SUMMARY
Background
Patients with methicillin-resistant Staphylococcus aureus (MRSA) infections caused by isolates with a high but ‘susceptible’ minimum inhibitory concentration (MIC) to vancomycin may suffer poor outcomes. The aim of this study was to determine the association of high compared to low vancomycin MICs and clinical outcomes (treatment failure and mortality) in patients with MRSA infections.
Methods
PubMed, the Cochrane Library, and electronic abstracts from meetings were queried from January 2000 to July 2010. Two reviewers independently screened titles and abstracts of studies evaluating outcomes of patients with MRSA infections, using broth microdilution (BMD) or the Etest to determine MIC, for full-text review. Patients participating in included studies were classified into two mutually exclusive groups: high MIC or low MIC. High MIC was defined as MIC ≥1 mg/l by BMD or ≥ 1.5 mg/l by Etest. Study-defined failure and mortality were assessed in each group.
Results
Fourteen publications and six electronic abstracts met the inclusion criteria, with 2439 patients (1492 high MIC and 947 low MIC). There was no evidence of publication bias or heterogeneity. An increased risk of failure was observed in the high MIC group compared to the low MIC group (summary risk ratio (RR) 1.40, 95% confidence interval (CI) 1.15–1.71). The overall mortality risk was greater in the high MIC group than in the low MIC group (summary RR 1.42, 95% CI 1.08–1.87). Sensitivity analyses showed similar findings for failure (summary RR 1.37, 95% CI 1.09–1.73) and mortality (summary RR 1.46, 95% CI 1.06–2.01) for patients with bacteremia. The study quality was poor-to-moderate, and study-defined endpoints were variable.
Conclusions
A susceptible but high MIC to vancomycin is associated with increased mortality and treatment failure among patients with MRSA infections.
Keywords: Methicillin-resistant Staphylococcus aureus, Microbial sensitivity testing, Bacteremia, Vancomycin, Mortality
1. Introduction
The emergence of methicillin-resistant Staphylococcus aureus (MRSA) in the late 1970s dramatically increased the use of vancomycin.1 For over 20 years, intravenous vancomycin has been the standard of care for serious MRSA infections. By 1996, the first isolate of MRSA with reduced susceptibility to vancomycin was reported.2 Although frank resistance to vancomycin is rare in S. aureus, intermediate resistance is more common.3 In 2006 the Clinical and Laboratory Standards Institute lowered the minimum inhibitory concentration (MIC) breakpoint for vancomycin susceptibility from >4 mg/l to >2 mg/l, partially based on reports of clinical failures in patients with infections caused by MRSA isolates with MICs of ≥4 mg/l.4,5 However, based on growing clinical experience of treatment failure against MRSA with vancomycin MICs in the higher end of the new ‘susceptible’ range, clinicians have begun to question the efficacy of vancomycin in patients with serious MRSA infections.6
The current literature provides inconsistent information on clinical outcomes of patients with infections caused by MRSA with high ‘susceptible’ MICs to vancomycin compared to low ‘susceptible’ MICs. Some studies have shown no significant association between higher MICs and poor outcomes,7,8 but others have suggested an association with increased treatment failures and higher mortality.9-13 Unfortunately, there are many differences in variables between individual studies (different patient populations, laboratory methods, outcome measures, etc.) making it difficult to interpret their findings and adding fuel to the ongoing debate regarding the efficacy of vancomycin against MRSA isolates with MICs at the higher end of the ‘susceptible’ range.
In order to comprehensively assess the available evidence addressing the question of whether high ‘susceptible’ vancomycin MICs are associated with poor clinical outcomes in patients with serious MRSA infections, we performed a systematic review and meta-analysis.
2. Methods
2.1. Data sources and searches
Studies reported between January 2000 and July 2011 in Medline and the Cochrane Library were identified by two infectious diseases subspecialists (JTJ, CAD); three search strategies were used, all applying the Boolean connector ‘and’ with the term ‘microbial sensitivity tests’: (1) ‘methicillin resistance’ and ‘staphylococcal infections’; (2) ‘methicillin resistance’ and ‘Staphylococcus aureus’; and (3) ‘methicillin-resistant Staphylococcus aureus’. Results were limited to ‘Humans’ and ‘All Adults’ (age ≥18 years) without any restriction on language. References in these studies were also reviewed to identify further sources. In addition, the electronic abstracts of the 2007, 2008, and 2009 Infectious Diseases Society of America (IDSA) annual meetings were reviewed using a similar methodology. The search was last run on August 12, 2011.
2.2. Study selection
Studies evaluating outcomes (failure and/or mortality) of patients with MRSA infections, stratified by MIC determined using broth microdilution (BMD) or the Etest, were considered candidates for inclusion; study designs that addressed this question, including case–control studies, cohort studies, and randomized control trials, were evaluated. Studies using only automated instruments were excluded, as were those exclusively focused on vancomycin-intermediate S. aureus (VISA) or hetero-resistant VISA (hVISA), or comparing isolates at extreme ranges of MIC (i.e., ≤0.5 vs. ≥2).
