Abstract
The discovery of JAK2 mutations in Philadelphia-negative myeloproliferative neoplasms has prompted investigators to evaluate mutation-targeted treatments to restore hematopoietic cell functions in these diseases. However, the results of the first clinical trials with JAK2 inhibitors are not as promising as expected, prompting a search for additional drugable targets to treat these disorders. In this paper, we used the hypomorphic Gata1low mouse model of primary myelofibrosis (PMF), the most severe of these neoplasms, to test the hypothesis that defective marrow hemopoiesis and development of extramedullary hematopoiesis in myelofibrosis is due to insufficient p27Kip 1 activity and is treatable by Aplidin®, a cyclic depsipeptide that activates p27 kip 1 in several cancer cells. Aplidin® restored expression of Gata1 and p27Kip 1 in Gata1 low hematopoietic cells, proliferation of marrow progenitor cells in vitro and maturation of megakaryocytes in vivo (reducing TGF-β/VEGF levels released in the microenvironment by immature Gata1 low megakaryocytes). Microvessel density, fibrosis, bone growth, and marrow cellularity were normal in Aplidin®-treated mice and extramedullary hematopoiesis did not develop in liver although CXCR4 expression in Gata1low progenitor cells remained low. These results indicate that Aplidin® effectively alters the natural history of myelofibrosis in Gata1low mice and suggest this drug as candidate for clinical evaluation in PMF.
Primary myelofibrosis (PMF) is a myeloproliferative neoplasm characterized by abnormalities in the interaction between the hematopoietic stem/progenitor cells and their bone marrow niches which lead to increased stem/progenitor cell trafficking and development of extramedullary hematopoiesis (Tefferi, 2000). Although the dominant positive JAK2 V617F mutation recently described in patients with myeloproliferative neoplasms is present only in approximately 50%of PMF patients (Zhan and Spivak, 2009), new data are emerging on additional molecular abnormalities associated with the disease. The molecular signature of PMF includes epigenetic absent/reduced expression of CXCR4 in stem/progenitor cells (Shi et al., 2007; Bogani et al., 2008) [CXCR4 is the receptor for the chemokine SDF1, also known as CXCL12, required for interaction of these cells with the marrow vascular niche (Broxmeyer, 2008)] and reduced levels of GATA1 in megakaryocytes (MK) (Vannucchi et al., 2005b) [GATA1 is a transcription factor essential for MK maturation (Orkin and Zon, 2008)]. Therefore in PMF, MK proliferate and accumulate in great numbers in the marrow releasing numerous cytokines (TGF-β, VEGF, osteoprotegerin/ BMP4, etc.) which activate stromal cells leading to fibrosis (fibroblasts), angiogenesis (endothelial cells), and osteosclerosis (osteoblasts) (Lataillade et al., 2008). These observations suggest that treatment of PMF may require drugs that target both stem cell and microenvironmental functions (Lataillade et al., 2008; Lane et al., 2009).
In an attempt to understand the pathogenesis of PMF, several mouse mutants have been generated as models for the disease (reviewed in Varricchio et al., 2009). Transgenic mice carrying the JAK2 V617F mutation readily develop, based on genetic background and dosage of JAK2 V617F expression, the myeloproliferative neoplasms polycythemia vera and essential thrombocythemia (Varricchio et al., 2009). These mice may develop secondary myelofibrosis but do not represent good models for primary PMF. Models more clearly resembling PMF have been developed based on mutations that interfere with either the extrinsic (thrombopoietin, TPO, its receptor, MPL and LNK, a protein that sequesters MPL preventing its trafficking to the cell membrane) or intrinsic (the transcription factor GATA1) control of megakaryopoiesis. The TPOhigh model dies of aggressive myelofibrosis within a few months (Varricchio et al., 2009) and is therefore unsuitable as a tool to identify treatment strategies. The observation that the TPOhigh mutation reduces Gata1 expression in MK (Vannucchi et al., 2005a) led to the discovery that deletion of the regulatory sequences that control Gata1 expression in these cells (hypomorphic Gata1low mutation) also induces myelofibrosis with age (Martelli et al., 2005). Gata1low mice have a life span more than 2 years and develop the disease in precise sequential stages (Martelli et al., 2005) (Fig. 1). Mice are born anemic and thrombocytopenic and recover from anemia at 1 month by developing extramedullary hematopoiesis in the spleen. The mice remain thrombocytopenic and develop increased bone formation at 1 month, fibrosis and increased angiogenesis at 6–8 months, and increased hematopoietic stem/progenitor cell trafficking and extramedullary hematopoiesis in liver at 10 months (Martelli et al., 2005). The normal life span of Gata1low mice in spite of hemopoietic failure in their marrow is probably due to the efficiency of the spleen as an extramedullary hematopoietic site in response to stress (Migliaccio et al., 2009). This precisely timed disease progression makes these mice suitable for identification of drugs that can alter the natural history of the disease. In fact, although the initiating lesion of PMF may not involve the Gata1 gene (Vannucchi et al., 2005b), the phenotype of these mice closely recapitulates the human disease because Gata1low mice express the same MK, stem/progenitor cell, and microenvironmental abnormalities expressed by PMF patients (Migliaccio et al., 2008). Gata1low stem/progenitor cells, in particular, lack CXCR4 expression (Migliaccio et al., 2008) while their marrow microenvironment expresses high levels of VEGF, TGF-β, CXCL12, and other growth factors known to activate stromal cells (Varricchio et al., 2009). Therefore, in these mice, as in PMF patients, defective hematopoiesis in the marrow, abnormal stem/progenitor cell trafficking, and development of hematopoiesis in extramedullary sites is probably the result of alterations in both stem cells (that lack CXCR4 expression) (Migliaccio et al., 2008) and their microenvironment (increased osteoblast and endothelial cell proliferation in the bone marrow) (Varricchio et al., 2009). The cyclic depsipeptide Aplidin® originally isolated from a Mediterranean marine tunicate, Aplidium albicans and presently producted by synthesis, is undergoing late phase II clinical trials for solid tumors, myeloma and T-cell lymphoma, with no reported marrow toxicity (Ferme et al., 2008; Mateos et al., 2008). Aplidin® does not inhibit colony formation from normal human progenitor cells (Depenbrock et al., 1998; Albella et al., 2002; Gomez et al., 2003) but induces cell cycle arrest and apoptosis of many tumor cell types (Munoz-Alonso et al., 2009). The ability of tumor cells to respond to Aplidin® is predicted by reduced expression of p27kip1 (Moneo et al., 2007), an inhibitor of cyclin D (Ray et al., 2009) necessary to maintain stem cells in quiescence (Cheng et al., 2000). Low concentrations of Aplidin® blocked G1-to-S transition by decreasing the level of the cyclin D1/cyclin-dependent kinase (Cdk)4/p21 complex, leading to an increase in the levels of hypophosphorylated retinoblastoma protein (Rb-1) in undifferentiated, anaplastic thyroid carcinoma cells (Bravo et al., 2005), induced G1 and G2/ Marrest and decreased the expression of cyclins A and B in SK-MEL-28 and UACC-257 melanoma cell lines (Munoz-Alonso et al., 2008), and decreased cyclin D1 and Cdk4 in murine myeloma 5T33MM cells (Caers et al., 2008). The direct effects of Aplidin® include rapid (within 1 min) activation of Rac1 (Gonzalez-Santiago et al., 2006; Munoz-Alonso et al., 2008), a member of the guanine triphosphatase family activated by the canonical Wnt signaling (Wu et al., 2008) that exerts multiple functions on hematopoietic stem/progenitor cells. Rac1 facilitates stem cell engraftment in the marrow and increases p27Kip1 expression (Gu et al., 2003; Walmsley et al., 2003; Cancelas et al., 2005). Indirect effects of Aplidin® on cancer growth are related to anti-angiogenic properties (Broggini et al., 2003; Biscardi et al., 2005; Straight et al., 2006). In vivo, Aplidin® inhibits spontaneous as well as VEGF- and FGF-2-induced angiogenesis in the chick allantoid assay (Taraboletti et al., 2004; Straight et al., 2006; Mitsiades et al., 2008). In vitro, Aplidin® interferes with endothelial cell growth by inhibiting both expression and signaling of VEGF (Broggini et al., 2003; Mitsiades et al., 2008). Since Gata1low stem/ progenitor cells were found to express low levels of p27kip1 (this article), we hypothesized that Aplidin®, by activating Rac1 and p27kip1 in stem/progenitor cells and by reducing the number of vascular niches, might restore both stem cell and microenvironmental function in this mouse model of PMF.
