Abstract
Deep brain stimulation of the subthalamic nucleus (STN DBS) provides a unique window into human brain function since it can reversibly alter the functioning of specific brain circuits. Basal ganglia–cortical circuits are thought to be excessively noisy in patients with Parkinson’s disease (PD), based in part on the lack of specificity of proprioceptive signals in basal ganglia–thalamic–cortical circuits in monkey models of the disease. PD patients are known to have deficits in proprioception, but the effects are often subtle, with paradigms typically restricted to one or two joint movements in a plane. Moreover, the effects of STN DBS on proprioception are virtually unexplored. We tested the following hypotheses: first, that PD patients will show substantial deficits in unconstrained, multi-joint proprioception, and, second, that STN DBS will improve multi-joint proprioception. Twelve PD patients with bilaterally implanted electrodes in the subthalamic nucleus and 12 age-matched healthy subjects were asked to position the left hand at a location that was proprioceptively defined in 3D space with the right hand. In a second condition, subjects were provided visual feedback during the task so that they were not forced to rely on proprioception. Overall, with STN DBS switched off, PD patients showed significantly larger proprioceptive localization errors, and greater variability in endpoint localizations than the control subjects. Visual feedback partially normalized PD performance, and demonstrated that the errors in proprioceptive localization were not simply due to a difficulty in executing the movements or in remembering target locations. Switching STN DBS on significantly reduced localization errors from those of control subjects when patients moved without visual feedback relative to when they moved with visual feedback (when proprioception was not required). However, this reduction in localization errors without vision came at the cost of increased localization variability.
Keywords: human, proprioception, deep brain stimulation, subthalamic nucleus, Parkinson’s disease
INTRODUCTION
It has recently become clear that Parkinson’s disease (PD) involves impairments in sensory as well as in motor functions (Chaudhuri and Schapira, 2009; Juri et al., 2010). In particular, a growing body of literature demonstrates that PD patients show deficits in proprioception (Konczak et al., 2009), a sensory modality critically important for the control of movement (Sainburg et al., 1995; Sober and Sabes, 2003). Loss of proprioception leads to a marked dependence on external stimuli to guide movement (Ghez and Sainburg, 1995; Sainburg et al., 1995, 2003), a known characteristic of PD patients (Flowers, 1976; Flowers and Downing, 1978; Flash et al., 1992; Klockgether and Dichgans, 1994; Adamovich et al., 2001). Blocking PD patients from visually tracking their moving hand can be especially disabling, even more than requiring spatial memory to localize a spatial target (Flash et al., 1992; Klockgether and Dichgans, 1994; Adamovich et al., 2001). Indeed, PD patients show impaired proprioception when discriminating bilateral elbow joint angles (Zia et al., 2000) or in detecting changes in either proximal or distal limb position (Maschke et al., 2003; Putzki et al., 2006). They show a reduced vibration-induced movement (Rickards and Cody, 1997; Schrader et al., 2008), elevated thresholds in judging the curvature of their arm paths when their arm is passively moved (Konczak et al., 2008), and impaired dynamic estimation of hand position during multi-joint reaching movements (Contreras-Vidal and Gold, 2004). Axial proprioception is impaired as well as limb proprioception (Wright et al., 2010). Furthermore, PD patients show deficits in the temporal as well as spatial discrimination of proprioceptive inputs (Fiorio et al., 2007). Electroencephalographic studies reveal altered late, but not early, cortical potentials to passive limb movement in PD, reflecting impaired cortical processing of proprioception (Seiss et al., 2003). Importantly, these deficits in proprioception are thought to contribute to the motor impairments in PD (Keijsers et al., 2005; Konczak et al., 2009; Wright et al., 2010).
Several studies have examined whether dopaminergic therapy reverses proprioceptive deficits in PD, but the results are conflicting. O’Suilleabhain (2001) found that dopaminergic therapy acutely worsened limb proprioception. Mongeon et al. (2009) likewise found that it worsened limb proprioception, but only in some patients. In contrast, Maschke et al. (2003) found that dopaminergic therapy had no effect on limb proprioception, while Li et al. (2010) found that it improved limb proprioception.
Even less is known about the effects of deep brain stimulation of the subthalamic nucleus (STN DBS) on proprioception. In this surgical therapy that is gaining wide acceptance, stimulating electrodes are implanted bilaterally in the STN. As with dopaminergic therapy, STN DBS markedly improves PD motor function (Deep Brain Stimulation for Parkinson’s Disease Study Group, 2001). Yet, only one study that we are aware of has directly examined the effects of STN DBS on proprioception. Maschke et al. (2005) found that STN DBS produced a small but significant improvement in proprioceptive acuity in a task in which patients’ had to detect passive forearm displacements.
Although PD patients have consistently shown proprioceptive deficits in a variety of experimental studies, the deficits have been subtle. Therefore, in order to examine the effects of STN DBS on proprioception, we wanted to use a task that challenged proprioceptive processing. The one previous study examining the effects of STN DBS on proprioception used a single joint elbow displacement task (Maschke et al., 2005). Control subjects were extremely accurate in that task, having a detection threshold of 0.9 degree arm displacement. PD patients off DBS had an elevated threshold, but its magnitude was only 2.5 degrees. This created something of a ceiling effect against which to show any possible effect of DBS. In the present study, we selected a psychophysical paradigm previously used to test proprioception and known to elicit large errors in healthy subjects (Tillery et al., 1994). Thus, we hoped to avoid baseline ceiling (or floor) effects in the evaluation of STN DBS modulation of proprioception.
Since the STN receives direct afferents from primary and secondary somatosensory cortices (Canteras et al., 1988; Juri et al., 2010) as well as from sensory processing areas of the thalamus (Lanciego et al., 2004) STN DBS could stimulate these somatosensory-related areas antidromically and interfere with abnormal firing patterns and pathological oscillations (Gradinaru et al., 2009), as well as orthodromically override faulty oscillatory and firing pattern activity in target structures (Xu et al., 2008). Moreover, STN DBS is thought to reduce pathological noise in basal ganglia–cortical circuits (Montgomery and Gale, 2008) and thus may improve behavioral variability (Guehl et al., 2006).
The hypotheses of the study were twofold. First, we hypothesized that PD patients would show substantial proprioceptive deficits in a challenging proprioceptive processing task. We tested this hypothesis by comparing the accuracy and precision (variability) of multi-joint localization of the limb in 3D space in PD patients off stimulation with those of control subjects. If PD patients show proprioception-specific deficits, they should show a relatively greater benefit from receiving visual feedback of the limbs than would control subjects. Second, since STN DBS is thought to reduce pathological noise in basal ganglia-cortical circuits, and since there is initial evidence that STN DBS improves single joint proprioception (Maschke et al., 2005), we hypothesized that STN DBS would produce improvements in the accuracy and precision of limb localization. If so, then switching on STN DBS should produce relatively greater improvements in limb localization when visual feedback of the limb is not provided, than when visual guidance of movement is possible.
A preliminary analysis of this experiment was presented in abstract form (Lee et al., 2009).
EXPERIMENTAL PROCEDURES
Subjects
Twelve patients with PD (mean age ± SD = 68 ± 7.5, 11 males) who had bilaterally implanted stimulating electrodes in the STN, and 12 controls (70 ± 6.3, five males) participated and ages of two groups did not significantly differ (t(22) = 0.72, p = 0.48). All PD patients had moderate PD (stages II to III of the Hoehn and Yahr scale (Hoehn and Yahr, 1967)), were clinically typical, and their motor disabilities were responsive to anti-parkinsonian medications. STN DBS produced a significant improvement in motor symptoms (mean Unified Parkinson’s Disease Rating Scale, UPDRS (Goetz and Stebbins, 1995), score of 35.7 for ON and 48.2 for OFF stimulation (t(11) = 9.68, p < 0.001)). Patients were screened for major cognitive impairment (Mini Mental State Examination (Folstein et al., 1975)) and depression (Beck Depression Inventory, Psychological Corporation, Boston, MA, USA). Table 1 presents the clinical characteristics of the patients. All patients but one were tested on their regular anti-parkinsonian medications (this subject was not on any anti-parkinsonian medication following surgery). Doses of such medications were markedly reduced from the levels used prior to STN DBS surgery. All subjects were free from significant upper limb or trunk arthritis or pain, and were without any significant neurological or psychiatric disease, except for Parkinson’s disease in the PD patients. Subjects were tested for hand dominance based on the Edinburgh Handedness Inventory (Oldfield, 1971). All PD and control subjects were right-handed except for one female control who was left-handed. After detailed explanation of the procedures, all subjects signed a consent form approved by the institutional review board of the University of California at San Diego.
Table 1.