2.3. Data extraction and quality assessment
Data in tables, figures, or the text from included studies were independently extracted by two reviewers (JTJ, CAD); if estimates of outcome were not provided in the available study reports (abstracts, posters, or manuscripts), authors were contacted by e-mail at least twice in an attempt to obtain the required information. Differences in opinion between reviewers were resolved by consensus. Patients were classified into two mutually exclusive groups: low MIC or high MIC. Patients with MRSA isolates with a vancomycin MIC <1 mg/l by BMD or <1.5 mg/l by Etest were categorized in the low MIC group; patients with MRSA isolates with a vancomycin MIC ≥1 mg/l by BMD or ≥1.5 mg/l by Etest were classified into the high MIC group.
For the primary outcomes of treatment failure and mortality, individual study definitions were adapted for the review. If mortality was the only outcome assessed in a particular study, all deaths were included as treatment failures for the assessment of the treatment failure outcome. Whatever the mortality estimate provided by a study (hospital mortality, 14-day mortality, 30-day mortality, etc.), this was used for the assessment of the mortality outcome in the review. Similarly, whatever the treatment failure outcome defined by a study, this was used for the assessment of the treatment failure outcome in the review.
2.4. Data synthesis and analysis
Publication bias was explored graphically using funnel plots. Individual study quality was assessed by exploration of possible selection and misclassification bias, as well as confounding. Heterogeneity was assessed with the heterogeneity test and I2 estimation. A p-value of ≤0.05 for the heterogeneity Chi-square test or an I2 ≥50% were considered evidence of heterogeneity.
Three predetermined different sensitivity analyses were performed: (1) studies that reported failure in patients with MRSA bloodstream infection (BSI); (2) studies that reported mortality in patients with MRSA BSI; and (3) studies that used the Etest for MIC classification and reported mortality of patients with MRSA BSI. Risk ratios (RR) with 95% confidence intervals (CI) were calculated using random-effects models (DerSimonian and Laird).14 Review Manager (RevMan) 5.1 software was used for the analysis and to create funnel and Forest plots.15
3. Results
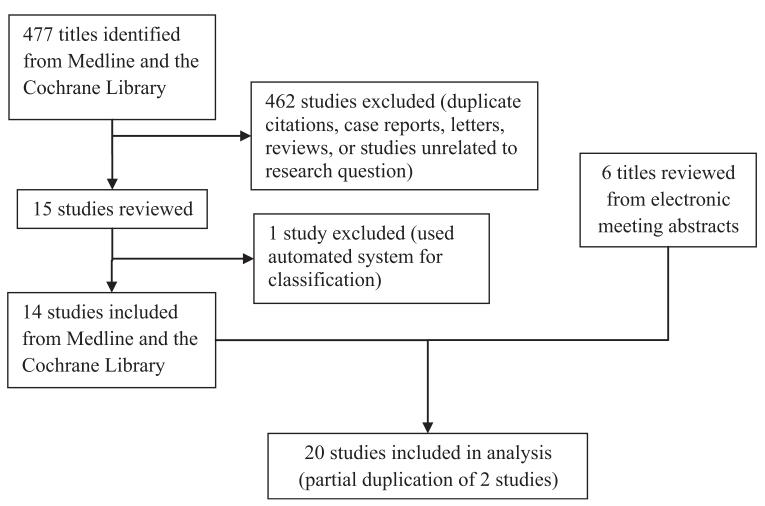
Of the 477 studies identified from Medline and the Cochrane Library, 462 were excluded because they were case reports, letters, reviews, studies not related to the research question, or duplicates (Figure 1). One study was subsequently excluded because it used an automated system alone for MIC classification. Of the14 remaining studies identified,10,12,13,16-32 two studies had partial duplication of data,10,12 but only one set of data from the overlapping studies was used in each analysis. Review of the electronic abstracts of meetings yielded another six studies.27-32 No randomized control trials were identified. Overall study quality was poor-to-moderate since most studies were retrospective (Table 1) and therefore perceived to have a moderate-to-high risk of bias.
Figure 1.
Results of the search strategy for the meta-analysis.
Table 1.