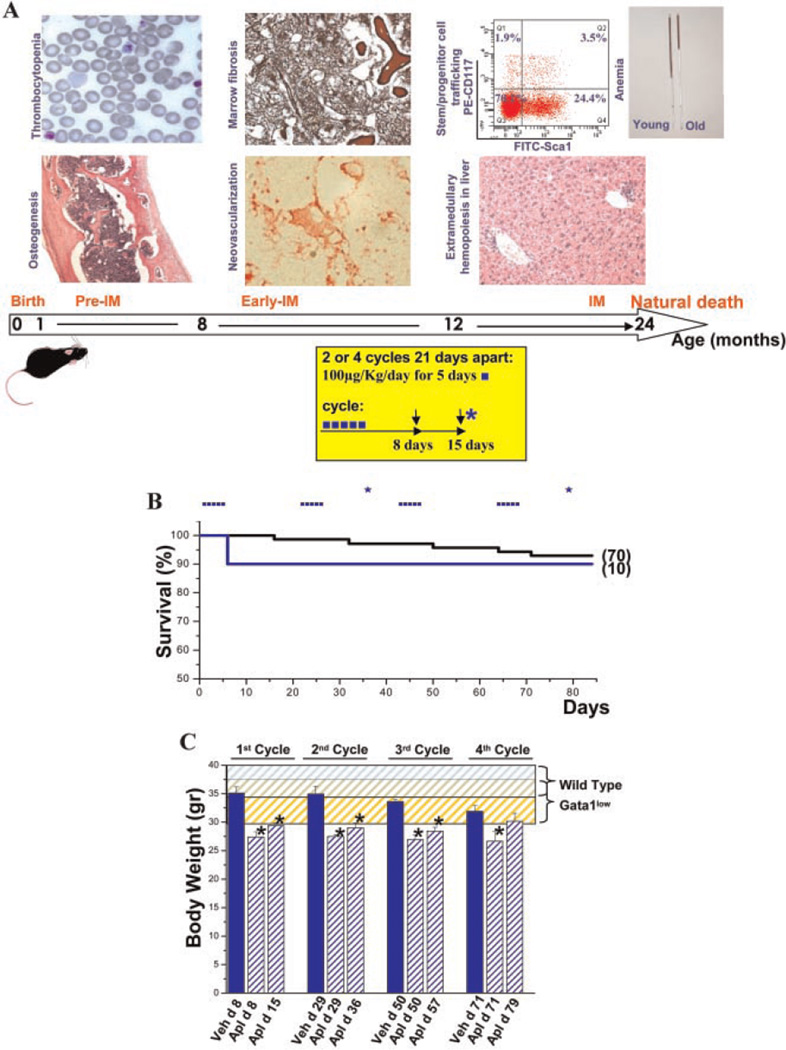
Fig. 1.
Aplidin® treatment is well tolerated in mice and modestly affects body weight. A: Natural history of myelofibrosis in Gata1low mice (modified from Varricchio et al., 2009) and schedule of Aplidin® treatment used in the experiments presented. Treatment of Gata1low mice was initiated at 10 months of age. The arrows and asterisk indicate day of blood sampling and mice sacrifice, respectively. B:Kaplan–Meier analyses of survival of control Gata1low mice (untreated or injected with vehicle, black) and of Gata1low mice treated with Aplidin® for four cycles (blue). The number of mice included in each group is indicated in parenthesis. C: Body weight of Gata1low mice treated either with vehicle (veh, blue) or with Aplidin® (Apl, shaded blue) during the course of the experiment. The day (d) of Aplidin® treatment is indicated on the x-axis. Results are presented as mean (±SD) determinations of at least three mice per experimental group and are compared to historic determinations of body weight of untreated wild-type (blue shaded area) and Gata1low (yellow shaded area) mice of comparable age. Values statistically different from the corresponding vehicle group are indicated by * (P <0.05).
To test this hypothesis, Gata1low mice were treated with consecutive Aplidin® cycles and evaluated for disease progression. Aplidin® treatment increased expression of p27kip1 and Gata1 in stem/progenitor cells, reduced TGF-β and VEGF production, and inhibited angiogenesis. In addition, Gata1low stem/progenitor cells from treated mice generated Gata1pos MK in the marrow, increasing peripheral blood platelets counts by threefold. Normal cellularity and reduced fibrosis and bone formation were also observed in Aplidin®-treated animals. These results suggest that Aplidin® altered the natural history of myelofibrosis in Gata1low mice by restoring both the microenvironmental and stem/progenitor cell defects, providing a rationale for clinical evaluation of Aplidin® in PMF patients.
Materials and Methods
Mice
Male wild-type and Gata1low mice were generated in the animal facility of Istituto Superiore di Sanità as described (Martelli et al., 2005). All the experiments were performed with sex- and age-matched mice under protocols approved by the institutional animal care committee.
Drug formulation
Vials containing Aplidin® (0.5 mg/vial) and mannitol (as additive, 25 mg/vial) were reconstituted with 1 ml of reconstitution solution (cremophor EL/ethanol/water, 15:15:70 v/v/v) that was further diluted with 9 mg/ml (0.9% w/v) of sodium chloride.
Drug treatment
Gata1low mice were divided into three age groups: disease-free (1–6 months old), early myelofibrosis (8–12 months old, when presence of the disease is detectable only in bone marrow), and myelofibrosis (15 months old to natural death, when the complete clinical picture of the human disease is manifested) (Martelli et al., 2005). Three experiments with slightly different doses and schedules were performed (see Fig. 1 and Supplemental Fig. 1 for details). Briefly, Gata1low mice were individually weighed and injected intraperitoneally (i.p.) with Aplidin® or with equivalent volumes of vehicle. The dose of Aplidin® administered was adjusted on the basis of weight with each animal receiving 60 µg of Aplidin®/kg/day for 9 days for either one cycle (experiment 1) or two cycles 28 days apart (experiment 2), or 100 µg of Aplidin®/kg/ day for five consecutive days for two or four cycles 21 days apart (experiment 3). Since the three separate experiments gave similar results, only the third experiment is described in detail for clarity. Aplidin® reduced animal mobility and normal drinking behavior. Therefore, in the second and third experiments mice were injected with 200 µl of sodium chloride (0.9% w/v) subcutaneously to prevent dehydration.
Hematological parameters
Blood was collected from the retro-orbital plexus into ethylenediaminetetraacetic acid (EDTA)-coated microcapillary tubes (20–40 µl/sampling). Hematocrit (Hct), platelet (plt), and white blood cell (WBC) counts were determined manually.
Flow cytometry and cell sorting
Cells obtained from bone marrow were counted manually by Trypan Blue (0.4% w/v, Sigma, St. Louis, MO) staining and incubated for 30 min on ice first with a Fcγ blocker (CD16/CD32) and then with phycoerythrin (PE)-conjugated anti-CD34, fluorescein isothiocyanate (FITC)-conjugated anti-CXCR4, and allophycocyanin (APC)-conjugated anti-CD117 (cKit) (all from PharMingen, San Diego, CA). Dead cells and non-specific signals were excluded by propidium iodide staining (5 µg/ml, Sigma) and appropriate isotype controls (PharMingen). Cells in the prospective stem/progenitor cell gates deprived of lineage positive cells (cKitpos/CD34pos or CD34neg) were isolated by sorting with an ARIA cell sorter (Becton Dickinson, Franklin Lakes, NJ) (>90% pure based upon reanalysis) (Migliaccio et al., 2008).
Progenitor cell determination
Prospectively isolated progenitor cells (1,000 cells/ml) and unfractionated liver cells (5 × 104 cells/ml) were cultured in methylcellulose cultures (0.9% w/v) containing fetal bovine serum (30% v/v, Sigma) and ratSCF (100 ng/ml), mouseIL3 (10 ng/ml), G-CSF and GM-CSF (50 ng/ml in both cases) (all from Sigma), and human erythropoietin (2 U/ml) (Roche Welwyn Garden City, Hertfordshire, UK). Cultures were incubated at 37°C in a humidified incubator containing 5% CO2 in air and scored for the presence of hematopoietic colonies on day 8 (Ghinassi et al., 2007; Migliaccio et al., 2009).
