Abstract
Alemtuzumab (Campath-1H) is a humanized monoclonal antibody (Ab) directed against CD52 that depletes lymphocytes and other leukocytes, mainly by complement-dependent mechanisms. We investigated the influence of alemtuzumab (i) on ex vivo-expanded cynomolgus monkeys regulatory T cells (Treg) generated for prospective use in adoptive cell therapy and (ii) on naturally-occurring Treg following alemtuzumab infusion. Treg were isolated from PBMC and lymph nodes and expanded for two rounds. CD52 expression, binding of alemtuzumab, and both complement-mediated killing and Ab-dependent cell-mediated cytotoxicity (ADCC) were compared between freshly-isolated and expanded Treg and effector T cells. Monkeys undergoing allogeneic heart transplantation given alemtuzumab were monitored for Treg and serum alemtuzumab activity. Ex vivo-expanded Treg showed progressive downregulation of CD52 expression, absence of alemtuzumab binding, minimal change in complement inhibitory protein (CD46) expression and no complement-dependent killing or ADCC. Infusion of alemtuzumab caused potent depletion of all lymphocytes, but a transient increase in the incidence of circulating Treg. After infusion of alemtuzumab, monkey serum killed fresh PBMC, but not expanded Treg. Thus, expanded cynomolgus monkey Treg are resistant to alemtuzumab-mediated, complement-dependent cytotoxicity. Furthermore, our data suggest that these expanded monkey Treg can be infused into graft recipients given alemtuzumab without risk of complement-mediated killing.
Keywords: Alemtuzumab, Campath-1H, non-human primate, regulatory T cells, transplantation
Introduction
Alemtuzumab (Campath-1H) is a humanized IgG1 mAb directed against the CD52 molecule that is expressed by lymphocytes, natural killer (NK) cells, dendritic cells and monocytes (1). Its administration depletes these cells and it is consequently used to treat lymphoid malignancies (2) or suppress transplant rejection (3). Complement activation, as well as Ab-dependent, cell-mediated cytotoxicity (ADCC) are responsible for alemtuzumab-mediated killing of CD52+ cells (4, 5). The half-life of alemtuzumab in humans is approximately 8 days, but since low concentrations may be sufficient to cause cytotoxicity, depletion of CD52+ cells may persist for up to two months after its infusion (6).
Regulatory T cells (Treg), defined as CD4+CD25highForkhead box P3 (FoxP3+T cells, can promote allograft tolerance in mice and humanized mouse models following their adoptive transfer (7–11). This function of Treg has usually been demonstrated in lymphocyte-depleted graft recipients, although there is also evidence of their tolerogenic function in immunocompetent hosts (12). A relative increase in circulating Treg has been demonstrated after alemtuzumab induction in renal transplant patients (13, 14), although this effect appears to be transient (15).
Recently, clinical trials have been initiated to determine the safety and efficacy of Treg infusion in transplant recipients (16). Human naturally-occurring Treg display relatively high levels of CD52 compared with other lymphocyte subsets, and are susceptible to killing by alemtuzumab (5, 17). However, little is known about CD52 expression on Treg after their ex vivo expansion,- a pre-requisite for their therapeutic application. We posed the question whether, in the context of non-human primate (NHP) organ transplantation and efforts to promote immunological tolerance, expanded NHP Treg are susceptible to alemtuzumab and whether circulating/residual alemtuzumab might destroy administered Treg, thus negating their in vivo function.
We investigated this question in cynomolgus macaques of Indonesian origin. Unlike humans, most NHP species express CD52 on both white and red blood cells. The Indonesian sub-species of cynomolgus macaque, however, does not express CD52 on erythrocytes, and can be given alemtuzumab safely (18). Cynomolgus monkey CD52 shares 85% structural homology with its human counterpart (19, 20). Our study focused on the influence of alemtuzumab on ex vivo-expanded cynomolgus macaque Treg, as well as on Treg in vivo.
Materials and Methods
Cynomolgus monkeys
Healthy cynomolgus monkeys (Macaca fascicularis) of Indonesian origin (3–5 kg), were obtained from specific pathogen-free colonies at Alpha Genesis, Inc, or the NIAID NHP colony (both Yamassee, SC). Some monkeys (n=3) received a heterotopic heart transplant from an ABO-compatible, allogeneic donor on day (d) 0. On d -2, 5 and 12, the recipient was given an intravenous (i.v.) infusion of alemtuzumab (Campath-1H; Genzyme, Cambridge, MA) at doses of 20, 10, and 10 mg/kg, respectively. Maintenance immunosuppression consisted of mycophenolate mofetil (Genentech USA, Inc, South San Francicso, CA) from d -1 to 18 (target trough levels of 3–6 µg/ml), followed by rapamycin (LC Laboratories, Woburn, MA) monotherapy from days 19 to 54 (target trough levels of 10–15 ng/ml) after which rapamycin was weaned slowly and discontinued completely on d 84. All animal procedures were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and conducted under a University of Pittsburgh Institutional Animal Care and Use Committee-approved protocol. Specific environment enrichment was provided.
Sources of cells
Normal, untreated monkeys were used as blood donors for in vitro experiments. PBMC were used either immediately upon isolation or after storage in liquid N2. Blood was drawn weekly after alemtuzumab infusion to monitor lymphocyte subsets.
Treg and Teff cell isolation
Cryopreserved cells were thawed or PBMC isolated from fresh blood on d 0 of each experiment (Treg isolation) for 2 rounds of Treg and effector T cell (Teff) expansion. Cells from the same source were also thawed or isolated on d 20, and served as unexpanded controls, as well as responder cells in carboxyfluorescein diacetate succinimidyl ester (CFSE)-mixed leukocyte reactions (MLR). nTreg were isolated from PBMC or LN cells by flow sorting (BD Aria, BD Biosciences, San Jose, CA) based on CD4+CD25hiCD127− expression, as described (21); Supplementary Figure 1A. Simultaneously, Teff were sorted based on CD4+CD25− expression and served as controls for expanded Treg. FoxP3 expression was determined in separate samples.