Summary of included studies
| Authors | Study type | Year | Published | Site of isolation |
MIC method |
Eligible patients |
High MIC group (n) |
Low MIC group (n) |
Failure outcomea | Mortality outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Choi et al.16 | Retrospective | 2011 | Yes | Lung | Etest | 70 | 34 | 36 | Clinical non-response | 28-day mortality |
| Ferry et al.17 | Retrospective | 2010 | Yes | Bone/joint | BMD | 41 | 40 | 1 | Re-do surgery, relapse, limb loss, death |
End of follow-up |
| Haque et al.18 | Retrospective | 2010 | Yes | Lung | Etest | 158 | 115 | 43 | Clinical non-response | 28-day mortality |
| Hidayat et al.19 | Prospective | 2006 | Yes | Lung, blood, wound, urine |
Etest | 95 | 44 | 51 | Clinical non-response | End of follow-up |
| Kullar et al.20 | Retrospective | 2011 | Yes | Blood | Etest | 320 | 122 | 198 | Persistent infection, death |
30-day mortality |
| Lewis E et al.27 | Retrospective | 2008 | No | Blood | BMD | 106 | 25 | 81 | Persistent infection, relapse, death |
- |
| Lewis T et al.25 | Retrospective | 2011 | Yes | Blood | Etest | 142 | 3 | 139 | - | 30-day mortality |
| Lodise et al.21 | Retrospective | 2008 | Yes | Blood | Etest | 92 | 66 | 26 | Persistent infection, relapse, death |
30-day mortality |
| Lustberg et al.30 | Retrospective | 2008 | No | Blood | Etest | 113 | 66 | 47 | Relapse | In-hospital mortality |
| Moise-Broder et al.10,b |
Retrospective | 2004 | Yes | Blood | BMD | 63 | 42 | 21 | Persistent infection | - |
| Moore et al.31 | Retrospective | 2008 | No | Blood | Etest | 122 | 116 | 6 | Clinical non-response | In-hospital mortality |
| Musta et al.22 | Retrospective | 2009 | Yes | Blood | Etest | 285 | 249 | 36 | - | In-hospital mortality |
| Neuner et al.26 | Retrospective | 2010 | Yes | Blood | Etest | 195 | 185 | 10 | - | In-hospital mortality |
| Price et al.23 Sakoulas et al.12,b |
Prospective | 2009 | Yes | Blood | Etest | 31 | 8 | 23 | - | 3-month mortality |
| Retrospective | 2004 | Yes | Blood | BMD | 30 | 21 | 9 | Persistent infection | - | |
| Soriano et al.13 | Prospective | 2008 | Yes | Blood | Etest | 168 | 130 | 38 | - | 30-day mortality |
| Swami et al.28 | Retrospective | 2008 | No | Blood | Etest | 97 | 52 | 45 | - | 30-day mortality |
| Wang et al.24 | Prospective | 2010 | Yes | Blood | BMD | 123 | 120 | 3 | - | 30-day mortality |
| Wilhelm et al.29 | Retrospective | 2008 | No | Blood, lung |
