Abstract
The purpose was to evaluate the effect of the disease duration prior to treatment, thyroid hormones level, or both on the reversibility of dilated cardiomyopathy. Between January 2006 and December 2010, a longitudinal study with a 6 months follow-up was carried on. One hundred and seventy patients with hyperthyroidism were referred to the cardiologist, and 127 had a 6 months followup after antithyroid treatment and were evaluated by echocardiography. Dilated cardiomyopathy reversibility criteria were established according to echocardiographic parameters. Complete reversibility existed when all parameters were met, partial reversibility when LVEF was ≥55% plus two or three other parameters, and no reversibility when LVEF was ≤55% regardless of other parameters. The results showed that echocardiography parameters related to the regression of myocardial mass were associated with a disease duration shorter than 10.38 months. This was the main predictive variable for reversal of dilated cardiomyopathy, followed by β-blocker treatment, and the last predictive variable was the serum level of free triiodothyronine. This study showed that the effect on the myocardium related to thyrotoxicosis was associated with the disease duration before treatment.
1. Introduction
Hyperthyroidism's prevalence in Mexico is about 0.7%. Most patients are women between 30 and 50 years old. A hyperdynamic cardiovascular dysfunction is the result of excess thyroid hormone secretion by the thyroid gland, in these patients, manifested mainly by elevated levels of serum triiodothyronine. Thyrotoxicosis involves two sites of action of the thyroid hormone. It acts directly on the heart and on the systemic vasculature producing tachycardia, increased left ventricular mass (LVM), and unchanged systolic function at the beginning of the thyroid dysfunction. However, long-term effects are diastolic dysfunction, cardiac arrhythmias, heart dilation, and a high output heart failure [1, 2].
It is controversial as to whether these cardiovascular alterations due to hyperthyroidism are purely functional or if there is a structural damage. An experimental study in rabbits demonstrated the presence of histological changes in myocytes, and functional damage was more important and related to the hormone levels [3].
In follow-up studies, it has been observed that once hyperthyroidism treatment is started, cardiovascular function returns to normal. However, in a percentage of patients, the cardiac damage remains with poor myocardial function [4, 5].
The purpose of this study is to evaluate the effect of the disease duration, serum thyroid hormones levels, or both on the myocardium of patients with overt hyperthyroidism and the implication of these factors in the reversibility of dilated cardiomyopathy once patients were euthyroid after antithyroid treatment.
2. Methods
Between January 2006 and December 2010, a longitudinal 6 months follow-up study was carried on. A total of 2,351 patients were seen in the endocrinology clinic. Of them, 798 were diagnosed with hyperthyroidism in the presence of free triiodothyronine (T3) level >1.59 ng/mL and concomitant suppressed thyroid stimulating hormone (TSH) level <0.35 mUI/mL; [6] 170 of them were referred to the cardiologist with cardiac symptoms, of these, 127 patients were identified with dilated cardiomyopathy and recruited for a 6 months follow-up.
Hypertension, diabetes mellitus, and/or any cardiovascular disease (valvular heart disease, coronary artery disease) were excluded. The disease duration was established in months from the onset of the first symptoms according to the patient until euthyroid state was reached.
The study included four visits to the cardiology clinic: (a) the first visit at referral time, the patients were sent by the endocrinologist because he found cardiovascular alterations, (b) the second visit was when the endocrinologist found the patients euthyroid, (c) and the third and fourth visits were three and six months later, respectively. In each visit a clinical history, physical examination, chest X-ray, electrocardiography (ECG), and echocardiographic assessment were performed, and serum thyroid hormones were determined.
Echocardiographic assessment was carried out by an experienced operator (L.O.R) using the same echocardiography instrument (Philips Envisor C Philips transducer S3). All digital images were stored on X-Celera system in order to compare images. Left and right ventricular (LVD, RVD) and left and right auricular (LAD, RAD) diameters were measured, and left ventricular ejection fraction (LVEF), left ventricular shortening fraction (LVSF), pulmonary artery systolic pressure, (PASP), left ventricular mass index (LVMI), and left ventricular mass (LVM), left ventricular end-diastolic (LVEDV) and -systolic volumes (LVESV) were determined using standard M-mode echocardiography and Simpson's method according to the American Society of Echocardiography (ASE) recommendations [7].
Dilated cardiomyopathy reversibility criteria for women and men were established according to the following echocardiographic parameter cut points: left ventricular ejection fraction (LVEF) (≤55%), left ventricular diastolic diameter (LVDD) (women ≥47 mm and men ≥49 mm), left ventricular systolic diameter (LVSD) (women ≥31 mm and men ≥35 mm), left ventricular end-diastolic volume (LVDV) (women ≥97 mL and men ≥102 mL), and left ventricular end-systolic volume (LVSD) (women ≥32 (mL) and men ≥34 mL). Complete reversibility was established when all parameters were within established cut points, partial reversibility when LVEF was equal or above 55% plus two or three of the echocardiographic parameters, and no regression when LVEF was less than 55% regardless of other parameters.
Local Research Ethics Committee approved the study protocol, and all participants signed the informed consent form.