Clinical characteristics of PD patients
| N | Age | Handedness | Time post surgery (years) |
Disease durationa (years) |
UPDRSb (ON-DBS) |
H & Yc stage (ON-DBS) |
UPDRS (OFF-DBS) |
H & Y stage (OFF-DBS) |
Medications |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 77 | R | 1.5 | 6 | 39 | 3 | 45 | 3.0 | Lev, Sel, Rop, Am |
| 2 | 72 | R | 2.7 | 11 | 40 | 3 | 49 | 3 | St, Rop, Par |
| 3 | 79 | R | 4 | 8 | 28 | 2 | 44 | 2 | Lev; St |
| 4d | 76 | R | 0.8 | 13 | 37 | 3 | 52 | 3 | None |
| 5 | 68 | R | 3 | 16 | 31 | 2 | 42 | 2 | Lev; Pr; Ent |
| 6 | 60 | R | 4 | 15 | 44 | 2 | 63 | 3 | Lev; LevR; Rop;Ent |
| 7 | 68 | R | 2 | 14 | 34 | 3 | 42 | 3 | Lev; St; RopXL; Ras |
| 8 | 60 | R | 1 | 10 | 41 | 3 | 48 | 3 | Lev; Pr; Ras |
| 9 | 61 | R | 1 | 10 | 28 | 3 | 39 | 3 | St; Pr |
| 10 | 76 | R | 3.7 | 10 | 37 | 3 | 51 | 3 | Lev; Ras |
| 11 | 61 | R | 3 | 7 | 32 | 3 | 50 | 3 | Lev |
| 12 | 58 | R | 3 | 10 | 37 | 3 | 54 | 3 | St; Rop; Ras |
LevR, Carbidopa/levodopa sustained release; Lev, Carbidopa/levodopa (regular formulation); Pr, Pramipexole; Sel, Selegiline; Ent, Entacapone; Br, Bromocriptine; Rop, Ropinirole; St, Stalevo (Carbidopa/levodopa/entacapone); Ras, Rasagiline; Am, Amantadine; Rot, Rotigotine; Art, Artane (trihexyphenidyl) and Par, Parcopa.
Duration is years since the first remembered parkinsonian symptom.
UPDRS: United Parkinson’s Disease Rating Scale, Motor section (maximum score of 108). Higher scores indicate greater motor impairments.
H & Y stage: Hoehn and Yahr stage (maximum score of 5). Higher stages indicate more severe disease.
Tested off medications.
Apparatus and experimental setup
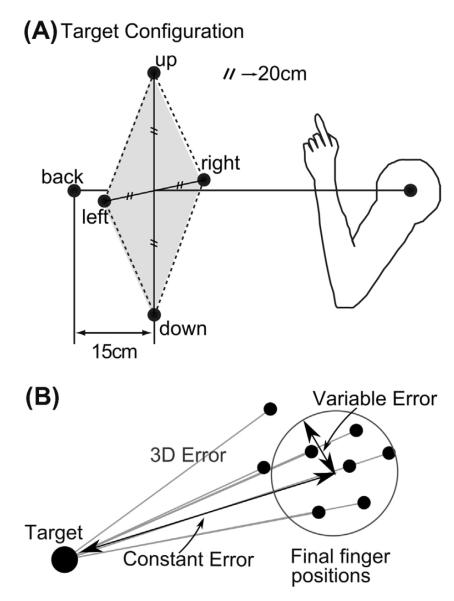
Subjects sat in front of a five degree-of-freedom robot arm (Thermo Fischer Scientific Inc., Catalyst 5, Waltham, MA, USA). The robot pseudorandomly presented one of five targets drawn from a pyramidal array centered on the subject’s midline (Fig. 1A). Four targets (up, down, left, and right) formed a diamond in a frontal plane. The length of the two diagonals was 40 cm. Target ‘back’ was located along the subject’s midline but 15 cm farther posterior than the first plane, at a distance approximately equal to the length of the subject’s arm with clenched fist with the arm extended. Thus, subjects were able to reach to the targets comfortably without fully extending their arm.
Fig. 1.
(A) Schematic diagram of the subject’s position and the five target locations shown in slightly rotated side view. Four targets (up, down, left, and right) formed a diamond (40-cm diagonal length) in a frontal plane, centered in the subject’s midline. A back target (back) was located 15 cm farther away from the first target plane along the subject’s midline. (B) The three main error measures. 3D error is the absolute distance in space of the final finger position from the target for each trial. Constant error is the distance of the mean of the endpoints to the target, and variable error is the dispersion of the endpoints around the endpoint mean location (see Methods).
The three dimensional shoulder and fingertip positions were recorded at 240 Hz using a Polhemus Liberty (Polhemus, Colchester, VT, USA) electromagnetic motion tracking system. Six degrees of freedom Polhemus sensors were placed on the nails of the subject’s right and left index fingertips, and on each shoulder (acromial process of the scapula). Custom software was used to control the robot arm and record the movement data.
Procedures
Each PD patient was tested both On and Off STN DBS. The order of stimulation testing was counterbalanced across patients. Patients were tested at least 1 h after turning the stimulator off, or on, to ensure that the majority of the stimulator effects had expired for off-stimulation testing, or returned for on-stimulation testing (Temperli et al., 2003).
There were two experimental conditions, one in which full visual feedback was provided (‘vision’) and one in which the subject was blindfolded (‘no vision’). In order to avoid any effect of experience with visual information of the target on proprioceptive localization, the no vision condition always preceded the vision condition. Subjects were asked to extend each index finger from a closed fist and place the ulnar side of each hand on their lap, 10 cm from the respective knee joint. The initial hand positions were marked with a small square of Velcro on each leg. Subjects returned their hands to the marked initial positions after each trial.
The task followed the general procedures described in Tillery et al. (1994) for testing proprioception during unconstrained two-hand apposition. The experimenter passively moved the subject’s dominant hand to the target, and then returned to its initial position. After a brief pause, the subject then reached with his/her non-dominant hand to “touch” the remembered 3D target.
No vision condition
The subject’s eyes were covered using a black blindfold. The robot arm moved to one of the five target locations in a pseudorandom order that was fixed across subjects and vision conditions. The experimenter then passively moved the subject’s dominant hand to touch the tip of the robot arm (the target) with the outstretched index finger. Throughout the session, the experimenter reminded the subject to remain passive during this time. After the subject’s index fingertip touched the tip of the robot, the experimenter released the subject’s arm, subjects held the hand in contact with the robot for approximately 1 s, and then the experimenter moved the subject’s passive arm back to the initial position. During the return of the subject’s hand to the initial position, the robot arm retracted to prevent contact with the robot during the subsequent localization movement with the non-dominant arm. After a brief pause following the hand’s return to the initial position, an auditory tone was given as a go signal, at which time subjects were to “touch” the remembered target location with their non-dominant hand. Subjects were instructed to make one smooth movement out, pause, and then return without corrections. They were also instructed to reach at a comfortable speed. The experimenter supported the weight of the subject’s arm throughout the target presentation, except during the brief interval that the subject’s hand was in contact with the target. The five targets were repeated eight times, for a total of 40 trials.
Vision condition
Subjects had full vision and were allowed to look anywhere they liked during the experiment. The same five target locations were presented in the same pseudorandom sequence and repeated eight times for each target location. The procedures and instructions were exactly same as the no vision condition.
Data processing
The movement data were processed using custom MATLAB (The MathWorks, Natick, MA, USA) scripts. The time course of the hand tangential velocity was calculated by linearly interpolating across the time and position data over 20 sample points around each time. Hand movement onset and offset were initially identified as when tangential velocity fell below 10% of its peak value for the first time before (for onset) or after (for offset) peak tangential velocity. Every trial was visually inspected and movement onsets and offsets were manually corrected if necessary. Trials that subjects initiated earlier than the go signal were removed, as were trials in which there were recording anomalies, such as segments of missing data. After removal of bad trials, at least five trials out of eight performed per target location remained for each subject, although rarely was there ever more than one trial per target missing. The overall trial rejection rate was a 1.7% (50/2880 total trials; five control trials removed, 13 removed from DBS on, and 32 removed from DBS Off).
Data analysis and statistics
Pointing errors
Three primary error measures were used to analyze the data (B). Denoting the medial–lateral direction as x, the inferior–superior direction as y, and the anterior–posterior as z, the error measures were defined as follows:
3D error is the absolute distance in space between the specified target and the reach endpoint for a given trial, providing an overall measure of performance. The formula is
where x, y, and z are the coordinates of the individual hand endpoints at every trial and tx, ty, and tz are the coordinates of the given target. This measure was calculated for every subject, trial, and vision/DBS/target condition without any explicit averaging for use in the linear mixed models.
Constant error is the length of the vector from the target to the mean position of reach endpoints to the target. The formula was
where ⟨x⟩, ⟨y⟩, and ⟨z⟩ are the individual hand endpoints averaged over all reaches to the given target at tx, ty, and tz. This measure was calculated for every subject and vision/DBS/target condition. Constant error in each spatial direction also was calculated by taking each term under the square root separately. These component constant errors were defined as positive if the final finger position was to the right of the target, higher than the target, or farther beyond the target with respect to the subject.
Variable error is the root mean square of the standard deviations of the endpoints along each direction to each target, providing an overall measure of endpoint variability to a given target. The formula is
where σx, σy, and σz are the standard deviations of the endpoints in each direction for each target. This measure was calculated for every subject and vision/DBS/target condition for use in the linear mixed models. Variable error in each direction was also considered independently by taking the standard deviation in each direction separately.