Histology
Femurs, tibias, and liver sections were stained with hematoxylin– eosin, Gomory-silver (MicroStain MicroKit, Diapath, Bologna, Italy) or Mallory-trichromic staining (Migliaccio et al., 2009). For immunohistochemistry, sections were incubated with an anti-Gata1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) (Migliaccio et al., 2009) and the immunoreaction detected with the avidin–biotin immunoperoxidase system (Vectastain Elite ABC Kit; Vector Laboratories, Burlingame, CA) on slides counterstained with hematoxylin–eosin. Histological observations were carried out using a ZEISS AXIOSKOPE light microscope (Jena, Germany) equipped with a Coolsnap Videocamera and the acquired images were analyzed with the MetaMorph 6.1 Software (Universal Imaging Corp, Downingtown, PA). Levels of Gata1 immunostaining per MK, numbers of reticulinic fibers and Mallory positive areas were determined by analyzing with the MethaMorph 6.1 program five randomly chosen non-overlapping areas per each section. For microvessel density determination, sections from the femur were incubated with a rabbit anti-mouse CD34 antibody (clone MEC 14.7, code CL8927AP, Cedarlane Laboratories, Burlington, NC) or a rabbit anti-mouse CD31 (Becton Dickinson) (dilution 1:200 for both). Antibody dilution and blocking of non-specific binding sites was performed with Ultra V-Block (code TA-060-UB, Thermo Scientific, Walthman, MA). Secondary labeled antibody incubation and stain development was performed using SuperPicTure Kit HRP Broad Spectrum (code 87–8963, Histo-Line Laboratories, Milano, Italy, dilution 1:1). Microvessel density was calculated as the mean number of stained vessels per 400× high power field, calculating the mean of five randomly chosen non-overlapping areas. Frequency of MK and percentage of Gata1pos MK were similarly obtained. Counting was performed by two separate investigators in a blinded fashion.
Quantitative RT-PCR analysis
Total RNA was prepared by lysing single erythroid colonies or prospectively isolated cell populations into Trizol (Gibco BRL, Paisley, UK). RNA was reverse transcribed with 2.5 µM random hexamers using the superscript kit (Invitrogen, Milan, Italy) and gene expression levels quantified by Real Time RT-PCR, as described (Ghinassi et al., 2007; Migliaccio et al., 2009). Beta-2 microglobulin cDNA was amplified as an internal standard. Reactions were performed in an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Carlsband, CA). Cycle threshold (Ct) were calculated with the SDS software and mRNA levels expressed as 2−Δct(ΔCt = target gene Ct – Beta – 2 microglobulin CtΔ).
Statistical analysis
Statistical analysis was performed by analysis of variance (ANOVA test) using Origin 3.5 software for Windows (Microcal Software, Inc., Northampton, MA) or by Kruskal–Wallis and Bonferroni–Dunn’s post hoc and Mann–Whiteny’s tests using GraphPad Prism 4.0, as appropriate.
Results
Aplidin® treatment is well tolerated by Gata1low mice and causes only modest reduction in body weight
Gata1low mice were treated for two or four cycles of Aplidin® and sacrificed for analysis 14 days after the second or fourth cycle (Fig. 1A and Supplemental Fig. 1). The slightly lower survival rate (1 death out of 10 treated mice, 85%) of the Aplidin®-treated mice with respect to controls (5 out of 70 mice, 93%) is not statistically significant. In addition, death in the treated group occurred during the first cycle of therapy while in the control mice mortality was observed throughout the period of observation and was consistent with the natural course of the disease (Martelli et al., 2005).
Body weight was monitored as a measure of morbidity/ toxicity (Teicher and Andrews, 2004). Gata1low mice are slightly smaller than wild-type littermates but the reduction is not statistically significant (P> 0.05) (Fig. 1C). Small (10–15%) but significant reductions in body weight were observed in the Aplidin®-treated Gata 1low animals which persisted throughout treatment (P< 0.05). These reductions are below the threshold that define toxicity (20%) and are likely due to the improvement in mobility observed in the treated animals. No sign of organ toxicity was observed upon post mortem examination of the sacrificed mice by the end of the treatment.
Progenitor cells prospectively isolated from the marrow of Gata1low mice express reduced levels of p27kip1 which predicts Aplidin® responsiveness
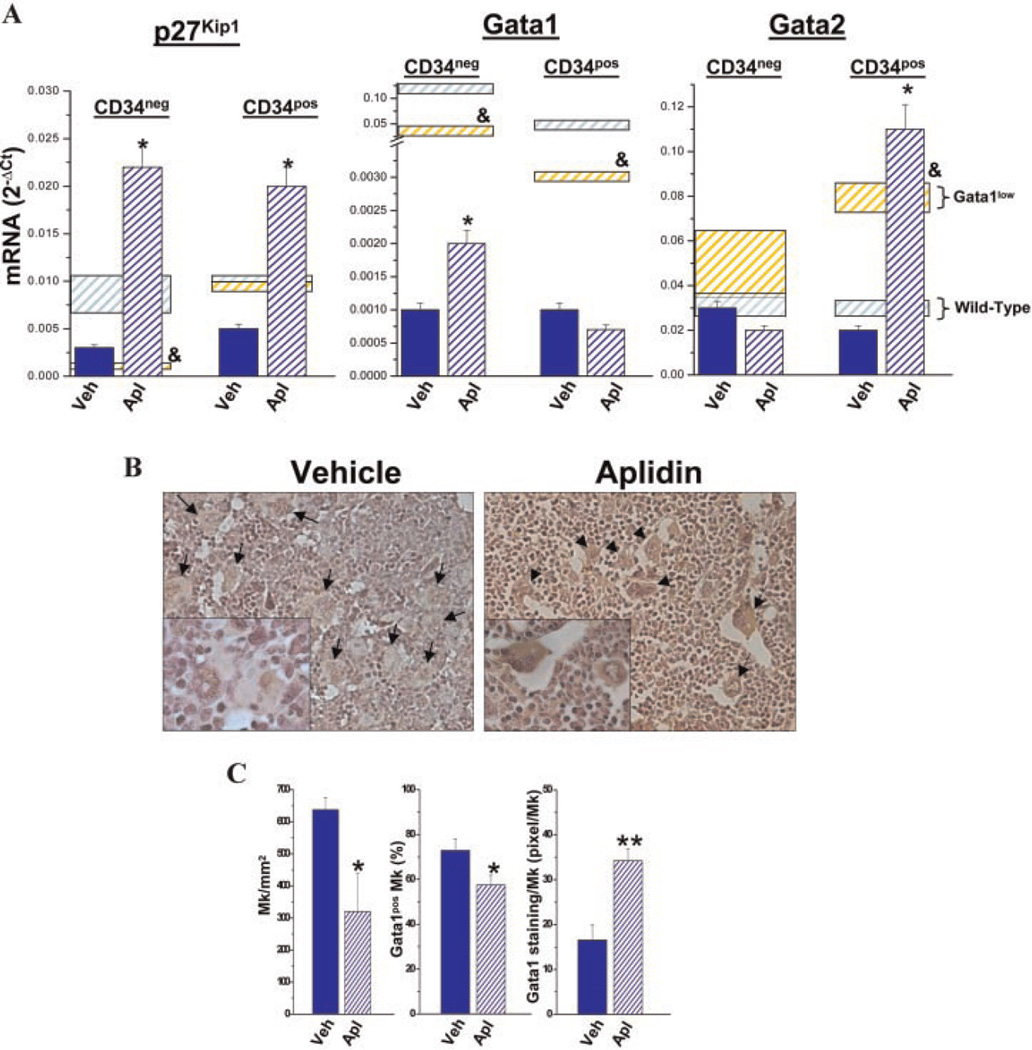
Since functional GATA binding sites are present in the regulatory regions of p27kip1 (Fukuchi et al., 2006), it is conceivable that progenitor cells carrying the hypomorphic Gata1low mutation express reduced levels of p27Kip1 and, therefore, meet the criteria for Aplidin® responsiveness. To test this hypothesis, progenitor cells were prospectively isolated from the marrow of Gata1low and wild-type littermates and the levels of p27Kip1 expressed by the various populations compared (Fig. 2A). Progenitor cells were identified on the basis of cKit and CD34 expression and divided into MK/ erythroid restricted (MEP, cKitposCD34neg) and common myeloid plus myeloid-restricted (CMP/GMP, cKitposCD34pos) progenitor cells (Ghinassi et al., 2007; Migliaccio et al., 2009). MEP purified from Gata1low mice expressed levels of p27Kip1 10-fold lower than those expressed by wild-type MEP (Fig. 2A). Gata1low CMP/GMP also expressed lower levels of p27Kip1 than the wild-type cells but the difference was modest (twofold) and not statistically significant (Fig. 2A). Aplidin®-treatment greatly increased the levels of p27Kip1 expressed by both Gata1low MEP and Gata1low CMP/GMP up to levels significantly higher (by 1-log) than those expressed by untreated wild-type cells.