Etest | 38 | 18 | 20 | - | ?In-hospital mortality |
| Yamaki and Wong- Beringer32 |
Retrospective | 2008 | No | Lung, wound, blood |
Etest | 180 | 57 | 123 | Clinical non-response | ?In-hospital mortality |
BMD, broth microdilution; MIC, minimum inhibitory concentration.
Mortality outcome used for failure outcome if not specified.
Partial duplication of data.
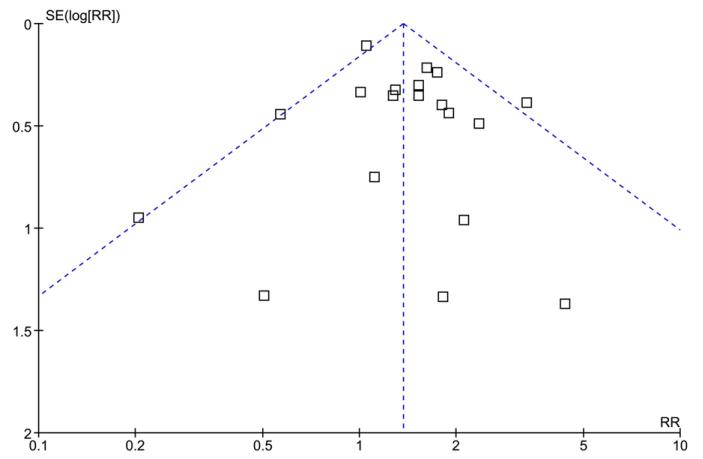
Among the 20 studies included, there was a total of 2439 unique patients, of whom 1492 (61.2%) had a high MIC and 947 (38.8%) had a low MIC. The funnel plot did not suggest publication bias (Figure 2). Most patients (1783/2439, 73.1%) in the meta-analysis came from published studies. There was no evidence of heterogeneity in all analyses.
Figure 2.
Funnel plot comparing the standard error (SE) of the logarithm of the risk ratio (RR) to the RR.
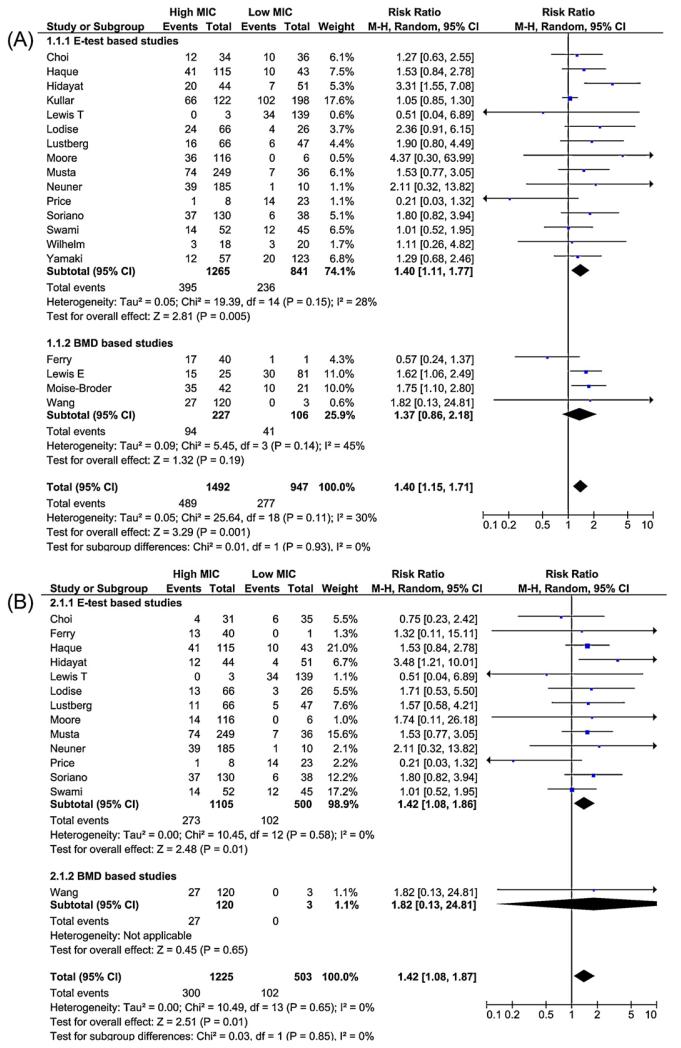
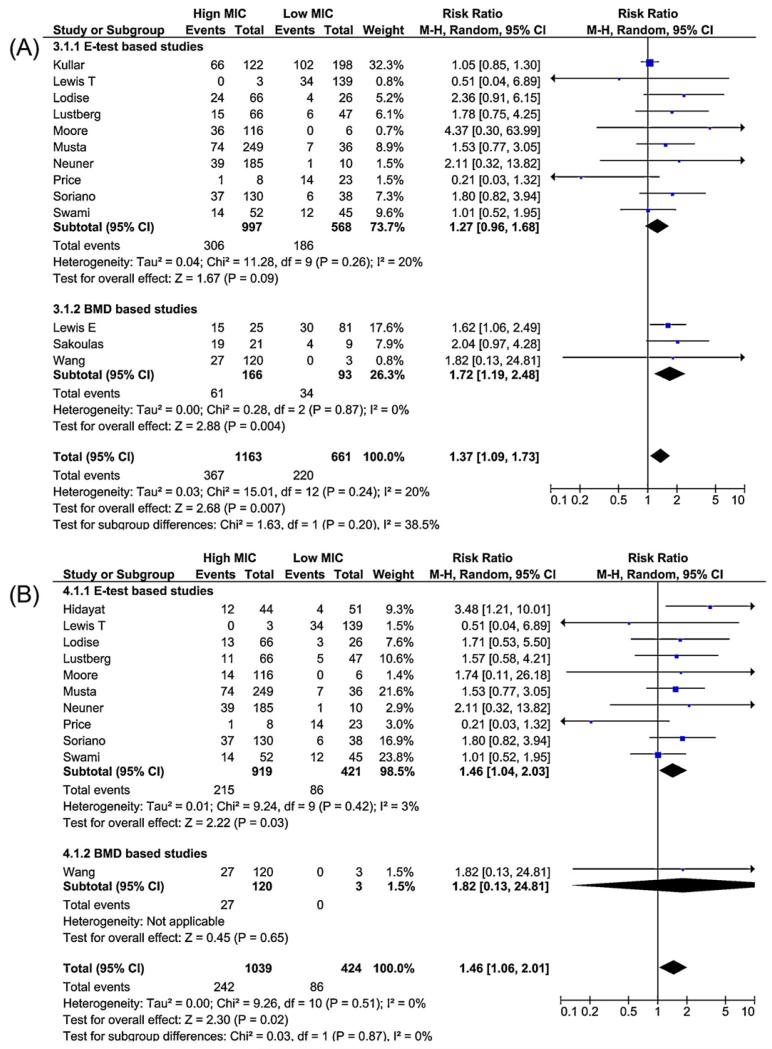
An increased risk of failure (Figure 3A) was observed in the high MIC group compared to the low MIC group (summary RR 1.40, 95% CI 1.15–1.71). The overall mortality risk was greater in the high MIC group compared to the low MIC group (summary RR 1.42, 95% CI 1.08–1.87; Figure 3B). Sensitivity analysis showed similar findings for failure (summary RR 1.37, 95% CI 1.09–1.73) and mortality (summary RR 1.46, 95% CI 1.06–2.01) in patients with BSI (Figure 4, A and B). Although there was variation in the precision of the estimates, the results were generally consistent in subgroup analyses restricted to each antimicrobial susceptibility testing method (Etest vs. BMD; Figures 3 and 4).
Figure 3.
Forest plot comparing risk ratios for the outcomes of (A) failure, and (B) mortality, comparing patients in the high minimum inhibitory concentration (MIC) group to patients in the low MIC group stratified by method of testing for MIC (Etest vs. broth microdilution (BMD)).
Figure 4.
Forest plot for the sensitivity analysis assessing only bloodstream infections, comparing risk ratios for the outcomes of (A) failure, and (B) mortality, comparing patients in the high minimum inhibitory concentration (MIC) group to patients in the low MIC group stratified by method of testing for MIC (Etest vs. broth microdilution (BMD)).
4. Discussion
This meta-analysis supports previous individual studies suggesting poorer outcomes in patients with serious MRSA infections with vancomycin MICs at the higher end of the current ‘susceptible’ range.