These three error measurements are interrelated in the following ways (Berkinblit et al., 1995; Adamovich et al., 1998, 2001; Poizner et al., 1998): if the constant error is considerably larger than the variable error, the mean 3D error will have a similar value as the constant error. If the constant error is small, that is, if the mean endpoint is near the target, and the variable error is large, the mean 3D error will have a value closer to the variable error.
In order to directly examine the effect of vision on performance, a no vision minus vision score was computed for each error measure. The higher the positive value of this measure, the greater the increase in error when vision was blocked.
Statistical analyses
To evaluate our first hypothesis of whether PD patients showed proprioceptive deficits, analyses compared control subjects to STN DBS Off patients. To evaluate whether STN DBS modulated proprioception, patients were compared On versus Off STN DBS. Finally, to examine the effects of reduction in PD severity following STN DBS therapy on the various pointing errors, the UPDRS score was added as a factor to the linear mixed-model comparing patients On and Off STN DBS, described below.
Linear mixed-models, maximum likelihood method, were used to examine the effect of group (e.g., control versus STN DBS Off), vision (no-vision versus vision), and target (up, down, right, left, and back) on each dependent variable. Vision, target, and DBS (for PD subjects) were identified as repeated effects. The random effects were first found on the full data set by examining combinations of vision, target, and subject (Pinheiro and Bates, 2000) and comparing their profiled deviance (Baayen et al., 2008). The random effect with the lowest profiled deviance had a vectorized vision component by subject, and nesting of target (vision∣subj/target in the lmer notation; Bates et al., 2012). Using 3D error as a sample-dependent parameter the profiled deviance was greatly reduced (1672 less, χ2(4) = 1671.7, p < 2.2e–16) over the scalar subject and target random effects. This random effect allows for subject and subject by target variation on the effect of vision, and was used for all possible cases. For analyses using single-vision components (i.e., no vision minus vision comparison) or post hoc examination of just vision or no vision, there was not enough data for the random effect to include the target interaction. Therefore, just the subject effect was included (1∣subj in lmer notation).
Significance of the fixed effects was then tested by adding single terms and comparing to the null model with one less level by the chi-square test on the profiled deviance (Baayen et al., 2008). For example, to test for a dependence on vision, the comparison was to a model fit with only an intercept term and one with the intercept plus vision. For interaction terms, if there were two main effects, A and B, then the model including all terms up to the interaction (1 + A + B + A:B) was compared to that with only the single terms (1 + A + B) and so on. All statistical analyses were done using R version 2.15.2 (R Core Team, 2012); linear mixed models were fit using lme4 version 0.999902344-0 (Bates et al., 2012).
All distributions were examined for normality by inspection of the QQ plots and density plots for each distribution. A number of distributions were skewed rather than normal (e.g., 3D errors, constant errors, variable errors, and peak speed). When this occurred, the data were log-transformed to reduce such skewing. A significance level of p < 0.05 was used.
RESULTS
PD subjects showed decreased peak speed
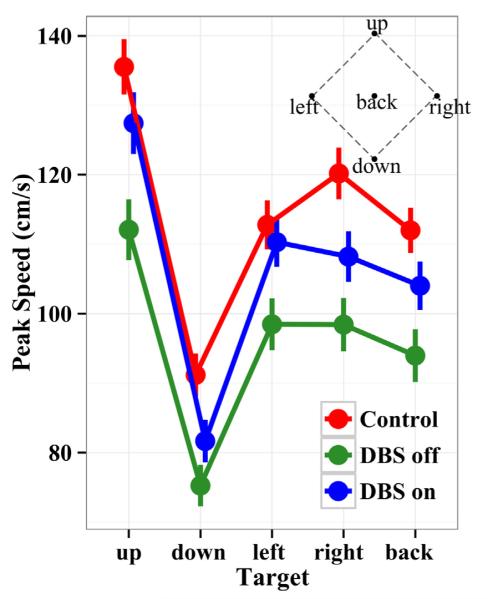
Fig. 2 presents peak speeds during reaches to each target location for each group. The speeds were pooled over the no vision and vision conditions, since the same pattern of speeds over targets held for all groups across vision conditions. STN DBS Off patients moved significantly more slowly than control subjects (χ2(1) = 13.3, p < 0.001). Averaged over targets, STN DBS Off patients’ peak speed was 95 ± 14 cm/s, which was 17% slower than controls, who moved with a mean peak speed of 115 ± 13 cm/s. When STN DBS was switched on, patients peak speed increased significantly (χ2(1) = 223, p < 0.001) to 104 ± 13 cm/s, a 9.5% increase. Over all groups there was a significant 10.7% increase in speed when eyes were opened (χ2(1) = 11.8, p = 0.001). As expected, since the targets varied in required movement amplitude, subjects’ speed was significantly different for the various targets (χ2(4) = 76.6, p < 0.001). The fastest movement was to the upper most target (the farthest), the slowest to the lowest target (the closest) and speeds were approximately the same across the left, right, and back targets (Fig. 2). Thus, STN DBS Off patients showed slower speeds than control subjects to all targets, reflecting the bradykinesia characteristic of PD. STN DBS On patients showed intermediate speeds to control subjects and STN DBS Off patients, reflecting the clinical benefit of the surgical therapy on this aspect of movement.
Fig. 2.
Peak tangential velocity of the hand during the reach averaged across vision conditions for the five targets. Error lines combine standard errors of the mean within and across subjects. The inset shows a frontal projection of the target space. Controls exhibit the fastest reaches, PD patients Off STN DBS the slowest, and patients On STN DBS show intermediate speeds. Thus, PD patients, as expected, show bradykinesia, which is significantly improved with STN DBS.
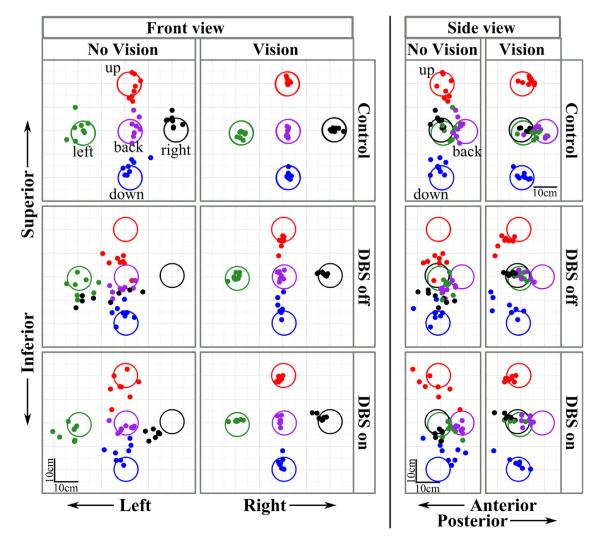
Endpoint distributions of PD patients and controls were qualitatively different
Fig. 3 presents endpoint distributions for a representative control subject (top row) and PD patient with STN switched off (middle row) and on (bottom row). The left-hand panels show a frontal view of the errors, and the right-hand panels a side view. Endpoints in the no vision and vision conditions are shown within each view. The size of the targets in the figure has been enlarged for clarity. Fig. 3 shows that the endpoints of the control subject in the no vision condition were much closer to the targets than the PD patient, On or Off STN DBS. The PD patient Off STN DBS showed a clustering of endpoints in the lower-central region of space. Without vision, the patient reached beneath the upper target and above the lower target, while making gross lateral errors to the rightmost target. The patient’s error distributions improved with STN DBS, although not to the level of the control subject. Fig. 3 further shows that with vision, the endpoint errors were reduced for both of the subjects, but markedly so for the PD patient.
Fig. 3.
Endpoint distributions of a representative control subject (top row) and a PD patient when STN DBS was switched off (middle row) and switched on (bottom row). Both front and side views are shown. Within each view, the no vision condition is on the left (first and third columns) and the vision condition is on the right (second and fourth columns). The large (10-cm diameter) circles represent the five targets with unique colors, and filled dots are individual trial endpoints with matching colors. Target size is enlarged for clarity. Scale bar = 10 cm. Note the markedly increased localization errors of the PD patient, particularly when vision was occluded and when STN DBS was switched off.
Comparison of control subjects and patients Off STN DBS
Patients Off STN DBS showed increased errors
3D and constant errors. Fig. 4 presents the mean 3D error, constant error, and variable errors across targets for each group in each vision condition. Fig. 4 shows that without vision, STN DBS Off patients had larger 3D and constant errors than control subjects for all targets except the left target. Pooled across targets, control subjects had a mean 3D error of 8.7 ± 1.3 cm and a mean constant error of 7.8 ± 1.2 cm, whereas, STN DBS Off patients had a mean 3D error of 12 ± 1.8 cm and a mean constant error of 10 ± 1.6 cm (Table 2). Both STN DBS Off patients and control subjects showed a strong reduction of the errors with vision (χ2(1) = 51.3, p < 0.001). With vision, STN DBS Off patients also had larger 3D (5.9 ± 1.0 cm) and constant errors (5.1 ± 1.0 cm) than control subjects (4.9 ± 0.77 cm 3D error and 4.2 ± 0.92 cm constant error). Pooled across targets and vision conditions, STN DBS Off patients had significantly larger 3D (χ2(1) = 4.95, p < 0.03) and constant errors (χ2(1) = 4.18, p < 0.05) than control subjects. The group × vision interaction was not significant for either 3D errors (χ2(1) = 1.2, ns) or constant errors (χ2(1) = 0.44, ns).