Treg expansion
The protocol used for Treg and Teff expansion and investigation is shown in Figure 1A. Treg and Teff were cultured in AIM-V medium, with 10% v/v heat-inactivated human AB serum. Treg were expanded using NHP-specific anti-CD2/3/28 microbeads (Miltenyi, Biotec, Bergisch Gladbach, Germany) at a cell:bead ratio of 1:2, with high-dose recombinant human (rhu)IL-2 (1000 U/ml) and rhu transforming growth factor β (TGF-β; 5ng/ml). Teff were expanded similarly at a cell:bead ratio of 2:1, with IL-2 (500 U/ml), but without TGF-β. When sufficient cells were obtained, they were tested for suppressive function in CFSE-MLR.
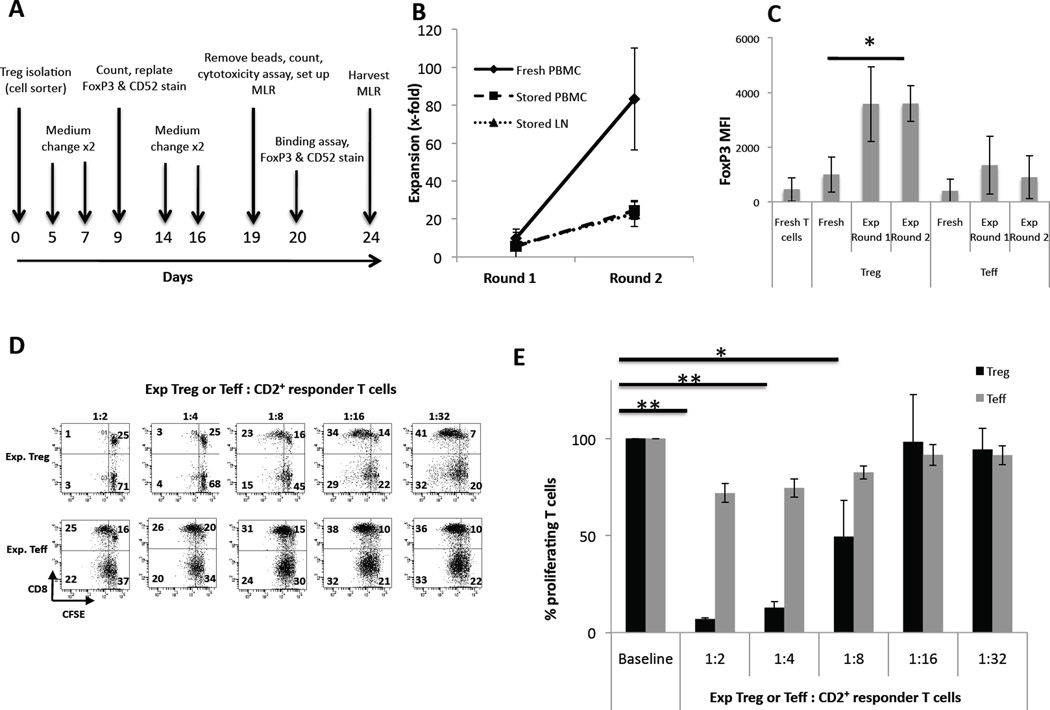
Figure 1. Expansion of cynomolgus monkey FoxP3+ Treg.
(A) Treg expansion protocol. Treg (CD4+CD25hiCD127−) were flow-sorted from fresh PBMC or cryopreserved PBMC or LN cells on d 0, and expanded using NHP-specific expansion beads, high dose rhu IL-2 and rhu TGF-β. Simultaneously, conventional T effector cells (Teff; CD4+CD25−) were sorted and expanded using beads and IL-2, but without TGF-β. Assays were carried out on the days indicated by arrows.
(B) Strong expansion of Treg from fresh PBMC. Treg were flow-sorted from fresh PBMC (n=3 experiments), or cryopreserved (stored) PBMC (n=3) or LN cells (n=4), then expanded following the protocol indicated in (A). Treg isolated from fresh PBMC (continuous line) expanded at a much faster rate than Treg sorted from either stored PBMC (dashed line) or stored LN cells (broken lines).
(C) Significant up-regulation of FoxP3 in expanded Treg. Fresh PBMC were stained for CD3, CD4, CD25, CD127, and FoxP3. FoxP3 MFI was analyzed on fresh CD3+CD4+ T cells, on fresh CD4+CD25hiCD127− Treg and fresh CD4+CD25− Teff. In the same experiment, expanded Treg and Teff were also stained, gating on CD4+ cells, and analyzed for FoxP3 at the end of round 1 and 2 of expansion. Expanded T cells showed increased FoxP3 expression, that was greater in expanded Treg than in expanded Teff (n=3 experiments for all conditions). *p<0.05.
(D, E) Expanded Treg exhibit strong suppressive function on CD4+ and CD8+ T cell proliferation. When sufficient cells were available, Treg were tested for suppressive function in CFSE-MLR, as described in the Materials and Methods. Expanded Treg (upper panels in D and black bars in E) showed strong suppressive capacity when added to bead-stimulated CD2+ autologous T cells, whereas expanded Teff (lower panels in D and gray bars in E) did not. Treg were strongly suppressive at ratios of up to 1Treg:4 CD2+ T cells. *p<0.05; **p<0.01. Data are representative of 3 experiments (D) and analyzed across experiments (E).
Expression of cell surface markers and intracellular staining
Fresh and expanded T cells were stained for cell surface antigens using fluorochrome-labeled mAbs directed against CD3, CD4, CD8 (all BD Biosciences), CD25 (eBioscience), CD46, CD52 (both AbD Serotec) or CD127 (BD Biosciences). Intracellular FoxP3 staining was performed using the protocol provided by eBioscience® (San Diego, CA).
Treg suppressive function: CFSE-MLR
CD2+T cells stained with CFSE were stimulated with NHP-specific anti-CD2/3/28 beads (Miltenyi Biotec) at a cell:bead ratio of 10:1. Expanded T cells stained with Violet Trace (to distinguish them from CD4+CFSE-proliferating responder cells) were added to the responder cells in responder:T cell ratios of 1:2, 1:4, 1:8, 1:16 and 1:32. CFSE-MLR were harvested on d 5. Proliferation was determined as the percentage of CFSE− cells within the CD3+CD4+ and CD3+CD8+ populations.
