Abstract
Nitric oxide (NO)-releasing non-steroidal anti-inflammatory drugs (NO-NSAIDs) which have been synthesized to reduce gastro-intestinal and cardiovascular toxicities of NSAIDs, possess anti-proliferative, pro-apoptotic and anti-cancer activities. Here, we show that NO-sulindac inhibited UVB-induced skin tumorigenesis in SKH-1 hairless mice. Topical application of NO-sulindac reduced tumor incidence, number (p<0.05) and volume (p<0.005) as compared to UVB (alone)-irradiated vehicle-treated mice. An increase in TUNEL-positive cells in skin lesions was accompanied by the enhanced Bax:Bcl-2 ratio. The expression of pro-apoptotic Bax was increased whereas anti-apoptotic Bcl-2 reduced. However, proliferation was identified as the major target of NO-sulindac in this study. A reduced expression of PCNA and cyclin D1 associated with the dampening of cell cycle progression was observed. The mechanism of this inhibition was related to the reduction in UVB-induced Notch signaling pathway. UVB-induced inflammatory responses were diminished by NO-sulindac as observed by a remarkable reduction in the levels of phosphorylated MAP Kinases Erk1/2, p38 and JNK1/2. In this regard, NO-sulindac also inhibited NFκB by enhancing IκBα as evidenced by the reduced expression of iNOS and COX-2, the direct NFκB transcription target proteins. NO-sulindac significantly diminished the progression of benign lesions to invasive carcinomas by suppressing the tumor aggressiveness and retarding epithelial-mesenchymal transition. A marked decrease in the expression of mesenchymal markers such as Fibronectin, N-cadherin, SNAI, Slug and Twist and an increase in epithelial cell polarity marker E-cadherin were noted in NO-sulindac-treated tumors. Our data suggest that NO-sulindac is a potent inhibitor of UVB-induced skin carcinogenesis and acts by targeting proliferation-regulatory pathways.
Keywords: NSAIDs, NO-sulindac, cell cycle, photocarcinogenesis, skin, UVB
Introduction
More than 1,000,000 new cases of skin cancers are reported every year in the United States which is approximately 40% of all diagnosed cancers (Ricotti et al., 2009). The pathogenesis of UVB-induced nonmelanoma skin cancer (NMSC) involves disruption of multiple signaling pathways that besides driving the initiation of these cancers lead to inflammation, proliferation of neoplastic lesions and suppression of apoptosis (Bickers and Athar, 2006). In this regard, we and others have shown that cyclooxygenases (COXs) particularly COX-2 is consistently upregulated in NMSCs (An et al., 2002; Chun et al., 2007). Therefore, non-steroidal anti-inflammatory drugs (NSAIDs) which are the known inhibitors of these enzymes have been reported to reduce the risk of cancer development in the skin and in other organs as well both in humans and experimental animals (Ulrich et al., 2006; Thun et al., 2012). However, many of these NSAIDs particularly COX-2 inhibitors are known to manifest severe cardiovascular (CV) and gastrointestinal (GI) toxicities. These serious CV and GI effects dampen enthusiasm for their use as chemopreventive drugs particularly for reducing skin cancer since majority of them are not lethal and can be surgically removed (Conaghan, 2012). However, in organ transplant receipts who are at very high risk of skin cancer induction, frequent surgical removal of these lesions causes significant morbidity. Therefore, potentially safer agents with minimal or no toxic effect are needed for their use as chemopreventive agents. To attenuate the toxicity associated with these agents, nitric oxide-releasing NSAIDs (NO-NSAIDs), which structurally consist of a traditional NSAID connected with a spacer linker covalently bound to NO moiety have been synthesized (Williams et al., 2004). The ability of NO-NSAIDs to release NO is believed to circumvent both CV and GI toxicities.
Interestingly, similar to their parent agents, NO-NSAIDsalso have anti-proliferative and anti-tumor effects (Williams et al., 2004; Stewart et al., 2009; Singh et al., 2012). These agents showed efficacy against a variety of cancer-types. Our laboratory has continued interest in investigating chemopreventive potential of NSAIDs and defining molecular mechanisms involved in the inhibition of tumorigenesis employing various murine models of NMSCs (Lee et al., 2003; Kim et al., 2005; Lee et al., 2005; Tang et al., 2008; Tang et al., 2010). Sulindac and its derivative, sulindac sulfide is known to inhibit the activities of COXs and act in a COX-dependent manner (Athar et al., 2004) and also in COX-independent manner (Piazza et al., 2009). These agents have also been demonstrated to be effective chemopreventive drugs when administered alone or in combination with other agents both in experimental models and in humans (Meyskens et al., 2008; Tang et al., 2010; Li et al., 2012). In the skin, we showed that sulindac administration reduces UVB-induced cutaneous phototoxicity (Athar et al., 2004). Recently, sulindac and its structural analogs are found to inhibit colon tumor growth in xenograft murine model (Zhou et al., 2010). We, therefore tested the notion that NO-sulindac may be a potentially effective agent against UVB-induced skin carcinogenesis.