Fig. 4.
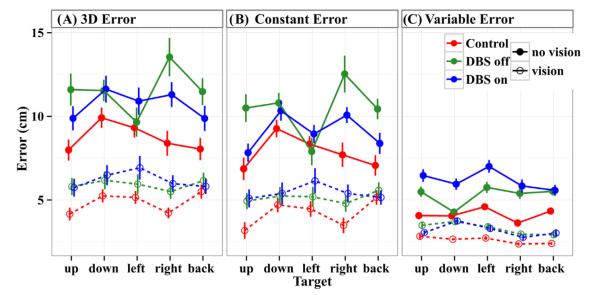
3D error, constant error, and variable errors across targets. Filled circles (upper set, solid lines) indicate no-vision, and open circles (lower set, dashed lines) indicate vision. All error lines indicate standard errors combined within and across subjects. 3D error (A) and constant error (B) show similar patterns. Without vision, control subjects had the smallest errors, STN DBS On patients had intermediate errors, and STN DBS Off patients had the largest errors. With vision, errors are markedly reduced for all groups. Controls subjects still have the smallest errors, but the error difference between control subjects and STN DBS Off patients is reduced from that of no vision. (C) Variable errors are smaller than constant errors, and show a different pattern within the PD group. Without vision, control subjects have the smallest variable errors, but unlike for 3D and constant errors, STN DBS Off patients have intermediate errors while STN DBS On patients have the largest errors. With vision, errors are reduced and similar across groups, although PD patients have slightly higher errors than control subjects.
Table 2.
Mean ± standard errors across groups
| Measure | Vision | Control | DBS Off | DBS On |
|---|---|---|---|---|
| 3D error (cm) | Blockked | 8.7±1.3 | 12±1.8 | 11±1.5 |
| Free | 4.9±0.77 | 5.9±1.0 | 6.2±1.1 | |
| Constant error (cm) | Blocked | 7.8±1.2 | 10±1.6 | 9.1±1.1 |
| Free | 4.2±0.92 | 5.1±1.0 | 5.4±1.2 | |
| Variable error (cm) | Blocked | 4.1±0.35 | 5.3±0.55 | 6.2±0.73 |
| Free | 2.6±0.22 | 3.3±0.35 | 3.2±0.34 | |
| Peak speed (cm/s) | Blocked | 110±9.3 | 90±9.5 | 98±8.5 |
| Free | 120±8.4 | 100±9.9 | 110±9.6 | |
| Constant error x (cm) medial–lateral: + is to the right of the target | Blocked | −0.43±1.4 | −1.2±2.4 | −2.4±1.9 |
| Free | −0.5±0.54 | −0.37±0.73 | −0.52±0.74 | |
| Constant error y (cm) superior–inferior: + is above the target | Blocked | 3.3±1.4 | 2.4±1.8 | 3.4±1.8 |
| Free | 0.33±0.7 | 0.56±1.1 | 0.41±0.96 | |
| Constant error z (cm) anterior–posterior: + is beyond the target | Blocked | −1.3±1.6 | −3.6±1.7 | −2±1.9 |
| Free | −0.51±1.1 | −0.27±1.3 | +0.96±1.6 | |
| Variable error x (cm) | Blocked | 2.2±0.2 | 3.3±0.47 | 3.3±0.43 |
| Free | 0.91±0.095 | 1.3±0.19 | 1.2±0.15 | |
| Variable error y (cm) | Blocked | 2.5±0.32 | 3±0.36 | 3.4±0.4 |
| Free | 1.4±0.14 | 1.9±0.26 | 1.8±0.27 | |
| Variable error z (cm) | Blocked | 2.1±0.31 | 2.5±0.3 | 3.4±0.68 |
| Free | 1.8±0.22 | 2.1±0.24 | 2.1±0.28 |
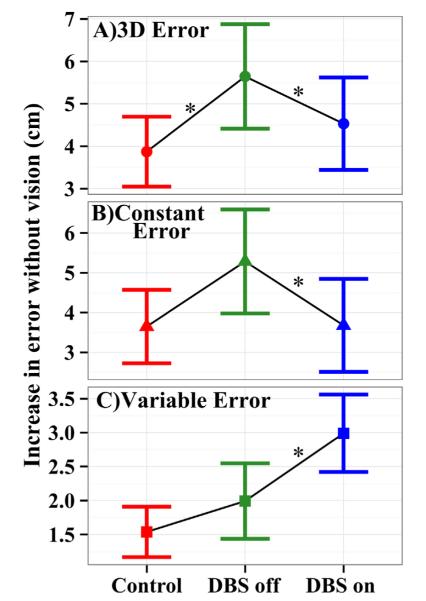
In order to directly examine the effect of vision on performance, a no vision minus vision score was computed for each error measure (see Methods). Fig. 5 presents the error change without vision for each group pooled across targets. All error changes are positive, reflecting larger errors without vision for each group. The greater the change in error, the greater the improvement in performance due to providing vision. The improvement of 3D error with vision for STN DBS Off patients was 61% (2.3 cm) greater than that for controls (χ2(1) = 3.96, p < 0.05). Thus, vision differentially improved the 3D errors of STN DBS Off patients compared to control subjects. The improvement in constant errors with vision for STN DBS patients was 36% (1.3 cm) greater than that of controls; however, this difference did not reach significance.
Fig. 5.
Difference in error across the vision conditions (no vision minus vision). Increasing values indicate increasingly larger errors in the no vision than vision condition, implying increased difficulty in localizing the limb using proprioception. Whisker bars indicate 95% confidence intervals across subjects. Asterisks indicate a significant difference between groups (p < 0.05). (A) 3D errors show disproportionately increased localization errors for STN DBS Off patients than control subjects when vision is occluded; this deficit is partially reversed when STN DBS is turned on. (B) Constant errors show the same pattern although the group difference is significant only for STN DBS On versus Off. (C) Variable errors show a small increase for STN DBS Off patients over that of control subjects, which is exacerbated by switching DBS on.
Variable errors. Fig. 4C shows that, as with 3D and constant errors, STN DBS Off patients had larger variable errors than control subjects across targets and vision conditions. Pooled across targets, STN DBS Off patients had a mean variable error of 5.3 ± 0.55 cm without vision, and 3.3 ± 0.35 cm with vision, whereas, those for control subjects were 4.1 ± 0.35 cm and 2.6 ± 0.22 cm, respectively (Table 2). Pooled across conditions, STN DBS Off patients had significantly larger variable errors than controls (χ2(1) = 6.76, p < 0.01). However, the Group × Vision interaction was not significant (χ2(1) = 0.01, ns). Fig. 5C shows that there was a modestly larger increase in variable error without vision for STN DBS Off patients than controls, although this difference was not significant. However, the variable error in the medial–lateral direction did show significantly more improvement (0.7 ± 0.3 cm) for STN DBS Off patients than controls (χ2(1) = 5.60, p < 0.02).
No vision–vision differences. We further isolated these dependencies by looking at the vision and no vision conditions separately. For 3D error, STN DBS Off patients had a mean 3D error that was significantly larger (by 38%) than that of controls without vision (χ2(1) = 6.04, p < 0.02), but was not significantly larger with vision (χ2(1) = 3.68, ns). Similarly, the mean constant error was significantly greater for STN DBS Off patients than for controls without vision (by 28%) (χ2(1) = 4.15, p < 0.05), but not significantly different with vision. On the other hand, the variable error was larger in PD subjects than of controls for both vision conditions (χ2(1) > 4.44, p < 0.05). Thus, although variability in the medial–lateral direction increased disproportionately in STN DBS Off patients compared to control subjects when vision was blocked, the overall variable error was larger in STN DBS Off patients than control subjects both with vision and without vision. Moreover, the 3D and constant errors of STN DBS Off patients were similar to those of controls with vision, but significantly larger without.
Comparison of patients On versus Off STN DBS
STN DBS selectively altered errors across conditions
3D and constant errors. Fig. 4 shows that there was a robust effect of vision for patients On as well as Off STN DBS. When patients were Off STN DBS (green lines1 in Fig. 4), their 3D errors dropped from an average of 12 ± 1.8 cm without vision to 5.9 ± 1.0 cm with vision, and when they were On STN DBS, 3D errors dropped from 11 ± 1.5 cm without vision to 6.2 ± 1.1 cm with vision (Table 2). Similarly, constant errors dropped from 10 ± 1.6 to 5.1 ± 1.0 cm when vision was provided for patients Off STN DBS, and from 9.1 ± 1.1 to 5.4 ± 1.2 cm when On STN DBS. For 3D error, there was a significant Group × Vision (χ2(1) = 7.94, p < 0.006 and Group × Target interaction (χ2(4) = 25.41, p < 0.001). Fig. 4A and Table 2 show that providing vision reduced the 3D error more when patients were Off STN DBS than when they were On (also see below), and that STN DBS acted selectively to decrease the 3D error for the upper, right, and back targets. Constant error showed the same overall pattern (Fig. 4B), but there were no significant interactions with Group or a main effect of Group. However, looking at the individual components of the constant error, there were significant interactions of DBS and Vision (χ2(1) = 4.87, p < 0.03) and DBS and Target in the superior–inferior direction (χ2(4) = 12.2, p < 0.02). For the DBS × Vision interaction, patients Off STN DBS pointed farther beneath the targets than they did when On STN DBS when the vision was blocked compared to when it was available. The DBS × Target interaction was dominated by patients Off STN DBS reaching 2.3 ± 2.7 cm farther below the upper target and 1.0 ± 2.0 cm farther above the lower target than when they were On STN DBS.