These findings have potential implications for clinicians involved in the management of serious MRSA infections. Some would consider the use of alternative agents for empiric treatment of suspected MRSA infections, arguing that administration of an active antimicrobial during the first 48–72 h of therapy is essential to prevent poor outcomes. However, in the authors’ opinion, current evidence is insufficient to support empiric use of alternative agents such as linezolid, daptomycin, telavancin, or quinupristin/dalfopristin for suspected serious MRSA infections for the following reasons: (1) broader use of these alternative agents will likely lead to increasing resistance to them; (2) frank vancomycin resistance remains rare; and (3) only a fraction of the patients with suspected MRSA infections are proven to have MRSA, and an even smaller proportion of these will be caused by organisms with a vancomycin MIC in the high-susceptible range.
Although some studies suggest that alternative antimicrobials may have therapeutic advantages over vancomycin, no definitive evidence from randomized trials indicates the superiority of any other agent.33-36 Unfortunately, few investigational agents are in the drug pipeline, and for the foreseeable future, clinicians will need to rely on currently available agents.37 Evidence assessing the effectiveness of antimicrobials other than vancomycin for the treatment of serious infections caused specifically by isolates with vancomycin MICs in the high ‘susceptible’ range is even more limited. Only a few of the reviewed studies, all with methodological limitations, attempted to address the question. Soriano et al. concluded that a MIC >1 mg/l by Etest was one of several risk factors for increased mortality, but only in patients treated empirically with vancomycin.13 Hidayat et al. reported that 12 out of 15 patients (80%) with MRSA infections with high vancomycin MICs and failing vancomycin therapy had successful outcomes when switched to other antimicrobial agents.19
A recently published study questions the therapeutic relevance of the association of poor outcomes and high vancomycin MIC: the association was demonstrated for S. aureus isolates overall, but there was no association with methicillin resistance or vancomycin therapy, suggesting that the poor outcomes may not actually be caused by an antibiotic failure.38 In contrast, the above-mentioned study by Soriano et al. suggests the opposite, given that increased mortality was found only in patients who received empiric therapy with vancomycin. In addition, the study by Kullar et al. indicates that antibiotic therapy failure may be at least partially responsible for poor outcomes, given that a pharmacodynamic parameter specific to vancomycin and difficult to overcome in the presence of isolates with higher vancomycin MICs (an area under the curve (AUC)/MIC of <421) was found to be associated with failure according to a classification and regression tree (CART) analysis.20 Finally, the observation from several studies that vancomycin MIC is associated with poor outcomes despite controlling for other possible determinants of failure or mortality, may further suggest that the association of these outcomes with high ‘susceptible’ MICs to vancomycin may be due to antibiotic treatment failure as opposed to other variables; only a randomized controlled trial would allow for adequate control of potential known and unknown confounders.
Because of the limitations of the available evidence, professional societies play an especially important role in guiding physicians in practice. To date, two US guidelines have addressed the management of serious MRSA infections caused by isolates with vancomycin MICs in the high ‘susceptible’ range. The vancomycin therapeutic monitoring guidelines from the American Society of Health-System Pharmacists, IDSA, and the Society for Infectious Disease Pharmacists recommend considering alternative therapies for MRSA infections if the MIC is ≥ 2 mg/l.39 In contrast, the more recent IDSA guidelines for the treatment of MRSA infections recommend that for isolates with a vancomycin MIC ≤2 mg/l, the patient’s clinical response should determine the continued use of vancomycin, independent of the MIC.40 The two guidelines give, therefore, potentially discordant recommendations for the management of infections caused by isolates with a MIC of 2 mg/l. Given that the manufacturer recommends that “an Etest MIC value which falls between standard two-fold dilutions must be rounded up to the next upper two-fold value before categorisation”41, and that according to this review a large proportion of recent MRSA isolates (around 60%) have a MIC ≥1.5 mg/l, many serious MRSA infections may be classified nowadays as caused by isolates for which consistent therapeutic guidance from professional societies is lacking.
In the authors’ opinion, rational decision-making should take into account clinical response, severity of illness, and potential side effects, as well as the other principles of antibiotic stewardship. An evaluation of the patient should include clinical factors and ensure that the appropriate interventions, such as adequate drainage and repeat cultures if appropriate, are performed. Awaiting further evidence from more methodologically sound studies, the authors suggest that a known vancomycin MIC of ≥1.5 mg/l by Etest (or ≥ 1 mg/l by BMD) should lower the clinician’s threshold to switch to alternative therapies in moderately to severely ill MRSA-infected patients without a rapid clinical or microbiological response to adequate vancomycin therapy and source control (if applicable).
A recently published meta-analysis on vancomycin MIC in S. aureus infections by van Hal et al. used different search terms, excluded abstracts from scientific conferences, and included studies using MIC determined by automated broth microdilution and patients with methicillin-susceptible S. aureus (MSSA) in their analysis.42 van Hal et al. classified MICs using a single breakpoint (1.5 μg/ml) regardless of the method of determination, whereas this analysis used established breakpoints that differ between the Etest and BMD, based on previous reports indicating that there is inconsistent correlation, compared to reference BMD, of MIC using different testing modalities, especially with automated instruments.43,44 These methodological differences led to variation in the studies included in the mortality analysis (both included twelve studies,10,12,13,16,18,19,21-24,26 but van Hal et al. selected twelve MRSA studies7,9,23,45-53 not in this review, and the current review incorporated eight studies17,20,25,27-32 that Van Hal did not). Despite these differences, it is reassuring that these two independently performed reviews both found the same associations between high vancomycin MIC and treatment failure and mortality.