In this study, we investigated the ability of NO-sulindac to inhibit the pathogenesis of UVB-induced skin tumors in SKH-1 hairless mice. Our data show that NO-sulindac significantly attenuated tumor cell proliferation by down-regulating cell cycle proteins via targeting Notch and RXR-PI3k/Akt signaling. It also reduced the progression of papilloma to carcinoma by modulating epithelial to mesenchymal transition markers during tumor growth. Alterations in tyrosine (tyr) nitrosylation of proteins, reduction in the expression of NFkB transcriptional targets iNOS/COX-2 and -dependent reduction in the inflammatory response were also associated with the diminution of tumor growth.
Material and Methods
Reagents and Antibodies
NO-sulindac (Batch number: NXS-M0002-SD-1-11-36) used in this study was procured from Magellan Laboratories Inc. (Morrisville, NC). NO-sulindac (C24H24FNO7S), a yellow crystalline powder was stored at room temperature. The details and the list of antibodies are provided as supplemental table 1.
Animals
SKH-1 hairless mice (6-8 weeks old) used in this study were purchased from Charles River Laboratories (Hartford, CT) and maintained under standard conditions of a 12 h dark/12 h light cycle, temperature of 24 ± 2°C and relative humidity of 50 ± 10%. They were given chow and water ad libitum. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Alabama at Birmingham.
UV light source
A UV Irradiation Unit (Daavlin Co., Bryan, OH) with an electronic controller to regulate dose of irradiated UVB was used in the study. The UV irradiation unit was equipped with six Philips Ultravoilet B TL 40W/12RS lamps. Furthermore, we also used a Kodacel cellulose film (Kodacel TA401 / 407) to eliminate any UVC as described earlier (Singh et al., 2012).
Experimental Protocol
For this experiment, animals were divided into three groups of fifteen mice each. Group-I animals received only vehicle (acetone) and served as an age-matched negative control. Group-II and III mice were irradiated with UVB (180mJ/cm2; twice/week) for 30 weeks. In addition to UVB irradiation, group II and III mice received twice weekly topical treatments (30 min prior to UVB irradiation) with either vehicle (acetone) or NO-sulindac (5 mg/mouse dissolved in 200μl acetone) respectively. The numbers and volume (using electronic Vernier Calipers) of tumors in each animal were recorded on a weekly basis as described earlier (Singh et al., 2012). The selection of the dose of NO-sulindac is based on our previously published study related to photo-protective effects of sulindac (Athar et al., 2004). Data are presented as mean ± SE and plotted as a function of weeks on test. Following irradiation for 30 weeks, the experiment was terminated and all mice were euthanized as per IACUC recommendations.
Histology, Immunohistochemistry, Immunofluorescence staining and Terminal deoxynucleotidyl transferase–mediated nick end labeling (TUNEL) assay
Skin and tumor tissues were fixed in 10% buffered formalin, embedded in paraffin, and cut into 5 μm thick sections. Paraffin sections of tissues were stained with H&E and examined for tumor histology as described earlier (Singh et al., 2012). For immunohistochemistry, the deparaffinized slides were placed in antigen unmasking solution (Vector laboratories) for 20 min at 95°C. After peroxidase blocking, the nonspecific sites were blocked in 2% BSA for 45 min at RT, followed by overnight incubation in primary antibody at 4°C. The sections were washed and incubated with biotinylated secondary antibody and then with HRP conjugated streptavidin. Color reaction was observed using 3, 3′-diaminobenzidine. Sections were counterstained with Harris hematoxylin (Sigma-Aldrich) and mounted using Permount (Fisher Scientific). However, for immunofluorescence staining, the sections were incubated with Alexa Fluor 594 (Invitrogen, Carlsbad, CA, USA), Dylight 488 (Pierce) or Fluorescein (Pierce)-coupled secondary antibody. Sections were mounted with Vectashield Mounting Medium with DAPI (H-1200; Vector Laboratories). TUNEL assay was performed using a kit from Roche Applied Science (Cat. no.1684795) according to manufacturer's guidelines.
Cell culture and treatment
Human epidermoid carcinoma, A431cells were obtained from the American Type Culture Corporation (Manassas, VA, USA) and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/ml of penicillin, and 100 μg/ml of streptomycin in a humidified atmosphere of 5% CO2 / 95% air at 37°C. Cells (50-60% confluent) were treated with NO-sulindac or vehicle (DMSO) in complete culture medium. After 24 h of drug exposure, the media was removed and the cells were harvested to prepare whole-cell lysates.
Flow cytometry
Briefly, A431 cells were treated with NO-sulindac or vehicle for 24 h. The cells were harvested by trypsinization, washed with PBS and were fixed with ice-cold 70% ethanol at -20°C overnight. Cells were then washed twice with PBS, incubated with 20 mg/ml RNase A and 200 mg/ml propidium iodide in PBS at room temperature for 30 min and subjected to flow cytometry using the FACSCalibur flow cytometer (San Jose, California). Data were analyzed and provided as percentage of G0, G1, S, and G2/M phase cells in each sample.