Variable errors. Surprisingly, switching DBS on increased variable errors without vision (but not with vision) (Fig. 4C). Without vision, pooled across targets the variable error was 5.3 ± 0.55 cm when DBS was Off and 6.2 ± 0.73 cm when DBS was On, respectively. However, with vision, the mean variable error dropped to 3.3 ± 0.35 cm and 3.2 ± 0.34 cm for STN DBS Off and On, respectively. This interaction of DBS (on versus off) and Vision (blocked versus free) for the variable error was significant (χ2(1) = 7.2, p < 0.002). There also was a significant Group × Vision interaction for the component of the variable error in the superior–inferior direction (χ2(1) = 6.19, p < 0.02). Patients On STN DBS had larger variable superior–inferior errors without vision (χ2(1) = 4.28, p < 0.04), but not significant with vision, than when they were Off STN DBS. Finally, there was a significant Group effect for the anterior–posterior component of the variable error (χ2(1) = 4.06, p < 0.05). STN DBS On patients had slightly larger variable errors in the anterior–posterior direction than STN DBS Off (Table 2, χ2(1) = 4.06, p < 0.05), but this effect did not significantly depend on vision. When directly examining the difference in variable error across the vision conditions (Fig. 5C), unlike the case for 3D and constant error, blocking vision significantly increased variable errors more (by 0.9 ± 0.9 cm) when patients were On STN DBS than when they were Off (χ2(1) = 7.10, p < 0.01).
Taking the vision conditions separately, there was no significant difference in variable error with vision when STN DBS was On versus Off. Without vision, variability increased by 15% (from 5.3 ± 0.55 to 6.2 ± 0.73 cm) when STN DBS was turned on (χ2(1) = 7.42, p < 0.01).
UPDRS scores predicted aspects of the spatial errors
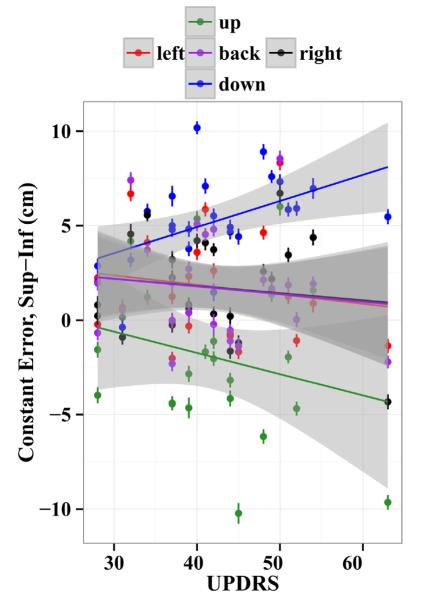
To examine the dependence of the measured errors on the UPDRS score, we included the UPDRS scores in linear mixed models as a factor that measured the slope of the dependence of the error on the UPDRS. There were no UPDRS main effects or interactions for the 3D error, constant error, or variable error (p > 0.054). This result indicates that changes in PD severity did not significantly predict changes in 3D error, constant error, or variable error when pooled over all conditions, or differentially do so within DBS and vision sub-conditions. However, PD severity did predict changes in specific components of the constant error. Within the separate spatial directions, there was a significant effect of UPDRS score on constant error in the anterior–posterior direction (χ2(1) = 18.01, p < 0.001). This effect indicated that with increasing PD severity, there was a modest anterior shift of endpoints (3 ± 2 cm between the least and most severe patients). There also were significant interactions between UPDRS scores and whether patients were On or Off STN DBS for the superior–inferior component of the constant error (χ2(1) = 11.9, p = 0.001), and the medial–lateral constant error (χ2(1) = 10.74, p = 0.001). STN DBS reduced the effect of PD severity on constant errors (medial–lateral: 0.21 ± 0.06 cm per UPDRS point less, superior–inferior: 0.16 ± 0.05 cm/point less). Finally, there was a significant UPDRS by target interaction (χ2(4) = 20.4, p < 0.001). This interaction indicated that with increasing UPDRS scores (i.e., with increasing PD severity), patients tended to point farther below the upper target (by 2 ± 3 cm from the least to most severe patients), and farther above the lower target (by 6 ± 3 cm from the least to most severe patients) (Fig. 6). This effect of PD severity on the superior–inferior constant error component is interesting, since localizing the arm in 3D space requires sensing and taking into account the effects of gravity on joint positions. However, since there were no interactions of UPDRS score with visual feedback condition and DBS, the degree of clinical improvement of patients with STN DBS switched on did not differentially improve limb localization when vision was occluded.
Fig. 6.
Constant error in the superior–inferior direction to each of the five targets separately for all subjects with STN DBS On and Off. Positive values are upward. Error lines represent standard errors over trials, the line is a least square fit within each target, and the shaded region represents 95% confidence of the fit. Constant error in the superior–inferior direction for the three targets at central height (left, back and right) show very little variability across UPDRS. However, the upper target shows an increasing downward deflection and the lower an increasing upward deflection with increasing UPDRS scores.
DISCUSSION
In this study we examined 3D reaching movements to proprioceptively defined targets in PD patients, and evaluated the effects of STN DBS on patients’ ability to localize these targets. We used a task that previously has been shown to elicit proprioceptive coding of spatial targets (Tillery et al., 1994). In this task, subjects must place the index finger of their non-dominant hand in the remembered 3D location of the dominant hand. When visual feedback is not provided, subjects are required to use exclusively proprioceptive cues in positioning their finger in space. When visual feedback is provided, subjects may code the target in visual coordinates, proprioceptive coordinates, or a combination of the two. If PD patients show proprioception-specific deficits, they should show a relatively greater benefit from receiving visual feedback of the limbs than would control subjects. Our first hypothesis was that with STN DBS switched off, PD patients would show larger localization errors than control subjects across conditions, and that providing vision would differentially improve limb localization in the PD patients relative to control subjects. This hypothesis was for the most part supported. We found that with STN DBS switched off, PD patients showed significantly larger 3D, constant and variable errors than control subjects across conditions (Fig. 4). Although providing visual feedback improved the performance of all subjects, it improved 3D errors and one component of the variable error (in the medial–lateral direction) for PD patients significantly more than for control subjects (Fig. 5A). Providing visual feedback also improved constant and variable errors in PD patients Off STN DBS more than for control subjects, but the magnitude of these improvements did not reach significance. 3D error, however, provides an overall measure of performance, incorporating both constant and variable errors (each of which trended toward differential improvement for PD patients Off STN DBS relative to controls when vision was provided). Since, providing vision significantly improved overall limb localization (3D error) in STN DBS patients more than control subjects, our first hypothesis is supported. These data add to the growing body of literature showing that PD patients have proprioceptive deficits, and do so across a wide range of tasks.
Our second hypothesis was that switching STN DBS on would improve the ability of patients to localize their limbs in space. This hypothesis was only partially supported. There was no main effect of switching STN DBS on pooled across vision conditions for 3D or constant errors. PD patients Off STN DBS had slightly larger 3D and constant errors than when On STN DBS without vision, and slightly lower errors with vision (Fig. 4). However, there was a significant interaction of STN DBS × Vision: switching STN DBS on improved 3D and constant errors more for patients reaching without vision than with vision (Fig. 5B). However it did so at the cost of increased variable errors (Fig. 5C).
PD patients show deficits in localizing the position of their limbs in 3D space based on proprioceptive cues
With STN DBS switched off, PD patients showed significantly larger localization errors across conditions than did control subjects, and providing visual feedback improved both 3D errors and medial–lateral variable errors more for PD patients than control subjects. We have previously found that PD patients off medication show increased variability specifically in the medial–lateral direction when reaching to 3D targets without vision, and that the origin of this variability was poor coordination of arm angles used to position the limb in the medial–lateral direction (Poizner et al., 1998). Interestingly, when vision was provided in the present study, the medial–lateral component of the variable error was specifically found to improve more in PD patients Off STN DBS than in control subjects. Both Poizner et al. (1998) and the present study used the same five spatial targets and the same initial posture of the arm (upper arm vertical, forearm horizontal). The results suggest that, at least under these experimental conditions, the lack of fidelity of the proprioceptive signal in PD leads to increased uncertainty in positioning the arm in the medial–lateral direction, and that this increased uncertainty may be due to the joint coordination requirements of precisely positioning the arm in this direction.