This study has several limitations. Despite the lack of heterogeneity in our statistical analysis, studies included used different patient populations, different definitions of failure, or different time-points for the assessment of mortality. Many of the studies were retrospective in nature, all were observational, and most were assessed to have at least a moderate risk for bias and confounding. Additionally, some studies were reports from medical meetings (abstracts or posters) and were therefore not subjected to a judicious peer-reviewed process.
A large, multicenter randomized controlled trial is ideally needed for solving the question of whether serious infections caused by MRSA isolates with high (but still ‘susceptible’) MICs should be treated with vancomycin as opposed to other therapies; such a study will likely be extremely complex, requiring a large sample size, incorporating the optimal pharmacodynamic dosing and monitoring, and assessing the toxicities possibly associated with the use of higher vancomycin doses. Unfortunately such a study is unlikely to be performed in the near future. In the absence of definitive evidence, the burden is on professional societies to provide clear overall recommendations for the management of MRSA infections and on clinicians to take responsibility for individualized therapeutic decisions at the bedside.
Acknowledgements
JTJ is supported in part by PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources.
Footnotes
Conflict of interest: JTJ has no relevant conflicts of interest to disclose. CDG is currently employed by Sanofi Pasteur, a company that does not make therapeutic agents or diagnostic testing for Staphylococcus aureus.
References
- 1.Moellering RC., Jr Vancomycin: a 50-year reassessment. Clin Infect Dis. 2006;42(Suppl 1):S3–4. doi: 10.1086/491708. [DOI] [PubMed] [Google Scholar]
- 2.Ploy MC, Grelaud C, Martin C, de Lumley L, Denis F. First clinical isolate of vancomycin-intermediate Staphylococcus aureus in a French hospital. Lancet. 1998;351:1212. doi: 10.1016/s0140-6736(05)79166-2. [DOI] [PubMed] [Google Scholar]
- 3.US Centers for Disease Control and Prevention . CDC reminds clinical laboratories and healthcare infection preventionists of their role in the search and containment of vancomycin-resistant Staphylococcus aureus (VRSA) CDC; Atlanta, GA: [accessed April 24, 2011]. 2011. Available at: http://www.cdc.gov/HAI/settings/lab/vrsa_lab_search_containment.html. [Google Scholar]
- 4.Clinical and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing. CLSI approved standard M100-S16. CLSI; Wayne, PA: 2006. [Google Scholar]
- 5.Tenover FC, Moellering RC., Jr. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis. 2007;44:1208–15. doi: 10.1086/513203. [DOI] [PubMed] [Google Scholar]
- 6.Hageman JC, Liedtke LA, Sunenshine RH, Strausbaugh LJ, McDonald LC, Tenover FC. Management of persistent bacteremia caused by methicillin-resistant Staphylococcus aureus: a survey of infectious diseases consultants. Clin Infect Dis. 2006;43:e42–5. doi: 10.1086/506568. [DOI] [PubMed] [Google Scholar]
- 7.Liao CH, Chen SY, Huang YT, Hsueh PR. Outcome of patients with meticillin-resistant Staphylococcus aureus bacteraemia at an Emergency Department of a medical centre in Taiwan. Int J Antimicrob Agents. 2008;32:326–32. doi: 10.1016/j.ijantimicag.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crompton JA, North DS, Yoon M, Steenbergen JN, Lamp KC, Forrest GN. Out-comes with daptomycin in the treatment of Staphylococcus aureus infections with a range of vancomycin MICs. J Antimicrob Chemother. 2010;65:1784–91. doi: 10.1093/jac/dkq200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maclayton DO, Suda KJ, Coval KA, York CB, Garey KW. Case–control study of the relationship between MRSA bacteremia with a vancomycin MIC of 2 microg/mL and risk factors, costs, and outcomes in inpatients undergoing hemodialysis. Clin Ther. 2006;28:1208–16. doi: 10.1016/j.clinthera.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Moise-Broder PA, Sakoulas G, Eliopoulos GM, Schentag JJ, Forrest A, Moellering RC., Jr Accessory gene regulator group II polymorphism in methicillin-resistant Staphylococcus aureus is predictive of failure of vancomycin therapy. Clin Infect Dis. 2004;38:1700–5. doi: 10.1086/421092. [DOI] [PubMed] [Google Scholar]
- 11.Neoh H-M, Hori S, Komatsu M, Oguri T, Takeuchi F, Cui L, et al. Impact of reduced vancomycin susceptibility on the therapeutic outcome of MRSA bloodstream infections. Ann Clin Microbiol Antimicrob. 2007;6:13. doi: 10.1186/1476-0711-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Jr, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004;42:2398–402. doi: 10.1128/JCM.42.6.2398-2402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soriano A, Marco F, Martinez JA, Pisos E, Almela M, Dimova VP, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46:193–200. doi: 10.1086/524667. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Review Manager. 5.1 ed. The Cochrane Collaboration; 2008. Copenhagen: The Nordic Cochrane Centre. [Google Scholar]