Preparation of tissue lysate for Western blot analysis
Skin/tumor samples were lysed in ice-cold lysis buffer (50mM Tris pH 7.5, 1% Triton X-100, 0.25% NaF, 10mM β-glycerol phosphate, 1mM EDTA, 5mM sodium pyrophosphate, 0.5mM Na3VO4, 0.1% 10mM DTT, 1% PMSF, and protease inhibitors) followed by centrifugation at 12,000×g for 20 min at 40C. The supernatant was collected and processed for immunoblot analysis. For immunoblotting, 60-80μg of proteins were resolved on 8-15% SDS-PAGE (BioRad, CA, USA) and transferred onto a nitrocellulose membrane. The membrane was probed with primary antibodies for 2 h at RT or overnight at 4°C followed by appropriate horseradish peroxidase-conjugated secondary antibodies. The bands were visualized using an enhanced chemiluminescence reagent (Pierce, Rockford, IL, USA). Membranes were stripped and re-probed with anti-b-actin antibody to verify equal protein loading. In instances where a blot was stripped multiple times and probed with different antibodies but the data are presented as a part of more than one figure, the same β-actin image was placed at the bottom of these different figures.
Statistical analysis
Statistical analysis was performed using Microsoft Excel software. The significance between two test groups was determined using Student's t test. A ‘p’ value of less 0.05 was considered to be significant.
Result
NO-sulindac reduces UVB-induced skin photocarcinogenesis
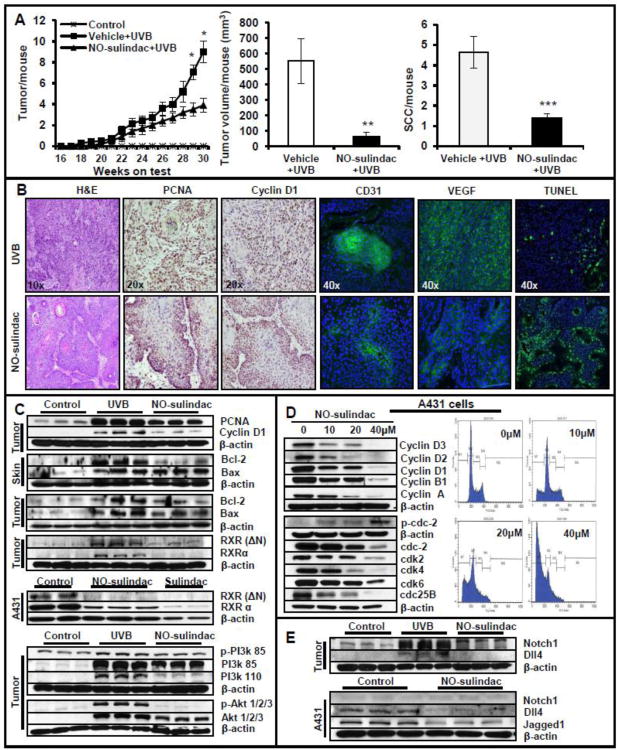
Topical application of NO-sulindac significantly reduced the development of UVB-induced skin tumor in SKH-1 hairless mice as compared to UVB (alone)-irradiated mice. As shown in Figure 1A, a substantial reduction in tumor number and tumor volume was noted in NO-sulindac-treated mice. At week 30th, the percentage tumor incidence of mice bearing tumors in NO-sulindac-treated group was 80% as compared to 100% of UVB-irradiated mice group (data not shown). Mice treated with NO-sulindac developed 3.9±0.67 tumors/mouse whereas UVB-irradiated mice induced 9.0±1.04 tumors/mouse (Fig. 1A). There was also a significant reduction in terms of tumor/tumor-bearing mouse (45%; p<0.05) (data not shown). The tumor volume was reduced by 88% (p<0.005) in mice administered with NO-sulindac treatment (Fig. 1A). NO-sulindac also slightly increased the latency period of tumor induction. To further analyze the tumor burden in these animals, we divided mice in groups bearing increasing number of tumors viz. 0-5, 6-10, 11-15 and 16-25 tumors/mouse. In NO-sulindac treatment group, 90% of mice had tumor burden between 0-5 tumors/mouse whereas only 10% had 6-10 tumors/mouse. However, only 15% of UVB (alone)-irradiated mice had 0-5 tumors/mouse, 50% 6-10 tumors/mouse, 25% 11-15 tumors/mouse and remaining 10% had 16-25 tumors/mouse (data not shown). These data indicate that NO-sulindac treatment reduced the development of UVB-induced skin tumors. Interestingly, the number of SCCs/mouse in UVB-irradiated mice was much higher than in NO-sulindac-treated group. Thus, NO-sulindac significantly reduced the progression of papillomas to SCCs (Fig. 1A). Histological analysis of tumor tissues obtained from UVB-irradiated mice at 30th weeks showed a substantial proportion of these SCCs were poorly differentiated SCCs characterized by the absence of keratin pearls, or often highly aggressive spindle cell carcinoma with hyperchromatic pleomorphic nuclei and considerably mitotic activity and invasion of dermis, and a few invasive keratoacanthoma whereas mostly highly differentiated SCCs carrying well-developed keratin pearls were present in NO-sulindac group (Fig. 1B). We also determined the expression of angiogenesis biomarkers in UVB-induced tumors in control and NO-sulindac treatment groups. As shown in Fig. 1B, vascular endothelial growth factor (VEGF)/CD31 staining was diminished following NO-sulindac treatment. Moreover, the expression of CD31 was reduced drastically in NO-sulindac-treated tumors as compared to UVB (alone)-irradiated tumors.
Figure 1.