Binding of alemtuzumab to target cells
Alemtuzumab was titrated to final concentrations of 100 – 0.001 µg/ml and cells incubated for 30 min at 4°C, washed, then blocked with normal goat serum to prevent non-specific binding. Washed cells were then stained with FITC-goat anti-hu IgG-γ (Invitrogen, Carlsbad, CA) and PerCP-Cy5.5 anti-CD3 (BD PharMingen, San Diego, CA). Fresh cells were stained additionally for APC-H7 anti-CD4 (BD PharMingen) and PE-Cy7 anti-CD25 (eBioscience) to enable analysis of binding to Treg and Teff cells within the total cell population. Analysis of binding was based on the MFI of FITC+ cells within live (DAPI−) CD3+ cells, setting the gate based on cells incubated with PBS alone.
Killing of target cells by alemtuzumab
To determine complement-mediated killing, cells were incubated with alemtuzumab in autologous serum (final concentration 100 – 0.01 µg/ml) for 30 min (at 37°C), washed, and stained for Annexin-V (BD Biosciences) to detect early apoptosis. Before analysis, cells were also stained with 7-AAD to detect dying/dead cells. CountBright counting beads (Invitrogen) (to determine absolute cell numbers) were added before flow cytometry. In addition, normal cynomolgus serum was compared with heat-inactivated serum (HI serum), RPMI-1640 (no serum) and RPMI-1640 + rabbit complement (10 µg/ml), with or without alemtuzumab (30 µg/ml).
To quantify ADCC, freshly-isolated normal cynomolgus PBMC and violet proliferation dye (VPD450)-labeled expanded Treg (ratio 4:1) were incubated with the same range of alemtuzumab concentrations, in the presence of heat-denatured autologous serum for 4 h at 37°C. The cells were then washed with PBS and stained with CD3, CD4, CD20 and FoxP3 mAbs. CountBright counting beads were added to each tube so that absolute cell numbers could be calculated. Background cell death was <2%. Positive controls for alemtuzumab killing of T cells were human PBMC in place of monkey cells together with heat-denatured autologous human serum.
Statistical analysis
Differences between means were evaluated using Student’s paired ‘t’-test or the non-parametric Mann-Whitney U test, as appropriate. Statistical analyses were conducted using the standard formula in Microsoft Excel software.
Results
Treg isolation and expansion from cynomolgus monkey blood and LN
The incidence of circulating Treg (CD4+CD25hiCD127−) in healthy, untreated cynomolgus monkeys was similar to that in healthy humans (i.e. ~5% of total CD4+ T cells), but was comparatively higher (~10%) in LN. The size of the monkeys (3–5 kg) allowed for a maximum blood draw of 30–50 ml per month. A large difference was noted in the number of Treg that could be obtained from the different cell sources (Table 1). Cryopreserved and fresh PBMC (both n=3 experiments) yielded 0.23% and 0.13% Treg, respectively, from total isolated cells, whereas LN cells (n=4 experiments) yielded 1.05% Treg from total isolated cells (Table 1). Although LN cells yielded higher absolute numbers of Treg, those isolated from fresh blood could be expanded at a much higher rate than Treg isolated from either cryopreserved PBMC or LN cells, with a mean 80-fold expansion after two rounds versus 24- and 22-fold expansion for stored PBMC and LN cells, respectively (Figure 1B).
Table 1.
Recovery of cynomolgus monkey Treg from various sources by cell sorting
| Treg source | Absolute cell # (x106) | Percentage of total cells |
|---|---|---|
| Fresh PBMC (n=3) | 0.12±0.06 | 0.13±0.05 |
| Cryopreserved PBMC (n=3) | 0.19±0.14 | 0.23±0.11 |
| Cryopreserved LN cells (n=4) | 1.20±0.66 | 1.05±0.62 |
Absolute numbers of cynomolgus monkey Treg (CD4+CD25hiCD127−), as well as Treg percentages obtained by flow sorting from a single blood draw (cryopreserved or fresh PBMC) were generally low (0.13–0.23% of total cells) compared to those obtained from LN (Treg: 1% of total cells). Values are means ±1SD (n=10 experiments).
FoxP3 expression was determined at the start of culture and after each round of expansion. Teff (CD4+CD25−) from the same source were expanded simultaneously and served as controls. Although not statistically significant, fresh Treg exhibited higher intracellular FoxP3 expression (MFI) than fresh Teff (p=0.060). FoxP3 expression (MFI) increased in both populations, especially Treg, upon expansion and was statistically significant for Treg (p=0.048) (Figure 1C and Supplementary Figure 1B). Whereas Treg exhibited comparatively high FoxP3 levels throughout culture, FoxP3 intensity in Teff was much lower (Figure 1C and Supplementary Figure 2) and diminished after the first round of expansion (Figure 1C).
To verify their suppressive function, when sufficient Treg were recovered at the end of the expansion period (d 20), CFSE-MLR were set up to determine their ability to inhibit CD2+ T cell proliferation. Expanded Treg were strongly suppressive at Treg:Tcell ratios up to 1:4, whereas expanded Teff were comparatively ineffective (Figure 1D,E). These data on FoxP3 expression and suppressive function confirmed that the expanded Treg retained their phenotypic and functional identity.
Expression of CD52
We next determined CD52 expression (MFI) on fresh total T cells (isolated from either PBMC or LN), as well as on fresh and expanded Treg and Teff (Supplementary Figure 2). CD52 expression on fresh Treg was higher than on fresh Teff (p=0.095) and on the fresh bulk T cell population before expansion. During expansion, CD52 expression decreased progressively on both Treg and Teff. Downmodulation of CD52 was greatest on expanded Treg, as shown in Figure 2A (p=0.041) and in Supplementary Figure 2.