Fig. 2.
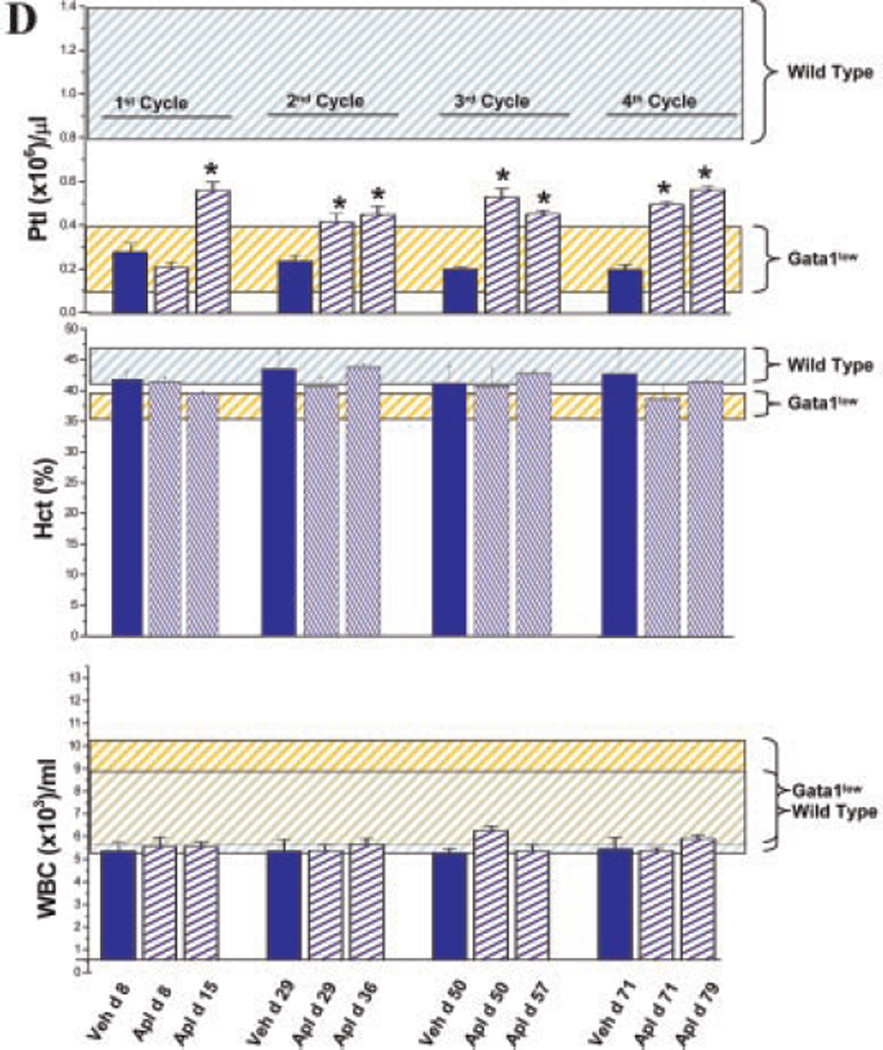
Aplidin® treatment increases the low levels of p27Kip1 and Gata1 expressed by progenitor cells prospectively isolate from the marrow of Gata1low mice and restore the ability of these cells to mature into Gata1pos megakaryocytes in vivo. A: Levels of p27Kip1, Gata1, and Gata2 expressed by cKitpos/CD34neg (MEP) and cKitpos/CD34pos (CMP/GMP) from the marrow of Gata1low mice treated either with vehicle or with Aplidin® for fourth cycles (day 79). Results are presented as mean (±SD) determinations of at least three mice per experimental group. Values statistically different (P < 0.05) between untreated wild-type and Gata1low mice are indicated by & while those statistically different between vehicle- and Aplidin®-treated Gata1low mice are indicated by *. B: Gata1 immunostaining of bone marrow sections from representative Gata1low mice treated either with vehicle or Aplidin® for four cycles. MKs are indicated by arrows and are presented at higher magnification in the insets. Magnification 20× in the parts and 40× in the insets. C: Frequency of MKs and of Gata1pos MKs and intensity of Gata1 immunostaining per Gata1pos MK observed in the marrow of three individual animals treated with vehicle or with Aplidin® for four cycles. Values are expressed as mean (±SD) of five determinations in randomly chosen sections for each of three mice included per experimental group. Values statistically different (P < 0.05 or <0.001) between vehicle- and Aplidin®-treated mice are indicated by * and **, respectively. D: Number of platelets (Ptl), hematocrit (Hct), and number of white blood cells (WBC) in the blood of vehicle-and Aplidin®-treated Gata1low mice during the course of the experiment. Results are presented as mean (±SD) determinations of at least three mice per experimental group and are compared to those observed in untreated wild-type (blue shaded area) and Gata1low (yellow shaded area) mice of comparable age. Values statistically different from the corresponding vehicle group are indicated by * (P < 0.05).
Aplidin® treatment increases Gata1 expression in progenitor cells prospectively isolated from the marrow of Gata1low mice and restores the ability of the cells to form hematopoietic colonies in vitro and to generate maturing MK in vivo
Progenitor cells from the marrow of Gata1low mice express reduced levels of Gata1 (Migliaccio et al., 2009) and fail to mature into Gata1pos MK in vivo (Martelli et al., 2005) and to form hematopoietic colonies in vitro (Ghinassi et al., 2010; Migliaccio et al., 2009). Aplidin® treatment significantly increased (by twofold) the levels of Gata1 expressed by MEP (Fig. 2A). CMP/GMP purified from the marrow of Gata1low mice also express reduced levels of Gata1 but have increased levels of Gata2 which when overexpressed may compensate for Gata1 deficiency (Huang et al., 2009). In CMP/GMP, Aplidin® treatment did not affect Gata1 expression but further increased Gata2 expression (Fig. 2A). These progenitor cells from the marrow of Aplidin®-treated Gata1low mice generated hematopoietic colonies in vitro while the number of colonies generated by the corresponding cells purified from the control vehicle-treated animals was below detection (Table 1).
TABLE 1.
Number of hematopoietic colonies generated in limiting dilution cultures of progenitor cells (cKitposCD34neg and cKitposCD34pos) prospectively isolated from the marrow of untreated wild-type mice (as control) and from Gata1low mice treated either with vehicle or with Aplidin® for four cycles
| Cloning efficiency (colonies/103 plated cells) |
||
|---|---|---|
| cKitposCD34neg | cKitposCD34pos | |
| Wild-type | 136 ± 52 | 595 ± 54 |
| Gata1low Vehicle | b.d. | b.d. |
| Gata1low Aplidin® | 47±5 | 23±2 |
Results are presented as mean (±SD) determinations of at least three mice per experimental group.