NO-sulindac reduces UVB-induced skin tumorigenesis by inhibiting proliferation, angiogenesis and by inducing cell cycle arrest/apoptosis via Notch/RXR-PI3K/Akt axis. (A) Data showing tumors/mouse, tumor volume/mouse (mm3) and SCCs/mouse; (B) histology, immunostaining for PCNA, cyclin D, VEGF and CD31 & TUNEL staining; (C) western blot analysis showing expression of PCNA, cyclin D1, Bax and Bcl-2 in tumors and tumor-adjacent skins. Shown here also is the expression of RXRs in murine SCCs and human A431 cells, & PI3K/Akt proteins in murine tumors; (D) western blot analysis showing expression of cyclins/cyclin dependent kinases and Flow cytometry analysis of A431 cells treated with NO-sulindac; (E) western blot analysis showing expression of Notchl, DII4 and Jaggedl in murine tumors and in A431 cells. *P < 0.05; **P < 0.005 and ***P < 0.001.
NO-sulindac treatment induces apoptosis in tumors and tumor-adjacent skin
NO-sulindac treatment induced apoptosis in tumors and tumor-adjacent skin. Number of TUNEL-positive cells both in tumors and tumor-adjacent skin were much higher in NO-sulindac treatment group as compared to UVB (alone) group (Fig. 1B). Bax/Bcl-2 ratio which determines death to survival signal was also found to be higher in NO-sulindac group which was associated with the decreased expression of Bcl-2 (Fig. 1C and Table 1). The parental compound of NO-sulindac, sulindac has been reported to exert its anticancer effects through binding with retinoid × receptor-α (RXRα) and subsequently interacting with p85α subunit of PI3K, leading to activation of death receptor-mediated apoptosis (Zhou et al., 2010). We, therefore, first confirmed the expression of RXR(ΔN) and RXRα in UVB-induced tumors and human epidermoid A431 cells. A431 cells are human epidermoid carcinoma cells carrying UVB-signature mutations in p53. Therefore, the responses of NO-sulindac shown here appear to be p53 independent. The expression of these proteins was induced in both murine and human SCC cells (Fig. 1C and Table 1). In this experiment, we included an additional treatment group of sulindac to confirm the earlier published data (Zhou et al., 2010). NO-sulindac similar to sulindac abrogated the expressions of these proteins. Immunoblotting assays showed that the UVB-induced phosphorylation of PI3K and Akt were reduced in response to NO-sulindac (Fig. 1C and Table 1). These data reveal that the anti-cancer activity of NO-sulindac occurs by apoptosis induction regulated possibly via inhibition of the RXR-PI3K/Akt axis.
Table 1.
Relative Expression of Proteins in Age-matched control, UVB-irradiated and NO-sulindac-treated Mouse.
| Proteins | Control (age-matched skin) | Acetone + UVB | NO-sulindac + UVB |
|---|---|---|---|
| PCNA | 2.34±0.24 | 7.64±0.26 | 5.31±0.18# |
| Cyclin D1 | 0.53±0.005 | 2.88±042 | 1.18±0.18* |
| Bcl-2 (skin) | 1.7±0.11 | 7.1±0.27 | 3.63±0.28# |
| Bax (skin) | 1.51±0.17 | 6.98±1.54 | 8.30±0.32 |
| Bcl-2 | 2.98±0.49 | 7.55±147 | 3.82±0.38† |
| Bax | 2.76±0.28 | 8.13±1.26 | 7.40±0.87 |
| Bax:Bcl-2 ratio (skin) | -- | 0.99±024 | 2.30±0.16† |
| Bax:Bcl-2 ratio (tumor) | -- | 1.10±0.07 | 1.96±0.29* |
| RXR(ΔN) | 2.05±0.25 | 5.39±027 | 3.05±0.26† |
| RXRα | 1.52±0.26 | 2.97±0.29 | 1.55±0.18† |
| p-PI3K 85 | 1.60±0.065 | 2.31±0.09 | 1.66±0.10* |
| PI3K 110 | 0.40±0.035 | 2.67±0.09 | 1.24±0.08# |
| p-Akt1/2/3 | 1.21±0.10 | 6.45±0.35 | 1.46±0.17# |
| Notch1 | 2.44±0.25 | 8.81±0.33 | 5.72±0.39† |
| Dll4 | 2.02±0.22 | 5.22±0.51 | 2.08±0.65† |
| iNOS | 0.46±0.14 | 3.11±0.18 | 1.00±0.31† |
| p-Erk1/2 | 4.23±0.08 | 11.58±0.19 | 10.69±0.17 |
| p-p38 | 12.48±0.55 | 27.44±1.58 | 20.76±0.22* |
| p-JNK1/2 | 1.75±0.23 | 11.14±0.30 | 8.58±0.67* |
| E-cadherin | 4.11±0.45 | 0.65±0.05 | 2.05±0.18* |
| N-cadherin | 0.53±0.08 | 3.43±0.24 | 0.61±0.03† |
| SNAI | 0.58±0.06 | 3.54±0.18 | 2.03±0.08† |
| Slug | 0.75±0.09 | 1.40±0.09 | 0.87±0.06† |
Data is represented in arbitrary scale generated by assessing band density in western blot normalized to the respective β-actin loading controls. Data are represented as mean ±SE of at least of three independent samples.
