelial organs] [DOI] [PubMed] [Google Scholar]
- 15.Affolter M, Zeller R, Caussinus E. Tissue remodelling through branching morphogenesis. Nat Rev Mol Cell Biol. 2009;10:831–842. doi: 10.1038/nrm2797. [DOI] [PubMed] [Google Scholar]
- 16.Andrew DJ, Ewald AJ. Morphogenesis of epithelial tubes: Insights into tube formation, elongation, and elaboration. Dev Biol. 2010;341:34–55. doi: 10.1016/j.ydbio.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim J, Thiery JP. Epithelial-mesenchymal transitions: insights from development. Development. 2012;139:3471–3486. doi: 10.1242/dev.071209. [DOI] [PubMed] [Google Scholar]
- 18.Lee K, Gjorevski N, Boghaert E, Radisky DC, Nelson CM. Snail1, Snail2, and E47 promote mammary epithelial branching morphogenesis. EMBO J. 2011;30:2662–2674. doi: 10.1038/emboj.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Ewald AJ, Huebner RJ, Palsdottir H, Lee JK, Perez MJ, Jorgens DM, Tauscher AN, Cheung KJ, Werb Z, Auer M. Mammary collective cell migration involves transient loss of epithelial features and individual cell migration within the epithelium. J Cell Sci. 2012;125:2638–2654. doi: 10.1242/jcs.096875. [In the above two studies, Ewald and colleagues utilize long-term confocal time-lapse microscopy to delineate the normal sequence of cellular events during duct initiation and elongation in the mammary gland. Notably, these authors also compare normal development to ductal carcinoma in situ and uncover some striking similarities, thereby suggesting that cancer progression may involve normal developmental mechanisms reactivated in the wrong context] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baneyx G, Baugh L, Vogel V. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc Natl Acad Sci U S A. 2002;99:5139–5143. doi: 10.1073/pnas.072650799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q, Magnusson MK, Mosher DF. Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Mol Biol Cell. 1997;8:1415–1425. doi: 10.1091/mbc.8.8.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daley WP, Gulfo KM, Sequeira SJ, Larsen M. Identification of a mechanochemical checkpoint and negative feedback loop regulating branching morphogenesis. Dev Biol. 2009;336:169–182. doi: 10.1016/j.ydbio.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daley WP, Kohn JM, Larsen M. A focal adhesion protein-based mechanochemical checkpoint regulates cleft progression during branching morphogenesis. Dev Dyn. 2011;240:2069–2083. doi: 10.1002/dvdy.22714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernfield M, Banerjee SD. The turnover of basal lamina glycosaminoglycan correlates with epithelial morphogenesis. Dev Biol. 1982;90:291–305. doi: 10.1016/0012-1606(82)90378-5. [DOI] [PubMed] [Google Scholar]
- 27.Spooner BS, Thompson-Pletscher HA, Stokes B, Bassett KE. Extracellular matrix involvement in epithelial branching morphogenesis. Dev Biol (N Y 1985) 1986;3:225–260. doi: 10.1007/978-1-4684-5050-7_12. [DOI] [PubMed] [Google Scholar]
- 28**.Haigo SL, Bilder D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science. 2011;331:1071–1074. doi: 10.1126/science.1199424. [In this study, the authors uncover a novel morphogenetic behavior involving the coordinated rotation of follicular epithelial cells about the circumferential axis of the Drosophila egg chamber. Such rotation is required for the assembly of a polarized basement membrane, which constricts the direction of tissue expansion so that it can only occur along the anterior-posterior axis] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Lewellyn L, Cetera M, Horne-Badovinac S. Misshapen decreases integrin levels to promote epithelial motility and planar polarity in Drosophila. J Cell Biol. 2013;200:721–729. doi: 10.1083/jcb.201209129. [Here, the authors demonstrate a role for the planar cell polarity signaling molecule Misshapen in the circumferential rotation of follicular epithelial cells during Drosophila egg chamber development. Misshapen kinase promotes integrin detachment at the rear of migrating cells, thereby facilitating cell migration] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Tanner K, Mori H, Mroue R, Bruni-Cardoso A, Bissell MJ. Coherent angular motion in the establishment of multicellular architecture of glandular tissues. Proc Natl Acad Sci U S A. 2012;109:1973–1978. doi: 10.1073/pnas.1119578109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Wang H, Lacoche S, Huang L, Xue B, Muthuswamy SK. Rotational motion during three-dimensional morphogenesis of mammary epithelial acini relates to laminin matrix assembly. Proc Natl Acad Sci U S A. 2013;110:163–168. doi: 10.1073/pnas.1201141110. [The above two studies demonstrate the relevance of the coordinated rotational epithelial cell motility initially discovered in Drosophila to the development of MDCK epithelial cell cysts in 3D culture, an in vitro model for mammalian epithelial morphogenesis] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grobstein C, Cohen J. Collagenase: effect on the morphogenesis of embryonic salivary epithelium in vitro. Science. 1965;150:626–628. doi: 10.1126/science.150.3696.626. [DOI] [PubMed] [Google Scholar]
- 33.Nakanishi Y, Nogawa H, Hashimoto Y, Kishi J, Hayakawa T. Accumulation of collagen III at the cleft points of developing mouse submandibular epithelium. Development. 1988;104:51–59. doi: 10.1242/dev.104.1.51. [DOI] [PubMed] [Google Scholar]