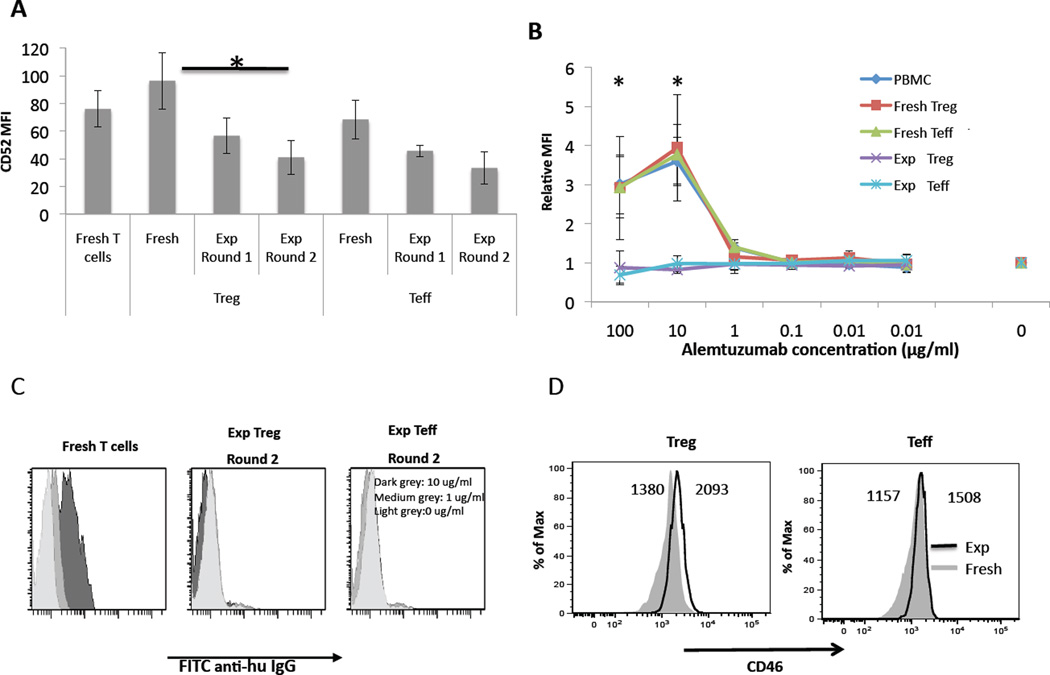
Figure 2. Expanded cynomolgus Treg significantly down-regulate CD52 expression, are not bound by alemtuzumab, and show little change in CD46 expression.
(A) T cells from peripheral blood or LN were stained for CD3, CD4, CD25 and CD52 (n=3). CD52 MFI was analyzed for bulk T cells, fresh (unexpanded) Treg (gated on CD4+CD25hiCD127−) and fresh (unexpanded) Teff (gated on CD4+CD25−). In the same experiment, Treg and Teff cells were analyzed for CD52 after round 1 and 2 of expansion (all conditions n=3). Expanded cells showed a decrease in CD52 expression after the first round of expansion, which was similar for Treg and Teff, with a further decrease after the second round (n=3 experiments for all conditions). *p<0.05. (B) Alemtuzumab does not bind to expanded Treg. Cells were incubated with alemtuzumab at concentrations from 0.001–100 µg/ml then stained with FITC-anti-human IgGγ, as described in the Materials and Methods to determine binding of alemtuzumab. Binding was expressed as relative MFI. Alemtuzumab exhibited concentration-dependent binding to freshly-isolated PBMC, as well as to freshly-isolated Treg and Teff (n=3 experiments). By contrast, alemtuzumab showed no binding to expanded Treg (n=3) or expanded Teff (n=3) at any concentration tested. *p<0.05; significance calculated between fresh T cells (n=3) and expanded Treg (n=3). (C), Representative histograms showing absence of binding to expanded cells. (D) Expanded Treg and Teff showed little change in surface expression of the complement inhibitory protein CD46 compared with fresh cells. Fresh and expanded Treg/Teff from the same monkey were stained for CD3, CD4, Foxp3, and CD46. Treg were gated on CD3+CD4+Foxp3+ while Teff were gated on CD3+CD4+Foxp3−. Compared to fresh cells, both Treg and Teff increased their expression of CD46 only modestly after two rounds of expansion. Data are representative of 3 separate experiments.
Binding of alemtuzumab to fresh and expanded T cells
To ascertain the relationship between cell surface expression of CD52 and binding of alemtuzumab, cells were incubated with various concentrations of alemtuzumab, then stained with secondary Ab. All freshly-isolated cells (total lymphocytes from PBMC or LN, fresh Treg and fresh Teff) bound alemtuzumab, with a peak at 10 µg/ml. Consistent with their higher CD52 expression, fresh Treg bound alemtuzumab slightly more strongly than fresh Teff, although this difference was not statistically significant. Importantly, alemtuzumab did not bind to expanded cells (either Treg or Teff) at any mAb concentration tested (Figure 2B, C). The difference in binding between expanded and fresh T cells was significant at alemtuzumab concentrations of 100 µg/ml (p=0.044) and 10 µg/ml (p=0.014). This finding correlates with the markedly reduced CD52 expression on expanded cells.
Cytotoxicity of alemtuzumab
After observing that expanded Treg had markedly reduced CD52 expression and were not bound by alemtuzumab, we quantified the cytotoxic activity of alemtuzumab against these cells. Notably, neither expanded Treg nor Teff upregulated expression of the complement inhibitory protein CD46 compared with fresh cells (Figure 2D). Fresh T cells (from PBMC) and expanded Treg and Teff were incubated with alemtuzumab at different concentrations, and then analyzed for apoptosis/cell death using a combination of Annexin-V and 7-AAD staining, as well as by total cell counts via the CountBright bead percentage (see Materials and Methods). When fresh T cells were incubated with high concentration alemtuzumab (30 µg/ml) in either normal cynomolgus serum (containing complement) or RPMI-1640 supplemented with rabbit complement for 30 min, killing was markedly enhanced compared to the absence of alemtuzumab (Figure 3A). However, when fresh T cells were incubated with either heat-inactivated serum or RPMI-1640, there was no difference in killing between 0 and 30 µg/ml alemtuzumab. Consequently, we used autologous serum for all subsequent experiments.
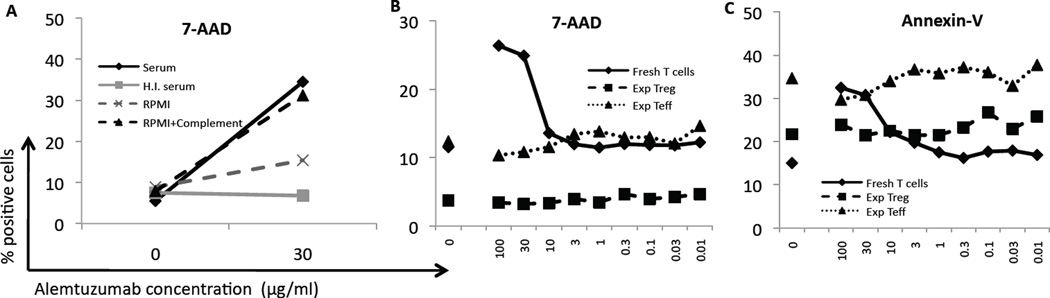
Figure 3. Alemtuzumab does not kill expanded Treg.