P < 0.05;
P < 0.005 and
P < 0.001.
NO-sulindac blocks proliferation of UVB-induced cutaneous lesions
We assessed the expression levels of proliferative cell nuclear antigen (PCNA) and cyclin D1 in these tumors by immunohistochemical and immunoblot analysis (Fig 1B and C). UVB (alone) group tumors and tumor-adjacent skin showed extensive PCNA and cyclin D1 staining, whereas it was significantly (p<0.05) reduced in tumor cells treated with NO-sulindac. To examine the effect of NO-sulindac on proliferation, we determined its effect on cell cycle progression in A431 cells. These cells carry UVB signature mutations in p53 (Tang et al., 2007). NO-sulindac treatment inhibited cell cycle progression which correlated with a reduction in the expression of cyclins (A, B1, D1, D2 and D3); cyclin-dependent kinases (cdk)s 2, 4 and 6 and cdc-2 and phosphatase cdc25B (Fig. 1D). NO-sulindac treatment arrested the cells in the G0/G1 phase which thereafter get apoptosed leading to a dramatic increase in sub-Go population (59.9% of cells at 40μM) as compared to control cells (0.53% of cells) (Fig. 1D). NO-sulindac treatment reduced the UVB-induced activation of Notch1 and Dll4 in tumors (Fig. 1E). To verify the role of Notch1 in the pathogenesis of human SCCs, we evaluated the expression of Notch1 and its ligands in A431 cells with and without NO-sulindac treatment. In this study, although Notch1 expression was not drastically reduced, the expressions of Dll4 and Jagged 1 were down-regulated following NO-sulindac treatment (Fig. 1E).
NO-sulindac diminishes pro-inflammatory signaling
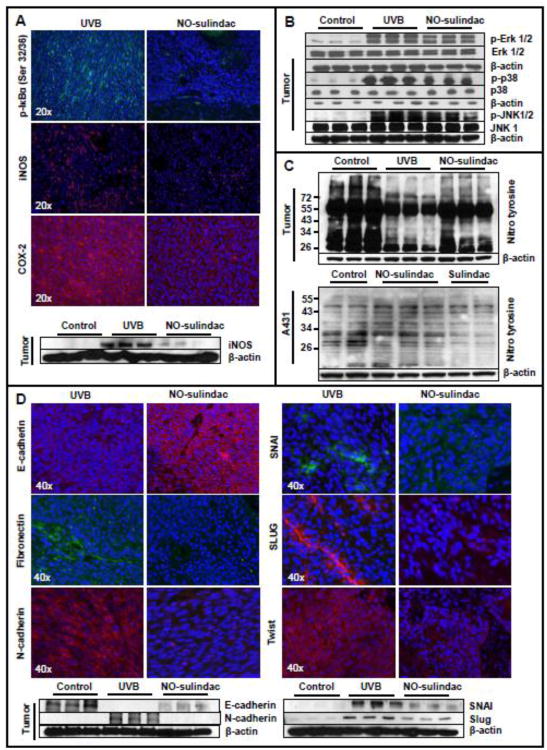
NFκB is a transcription factor, which is known to underlie the pathogenesis of inflammation. In cytoplasm, transcriptionally inactive NFκB exists as heterotrimeric complex consisting of p65, p52/p50 and inhibitory kappa B (IkB). During pro-inflammatory process this gets dissociated by the phosphorylation of IkB through the upstream kinases known as IKKs. Phosphorylated IkB is then degraded via ubiquitinization. The dissociated p50-p65/p52-p65 complexes translocate to the nucleus to activate its transcription functions (Gilmore, 2006; DiDonato et al., 2012). A large number of proteins are transcriptionally regulated by NFκB. The inflammation regulatory proteins COX-2 and iNOS are also direct transcriptional targets of NFκB (Gilmore, 2006). We observed a uniform nuclear staining for p65 in both tumor and stromal cells (data not shown). However, NO-sulindac treatment reduced UVB-induced phosphorylation of IkB (Fig. 2A) and expression of nuclear NFκBp65 in neoplastic lesions. The p65 staining was discernible only in small tumor islands whereas stromal staining was mainly abolished (data not shown). Consistently, the expression levels of pro-inflammatory proteins COX-2 and iNOS in UVB (alone)-induced tumors were reduced by NO-sulindac treatment (Fig. 2A and Table 1). Phosphorylation-dependent activation of MAPK proteins such as ERK1/2, p38 and JNK are thought to play an important regulatory role in UVB-induced inflammation during the progression of the skin carcinogenesis (Bickers and Athar, 2006). NO-sulindac treatment reduced phosphorylation-dependent activation of ERK1/2, p38 and JNK1/2 in chronic UVB-irradiated skin as confirmed by immunofluorescence staining (data not shown) and immunoblotting (Fig. 2B and Table 1). However, the reduction in ERK1/2 activities was not significant. We also investigated the effect of NO-sulindac on tyr nitrosylation of proteins in UVB-induced tumor and in A431 cells (Fig. 2C). We observed that NO-sulindac alters the pattern of tyr nitrosylation of protein both in tumor tissue and in A431 cellular proteins.
Figure 2.