In Gata1low mice, defective maturation results in accumulation of MK that are either negative (30%) or slightly positive (15 pixel/MK) for Gata1 by immunostaining in the marrow [(Martelli et al., 2005) and data not shown]. Marrow from Aplidin®-treated mice contained significantly fewer MK than the marrow from vehicle-treated animals (Fig. 2B,C). By immunostaining, Gata1pos MKs were significantly less frequent in marrow sections from the Aplidin®-treated group than in sections from the vehicle-treated animals. However, the intensity of the Gata1 immunostaining of each Gata1pos MK in the Aplidin®-treated animals was significantly higher (by >2-fold) than that of the MK of the vehicle-treated group (Fig. 2C). The greater Gata1 content of MK, associated with reduced frequency of Gata1pos MK in marrow sections from Aplidin®-treated mice suggests that Aplidin®-treatment restored MK maturation inducing these cells to mature into platelets. In agreement with this hypothesis, significant increases (by 20–30%, P<0.05) in platelet counts were observed in the blood of Gata1low mice after the first cycle and persisted for the duration of Aplidin® treatment (Fig. 2D and Supplemental Fig. 2). By comparison, modest changes in hematocrit (Hct) and WBC counts were observed in both Aplidin®- and vehicle-treated animals (Fig. 2D).
Aplidin® treatment restores the marrow microenvironmental abnormalities of Gata1low mice
The numerous immature MK present in the marrow of Gata1low mice release high levels of cytokines (Kacena et al., 2004; Martelli et al., 2005; Garimella et al., 2007) that activate endothelial cells, fibroblasts, and osteoblasts resulting in increased vessel density, fibrosis, and formation of bone trabeculae in the marrow cavity and reduced space for hematopoietic cells which decline in number with age (Fig. 1A). To assess whether Aplidin® treatment, by restoring MK maturation also reversed these bone marrow microenvironmental traits, marrow cellularity, VEGF and TGF-β expression, microvessel density, fibrosis, and osteogenesis were assessed in Gata1low mice treated with Aplidin® for 2 and 4 cycles (Fig. 3).
Fig. 3.
Aplidin® treatment increases cellularity and decreases VEGF and TGF-β expression, vessel density, fiber and bone formation in the marrow of Gata1low mice. A: Number of cells and VEGF and TGF-β expression in the femur of vehicle-and Aplidin®-treated Gata1low mice after the second and fourth cycle. mRNA levels were determined by quantitative RT-PCR and expressed as ratio with the levels of expression in the vehicle control group. Results are presented as mean (±SD) determinations of at least three mice per experimental group. Values statistically different from the corresponding vehicle group are indicated by * (P<0.05). B: Microvessel density (by CD34-staining), (C) fibrosis (by Gomory-silver staining),and(D) bone formation (by Mallory staining) of the femur from Gata1low mice treated either with vehicle or with Aplidin® for four cycles. Results were quantified with the MetaMorph6.1 program and presented as mean (±SD) determinations with three mice per experimental group in the parts on the right. Magnification 20× in (B) and (C) and 10× in (E). Values statistically different (P <0.05 and 0.0001) from those observed in vehicle mice are indicated by * and **, respectively.
A trend toward increased numbers of marrow cells was observed in animals treated with Aplidin® although increases became statistically significant only after the fourth cycle (Fig. 3A and Supplemental Fig. 3). This increase in marrow cellularity was associated with statistically significant reductions in the levels of VEGF (by the second cycle) and TGF-β (by the second and fourth cycle) mRNA expressed by the marrow (Fig. 3A and data not shown). In addition, Aplidin®-treatment reduced microvessel density by 60% by the end of the second cycle (day 36), although this reduction did not reach statistical significance until the fourth cycle (day 79) (Fig. 3B). Aplidin®-treatment reduced the density of reticulinic fibers (Fig. 3C) and the areas of active osteogenesis, as revealed by Mallory staining, in the fourth cycle (Fig. 3D). Although, Aplidin®-treatment did not reduce the spleen weight of Gata1low mice which remained abnormally high (Supplemental Fig. 4), it decreased fibrosis, neo-angiogenesis, and expression of TGF-β and VEGF in this organ (data not shown).
Aplidin® treatment does not restore CXCR4 expression in progenitor cells but prevents development of extramedullary hematopoiesis in the liver
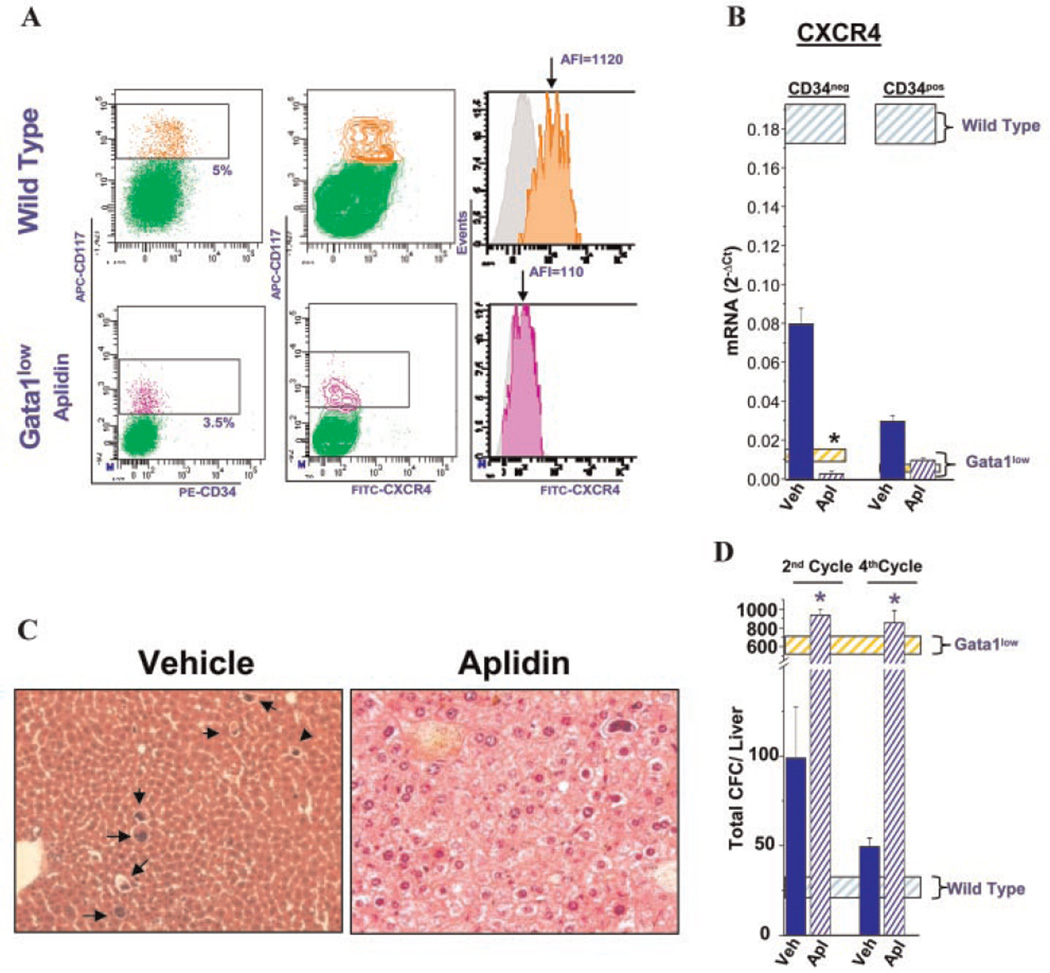
After four cycles of Aplidin®-treatment, the levels of CXCR4 mRNA and protein expressed by progenitor cells from the marrow of Gata1low mice remained barely detectable (Fig. 4A,B and Supplemental Table 1).
Fig. 4.
Aplidin® treatment does not restore CXCR4 expression in the stem/progenitor cells and does not halt progenitor cell trafficking but prevents development of extramedullary hematopoiesis in the liver of Gata1low mice. A:Flow cytometrical determinations of CXCR4 expression in progenitor cells (CD117pos) of the marrow of wild-type and Aplidin®-treated (4 cycles) Gata1low mice. CXCR4 expression is presented both as contour plot and as histogram to highlight the population profiles and the mean fluorescence intensity, indicated by an arrow, respectively. Isotype controls for CXCR4 analyses are presented in gray. See Table 2 for further details. B: Levels of CXCR4 mRNA expressed by progenitor cells (cKitposCD34neg and cKitposCD34pos) isolated from the marrow of vehicle-or Aplidin®-treated mice, respectively. The levels of CXCR4 expressed by cells purified from wild-type and Gata1low mice are indicated by the blue and yellow shaded areas, respectively. Values statistically significant (P<0.05) from those observed in untreated Gata1low mice are indicated by *. All the results observed in Gata1low mice are statistically lower (P<0.001) than those observed in wild- type mice. C: Hematoxylin–eosin staining of liver sections from a vehicle-and an Aplidin®-treated Gata1low mice, respectively. The presence of MK in the liver parenchyma is indicated by arrows. Magnification 20×. D:Number of hematopoietic progenitor cells (CFC) present in the liver from vehicle- and Aplidin®-treated Gata1low mice. Results are presented as mean (±SD) of those observed in three mice per experimental group. The number of progenitor cells present in the liver of untreated wild-type and Gata1low mice of comparable age are indicated by the shaded blue and yellow areas, respectively. Values statistically greater (P <0.01) than those observed in vehicle-treated mice are indicated by *.