Our results that PD patients Off STN DBS show proprioceptive deficits are consistent with numerous studies demonstrating deficits in proprioception in PD patients (see Konczak et al., 2009 for a review). In examining proprioception in PD patients, Demirci et al. (1997) found that without vision of the hand, PD patients needed greater displacement of a finger to perceive the same displacement as healthy individuals when the displacement was referenced to a visual target. Consistent with the common observation that PD patients do not perceive themselves as speaking softly, moving slowly, or making small movements (Fox et al., 2012), Demirci et al. (1997) proposed that motor commands, corollary discharges, and proprioceptive feedback are all reduced in PD. Indeed, behavioral training to recalibrate PD patients’ proprioceptive perception of movement amplitude by providing repeated comparisons of seen versus felt movement amplitudes leads to improved motor performance (Fox et al., 2012). Our results fit nicely within such a framework.
PD limb-localization deficits are not due to motor or memory impairments
When subjects had full visual as well as proprioceptive feedback during target presentation and response, they no longer were required to code targets in proprioceptive coordinates but instead could use external visual coding, or a combination of visual and proprioceptive coding. Thus, subjects could in large part bypass any proprioceptive processing deficits that they might have. Since exactly the same set of targets and response requirements were used in both conditions, the motor requirements of the task remained the same. Thus, the impaired performance of the PD patients in the no-vision condition cannot be due to subjects being impaired in moving to the various target locations, as errors were substantially reduced for all subjects with visual feedback. Indeed, the 3D errors of the PD patients Off STN DBS with visual feedback were on average 32% lower than those of the control subjects without vision. Likewise, the same memory interval was used between target presentation, retraction of the robot arm, and pointing response in the vision and no-vision conditions. Again, since PD patients’ performance with vision was superior to that of controls in the no-vision condition, the impaired performance of PD patients without vision cannot be due to a memory impairment. Previous findings also demonstrate that PD patients have no difficulty remembering the spatial locations of 3D targets in peripersonal space over these brief intervals (Poizner et al., 1998; Adamovich et al., 2001). Rather, our findings indicate that there is impaired proprioception in PD.
STN DBS improves proprioceptive accuracy in limb localization, but reduces its precision
We found that the effects of STN DBS on proprioception were mixed. Switching STN DBS on improved 3D and constant errors significantly more for patients reaching without vision than with vision, although we did not find a major benefit of STN DBS on proprioceptive accuracy. For 3D and constant errors, the DBS × Vision interaction depended upon target (Fig. 4). There was substantial variation in accuracy across spatial targets for both groups without vision. For PD patients Off STN DBS, the upper, right, and back targets showed the largest increase in error over that of control subjects. These three targets elicit greater intersegmental interactions than do the lower and left targets. Since proprioception is critical for compensating for intersegmental interactions (Sainburg et al., 1993, 1995; Messier et al., 2003), increased errors to these targets are consistent with PD patients having proprioceptive deficits. Interestingly, PD patients and control subjects showed a much more uniform pattern of accuracy across targets with vision. This differential pattern of errors across spatial targets in the no vision and vision conditions is consistent with very different processes underlying subjects’ localization of the limb in the two conditions, proprioceptive in the former and visual in the latter.
Surprisingly, we found that STN DBS increased variable errors specifically when patients reached without vision. We had hypothesized the opposite pattern would occur, since STN DBS is thought to decrease pathological noise in affected basal ganglia–thalamo–cortical circuits (Montgomery and Gale, 2008). Why STN DBS would increase a patient’s uncertainty in limb localization is unclear. STN DBS has been reported to act as a “two-edged sword,” in that its effects on motor performance interact with a patient’s residual basal ganglia function, improving performance in patients with low baseline function and impairing performance in patients with high baseline function (Chen et al., 2006; Joundi et al., 2012). However, in the present study, in general we found that changes in patients’ motor severity with STN DBS did not interact with visual feedback in predicting accuracy or precision in 3D limb localization. Whether baseline basal ganglia function or other factors contributed to the increased variability in limb localization with STN DBS remains to be determined.
Effects of DA therapy versus STN DBS on proprioception
As mentioned in the Introduction, the effects of dopaminergic therapy on proprioception have been conflicting. Some studies find that it worsens proprioception (O’Suilleabhain, 2001; Wright et al., 2010), others that it has no effect (Maschke et al., 2003), and yet others that it improves proprioception (Li et al., 2010). Since we tested 11 of the 12 patients while they were on their dopaminergic therapy, we cannot completely rule out the possibility that the medications contributed to the effects we found. However, patients receive markedly reduced doses of anti-parkinsonian medications following STN DBS surgery, with reductions typically being in the order of 50% (Benabid et al., 2009). Thus, it is unlikely that the residual dopaminergic medications that the patients were on following surgery were a determining factor in the findings.
Although, in general, there is a major overlap in the therapeutic benefits achieved by STN DBS and dopaminergic therapy, the two therapies act through different mechanisms (Montgomery and Gale, 2008), do not have congruent responses (Zaidel et al., 2010), and differentially modulate Parkinson’s disease related brain networks (Mure et al., 2012; Ko et al., 2013). Dopaminergic therapy is largely trophic and stimulates not only striatal activity, but also widespread regions throughout the central nervous system where there are dopamine receptors. DBS, in contrast, acts more locally, focuses on modulating the output of the basal ganglia through its effect on the STN or on other brain regions activated orthodromically, or especially antidromically from the STN (McIntyre et al., 2004; Li et al., 2007; Lalo et al., 2008; Gradinaru et al., 2009). Our data indicate that there is a beneficial effect of STN DBS on the accuracy of multi-joint proprioception. Since the STN receives direct afferents from primary and secondary somatosensory cortices (Canteras et al., 1988; Juri et al., 2010) as well as from sensory processing areas of the thalamus (Lanciego et al., 2004), STN DBS could stimulate these somatosensory-related areas antidromically and interfere with their abnormal firing patterns, as well as those of cortical microcircuits of which they are a part (Li et al., 2007). Thus, STN DBS could override pathological synchronization within the cortico-basal ganglia sensorimotor loops improving limb localization accuracy.
Models of the pathophysiology of PD and proprioception
The proprioceptive deficit in PD appears to be central rather than peripheral in origin, since muscle spindle function and early cortical processing of proprioceptive information is essentially unaffected (Dufresne et al., 1981; Seiss et al., 2003), but later cortical processing is impaired (Seiss et al., 2003). Indeed, a substantial proportion of cells in the STN and globus pallidus internal (GPi) of the monkey selectively respond to passive and active movements, and are tightly tuned to movement in one direction (flexion or extension) around a single joint, (DeLong et al., 1985; Filion et al., 1988). Recordings in PD patients also reveal cells in the human STN and GPi respond to passive and active movement (Rodriguez-Oroz et al., 2001; Abosch et al., 2002; Theodosopoulos et al., 2003). When monkeys are made parkinsonian through MPTP treatment, the number of GPi cells responding to passive or active movement increases, with most neurons now responding to the movement of several joints rather than just a single joint (Filion et al., 1988). This loss in neuronal response specificity to passive limb movement is also observed in the basal ganglia receiving area of the thalamus (Pessiglione et al., 2005) and in the supplementary motor area (Escola et al., 2002), a major cortical target of basal ganglia projections. Thus, in the parkinsonian state, much noisier and less differentiated proprioceptive information is propagated throughout the cortico-basal ganglia sensorimotor loop. This in turn may lead to decreased accuracy in sensing limb position in the absence of vision in PD patients as observed in the present experiment.
Classical models of the pathophysiology of PD have emphasized the anatomical segregation of multiple looping structures linking frontal cortex and the basal ganglia, distinct pathways within the basal ganglia, and excessive firing rates of basal ganglia output nuclei that lead to excessive tonic inhibition of thalamus and cortex (Albin et al., 1989; Bergman et al., 1990). However, it recently has become clear that temporal patterning within these looped structures is of critical importance, and that the deficiency in dopamine in PD results in excessive oscillations and pathological synchronization especially at beta band frequencies (13–30 Hz) (Brown, 2003; DeLong and Wichmann, 2007; Weinberger and Dostrovsky, 2011). Such pathological beta oscillations have been recorded from the subthalamic nucleus, globus pallidus and motor cortical areas in both primate models of PD and from PD patients (Brown and Williams, 2005; Brown, 2007). Although the mechanisms of action of DBS are not fully understood, high-frequency stimulation may override or “jam” pathological oscillations (Rivlin-Etzion et al., 2008; Eusebio et al., 2012), thereby reducing faulty information transmission and excessive noise in the circuit. This in turn could allow for higher fidelity proprioceptive information transmission throughout the loop.
CONCLUSIONS
Although the motor deficits of PD patients are the most clinically apparent, patients show sensory deficits as well. Proprioception is the key sensory requirement for multi-joint coordination (Sainburg et al., 1993, 1995; Messier et al., 2003), and is important not only for feedback correction but also for motor planning (Ghez and Sainburg, 1995). Thus, understanding deficits in proprioceptive processing in PD may help elucidate patients’ difficulties in motor behavior. Furthermore, STN DBS surgery for PD is being increasingly utilized, but its effects on proprioception are virtually unexplored. Using a challenging proprioceptive task, we found that PD patients OFF STN DBS showed substantial deficits in multi-joint proprioception. Such deficits may in part underlie PD patients’ reliance on external visual cues to guide their movements. We further found that STN DBS had mixed effects on proprioception. Switching STN DBS on improved proprioceptive accuracy, although it increased patients’ uncertainty about their limb positioning. The improvement in the accuracy of 3D limb localization based on proprioceptive cues may be the result of high-frequency stimulation overriding pathological oscillations in affected circuits, thereby reducing faulty information transmission.