NO-sulindac reduces the expression of pro-inflammatory MAP kinases, alters tyrnitrosylation and reduces the expression of epithelial-mesenchymal transition regulating proteins. (A) Immunofluroscence staining of paraffin-fixed tumor tissues showing the expression of p-lκBα (Ser32/36), iNOS & COX-2: (B) expression of p-Erk1/2, p-38 & p-JNK1/2; (C) tyrnitrosylation in murine SCCs & in A431 cells; (D) immunofluorescence staining/western Wot analysis showing the expression of E-cadherin, Fibronection, N-cadherin, Twist, SNAI & Slug.
Tumor progression is impaired by NO-sulindac
The progression of benign lesions to malignant SCCs, in which the polarized epithelial cells get motile and acquire mesenchymal characteristics, is mediated through the process known as epithelial-mesenchymal transitions (EMT). It is orchestrated via upregulation of various transcription factors such as SNAIs and twist (Kang and Massague, 2004; Lim and Thiery, 2012). Therefore in this study, we determined whether NO-sulindac reduces the progression of benign lesions to highly aggressive and invasive SCCs by reducing the UVB-induced expressions of N-cadherin, Fibronectin, Twist, Snail and Slug and upregulating E-cadherin. Immunostaining of tumors for these epithelial and mesenchymal biomarker proteins showed that mesenchymal proteins were significantly down-regulated with an increase in E-cadherin in tumors developed in NO-sulindac-treated animals (Fig. 2 D and Table 1). We also observed that NO-sulindac reduces cellular migration capacity of A431 cells as determined by scratch/wound healing assay (data not shown).
Discussion
The molecular pathogenesis of UVB-induced skin cancers is complex and multiple protein targets are overexpressed or are inactivated in these lesions. Among these COXs are considered important in augmenting the risk of UVB-induced skin cancer development as administration of both COX-2-specific and COX-1/COX-2 pan inhibitors are highly effective in attenuating this enhanced cancer risk (Lee et al., 2003). Sulindac, a COX-1/COX-2 inhibitor, is known to act by reducing prostaglandin production but also via COX-independent mechanisms (Athar et al., 2004; Piazza et al., 2009). In this study, our data show that NO-sulindac reduced skin photocarcinogenesis by reducing the number and size of UVB-induce tumors in NO-sulindac treatment group. No mouse had more than 10 tumor/mouse. In addition, unlike SCCs in UVB-alone treatment group which develop some highly invasive carcinomas, the tumors developed in NO-sulindac group were mainly small papillomas and a few highly differentiated and less vascularized SCCs. These data suggested that NO-sulindac retarded the progression of the disease significantly.
Earlier, we found that tumor-adjacent skin provides an important surrogate tissue to assess the effects of chemopreventive agents on various biomarkers predictors of the disease progression (Tang et al., 2007). NO-sulindac-induced apoptosis in tumors and tumor-adjacent skin was found to be dependent on the inhibition of RXR-PI3K/Akt axis which is similar to the earlier described sulindac-mediated apoptosis response (Zhou et al., 2010). Therefore, addition of NO moiety in sulindac does not alter the basic property of the parental molecule in terms of its ability to induce apoptosis. A significant reduction in the biomarker proteins depicting proliferation such as PCNA and cyclin D1 was noted in tumors induced in NO-sulindac treatment group as compared to UVB (alone) group suggesting that the major target of NO-sulindac remains tumor cell proliferation. A similar trend was reported in earlier studies where administration of other NSAIDs inhibited tumor cell proliferation both in the skin (Athar et al., 2004; Singh et al., 2012) and other organs (Suh et al., 2011). Consistent with the reduction in proliferation, here we also observed a blockade in the cell cycle progression in in vitro studies. NO-sulindac treatment resulted in slowing the progression of G0/G1 cells to S phase and also arrested cell cycle progression in G2/M. More importantly, these arrested cells were eventually apoptosed as evidenced by a dramatic increase in sub-G0 population in NO-sulindac-treated cells. This slowing of cell cycle progression was found to be due to a reduction in the expression of cell cycle-driving cyclins and their partner kinases. Earlier, we reported that during the pathogenesis of UVB-induced SCCs, the mutated tumor suppressor p53 is accumulated in tumors while expression of cell cycle regulatory proteins is significantly augmented in these tumor cells (Kim et al., 2002). Therefore, the observed reduction in cell cycle progression by NO-sulindac treatment and consequent inhibition of tumor cell proliferation seems to be the major mechanisms by which NO-sulindac impede tumor growth. Earlier, Notch signaling was associated with the pathogenesis of cutaneous SCCs. Notch signaling in the skin is considered crucial for maintaining a balance between proliferation, differentiation and apoptosis (Dotto, 2009). UVB exposure of the skin leads to induction of this pathway in keratinocytes (Mandinova et al., 2008). We found a significant decrease in the expression of genes regulating this pathway. We therefore propose here that NO-sulindac targets tumor cell proliferation and enhances susceptibility of these slowly proliferating tumor cells to apoptosis by blocking Notch signaling pathway in addition to targeting cell survival signaling. Although the direct demonstration of the exact mechanism by which NO-sulindac targets Notch signaling is beyond the scope of this manuscript but PGE2-dependent regulation of this pathway is known (Lee et al., 2009). Earlier, we showed that COX-2-dependent PGE2 acts through its receptors during progression of NMSCs (Lee et al., 2005). We also showed that MAPK signaling is important in the regulation of inflammation and tumorigenesis by UVB (Kim et al., 2005). Therefore, the observations in this study that NO-sulindac inhibits these regulators of inflammation and -dependent tumorigenesis suggests that NO-sulindac-dependent decrease in inflammation contributes significantly in reducing the UVB-induced photocarcinogenesis. Using pharmacological inhibitor of NFκB, we recently showed that pyrrolidinedithiocarbomate effectively reduces UVB-induced carcinogenesis, further signifying the importance of inflammation regulatory transcription factor in UVB-induced photocarcinogenesis (Kim et al., 2012). Inflammation is also known to augment EMT (Du et al., 2010). Thus, the observed decrease in EMT following NO-sulindac treatment in this study may be related to the inhibition in UVB-induced inflammation-regulatory signaling. In summary, these data suggest that NO-sulindac is a potential chemopreventive drug for UVB-induced photocarcinogenesis. It acts at multiple molecular targets particularly on those regulating proliferation.