Hematopoietic cells were not detected by histological analyses in the liver parenchyma of Gata1low mice treated with Aplidin® while numerous MK were observed in the parenchyma of the vehicle-treated control group (Fig. 4C). Because of the underlying extramedullary hematopoiesis, in untreated and vehicle-treated animals the liver increased in size and were spongy in consistency, while size and consistency of the liver in Aplidin®-treated mice were normal (result not shown). These observations indicate that Aplidin®-treatment prevented development of hematopoiesis in the liver of these mice. However, greater numbers of colony forming cells were detectable in the liver of Gata1low mice treated with Aplidin® (Fig. 4D). Given the extensive vascularization of the liver, the presence of such great numbers of progenitor cells in this organ indicates that Aplidin® treatment did not prevent, and may even have favored, stem/progenitor cell trafficking in these animals. These data suggest that increased stem/progenitor cell trafficking induced by lack of CXCR4 expression may be necessary but is not sufficient for extramedullary hematopoiesis to occur.
Discussion
Primary myelofibrosis is the most severe of the Philadelphianeg myeloproliferative neoplasms (Tefferi, 2000; Tefferi et al., 2007). The only effective treatment currently available for this disease is bone marrow transplantation (Cervantes et al., 2007), a therapy that can only be provided to eligible patients for whom suitable donors exist. Palliative treatment may include removal of the enlarged spleen. However, splenectomy in patients with PMF has been associated with a higher incidence of development of extramedullary hematopoiesis in liver and a higher rate of blast transformation (Cervantes et al., 2007). Clinical trials with JAK2 inhibitors in patients with PMF led to a dramatic rapid reduction of spleen size but did not improve other hematological parameters and even induced severe thrombocytopenia (reviewed in Pardanani, 2008). Alternative strategies to cure this disease are therefore warranted (Lataillade et al., 2008; Lane et al., 2009).
We have extensively characterized the myelofibrotic phenotype expressed by Gata1low mice (Varricchio et al., 2009). Previous studies have determined that the hematopoietic functions of Gata1low stem/progenitor cells are exquisitely rescued by the spleen microenvironment (Migliaccio et al., 2009). Therefore, removal of the spleen from heterozygous Gata1low/+ females [since Gata1 is on the X chromosome (Zon et al., 1990), due to the lionization process these females have both Gata1low and Gata1+ stem/progenitor cells] restored wild-type hematopoiesis in the marrow normalizing many of the myelofibrotic traits expressed by these animals (increased platelets counts in the blood, restored marrow cellularity, and decreased angiogenesis and bone formation) (Migliaccio et al., 2009). Therefore, removal of the spleen rescued the stem cell abnormalities of the Gata1low/+ females. However, splenectomy did not restore the cytokine expression profile of the marrow which remained abnormally high and, similar to the effect induced in PMF patients, promoted development of extramedullary hematopoiesis in liver (Ghinassi et al., 2010). Therefore restoration of wild-type hematopoiesis in the marrow of Gata1low/+ females did not prevent all of the disease manifestations indicating that, as suggested for PMF patients (Lataillade et al., 2008; Lane et al., 2009), also in this animal model treatment of myelofibrosis may require normalization of both stem/progenitor cell and microenvironmental functions.
The dual action of Aplidin® on cancer growth (induction of apoptosis of cancer cells and inhibition of angiogenesis) suggests that this drug may be effective in altering the natural history of myelofibrosis in Gata1low mice. Indeed, Aplidin®-treatment rescued both the microenvironmental and progenitor cell function in the marrow of Gata1low mice preventing these mice from developing myelofibrosis: it increased blood platelet counts, induced normal marrow cellularity, decreased fibrosis, angiogenesis, and bone formation and prevented, or at least delayed, development of extramedullary hematopoiesis in liver. Decreased angiogenesis, fibrosis, and bone formation were the result of reduced VEGF, TGF-β and, probably, bone-morphogenic proteins that are released by Gata1low MK (Kacena et al., 2004; Garimella et al., 2007) which are greatly decreased in number in the marrow of Aplidin®-treated animals. Restoration of MK maturation was due to increased Gata1 expression while increases in the expression of both p27Kip1 (at the mRNA level) and Gata1 (both as mRNA and protein) probably contributed to rescue the function of hematopoietic cells from the marrow. Although technical limitations prevented direct measurements of p27kip1 protein levels in this experimental model, Aplidin® has been reported to increase both p27kip1 mRNA and protein in sarcoma cell lines (Moneo et al., 2007). The protein p27Kip1 regulates stem/ progenitor cell fate decisions with low levels of p27Kip1 favoring proliferation and high levels promoting differentiation (Cheng et al., 2000; Messina et al., 2005) and, by affecting microtubule modeling, inhibits migration, and tissue invasion of sarcoma cells (Baldassarre et al., 2005), suggesting that increased levels of p27Kip1 expression contributed to rescue the hematopoietic functions of Gata1low progenitor cells from the marrow and prevented establishment of hematopoiesis in extramedullary sites. Increased levels of Gata1 expression may have cooperated with p27Kip1 to restore in vitro and in vivo hematopoietic functions of the progenitor cells from the marrow. Kinetic studies indicate that the first detectable (within 60 sec) change induced by Aplidin® in several cancer cells is represented by Rac1 activation, a protein that regulates p27kip1 expression (Gonzalez-Santiago et al., 2006; Munoz-Alonso et al., 2008). The observations that Gata1low stem/ progenitor cells express low levels of p27Kip1 and that Aplidin® restored p27Kip1 expression in these cells, suggest that the phenotype of Gata1low stem/progenitor cells includes insufficient levels of Rac1 activation. Unfortunately, due to its low level of basal activity (Gu et al., 2003), levels of Rac1 activation in the bone marrow of Gata1low and wild-type mice could not be directly compared. However, in agreement with the hypothesis that Rac1 is down regulated in Gata1low mice, stem/progenitor cells from Rac1 deficient mice display, as those from Gata1low mice, CXCR4-independent enhanced mobilization and engraft poorly when transplanted into NOD/ SCID mice (Gu et al., 2003). It is, therefore, conceivable that, similar to findings observed in other cell systems, Aplidin® induced p27Kip1 expression in Gata1low cells through Rac1 activation.
In conclusion, Aplidin®-treatment was well tolerated by Gata1low mice with acceptable toxicity (death) and morbidity (changes in body weight) and prevented development of myelofibrosis by partially restoring progenitor cell and microenvironmental functions in the marrow. On the basis of these encouraging results, the design for a clinical trial with Aplidin® in PMF patients is under development (Dr. A. Tefferi, personal communication, April 2, 2010).
Supplementary Material
Acknowledgments
This study was supported by Ministero per la Ricerca Scientifica, Alleanza sul Cancro (ARM and AMV), Toscana Life Foundation, Italy (AMV), National Cancer Institute (grant no. P01-CA108671), and NY STAR, NY, USA (ARM). MV was supported by a fellowship from Pharma Mar S.A.
Contract grant sponsor: Ministero per la Ricerca Scientifica, Alleanza sul Cancro.
Contract grant sponsor: Toscana Life Foundation, Italy.
Contract grant sponsor: National Cancer Institute;
Contract grant number: P01-CA108671.
Contract grant sponsor: NY STAR, NY, USA.
Contract grant sponsor: Pharma Mar S.A..
Footnotes
Conflict of interest disclosure: AP, FM, PG, MZ, BG, E D’A, AMV, and ARM declare no competing financial interest. MA and JJ are/ were employed by Pharma Mar and MV received a fellowship from Pharma Mar.
Additional Supporting Information may be found in the online version of this article.