- 34.Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 2004;6:1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Moore KA, Polte T, Huang S, Shi B, Alsberg E, Sunday ME, Ingber DE. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev Dyn. 2005;232:268–281. doi: 10.1002/dvdy.20237. [This study suggests a role for Rho-mediated actomyosin contraction in the establishment of local ECM force anisotropy, which is important for the regulation of localized epithelial cell proliferation and subsequent branch outgrowth in the embryonic lung] [DOI] [PubMed] [Google Scholar]
- 36*.Larsen M, Wei C, Yamada KM. Cell and fibronectin dynamics during branching morphogenesis. J Cell Sci. 2006;119:3376–3384. doi: 10.1242/jcs.03079. [DOI] [PubMed] [Google Scholar]
- 37*.Davidson LA, Dzamba BD, Keller R, Desimone DW. Live imaging of cell protrusive activity, and extracellular matrix assembly and remodeling during morphogenesis in the frog, Xenopus laevis. Dev Dyn. 2008;237:2684–2692. doi: 10.1002/dvdy.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Zamir EA, Rongish BJ, Little CD. The ECM moves during primitive streak formation--computation of ECM versus cellular motion. PLoS Biol. 2008;6:e247. doi: 10.1371/journal.pbio.0060247. [The above three studies were the first to use confocal time-lapse microscopy with fluorescently-labeled ECM molecules to analyze the relationship between cell motility and ECM translocation] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raymond K, Deugnier MA, Faraldo MM, Glukhova MA. Adhesion within the stem cell niches. Curr Opin Cell Biol. 2009;21:623–629. doi: 10.1016/j.ceb.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 41.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Tanentzapf G, Devenport D, Godt D, Brown NH. Integrin-dependent anchoring of a stem-cell niche. Nat Cell Biol. 2007;9:1413–1418. doi: 10.1038/ncb1660. [The above two studies elegantly demonstrate an integral requirement for integrin-mediated attachment in the stem cell niche. In reference 42, Shen and colleagues demonstrate that adult neural stem cell binding to the vascular basement membrane via α6β1 integrin is required for stem cell maintenance. In reference 43, Tanentzapf and colleagues uncover a role for β1 integrin-mediated attachment of Drosophila hub (niche) cells to the ECM which is required for germline stem cell maintenance] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunt GC, Singh P, Schwarzbauer JE. Endogenous production of fibronectin is required for self-renewal of cultured mouse embryonic stem cells. Exp Cell Res. 2012;318:1820–1831. doi: 10.1016/j.yexcr.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Bentzinger CF, Wang YX, von Maltzahn J, Soleimani VD, Yin H, Rudnicki MA. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell. 2013;12:75–87. doi: 10.1016/j.stem.2012.09.015. [This paper demonstrates how stem cell binding to a specific ECM protein such as FN can repopulate the satellite cell niche as stem cells undergo differentiation for muscle regeneration. FN binding to Syndecan 4 stimulates it to form a co-receptor with Frizzled 7 that supports downstream signaling via Wnt7a to increase satellite cell expansion within the niche] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 47.Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, Hartner A, Sekiguchi K, Reichardt LF, Watt FM. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. 2011;144:577–589. doi: 10.1016/j.cell.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 49.Xie T, Li L. Stem cells and their niche: an inseparable relationship. Development. 2007;134:2001–2006. doi: 10.1242/dev.002022. [DOI] [PubMed] [Google Scholar]
- 50*.Endo Y, Ishiwata-Endo H, Yamada KM. Extracellular matrix protein anosmin promotes neural crest formation and regulates FGF, BMP, and WNT activities. Dev Cell. 2012;23:305–316. doi: 10.1016/j.devcel.2012.07.006. [Here, the authors demonstrate that local deposition of the ECM protein anosmin in chick neural crest enhances FGF8 signaling in these cells while at the same time dampening BMP5 and Wnt3a, thereby positively regulating cranial neural crest formation. These results illustrate how a single ECM protein can coordinate multiple growth factor activities to affect the cell biology of a stem cell-like population] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51**.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 52**.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [The above two seminal studies demonstrate how ECM mechanical signals influence stem cell lineage commitment. Engler and colleagues show that ECM rigidity plays an important role in the determination of mesenchymal stem cell fate. Likewise, McBeath and colleagues demonstrate that cell shape is also a critical regulator of this process. In both cases, Rho-mediated contractility is a central signaling pathway for the regulation of lineage commitment] [DOI] [PubMed] [Google Scholar]
- 53.Tse JR, Engler AJ. Stiffness gradients mimicking in vivo tissue variation regulate mesenchymal stem cell fate. PLoS One. 2011;6:e15978. doi: 10.1371/journal.pone.0015978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pek YS, Wan AC, Ying JY. The effect of matrix stiffness on mesenchymal stem cell differentiation in a 3D thixotropic gel. Biomaterials. 2010;31:385–391. doi: 10.1016/j.biomaterials.2009.09.057. [DOI] [PubMed] [Google Scholar]