Highlights.
NO-sulindac is a potent chemopreventive agent for UVB-induced skin cancer.
NO-sulindac effectively blocks proliferation.
NO-sulindac targets Notch and RXR-PI3k/Akt pathway to achieve anti-tumor efficacy.
Acknowledgments
This work has been supported by NIH/NCI N01-CN-43300 274 and R01 CA138998 grants to M.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An KP, Athar M, Tang X, Katiyar SK, Russo J, Beech J, Aszterbaum M, Kopelovich L, Epstein EH, Jr, Mukhtar H, Bickers DR. Cyclooxygenase-2 expression in murine and human nonmelanoma skin cancers: implications for therapeutic approaches. Photochem Photobiol. 2002;76:73–80. doi: 10.1562/0031-8655(2002)076<0073:ceimah>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Athar M, An KP, Tang X, Morel KD, Kim AL, Kopelovich L, Bickers DR. Photoprotective effects of sulindac against ultraviolet B-induced phototoxicity in the skin of SKH-1 hairless mice. Toxicol Appl Pharmacol. 2004;195:370–378. doi: 10.1016/j.taap.2003.09.030. [DOI] [PubMed] [Google Scholar]
- Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- Chun KS, Akunda JK, Langenbach R. Cyclooxygenase-2 inhibits UVB-induced apoptosis in mouse skin by activating the prostaglandin E2 receptors, EP2 and EP4. Cancer Res. 2007;67:2015–2021. doi: 10.1158/0008-5472.CAN-06-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaghan PG. A turbulent decade for NSAIDs: update on current concepts of classification, epidemiology, comparative efficacy, and toxicity. Rheumatol Int. 2012;32:1491–1502. doi: 10.1007/s00296-011-2263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- Dotto GP. Crosstalk of Notch with p53 and p63 in cancer growth control. Nat Rev Cancer. 2009;9:587–595. doi: 10.1038/nrc2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Nakamura Y, Tan TL, Lee P, Lee R, Yu B, Jamora C. Expression of snail in epidermal keratinocytes promotes cutaneous inflammation and hyperplasia conducive to tumor formation. Cancer Res. 2010;70:10080–10089. doi: 10.1158/0008-5472.CAN-10-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Kim AL, Athar M, Bickers DR, Gautier J. Stage-specific alterations of cyclin expression during UVB-induced murine skin tumor development. Photochem Photobiol. 2002;75:58–67. doi: 10.1562/0031-8655(2002)075<0058:ssaoce>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kim AL, Labasi JM, Zhu Y, Tang X, McClure K, Gabel CA, Athar M, Bickers DR. Role of p38 MAPK in UVB-induced inflammatory responses in the skin of SKH-1 hairless mice. J Invest Dermatol. 2005;124:1318–1325. doi: 10.1111/j.0022-202X.2005.23747.x. [DOI] [PubMed] [Google Scholar]
- Kim H, Casta A, Tang X, Luke CT, Kim AL, Bickers DR, Athar M, Christiano AM. Loss of hairless confers susceptibility to UVB-induced tumorigenesis via disruption of NF-kappaB signaling. PLoS One. 2012;7:e39691. doi: 10.1371/journal.pone.0039691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Kim A, Kopelovich L, Bickers DR, Athar M. Differential expression of E prostanoid receptors in murine and human non-melanoma skin cancer. J Invest Dermatol. 2005;125:818–825. doi: 10.1111/j.0022-202X.2005.23829.x. [DOI] [PubMed] [Google Scholar]
- Lee JL, Mukhtar H, Bickers DR, Kopelovich L, Athar M. Cyclooxygenases in the skin: pharmacological and toxicological implications. Toxicol Appl Pharmacol. 2003;192:294–306. doi: 10.1016/s0041-008x(03)00301-6. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kim MH, Han HJ. Arachidonic acid potentiates hypoxia-induced VEGF expression in mouse embryonic stem cells: involvement of Notch, Wnt, and HIF-1alpha. Am J Physiol Cell Physiol. 2009;297:C207–216. doi: 10.1152/ajpcell.00579.2008. [DOI] [PubMed] [Google Scholar]
- Li X, Gao L, Cui Q, Gary BD, Dyess DL, Taylor W, Shevde LA, Samant RS, Dean-Colomb W, Piazza GA, Xi Y. Sulindac inhibits tumor cell invasion by suppressing NF-kappaB-mediated transcription of microRNAs. Oncogene. 2012 doi: 10.1038/onc.2011.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Thiery JP. Epithelial-mesenchymal transitions: insights from development. Development. 2012;139:3471–3486. doi: 10.1242/dev.071209. [DOI] [PubMed] [Google Scholar]