(A) Killing of cells was tested using normal cynomolgus monkey serum, heat-inactivated serum (HI serum), RPMI-1640 (no serum), or RPMI-1640 +complement, each with or without 30 µg/ml alemtuzumab. Killing is shown as the percentage of cells that became 7-AAD-positive. Normal serum and RPMI-1640 + complement were associated with increased killing of cells incubated with alemtuzumab. Heat-inactivated serum and RPMI-1640 were not associated with increased killing. (B, C) Cells were incubated with alemtuzumab at the concentrations shown to determine apoptosis and killing of cells. Fresh T cells showed increasing 7-AAD (B) and Annexin-V (C) staining as the concentration of alemtuzumab increased. Expanded Teff had a higher baseline level of Annexin-V and 7-AAD staining, but did not show any increase when alemtuzumab was added. Data are representative of 4 separate experiments.
Alemtuzumab was diluted to final concentrations ranging from 0.01 to 100 µg/ml to generate a titration curve. Freshly-isolated bulk T cells were killed in a dose-dependent manner (determined by higher incidences of Annexin-V+ and 7-AAD+ cells; Figure 3B, and Supplementary Figure 3) and CountBright bead percentage. Killing was evident at or above a mAb concentration of 10 µg/ml, correlating with maximal binding of the mAb to these cells (Figure 2B). No killing was detected at <1 µg/ml, where values returned to baseline (no alemtuzumab) level. Expanded Treg exhibited a lower baseline level of apoptosis than expanded Teff in each experiment, but both expanded Treg and Teff displayed no alemtuzumab-induced killing. Annexin-V and 7-AAD staining (Figure 3B,C and Supplementary Figure 3), and the percentage of CountBright beads remained at baseline levels at each concentration of alemtuzumab tested. Thus, even at high in vitro concentration, alemtuzumab does not kill expanded T cells through a complement-dependent mechanism.
Assessment of ADCC-mediated killing of fresh cynomolgus T cells or expanded Treg by alemtuzumab in the presence of heat-inactivated autologous serum for 4h as described in the Materials and Methods revealed very low levels of cytotoxicity (<5% cell killing) (data not shown).
Quantitation of alemtuzumab activity in monkey serum after intravenous infusion
Serum samples were obtained from 3 heart-transplanted monkeys pre-alemtuzumab and at several times after mAb infusion. They were tested for alemtuzumab-induced killing of fresh normal human T cells (control target cells) as well as of cynomolgus autologous expanded Treg. Similar to the in vitro studies, in which alemtuzumab was added to serum, when fresh T cells were incubated with serum drawn immediately after infusion of alemtuzumab, killing of the fresh T cells was clearly evident (Figure 4). In contrast, when incubated with serum drawn 7 d after infusion, Annexin-V and 7-AAD staining levels had returned to baseline (pre-alemtuzumab) (Figure 4 and Supplementary Figure 4), indicating that any alemtuzumab remaining in the serum 7 d after infusion did not kill the cells.
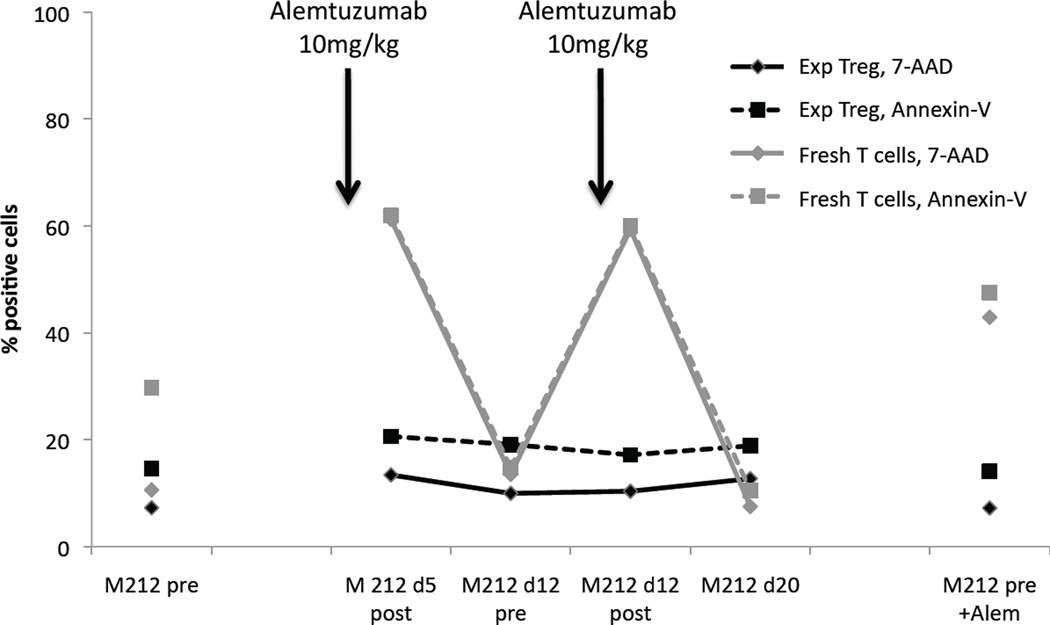
Figure 4. Alemtuzumab-containing serum, taken immediately after mAb infusion, does not kill expanded Treg.