- 16.Choi EY, Huh JW, Lim CM, Koh Y, Kim SH, Choi SH, et al. Relationship between the MIC of vancomycin and clinical outcome in patients with MRSA nosocomial pneumonia. Intensive Care Med. 2011;37:639–47. doi: 10.1007/s00134-011-2130-7. [DOI] [PubMed] [Google Scholar]
- 17.Ferry T, Uckay I, Vaudaux P, Francois P, Schrenzel J, Harbarth S, et al. Risk factors for treatment failure in orthopedic device-related methicillin-resistant Staphylococcus aureus infection. Eur J Clin Microbiol Infect Dis. 2010;29:171–80. doi: 10.1007/s10096-009-0837-y. [DOI] [PubMed] [Google Scholar]
- 18.Haque NZ, Zuniga LC, Peyrani P, Reyes K, Lamerato L, Moore CL, et al. Relationship of vancomycin minimum inhibitory concentration to mortality in patients with methicillin-resistant Staphylococcus aureus hospital-acquired, ventilator-associated, or health-care-associated pneumonia. Chest. 2010;138:1356–62. doi: 10.1378/chest.09-2453. [DOI] [PubMed] [Google Scholar]
- 19.Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166:2138–44. doi: 10.1001/archinte.166.19.2138. [DOI] [PubMed] [Google Scholar]
- 20.Kullar R, Davis SL, Levine DP, Rybak MJ. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacter-emia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52:975–81. doi: 10.1093/cid/cir124. [DOI] [PubMed] [Google Scholar]
- 21.Lodise TP, Graves J, Evans A, Graffunder E, Helmecke M, Lomaestro BM, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother. 2008;52:3315–20. doi: 10.1128/AAC.00113-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musta AC, Riederer K, Shemes S, Chase P, Jose J, Johnson LB, et al. Vancomycin MIC plus heteroresistance and outcome of methicillin-resistant Staphylococcus aureus bacteremia: trends over 11 years. J Clin Microbiol. 2009;47:1640–4. doi: 10.1128/JCM.02135-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price J, Atkinson S, Llewelyn M, Paul J. Paradoxical relationship between the clinical outcome of Staphylococcus aureus bacteremia and the minimum inhibitory concentration of vancomycin. Clin Infect Dis. 2009;48:997–8. doi: 10.1086/597359. [DOI] [PubMed] [Google Scholar]
- 24.Wang JL, Wang JT, Sheng WH, Chen YC, Chang SC. Nosocomial methicillin-resistant Staphylococcus aureus (MRSA) bacteremia in Taiwan: mortality analyses and the impact of vancomycin, MIC = 2 mg/l, by the broth microdilution method. BMC Infect Dis. 2010;10:159. doi: 10.1186/1471-2334-10-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis T, Chaudhry R, Nightingale P, Lambert P, Das I. Methicillin-resistant Staphylococcus aureus bacteremia: epidemiology, outcome, and laboratory characteristics in a tertiary referral center in the UK. Int J Infect Dis. 2011;15:e131–5. doi: 10.1016/j.ijid.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Neuner EA, Casabar E, Reichley R, McKinnon PS. Clinical, microbiologic, and genetic determinants of persistent methicillin-resistant Staphylococcus aureus bacteremia. Diagn Microbiol Infect Dis. 2010;67:228–33. doi: 10.1016/j.diagmicrobio.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 27.Lewis E, Welsh K, Abbot A, Gardiner J, Wanger A, Armitige L, et al. Risk factors for the failure of vancomycin in the treatment of MRSA bacteremia; Interscience Conference on Antimicrobial Agents and Chemotherapy/Infectious Diseases Society of America Joint Meeting; 2008. [Google Scholar]
- 28.Swami A, de Sanctis J, Gerasymchuk L, Sawarynski K, Powell K, Robinson-Dunn B, et al. Clinical association of vancomycin MIC creep, agr group II locus, and treatment failure in MRSA bacteremia; Interscience Conference on Antimicrobial Agents and Chemotherapy/Infectious Diseases Society of America Joint Meeting; 2008. [Google Scholar]
- 29.Wilhelm K, Patel G, Tahera L, Kelly M, Cooper T. Vancomycin: the impact of MIC on clinical outcomes in MRSA bacteremia and pneumonia; Interscience Conference on Antimicrobial Agents and Chemotherapy/Infectious Diseases Society of America Joint Meeting; 2008. [Google Scholar]
- 30.Lustberg M, Goff D, Pancholi P, Mangino J. Outcomes in patients with MRSA bacteremia and reduced susceptibility to vancomycin; Interscience Conference on Antimicrobial Agents and Chemotherapy/Infectious Diseases Society of America Joint Meeting; 2008. [Google Scholar]
- 31.Moore C, Cheema F, Chua T, Perri M, Davis S, Donabedian S, et al. Epidemiology and outcomes of vancomycin treatment for MRSA bacteremia in an urban tertiary healthcare system: preliminary results; Interscience Conference on Antimicrobial Agents and Chemotherapy/Infectious Diseases Society of America Joint Meeting; 2008. [Google Scholar]
- 32.Yamaki J, Wong-Beringer A. Predicting vancomycin susceptibility of infected MRSA strains based on clinical, epidemiological, and microbiologic variables; Interscience Conference on Antimicrobial Agents and Chemotherapy/Infectious Diseases Society of America Joint Meeting; 2008. [Google Scholar]
- 33.Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2002;34:1481–90. doi: 10.1086/340353. [DOI] [PubMed] [Google Scholar]