Literature Cited
- Albella B, Faircloth G, Lopez-Lazaro L, Guzman C, Jimeno J, Bueren JA. In vitro toxicity of ET-743 and aplidine, two marine-derived antineoplastics, on human bone marrow haematopoietic progenitors. Comparison with the clinical results. Eur J Cancer. 2002;38:1395–1404. doi: 10.1016/s0959-8049(01)00245-3. [DOI] [PubMed] [Google Scholar]
- Baldassarre G, Belletti B, Nicoloso MS, Schiappacassi M, Vecchione A, Spessotto P, Morrione A, Canzonieri V, Colombatti A. p27(Kip1)-stathmin interaction influences sarcoma cell migration and invasion. Cancer Cell. 2005;7:51–63. doi: 10.1016/j.ccr.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Biscardi M, Caporale R, Balestri F, Gavazzi S, Jimeno J, Grossi A. VEGF inhibition and cytotoxic effect of aplidin in leukemia cell lines and cells from acute myeloid leukemia. Ann Oncol. 2005;16:1667–1674. doi: 10.1093/annonc/mdi311. [DOI] [PubMed] [Google Scholar]
- Bogani C, Ponziani V, Guglielmelli P, Desterke C, Rosti V, Bosi A, Le Bousse-Kerdiles MC, Barosi G, Vannucchi AM. Hypermethylation of CXCR4 promoter in CD34+ cells from patients with primary myelofibrosis. Stem Cells. 2008;26:1920–1930. doi: 10.1634/stemcells.2008-0377. [DOI] [PubMed] [Google Scholar]
- Bravo SB, Garcia-Rendueles ME, Seoane R, Dosil V, Cameselle-Teijeiro J, Lopez-Lazaro L, Zalvide J, Barreiro F, Pombo CM, Alvarez CV. Plitidepsin has a cytostatic effect in human undifferentiated (anaplastic) thyroid carcinoma. Clin Cancer Res. 2005;11:7664–7673. doi: 10.1158/1078-0432.CCR-05-0455. [DOI] [PubMed] [Google Scholar]
- Broggini M, Marchini SV, Galliera E, Borsotti P, Taraboletti G, Erba E, Sironi M, Jimeno J, Faircloth GT, Giavazzi R, D’Incalci M. Aplidine, a new anticancer agent of marine origin, inhibits vascular endothelial growth factor (VEGF) secretion and blocks VEGF-VEGFR-1 (flt-1) autocrine loop in human leukemia cells MOLT-4. Leukemia. 2003;17:52–59. doi: 10.1038/sj.leu.2402788. [DOI] [PubMed] [Google Scholar]
- Broxmeyer HE. Chemokines in hematopoiesis. Curr Opin Hematol. 2008;15:49–58. doi: 10.1097/MOH.0b013e3282f29012. [DOI] [PubMed] [Google Scholar]
- Caers J, Menu E, De Raeve H, Lepage D, Van Valckenborgh E, Van Camp B, Alvarez E, Vanderkerken K. Antitumour and antiangiogenic effects of Aplidin in the 5TMM syngeneic models of multiple myeloma. Br J Cancer. 2008;98:1966–1974. doi: 10.1038/sj.bjc.6604388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancelas JA, Lee AW, Prabhakar R, Stringer KF, Zheng Y, Williams DA. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med. 2005;11:886–891. doi: 10.1038/nm1274. [DOI] [PubMed] [Google Scholar]
- Cervantes F, Mesa R, Barosi G. New and old treatment modalities in primary myelofibrosis. Cancer J. 2007;13:377–383. doi: 10.1097/PPO.0b013e31815a7c0a. [DOI] [PubMed] [Google Scholar]
- Cheng T, Rodrigues N, Dombkowski D, Stier S, Scadden DT. Stem cell repopulation efficiency but not pool size is governed by p27(kip1) Nat Med. 2000;6:1235–1240. doi: 10.1038/81335. [DOI] [PubMed] [Google Scholar]
- Depenbrock H, Peter R, Faircloth GT, Manzanares I, Jimeno J, Hanauske AR. In vitro activity of aplidine, a new marine-derived anti-cancer compound, on freshly explanted clonogenic human tumour cells and haematopoietic precursor cells. Br J Cancer. 1998;78:739–744. doi: 10.1038/bjc.1998.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferme C, Mateos MV, Szyldergemajn S, Zucca E, Espinoza J, Briones J, Morschhauser F, Gisselbrecht C, Ribrag V. Plitideps in is active in peripheral T-cell lynphoma (PTCL): A subset analysis from an ongoing multicenter phase II trial. Blood. 2008;112:1566. [Google Scholar]
- Fukuchi Y, Yamato K, Kawamura C, Ikeda Y, Kizaki M. p27KIP1 and GATA-1 are potential downstream molecules in activin A-induced differentiation and apoptosis pathways in CML cells. Oncol Rep. 2006;16:1099–1103. [PubMed] [Google Scholar]
- Garimella R, Kacena MA, Tague SE, Wang J, Horowitz MC, Anderson HC. Expression of bone morphogenetic proteins and their receptors in the bone marrow megakaryocytes of GATA-1(low) mice: A possible role in osteosclerosis. J Histochem Cytochem. 2007;55:745–752. doi: 10.1369/jhc.6A7164.2007. [DOI] [PubMed] [Google Scholar]
- Ghinassi B, Sanchez M, Martelli F, Amabile G, Vannucchi AM, Migliaccio G, Orkin SH, Migliaccio AR. The hypomorphic Gata1 low mutation alters the proliferation/ differentiation potential of the common megakaryocytic-erythroid progenitor. Blood. 2007;109:1460–1471. doi: 10.1182/blood-2006-07-030726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghinassi B, Martelli F, Verrucci M, D’Amore E, Migliaccio G, Vannucchi AM, Hoffman R, Migliaccio AR. Evidence for organ-specific stem cell microenvironments. J Cell Physiol. 2010;223:460–470. doi: 10.1002/jcp.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez SG, Bueren JA, Faircloth GT, Jimeno J, Albella B. In vitro toxicity of three new antitumoral drugs (trabectedin, aplidin, and kahalalide F) on hematopoietic progenitors and stem cells. Exp Hematol. 2003;31:1104–1111. [PubMed] [Google Scholar]
- Gonzalez-Santiago L, Suarez Y, Zarich N, Munoz-Alonso MJ, Cuadrado A, Martinez T, Goya L, Iradi A, Saez-Tormo G, Maier JV, Moorthy A, Cato AC, Rojas JM, Munoz A. Aplidin induces JNK-dependent apoptosis in human breast cancer cells via alteration of glutathione homeostasis, Rac1 GTPase activation, and MKP-1 phosphatase downregulation. Cell Death Differ. 2006;13:1968–1981. doi: 10.1038/sj.cdd.4401898. [DOI] [PubMed] [Google Scholar]
- Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, Harris CE, Lee AW, Prabhakar R, Atkinson SJ, Kwiatkowski DJ, Williams DA. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- Huang Z, Dore LC, Li Z, Orkin SH, Feng G, Lin S, Crispino JD. GATA-2 reinforces megakaryocyte development in the absence of GATA-1. Mol Cell Biol. 2009;29:5168–5180. doi: 10.1128/MCB.00482-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacena MA, Shivdasani RA, Wilson K, Xi Y, Troiano N, Nazarian A, Gundberg CM, Bouxsein ML, Lorenzo JA, Horowitz MC. Megakaryocyte-osteoblast interaction revealed in mice deficient in transcription factors GATA-1 and NF-E2. J Bone Miner Res. 2004;19:652–660. doi: 10.1359/JBMR.0301254. [DOI] [PubMed] [Google Scholar]
- Lane SW, Scadden DT, Gilliland DG. The leukemic stem cell niche: Current concepts and therapeutic opportunities. Blood. 2009;114:1150–1157. doi: 10.1182/blood-2009-01-202606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lataillade JJ, Pierre-Louis O, Hasselbalch HC, Uzan G, Jasmin C, Martyre MC, Le Bousse-Kerdiles MC. Does primary myelofibrosis involve a defective stem cell niche? From concept to evidence. Blood. 2008;112:3026–3035. doi: 10.1182/blood-2008-06-158386. [DOI] [PubMed] [Google Scholar]