Acknowledgements
This study was supported in part by NIH grant #2 R01 NS036449 (HP), NSF grants # ENG-1137279 (EFRI M3C) and SBE-0542013 to the Temporal Dynamics of Learning Center, an NSF Science of Learning Center, ONR MURI Award #N00014-10-1-0072 (HP), and J.P. Bickell Foundation (DYH). We thank Manuel Hernandez for help with data collection and Ed Vul for helpful discussions.
Abbreviations
- DBS
deep brain stimulation
- GPi
internal (medial) segment of the globus pallidus
- PD
Parkinson’s disease
- STN
subthalamic nucleus
- UPDRS
United Parkinson’s Disease Rating Scale
Footnotes
For interpretation of color in Fig. 4, the reader is referred to the web version of this article.
REFERENCES
- Abosch A, Hutchison WD, Saint-Cyr JA, Dostrovsky JO, Lozano AM. Movement-related neurons of the subthalamic nucleus in patients with Parkinson disease. J Neurosurg. 2002;97:1167–1172. doi: 10.3171/jns.2002.97.5.1167. [DOI] [PubMed] [Google Scholar]
- Adamovich SV, Berkinblit MB, Fookson O, Poizner H. Pointing in 3D space to remembered targets. I. Kinesthetic versus visual target presentation. J Neurophysiol. 1998;79:2833–2846. doi: 10.1152/jn.1998.79.6.2833. [DOI] [PubMed] [Google Scholar]
- Adamovich S, Berkinblit M, Hening W, Sage J, Poizner H. The interaction of visual and proprioceptive inputs in pointing to actual and remembered targets in Parkinson’s disease. Neuroscience. 2001;104:1027–1041. doi: 10.1016/s0306-4522(01)00099-9. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang. 2008;59:390–412. [Google Scholar]
- Bates D, Maechler M, Bolker B. lme4: Linear mixed-effects models using Eigen and S4. R package version 0.999902344-0. 2012 Available from: http://lme4.r-forge.r-project.org/
- Benabid AL, Chabardes S, Torres N, Piallat B, Krack P, Fraix V, Pollak P. Functional neurosurgery for movement disorders: a historical perspective. Prog Brain Res. 2009;175:379–391. doi: 10.1016/S0079-6123(09)17525-8. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong M. Reversal of experimental Parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- Berkinblit MB, Fookson OI, Smetanin B, Adamovich SV, Poizner H. The interaction of visual and proprioceptive inputs in pointing to actual and remembered targets. Exp Brain Res. 1995;107:326–330. doi: 10.1007/BF00230053. [DOI] [PubMed] [Google Scholar]
- Brown P, Williams D. Basal ganglia local field potential activity: character and functional significance in the human. Clin Neurophysiol. 2005;116:2510–2519. doi: 10.1016/j.clinph.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease. Mov Disord. 2003;18:357–363. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- Brown P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr Opin Neurobiol. 2007;17:656–664. doi: 10.1016/j.conb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Shammah-Lagnado SJ, Silva BA, Ricardo JA. Somatosensory inputs to the subthalamic nucleus: a combined retrograde and anterograde horseradish peroxidase study in the rat. Brain Res. 1988;458:53–64. doi: 10.1016/0006-8993(88)90495-7. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Schapira AHV. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8:464–474. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- Chen CC, Brücke C, Kempf F, Kupsch A, Lu CS, Lee ST, Tisch S, Limousin P, Hariz M, Brown P. Deep brain stimulation of the subthalamic nucleus: a two-edged sword. Curr Biol. 2006;16:R952–R953. doi: 10.1016/j.cub.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Contreras-Vidal JL, Gold DR. Dynamic estimation of hand position is abnormal in Parkinson’s disease. Parkinsonism Relat Disord. 2004;10:501–506. doi: 10.1016/j.parkreldis.2004.06.002. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Crutcher MD, Georgopoulos AP. Primate globus pallidus and subthalamic nucleus: functional organization. J Neurophysiol. 1985;53:530–543. doi: 10.1152/jn.1985.53.2.530. [DOI] [PubMed] [Google Scholar]
- Demirci M, Grill S, McShane L, Hallett M. A mismatch between kinesthetic and visual perception in Parkinson’s disease. Ann Neurol. 1997;41:781–788. doi: 10.1002/ana.410410614. [DOI] [PubMed] [Google Scholar]
- Dufresne JR, Soechting JF, Tolosa ES. Myotatic reflexes and the on–off effect in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1981;44:315–322. doi: 10.1136/jnnp.44.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escola L, Michelet T, Douillard G, Guehl D, Bioulac B, Burbaud P. Disruption of the proprioceptive mapping in the medial wall of parkinsonian monkeys. Ann Neurol. 2002;52:581–587. doi: 10.1002/ana.10337. [DOI] [PubMed] [Google Scholar]
- Eusebio A, Cagnan H, Brown P. Does suppression of oscillatory synchronisation mediate some of the therapeutic effects of DBS in patients with Parkinson’s disease? Front Integr Neurosci. 2012;6:47. doi: 10.3389/fnint.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion M, Tremblay L, Bédard PJ. Abnormal influences of passive limb movement on the activity of globus pallidus neurons in parkinsonian monkeys. Brain Res. 1988;444:165–176. doi: 10.1016/0006-8993(88)90924-9. [DOI] [PubMed] [Google Scholar]
- Fiorio M, Stanzani C, Rothwell JC, Bhatia KP, Moretto G, Fiaschi A, Tinazzi M. Defective temporal discrimination of passive movements in Parkinson’s disease. Neurosci Lett. 2007;417:312–315. doi: 10.1016/j.neulet.2007.02.050. [DOI] [PubMed] [Google Scholar]
- Flash T, Inzelberg R, Schechtman E, Korczyn AD. Kinematic analysis of upper limb trajectories in Parkinson’s disease. Exp Neurol. 1992;118:215–226. doi: 10.1016/0014-4886(92)90038-r. [DOI] [PubMed] [Google Scholar]
- Flowers KA, Downing AC. Predictive control of eye movements in Parkinson disease. Ann Neurol. 1978;4:63–66. doi: 10.1002/ana.410040112. [DOI] [PubMed] [Google Scholar]
- Flowers KA. Visual “closed-loop” and “open-loop” characteristics of voluntary movement in patients with Parkinsonism and intention tremor. Brain. 1976;99:269–310. doi: 10.1093/brain/99.2.269. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh P. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox C, Ebersbach G, Ramig L, Sapir S. LSVT LOUD and LSVT BIG: Behavioral treatment programs for speech and body movement in Parkinson disease. Parkinson’s Dis. 2012;2012:391946. doi: 10.1155/2012/391946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez C, Sainburg R. Proprioceptive control of interjoint coordination. Can J Physiol Pharmacol. 1995;73:273–284. doi: 10.1139/y95-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG, Stebbins GT. Mortality and hallucinations in nursing home patients with advanced Parkinson’s disease. Neurology. 1995;45:669–671. doi: 10.1212/wnl.45.4.669. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guehl D, Dehail P, de Sèze MP, Cuny E, Faux P, Tison F, Barat M, Bioulac B, Burbaud P. Evolution of postural stability after subthalamic nucleus stimulation in Parkinson’s disease: a combined clinical and posturometric study. Exp Brain Res. 2006;170:206–215. doi: 10.1007/s00221-005-0202-z. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Joundi RA, Brittain JS, Green AL, Aziz TZ, Jenkinson N. High-frequency stimulation of the subthalamic nucleus selectively decreases central variance of rhythmic finger tapping in Parkinson’s disease. Neuropsychologia. 2012;50:2460–2466. doi: 10.1016/j.neuropsychologia.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Juri C, Rodriguez-Oroz M, Obeso JA. The pathophysiological basis of sensory disturbances in Parkinson’s disease. J Neurol Sci. 2010;289:60–65. doi: 10.1016/j.jns.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Keijsers NL, Admiraal MA, Cools AR, Bloem BR, Gielen CC. Differential progression of proprioceptive and visual information processing deficits in Parkinson’s disease. Eur J Neurosci. 2005;21:239–248. doi: 10.1111/j.1460-9568.2004.03840.x. [DOI] [PubMed] [Google Scholar]
- Klockgether T, Dichgans J. Visual control of arm movement in Parkinson’s disease. Mov Disord. 1994;9:48–56. doi: 10.1002/mds.870090108. [DOI] [PubMed] [Google Scholar]
- Ko JH, Mure H, Tang CC, Ma Y, Dhawan V, Spetsieris P, Eidelberg D. Parkinson’s disease: increased motor network activity in the absence of movement. J Neurosci. 2013;33:4540–4549. doi: 10.1523/JNEUROSCI.5024-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konczak J, Li K-Y, Tuite PJ, Poizner H. Haptic perception of object curvature in Parkinson’s disease. PLoS One. 2008;3:e2625. doi: 10.1371/journal.pone.0002625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konczak J, Corcos DM, Horak F, Poizner H, Shapiro M, Tuite P, Volkmann J, Maschke M. Proprioception and motor control in Parkinson’s disease. J Mot Behav. 2009;41:543–552. doi: 10.3200/35-09-002. [DOI] [PubMed] [Google Scholar]