Monkey serum was drawn pre-alemtuzumab, immediately after a second dose (10mg/kg), immediately before and after a third dose (10mg/kg), and 1 week after the third dose. These sera were incubated with freshly-isolated monkey T cells and expanded autologous Treg. The percentages of Annexin-V and 7-AAD positive cells are shown. Arrows show time-points when alemtuzumab was infused. Fresh T cells showed an increase in apoptosis and cell killing in response to high concentrations of alemtuzumab in the blood (d5 post-alemtuzumab, d12 post-alemtuzumab), as well as when exposed to pre-alemtuzumab serum to which 10 µg/ml of alemtuzumab had been added (far right). Values returned to baseline levels of apoptosis and killing when fresh T cells were incubated with serum obtained 1 week after alemtuzumab infusion (d12, d20), indicating that serum contained high concentrations of alemtuzumab early after infusion. Expanded autologous Treg showed no increase in apoptosis or killing when exposed to a high concentration of alemtuzumab in serum, whether the serum was drawn from an alemtuzumab-treated monkey, or whether alemtuzumab had been added to the serum in vitro (far right).
These observations were confirmed by adding alemtuzumab (10 µg/ml) to the pre-alemtuzumab (normal) serum sample, which resulted in a similar increase in Annexin-V and 7-AAD staining (Figure 4 and Supplementary Figure 4). Similarly to the in vitro experiments, autologous expanded Treg showed no evidence of killing when incubated with either serum taken immediately after alemtuzumab infusion or serum supplemented with 10 µg/ml alemtuzumab (Figure 4).
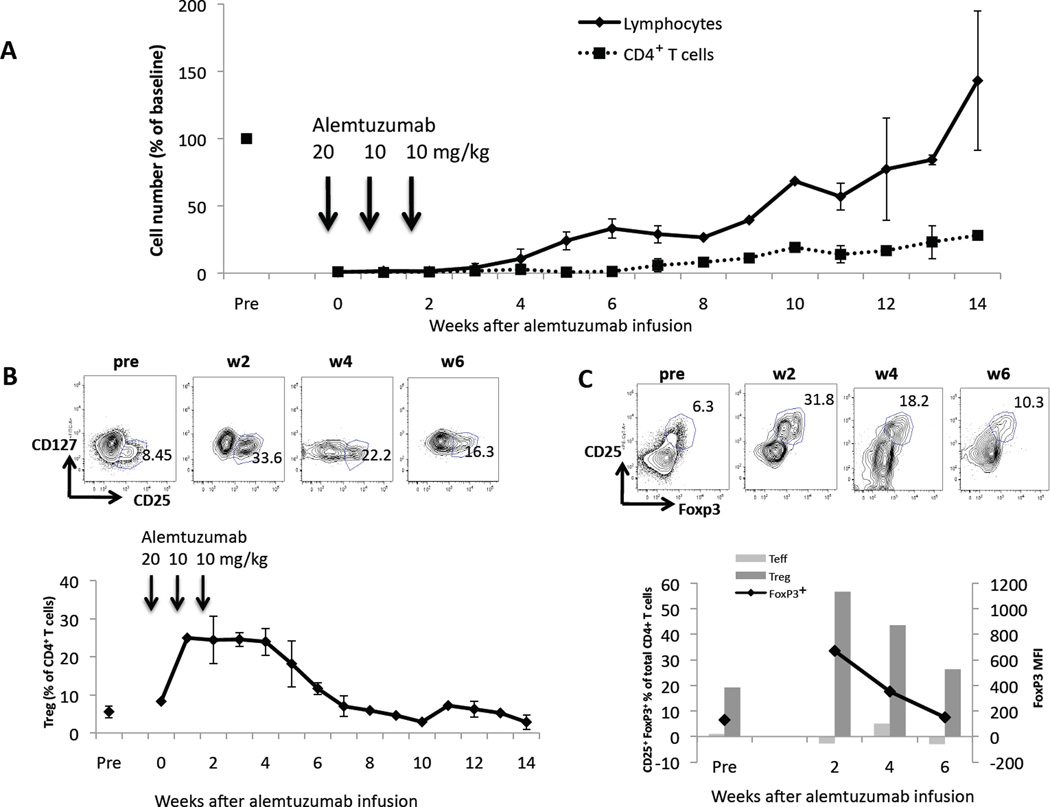
Alemtuzumab infusion: relative sparing of Treg
Since alemtuzumab appeared minimally cytotoxic to Treg after their ex vivo expansion, the influence of alemtuzumab infusion on Treg remaining in the circulation was investigated. Cynomolgus monkeys (n=3) received an allogeneic heterotopic heart transplant on d 0 under the immunosuppressive regimen described in the Methods. Pan T cell numbers were depleted >97% after the first dose of alemtuzumab and remained at this low level following subsequent doses. Recovery of total CD4+T cells began by week 7 (increase to >10% of pre-alemtuzumab numbers) (Figure 5A). All 3 monkeys maintained relatively high numbers of circulating Treg within this small population of CD4+T cells, as determined by the incidence of CD25hiCD127− cells (Figure 5B). In view of the overall very low number of circulating lymphocytes, we were able to perform intracellular FoxP3 staining only at bi-weekly intervals. FoxP3+ staining and analysis confirmed that the CD25hiCD127− cells were indeed FoxP3+ Treg (Figure 5C). The increase in the proportion of circulating Treg was transient, as it was evident only from weeks 2–6, after which Treg numbers returned to pre-alemtuzumab baseline levels (Figure 5C).
Figure 5. Relative increase in circulating Treg after infusion of alemtuzumab.
Monkeys that had received a heart transplant on d 0 and 3 doses of alemtuzumab on d -2, 5, and 12 (n=3 total; n=2 for each time-point) showed (A) a profound depletion of total lymphocytes and, in particular, CD4+T cells. (B) Within the remaining small circulating CD4+T cell population, the percentage of CD25hiCD127− Treg increased significantly after alemtuzumab infusion in all 3 monkeys (up to 35% in one case) and returned to baseline values (~5%) by week 7 post-transplant. Representative dot plots of CD127 vs. CD25 pre- and at 2, 4, 6 weeks after alemtuzumab infusion are shown. (C) Intracellular staining for FoxP3 (black line; left vertical axis in lower figure) pre- and 2, 4, and 6 weeks after alemtuzumab infusion shows a transient increase in FoxP3+ cells within the CD4+ cell population. Representative dot plots of CD25 vs. Foxp3 are shown above. The MFI for FoxP3 (dark grey bars; right vertical axis) in CD25hiCD127− Treg is markedly higher than that in the CD25− Teff subpopulation (light grey bars) at each time point. Representative values for one monkey are shown.