- Martelli F, Ghinassi B, Panetta B, Alfani E, Gatta V, Pancrazzi A, Bogani C, Vannucchi AM, Paoletti F, Migliaccio G, Migliaccio AR. Variegation of the phenotype induced by the Gata1 low mutation in mice of different genetic backgrounds. Blood. 2005;106:4102–4113. doi: 10.1182/blood-2005-03-1060. [DOI] [PubMed] [Google Scholar]
- Mateos MV, Cibeira MT, Richardson P, Blade J, Prosper F, Oriol A, Rubia J, Alegre A, Lahuerta JJ, Garcia-Sanz R, Mitsiades CS. Final results of a phase II trial with plitidepsin (Aplidin) alone and in combination with dexamethasone in patients with relapsed/refractory multiple myeloma. Blood. 2008:112–3700. [Google Scholar]
- Messina G, Blasi C, La Rocca SA, Pompili M, Calconi A, Grossi M. p27Kip1 acts downstream of N-cadherin-mediated cell adhesion to promote myogenesis beyond cell cycle regulation. Mol Biol Cell. 2005;16:1469–1480. doi: 10.1091/mbc.E04-07-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio AR, Martelli F, Verrucci M, Migliaccio G, Vannucchi AM, Ni H, Xu M, Jiang Y, Nakamoto B, Papayannopoulou T, Hoffman R. Altered SDF-1/CXCR4 axis in patients with primary myelofibrosis and in the Gata1 low mouse model of the disease. Exp Hematol. 2008;36:158–171. doi: 10.1016/j.exphem.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio AR, Martelli F, Verrucci M, Sanchez M, Valeri M, Migliaccio G, Vannucchi AM, Zingariello M, Di Baldassarre A, Ghinassi B, Rana RA, van Hensbergen Y, Fibbe WE. GATA1 expression driven by the alternative HS2 enhancer in the spleen rescues the hematopoietic failure induced by the hypomorphic GATA1 low mutation. Blood. 2009;114:2107–2120. doi: 10.1182/blood-2009-03-211680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsiades CS, Ocio EM, Pandiella A, Maiso P, Gajate C, Garayoa M, Vilanova D, Montero JC, Mitsiades N, McMullan CJ, Munshi NC, Hideshima T, Chauhan D, Aviles P, Otero G, Faircloth G, Mateos MV, Richardson PG, Mollinedo F, San-Miguel JF, Anderson KC. Aplidin, a marine organism-derived compound with potent antimyeloma activity in vitro and in vivo. Cancer Res. 2008;68:5216–5225. doi: 10.1158/0008-5472.CAN-07-5725. [DOI] [PubMed] [Google Scholar]
- Moneo V, Serelde BG, Leal JF, Blanco-Aparicio C, Diaz-Uriarte R, Aracil M, Tercero JC, Jimeno J, Carnero A. Levels of p27(kip1) determine Aplidin sensitivity. Mol Cancer Ther. 2007;6:1310–1316. doi: 10.1158/1535-7163.MCT-06-0729. [DOI] [PubMed] [Google Scholar]
- Munoz-Alonso MJ, Gonzalez-Santiago L, Zarich N, Martinez T, Alvarez E, Rojas JM, Munoz A. Plitidepsin has a dual effect inhibiting cell cycle and inducing apoptosis via Rac1/c-Jun NH2-terminal kinase activation in human melanoma cells. J Pharmacol Exp Ther. 2008;324:1093–1101. doi: 10.1124/jpet.107.132662. [DOI] [PubMed] [Google Scholar]
- Munoz-Alonso MJ, Gonzalez-Santiago L, Martinez T, Losada A, Galmarini CM, Munoz A. The mechanism of action of plitidepsin. Curr Opin Investig Drugs. 2009;10:536–542. [PubMed] [Google Scholar]
- Orkin SH, Zon LI. Hematopoiesis: An evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardanani A. JAK2 inhibitor therapy in myeloproliferative disorders: Rationale, preclinical studies and ongoing clinical trials. Leukemia. 2008;22:23–30. doi: 10.1038/sj.leu.2404948. [DOI] [PubMed] [Google Scholar]
- Ray A, James MK, Larochelle S, Fisher RP, Blain SW. p27Kip1 inhibits cyclin D-cyclin-dependent kinase 4 by two independent modes. Mol Cell Biol. 2009;29:986–999. doi: 10.1128/MCB.00898-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Ishii T, Hu W, Sozer S, Zhang W, Bruno E, Lindgren V, Xu M, Hoffman R. Effects of chromatin-modifying agents on CD34+ cells from patients with idiopathic myelofibrosis. Cancer Res. 2007;67:6417–6424. doi: 10.1158/0008-5472.CAN-07-0572. [DOI] [PubMed] [Google Scholar]
- Straight AM, Oakley K, Moores R, Bauer AJ, Patel A, Tuttle RM, Jimeno J, Francis GL. Aplidin reduces growth of anaplastic thyroid cancer xenografts and the expression of several angiogenic genes. Cancer Chemother Pharmacol. 2006;57:7–14. doi: 10.1007/s00280-005-0014-7. [DOI] [PubMed] [Google Scholar]
- Taraboletti G, Poli M, Dossi R, Manenti L, Borsotti P, Faircloth GT, Broggini M, D’Incalci M, Ribatti D, Giavazzi R. Antiangiogenic activity of aplidine, a new agent of marine origin. Br J Cancer. 2004;90:2418–2424. doi: 10.1038/sj.bjc.6601864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A. Myelofibrosis with myeloid metaplasia. N Engl J Med. 2000;342:1255–1265. doi: 10.1056/NEJM200004273421706. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Thiele J, Orazi A, Kvasnicka HM, Barbui T, Hanson CA, Barosi G, Verstovsek S, Birgegard G, Mesa R, Reilly JT, Gisslinger H, Vannucchi AM, Cervantes F, Finazzi G, Hoffman R, Gilliland DG, Bloomfield CD, Vardiman JW. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: Recommendations from an ad hoc international expert panel. Blood. 2007;110:1092–1097. doi: 10.1182/blood-2007-04-083501. [DOI] [PubMed] [Google Scholar]
- Teicher B, Andrews P. In vivo methods. In: Teicher BA, editor. Anticancer drug development guide. 2nd edition. Totama, NJ: Humana Press; 2004. p. 110. [Google Scholar]
- Vannucchi AM, Bianchi L, Paoletti F, Pancrazzi A, Torre E, Nishikawa M, Zingariello M, Di Baldassarre A, Rana RA, Lorenzini R, Alfani E, Migliaccio G, Migliaccio AR. A pathobiologic pathway linking thrombopoietin, GATA-1, and TGF-beta1 in the development of myelofibrosis. Blood. 2005a;105:3493–3501. doi: 10.1182/blood-2004-04-1320. [DOI] [PubMed] [Google Scholar]
- Vannucchi AM, Pancrazzi A, Guglielmelli P, Di Lollo S, Bogani C, Baroni G, Bianchi L, Migliaccio AR, Bosi A, Paoletti F. Abnormalities of GATA-1 in megakaryocytes from patients with idiopathic myelofibrosis. Am J Pathol. 2005b;167:849–858. doi: 10.1016/S0002-9440(10)62056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varricchio L, Mancini A, Migliaccio AR. Pathological interactions between hematopoietic stem cells and their niche revealed by mouse models of primary myelofibrosis. Expert Rev Hematol. 2009;2:315–334. doi: 10.1586/ehm.09.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley MJ, Ooi SK, Reynolds LF, Smith SH, Ruf S, Mathiot A, Vanes L, Williams DA, Cancro MP, Tybulewicz VL. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science. 2003;302:459–462. doi: 10.1126/science.1089709. [DOI] [PubMed] [Google Scholar]
- Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell. 2008;133:340–353. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan H, Spivak JL. The diagnosis and management of polycythemia vera, essential thrombocythemia, and primary myelofibrosis in the JAK2 V617F era. Clin Adv Hematol Oncol. 2009;7:334–342. [PubMed] [Google Scholar]
- Zon LI, Tsai SF, Burgess S, Matsudaira P, Bruns GA, Orkin SH. The major human erythroid DNA-binding protein (GF-1): Primary sequence and localization of the gene to the X chromosome. Proc Natl Acad Sci USA. 1990;87:668–672. doi: 10.1073/pnas.87.2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.