- Lalo E, Thobois S, Sharott A, Polo G, Mertens P, Pogosyan A, Brown P. Patterns of bidirectional communication between cortex and basal ganglia during movement in patients with Parkinson disease. J Neurosci. 2008;28:3008–3016. doi: 10.1523/JNEUROSCI.5295-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciego JL, Gonzalo N, Castle M, Sanchez-Escobar C, Aymerich MS, Obeso JA. Thalamic innervation of striatal and subthalamic neurons projecting to the rat entopeduncular nucleus. Eur J Neurosci. 2004;19:1267–1277. doi: 10.1111/j.1460-9568.2004.03244.x. [DOI] [PubMed] [Google Scholar]
- Lee D, Henriques DYP, Li X, Tillery S, Song D, Poizner H. Reaching to kinesthetically defined targets in Parkinson’s disease: effects of deep brain stimulation therapy. Society for Neuroscience Meeting; Chicago, IL: 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Arbuthnott GW, Jutras MJ, Goldberg JA, Jaeger D. Resonant antidromic cortical circuit activation as a consequence of high-frequency subthalamic deep-brain stimulation. J Neurophysiol. 2007;98:3525–3537. doi: 10.1152/jn.00808.2007. [DOI] [PubMed] [Google Scholar]
- Li K-Y, Pickett K, Nestrasil I, Tuite P, Konczak J. The effect of dopamine replacement therapy on haptic sensitivity in Parkinson’s disease. J Neurol. 2010;257:1992–1998. doi: 10.1007/s00415-010-5646-9. [DOI] [PubMed] [Google Scholar]
- Maschke M, Gomez CM, Tuite PJ, Konczak J. Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain. 2003;126:2312–2322. doi: 10.1093/brain/awg230. [DOI] [PubMed] [Google Scholar]
- Maschke M, Tuite PJ, Pickett K, Wächter T, Konczak J. The effect of subthalamic nucleus stimulation on kinaesthesia in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2005;76:569–571. doi: 10.1136/jnnp.2004.047324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Walter BL, Vitek JL. How does deep brain stimulation work? Present understanding and future questions. J Clin Neurophysiol. 2004;21:40–50. doi: 10.1097/00004691-200401000-00006. [DOI] [PubMed] [Google Scholar]
- Messier J, Adamovich S, Berkinblit M, Tunik E, Poizner H. Influence of movement speed on accuracy and coordination of reaching movements to memorized targets in three-dimensional space in a deafferented subject. Exp Brain Res. 2003;150:399–416. doi: 10.1007/s00221-003-1413-9. [DOI] [PubMed] [Google Scholar]
- Mongeon D, Blanchet P, Messier J. Impact of Parkinson’s disease and dopaminergic medication on proprioceptive processing. Neuroscience. 2009;158:426–440. doi: 10.1016/j.neuroscience.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Montgomery EB, Gale JT. Mechanisms of action of deep brain stimulation (DBS) Neurosci Biobehav Rev. 2008;32:388–407. doi: 10.1016/j.neubiorev.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Mure H, Tang CC, Argyelan M, Ghilardi M-F, Kaplitt MG, Dhawan V, Eidelberg D. Improved sequence learning with subthalamic nucleus deep brain stimulation: evidence for treatment-specific network modulation. J Neurosci. 2012;32:2804–2813. doi: 10.1523/JNEUROSCI.4331-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. Assessment and analysis of handedness – Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- O’Suilleabhain P. Proprioception in Parkinson’s disease is acutely depressed by dopaminergic medications. J Neurol Neurosurg Psychiatry. 2001;71:607–610. doi: 10.1136/jnnp.71.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessiglione M, Guehl D, Rolland A-S, François C, Hirsch EC, Féger J, Tremblay L. Thalamic neuronal activity in dopamine-depleted primates: evidence for a loss of functional segregation within basal ganglia circuits. J Neurosci. 2005;25:1523–1531. doi: 10.1523/JNEUROSCI.4056-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. Springer Verlag; 2000. [Google Scholar]
- Poizner H, Fookson OI, Berkinblit MB, Hening W, Feldman G, Adamovich S. Pointing to remembered targets in 3-D space in Parkinson’s disease. Motor Control. 1998;2:251–277. doi: 10.1123/mcj.2.3.251. [DOI] [PubMed] [Google Scholar]
- Putzki N, Stude P, Konczak J, Graf K, Diener H-C, Maschke M. Kinesthesia is impaired in focal dystonia. Mov Disord. 2006;21:754–760. doi: 10.1002/mds.20799. [DOI] [PubMed] [Google Scholar]
- R Core Team . R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. ISBN 3-900051-07-0, Available from: http://www.R-project.org/ [Google Scholar]
- Rickards C, Cody FW. Proprioceptive control of wrist movements in Parkinson’s disease. Reduced muscle vibration-induced errors. Brain. 1997;120:977–990. doi: 10.1093/brain/120.6.977. [DOI] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Marmor O, Saban G, Rosin B, Haber SN, Vaadia E, Prut Y, Bergman H. Low-pass filter properties of basal ganglia cortical muscle loops in the normal and MPTP primate model of Parkinsonism. J Neurosci. 2008;28:633–649. doi: 10.1523/JNEUROSCI.3388-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, Rodriguez M, Guridi J, Mewes K, Chockkman V, Vitek J, DeLong MR, Obeso JA. The subthalamic nucleus in Parkinson’s disease: somatotopic organization and physiological characteristics. Brain. 2001;124:1777–1790. doi: 10.1093/brain/124.9.1777. [DOI] [PubMed] [Google Scholar]
- Sainburg R, Poizner H, Ghez C. Loss of proprioception produces deficits in interjoint coordination. J Neurophysiol. 1993;70:2136–2147. doi: 10.1152/jn.1993.70.5.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Ghilardi MF, Poizner H, Ghez C. Control of limb dynamics in normal subjects and patients without proprioception. J Neurophysiol. 1995;73:820–835. doi: 10.1152/jn.1995.73.2.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Lateiner JE, Latash ML, Bagesteiro LB. Effects of altering initial position on movement direction and extent. J Neurophysiol. 2003;89:401. doi: 10.1152/jn.00243.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader C, Peschel T, Däuper J, Rollnik JD, Dengler R, Kossev AR. Changes in processing of proprioceptive information in Parkinson’s disease and multiple system atrophy. Clin Neurophysiol. 2008;119:1139–1146. doi: 10.1016/j.clinph.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Seiss E, Praamstra P, Hesse CW, Rickards H. Proprioceptive sensory function in Parkinson’s disease and Huntington’s disease: evidence from proprioception-related EEG potentials. Exp Brain Res. 2003;148:308–319. doi: 10.1007/s00221-002-1291-6. [DOI] [PubMed] [Google Scholar]
- Sober SJ, Sabes PN. Multisensory integration during motor planning. J Neurosci. 2003;23:6982–6992. doi: 10.1523/JNEUROSCI.23-18-06982.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temperli P, Ghika J, Villemure J-G, Burkhard PR, Bogousslavsky J, Vingerhoets FJG. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 2003;60:78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- Theodosopoulos PV, Marks WJ, Christine C, Starr PA. Locations of movement-related cells in the human subthalamic nucleus in Parkinson’s disease. Mov Disord. 2003;18:791–798. doi: 10.1002/mds.10446. [DOI] [PubMed] [Google Scholar]
- Tillery SI, Flanders M, Soechting JF. Errors in kinesthetic transformations for hand apposition. Neuroreport. 1994;6:177–181. doi: 10.1097/00001756-199412300-00045. [DOI] [PubMed] [Google Scholar]
- Weinberger M, Dostrovsky JO. A basis for the pathological oscillations in basal ganglia: the crucial role of dopamine. Neuroreport. 2011;22:151–156. doi: 10.1097/WNR.0b013e328342ba50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WG, Gurfinkel VS, King LA, Nutt JG, Cordo PJ, Horak FB. Axial kinesthesia is impaired in Parkinson’s disease: effects of levodopa. Exp Neurol. 2010;225:202–209. doi: 10.1016/j.expneurol.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Russo GS, Hashimoto T, Zhang J, Vitek JL. Subthalamic nucleus stimulation modulates thalamic neuronal activity. J Neurosci. 2008;28:11916–11924. doi: 10.1523/JNEUROSCI.2027-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel A, Bergman H, Ritov Y, Md ZI. Levodopa and subthalamic deep brain stimulation responses are not congruent. Mov Disord. 2010;25:2379–2386. doi: 10.1002/mds.23294. [DOI] [PubMed] [Google Scholar]
- Zia S, Cody F, O’Boyle D. Joint position sense is impaired by Parkinson’s disease. Ann Neurol. 2000;47:218–228. [PubMed] [Google Scholar]