Discussion
We examined whether ex vivo-expanded NHP (cynomolgus monkey) Treg are resistant to alemtuzumab-mediated killing and whether it might be feasible to administer these cells to transplant recipients without their being killed by circulating mAb at the time of infusion. We used Indonesian cynomolgus macaques specifically, since CD52 is not expressed on the erythrocytes of this species. A significant finding was that the expanded Treg exhibited considerably lower CD52 expression than either fresh bulk T cells or freshly-isolated Treg. Alemtuzumab showed strong binding to freshly-isolated pan T cells and Treg, with maximum activity at a concentration of 10 µg/ml. Therapeutic levels in humans have been reported to peak at 13.7 µg/ml after an initial dose of 20 mg (6). We could not identify a laboratory to measure alemtuzumab in serum that would have allowed us determine mAb concentrations after infusion. However, the killing assay that we performed using monkey serum taken immediately and 7d after mAb infusion suggests that most cytotoxic activity of circulating alemtuzumab has disappeared after 7d. Importantly, alemtuzumab did not bind to expanded Treg at concentrations up to 100 µg/ml, and whereas T cells freshly-isolated from blood were susceptible to concentration-dependent killing by alemtuzumab, expanded Treg were not killed at any concentration tested. These findings suggest that if Treg are isolated and expanded from a cynomolgus graft recipient before alemtuzumab administration, then the expanded cells can be administered to lymphocyte-depleted monkeys at any time after transplantation in the presence of circulating alemtuzumab, without risk of complement-mediated killing.
We verified complement-dependent killing of freshly-isolated cynomolgus T cells by alemtuzumab. No killing was observed (compared to baseline) when monkey serum was heat-inactivated, but cytotoxicity was restored when complement was added. By contrast, only very low levels of ADCC were mediated by alemtuzumab against freshly-isolated or expanded cynomolgus T cells. Our findings indicate a major complement-dependent component of cynomolgus T cell killing by alemtuzumab. This contrasts with the report of Hu et al (4), who claimed that, using a transgenic mouse expressing human CD52, killing of circulating lymphocytes by alemtuzumab was largely independent of complement. The principal mechanism identified in the latter study was ADCC mediated by neutrophils and NK cells. In the present study, complement contributed significantly to the death of fresh, but not expanded T cells in the absence of neutrophils and NK cells. Notably, Lowenstein et al (5) reported predominantly complement-mediated killing of human CD4+ T cells by alemtuzumab, which was related to expression of CD52, although only unexpanded cells were studied.
Analyses of the influence of alemtuzumab on human Treg in vivo have demonstrated a transient increase in circulating CD4+CD25+FoxP3+ cells after kidney transplantation (13). The mechanism responsible for the initial increase in Treg is not understood, but de novo generation was suggested and could be explained by the induction of Treg in vivo (22). In vitro studies of Treg proliferation have shown that Treg expand preferentially in allogeneic MLR in the presence of alemtuzumab (23). However, in a similar study, incubation of human PBMC with anti-thymocyte globulin was associated with Treg expansion whereas, by contrast, alemtuzumab depleted CD25+ Treg (17).
An explanation for the increased incidence of Treg observed in the circulation of cynomolgus monkeys after alemtuzumab administration (Figure 5B) may be that lymphocyte depletion may occur mainly in blood and least in bone marrow (4). Human bone marrow, where the cells are relatively spared from alemtuzumab-mediated killing (24), has a much higher proportion of Treg than blood, LN, and thymus. This could explain the relatively high incidence of Treg in human blood during the first 6 weeks after starting alemtuzumab. Interestingly, when alemtuzumab was added to ex vivo cultures of human umbilical cord blood stem cells, a significant increase in stem cells was observed (25).
Generation or preferential expansion of Treg after alemtuzumab administration in humans appears to be transient in vivo. Bloom et al (13) reported peak Treg numbers (% total CD4+T cells) 6 months after renal transplantation, with a subsequent decline, although the percentage remained elevated for 36 months post-transplant. Long-term follow-up of human kidney transplant recipients has indicated that alemtuzumab-based immunosuppression decreases the proportion of CD4+Treg when measured several years after transplantation, which correlates with increased anti-donor reactivity in these patients (15). The present results in cynomolgus monkeys indicate a much shorter period of increased incidence of Treg of only 2–7 weeks. This difference may be related to different binding affinity of alemtuzumab in monkeys compared with humans (18).
Although we did not determine the half-life of alemtuzumab (a humanized mAb) in cynomolgus monkeys, our finding that ex vivo-expanded cynomolgus Treg are not affected by alemtuzumab-mediated complement-dependent killing has important relevance to the testing of adoptively-transferred Treg for therapy of allograft rejection in alemtuzumab-depleted cynomolgus monkey hosts.
In conclusion, we have demonstrated that, following their ex vivo expansion, cynomolgus monkey Treg downregulate CD52 expression and are not susceptible to complement-mediated killing by alemtuzumab. This suggests that expanded cynomolgus Treg can be infused into transplant recipients at any time after alemtuzumab-mediated lymphocyte depletion without their destruction by residual mAb. In cynomolgus monkeys, a transient, relative increase in the incidence of Treg occurs in vivo, starting about two weeks after the initial alemtuzumab dose, and persists for approximately 4–5 further weeks.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health grant U01-AI091197 (AWT and DKCC). Eefje M. Dons is the recipient of fellowships from the Ter Meulen Fund of the Royal Netherlands Academy of Arts and Sciences and the Stichting Professor Michael van Vloten Fund, The Netherlands. Giorgio Raimondi is in receipt of an American Heart Association Beginning Grant-in-Aid, an American Diabetes Association Junior Faculty Grant and the Thomas E. Starzl Transplantation Institute Joseph A. Patrick Fellowship. HZ is in receipt of an American Society of Transplantation Basic Science Fellowship.