- Mandinova A, Lefort K, Tommasi di Vignano A, Stonely W, Ostano P, Chiorino G, Iwaki H, Nakanishi J, Dotto GP. The FoxO3a gene is a key negative target of canonical Notch signalling in the keratinocyte UVB response. Embo J. 2008;27:1243–1254. doi: 10.1038/emboj.2008.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyskens FL, Jr, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, Kelloff G, Lawson MJ, Kidao J, McCracken J, Albers CG, Ahnen DJ, Turgeon DK, Goldschmid S, Lance P, Hagedorn CH, Gillen DL, Gerner EW. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila) 2008;1:32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza GA, Keeton AB, Tinsley HN, Gary BD, Whitt JD, Mathew B, Thaiparambil J, Coward L, Gorman G, Li Y, Sani B, Hobrath JV, Maxuitenko YY, Reynolds RC. A novel sulindac derivative that does not inhibit cyclooxygenases but potently inhibits colon tumor cell growth and induces apoptosis with antitumor activity. Cancer Prev Res (Phila) 2009;2:572–580. doi: 10.1158/1940-6207.CAPR-09-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricotti C, Bouzari N, Agadi A, Cockerell CJ. Malignant skin neoplasms. Med Clin North Am. 2009;93:1241–1264. doi: 10.1016/j.mcna.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Singh T, Chaudhary SC, Kapur P, Weng Z, Elmets CA, Kopelovich L, Athar M. Nitric oxide donor exisulind is an effective inhibitor of murine photocarcinogenesis(dagger) Photochem Photobiol. 2012;88:1141–1148. doi: 10.1111/j.1751-1097.2012.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart GD, Nanda J, Brown DJ, Riddick AC, Ross JA, Habib FK. NO-sulindac inhibits the hypoxia response of PC-3 prostate cancer cells via the Akt signalling pathway. Int J Cancer. 2009;124:223–232. doi: 10.1002/ijc.23934. [DOI] [PubMed] [Google Scholar]
- Suh N, Reddy BS, DeCastro A, Paul S, Lee HJ, Smolarek AK, So JY, Simi B, Wang CX, Janakiram NB, Steele V, Rao CV. Combination of atorvastatin with sulindac or naproxen profoundly inhibits colonic adenocarcinomas by suppressing the p65/beta-catenin/cyclin D1 signaling pathway in rats. Cancer Prev Res (Phila) 2011;4:1895–1902. doi: 10.1158/1940-6207.CAPR-11-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JY, Aszterbaum M, Athar M, Barsanti F, Cappola C, Estevez N, Hebert J, Hwang J, Khaimskiy Y, Kim A, Lu Y, So PL, Tang X, Kohn MA, McCulloch CE, Kopelovich L, Bickers DR, Epstein EH., Jr Basal cell carcinoma chemoprevention with nonsteroidal anti-inflammatory drugs in genetically predisposed PTCH1+/- humans and mice. Cancer Prev Res (Phila) 2010;3:25–34. doi: 10.1158/1940-6207.CAPR-09-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Kim AL, Kopelovich L, Bickers DR, Athar M. Cyclooxygenase-2 inhibitor nimesulide blocks ultraviolet B-induced photocarcinogenesis in SKH-1 hairless mice. Photochem Photobiol. 2008;84:522–527. doi: 10.1111/j.1751-1097.2008.00303.x. [DOI] [PubMed] [Google Scholar]
- Tang X, Zhu Y, Han L, Kim AL, Kopelovich L, Bickers DR, Athar M. CP-31398 restores mutant p53 tumor suppressor function and inhibits UVB-induced skin carcinogenesis in mice. The Journal of clinical investigation. 2007;117:3753–3764. doi: 10.1172/JCI32481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nature reviews. Clinical oncology. 2012;9:259–267. doi: 10.1038/nrclinonc.2011.199. [DOI] [PubMed] [Google Scholar]
- Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer. 2006;6:130–140. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- Williams JL, Kashfi K, Ouyang N, del Soldato P, Kopelovich L, Rigas B. NO-donating aspirin inhibits intestinal carcinogenesis in Min (APC(Min/+)) mice. Biochem Biophys Res Commun. 2004;313:784–788. doi: 10.1016/j.bbrc.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Zhou H, Liu W, Su Y, Wei Z, Liu J, Kolluri SK, Wu H, Cao Y, Chen J, Wu Y, Yan T, Cao X, Gao W, Molotkov A, Jiang F, Li WG, Lin B, Zhang HP, Yu J, Luo SP, Zeng JZ, Duester G, Huang PQ, Zhang XK. NSAID sulindac and its analog bind RXRalpha and inhibit RXRalpha-dependent AKT signaling. Cancer Cell. 2010;17:560–573. doi: 10.1016/j.ccr.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]