- 34.Kalil AC, Murthy MH, Hermsen ED, Neto FK, Sun J, Rupp ME. Linezolid versus vancomycin or teicoplanin for nosocomial pneumonia: a systematic review and meta-analysis. Crit Care Med. 2010;38:1802–8. doi: 10.1097/CCM.0b013e3181eb3b96. [DOI] [PubMed] [Google Scholar]
- 35.Fowler VG, Jr, Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355:653–65. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 36.Rubinstein E, Lalani T, Corey GR, Kanafani ZA, Nannini EC, Rocha MG, et al. Telavancin versus vancomycin for hospital-acquired pneumonia due to Gram-positive pathogens. Clin Infect Dis. 2010;52:31–40. doi: 10.1093/cid/ciq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 38.Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O’Sullivan MV, et al. Antibiotic choice may not explain poorer outcomes in patients with Staphylococcus aureus bacteremia and high vancomycin minimum inhibitory concentrations. J Infect Dis. 2011;204:340–7. doi: 10.1093/infdis/jir270. [DOI] [PubMed] [Google Scholar]
- 39.Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Jr, Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 40.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 41.Etest® package, insert. Solna. AB bioMérieux; Sweden: 2003. [Google Scholar]
- 42.van Hal SJ, Lodise TP, Paterson DL. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis. 2012;54:755–71. doi: 10.1093/cid/cir935. [DOI] [PubMed] [Google Scholar]
- 43.Keel RA, Sutherland CA, Aslanzadeh J, Nicolau DP, Kuti JL. Correlation between vancomycin and daptomycin MIC values for methicillin-susceptible and methicillin-resistant Staphylococcus aureus by 3 testing methodologies. Diagn Microbiol Infect Dis. 2010;68:326–9. doi: 10.1016/j.diagmicrobio.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Swenson JM, Anderson KF, Lonsway DR, Thompson A, McAllister SK, Limbago BM, et al. Accuracy of commercial and reference susceptibility testing methods for detecting vancomycin-intermediate Staphylococcus aureus. J Clin Microbiol. 2009;47:2013–7. doi: 10.1128/JCM.00221-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bae IG, Federspiel JJ, Miro JM, Woods CW, Park L, Rybak MJ, et al. Heterogeneous vancomycin-intermediate susceptibility phenotype in bloodstream methicillin-resistant Staphylococcus aureus isolates from an international cohort of patients with infective endocarditis: prevalence, genotype, and clinical significance. J Infect Dis. 2009;200:1355–66. doi: 10.1086/606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lalueza A, Chaves F, San Juan R, Daskalaki M, Otero JR, Aguado JM. Is high vancomycin minimum inhibitory concentration a good marker to predict the outcome of methicillin-resistant Staphylococcus aureus bacteremia? J Infect Dis. 2009;201:311–2. doi: 10.1086/649572. author reply 312-3. [DOI] [PubMed] [Google Scholar]
- 47.Schweizer ML, Furuno JP, Sakoulas G, Johnson JK, Harris AD, Shardell MD, et al. Increased mortality with accessory gene regulator (agr) dysfunction in Staphylococcus aureus among bacteremic patients. Antimicrob Agents Chemother. 2010;55:1082–7. doi: 10.1128/AAC.00918-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takesue Y, Nakajima K, Takahashi Y, Ichiki K, Ishihara M, Wada Y, et al. Clinical characteristics of vancomycin minimum inhibitory concentration of 2 mug/ml methicillin-resistant Staphylococcus aureus strains isolated from patients with bacteremia. J Infect Chemother. 2011;17:52–7. doi: 10.1007/s10156-010-0086-0. [DOI] [PubMed] [Google Scholar]
- 49.van Hal SJ, Wehrhahn MC, Barbagiannakos T, Mercer J, Chen D, Paterson DL, et al. Performance of various testing methodologies for detection of heteroresistant vancomycin-intermediate Staphylococcus aureus in bloodstream isolates. J Clin Microbiol. 2011;49:1489–94. doi: 10.1128/JCM.02302-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang WC, Lee CH, Liu JW. Clinical characteristics and risk factors for mortality in patients with meningitis caused by Staphylococcus aureus and vancomycin minimal inhibitory concentrations against these isolates. Journal Microbiol Immunol infectcehsp sp025/cehsp sp025/Wei mian yu gan ran za zhi. 2010;43:470–7. doi: 10.1016/S1684-1182(10)60073-4. [DOI] [PubMed] [Google Scholar]
- 51.Hsu DI, Hidayat LK, Quist R, Hindler J, Karlsson A, Yusof A, et al. Comparison of method-specific vancomycin minimum inhibitory concentration values and their predictability for treatment outcome of meticillin-resistant Staphylococcus aureus (MRSA) infections. Int J Antimicrob Agents. 2008;32:378–85. doi: 10.1016/j.ijantimicag.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Moise PA, Sakoulas G, Forrest A, Schentag JJ. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2007;51:2582–6. doi: 10.1128/AAC.00939-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoon YK, Kim JY, Park DW, Sohn JW, Kim MJ. Predictors of persistent methicillin-resistant Staphylococcus aureus bacteraemia in patients treated with vancomycin. J Antimicrobial Chemother. 2010;65:1015–8. doi: 10.1093/jac/dkq050. [DOI] [PubMed] [Google Scholar]