Abbreviations
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- FoxP3
Forkhead box P3
- LN
lymph node(s)
- MFI
mean fluorescence intensity
- MMF
mycophenolate mofetil
- NHP
non-human primate
- NK
natural killer
- PBMC
peripheral blood mononuclear cells
- Teff
effector T cells
- Treg
regulatory T cells
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Hale G, Xia MQ, Tighe HP, Dyer MJ, Waldmann H. The CAMPATH-1 antigen (CDw52) Tissue Antigens. 1990;35(3):118–127. doi: 10.1111/j.1399-0039.1990.tb01767.x. [DOI] [PubMed] [Google Scholar]
- 2.Hale G, Dyer MJ, Clark MR, Phillips JM, Marcus R, Riechmann L, et al. Remission induction in non-Hodgkin lymphoma with reshaped human monoclonal antibody CAMPATH-1H. Lancet. 1988;2(8625):1394–1399. doi: 10.1016/s0140-6736(88)90588-0. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro R, Basu A, Tan H, Gray E, Kahn A, Randhawa P, et al. Kidney transplantation under minimal immunosuppression after pretransplant lymphoid depletion with Thymoglobulin or Campath. J Am Coll Surg. 2005;200(4):505–515. doi: 10.1016/j.jamcollsurg.2004.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu Y, Turner MJ, Shields J, Gale MS, Hutto E, Roberts BL, et al. Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model. Immunology. 2009;128(2):260–270. doi: 10.1111/j.1365-2567.2009.03115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowenstein H, Shah A, Chant A, Khan A. Different mechanisms of Campath-1H-mediated depletion for CD4 and CD8 T cells in peripheral blood. Transpl Int. 2006;19(11):927–936. doi: 10.1111/j.1432-2277.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 6.Morris EC, Rebello P, Thomson KJ, Peggs KS, Kyriakou C, Goldstone AH, et al. Pharmacokinetics of alemtuzumab used for in vivo and in vitro T-cell depletion in allogeneic transplantations: relevance for early adoptive immunotherapy and infectious complications. Blood. 2003;102(1):404–406. doi: 10.1182/blood-2002-09-2687. [DOI] [PubMed] [Google Scholar]
- 7.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3(3):199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 8.Bluestone JA. Regulatory T-cell therapy: is it ready for the clinic? Nat Rev Immunol. 2005;5(4):343–349. doi: 10.1038/nri1574. [DOI] [PubMed] [Google Scholar]
- 9.Walsh PT, Taylor DK, Turka LA. Tregs and transplantation tolerance. J Clin Invest. 2004;114(10):1398–1403. doi: 10.1172/JCI23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang S, Lechler RI. Regulatory T cells in the control of transplantation tolerance and autoimmunity. Am J Transplant. 2003;3(5):516–524. doi: 10.1034/j.1600-6143.2003.00124.x. [DOI] [PubMed] [Google Scholar]
- 11.Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol. 2012;12(6):417–430. doi: 10.1038/nri3227. [DOI] [PubMed] [Google Scholar]
- 12.Raimondi G, Sumpter TL, Matta BM, Pillai M, Corbitt N, Vodovotz Y, et al. Mammalian target of rapamycin inhibition and alloantigen-specific regulatory T cells synergize to promote long-term graft survival in immunocompetent recipients. J Immunol. 2010;184(2):624–636. doi: 10.4049/jimmunol.0900936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloom DD, Chang Z, Fechner JH, Dar W, Polster SP, Pascual J, et al. CD4+ CD25+ FOXP3+ regulatory T cells increase de novo in kidney transplant patients after immunodepletion with Campath-1H. Am J Transplant. 2008;8(4):793–802. doi: 10.1111/j.1600-6143.2007.02134.x. [DOI] [PubMed] [Google Scholar]
- 14.Pascual J, Bloom D, Torrealba J, Brahmbhatt R, Chang Z, Sollinger HW, et al. Calcineurin inhibitor withdrawal after renal transplantation with alemtuzumab: clinical outcomes and effect on T-regulatory cells. Am J Transplant. 2008;8(7):1529–1536. doi: 10.1111/j.1600-6143.2008.02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macedo C, Walters JT, Orkis EA, Isse K, Elinoff BD, Fedorek SP, et al. Long-term effects of alemtuzumab on regulatory and memory T-cell subsets in kidney transplantation. Transplantation. 2012;93(8):813–821. doi: 10.1097/TP.0b013e318247a717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117(3):1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez M, Clarkson MR, Albin M, Sayegh MH, Najafian N. A novel mechanism of action for anti-thymocyte globulin: induction of CD4+CD25+Foxp3+ regulatory T cells. J Am Soc Nephrol. 2006;17(10):2844–2853. doi: 10.1681/ASN.2006050422. [DOI] [PubMed] [Google Scholar]
- 18.van der Windt DJ, Smetanka C, Macedo C, He J, Lakomy R, Bottino R, et al. Investigation of lymphocyte depletion and repopulation using alemtuzumab (Campath-1H) in cynomolgus monkeys. Am J Transplant. 2010;10(4):773–783. doi: 10.1111/j.1600-6143.2010.03050.x. [DOI] [PubMed] [Google Scholar]
- 19.Perry AC, Jones R, Hall L. Identification of an abundant monkey epididymal transcript encoding a homologue of human CAMPATH-1 antigen precursor. Biochim Biophys Acta. 1992;1171(1):122–124. doi: 10.1016/0167-4781(92)90152-p. [DOI] [PubMed] [Google Scholar]
- 20.Yeung CH, Schroter S, Wagenfeld A, Kirchhoff C, Kliesch S, Poser D, et al. Interaction of the human epididymal protein CD52 (HE5) with epididymal spermatozoa from men and cynomolgus monkeys. Mol Reprod Dev. 1997;48(2):267–275. doi: 10.1002/(SICI)1098-2795(199710)48:2<267::AID-MRD15>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 21.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203(7):1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dons EM, Raimondi G, Cooper DK, Thomson AW. Induced regulatory T cells: mechanisms of conversion and suppressive potential. Hum Immunol. 2012;73(4):328–334. doi: 10.1016/j.humimm.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levitsky J, Leventhal JR, Miller J, Huang X, Chen L, Chandrasekaran D, et al. Favorable effects of alemtuzumab on allospecific regulatory T-cell generation. Hum Immunol. 2012;73(2):141–149. doi: 10.1016/j.humimm.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, et al. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64(22):8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 25.Lim CK, Sun L, Feng Q, Law P, Chua WT, Lim SN, et al. Effect of anti-CD52 antibody alemtuzumab on ex-vivo culture of umbilical cord blood stem cells. J Hematol Oncol. 2008;1:19. doi: 10.1186/1756-8722-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.