2.1. Data Analysis
Numerical variables showed a different distribution from normal standards (Gaussian distribution) (test of normality Shapiro-Wilk's, P > 0.05). Data are presented as percentiles 25 and 75 and median. Relative frequencies and proportions were estimated for the nominal and categorical variables. Stata 10.0 for Windows software was used for the statistical analysis.
To estimate the effect of disease duration and/or the thyroid hormone levels on myocardium at the referral, regression coefficients were estimated for each echocardiographic parameter (the dependent variables) against disease duration or the level of thyroid hormone (the independent variables). Natural log transformed was used to normalize a skewed distribution. Because a bimodal distribution was identified, LVMI and LVM were divided into low (46–52 and 73–81, resp.) and high values (62–67 and 123–128, resp.). No interaction was identified between the disease duration and the thyroid hormone levels (P > 0.20).
The percentage of patients' reversibility status was estimated according to established criteria and compared against disease duration, heart rate, serum thyroid hormones (T3, T4, and TSH), and echocardiographic parameters at referral time (LVDD, LVSD, LVEDV, LVESV, LVEF, LVSF, LVMI, LVM, and PASP). The Kruskal Wallis test was used for the numerical variables and Chi2 test or Fisher's exact test as required for the categorical.
Echocardiographic parameters at referral time and at six months by the reversibility status were compared with the two way ANOVA (natural log-transformed was used to normalize a skewed distribution). The percentage of change of the echocardiographic parameters between referral time and at 6 months was calculated: (the values of the echocardiographic parameters at 6 months minus the parameter at referral time divided by the values of the echocardiographic at referral time) ∗ 100. Statistical significance was set at an alpha level ≤0.05.
Multivariable analysis was carried out using the classification and regression tree (CART) modeling [8]. This method allows the construction of a binary tree structure classifier that starts with the whole measurement space itself, which by definition is the matrix containing all measurement vectors, and proceeds by repeated splits of subsets of the measurement space into two descendant subsets. The fundamental idea is to select such split in which the data in the descendant subsets are “purer” according to the classification problem. The response variable was the dilated cardiomyopathy recovery classification (complete, partial, or none). The CART was estimated using disease duration, pharmacological treatment, antithyroid treatment, and hormone serum values (T3 and TSH). In order to clarify CART's results, a different geometric figure was used to identify each predictive variable, and the terminal nodes are identified with an asterisk.
3. Results
3.1. Findings at Referral Time
Most patients had diffuse toxic goiter (79 patients (62%)), multinodular goiter was found in 32 patients (25%), and only 16 patients (13%) had an autonomously functioning thyroid nodule. Sixty-six (52%) were treated with radioactive Iodine. In 32 patients (25%), the thyroid was removed surgically, and the remaining 29 (23%) received antithyroid drug treatment. Sixty-six patients (52%) were in sinus rhythm, 49 (39%) in atrial fibrillation, and the remaining had flutter or supraventricular tachycardia. Cardiovascular symptoms identified were tachycardia (90%), palpitations (85%), dyspnea (65%), atrial fibrillation (45%), or systolic hypertension (30%). One hundred and two were women (81%), and 25 were men (19%).
Clinical data, thyroid hormones, and echocardiographic parameters at the first visit at referral time are shown in Table 1. Most patients were young, with a normal to low body mass index (BMI), a long disease duration, and tachycardia. TSH (0.004–0.32 mU/mL) was within abnormal limits, and 83 patients (69%) also had abnormal levels of free T3. Echocardiographic parameters confirmed dilated cardiomyopathy in all patients.
Table 1.
Clinical conditions, thyroid hormones, and echocardiography parameters at referral.
| 25th percentile | Median | 75th percentile | |
|---|---|---|---|
| Clinical variables | |||
| Age (years) | 28 | 33 | 42 |
| BMI (Kg/m2) | 22 | 22.5 | 23 |
| Disease duration (months) | 11 | 16 | 25 |
| Heart rate (bpm) | 108 | 120 | 133 |
| Thyroid hormones | |||
| Free triiodothyronine (T3L) (pg/mL) | 3.6 | 4.8 | 6.21 |
| Total thyroxine (T4) (ng/dL) | 6.8 | 9.3 | 12.1 |
| Thyroid stimulating hormone (TSH) (mU/mL) | 0.14 | 0.060 | 0.08 |
| Echocardiographic parameters | |||
| Left ventricular diastolic diameter (mm) | 52 | 53 | 56 |
| Left ventricular systolic diameter (mm) | 31 | 32 | 34 |
| Left ventricular ejection fraction (%) | 37 | 45 | 48 |
| Shortening fraction (%) | 27 | 29 | 32 |
| Telediastolic volume (mL) | 85 | 100 | 105 |
| Telesystolic volume (mL) | 33 | 37 | 41 |
| Pulmonary pressure (mmHg) | 35 | 43 | 56 |
| Left ventricular mass index (g/m2) | 47 | 49 | 50 |
| Left ventricular mass (g) | 77 | 78 | 79 |
Note: normal thyroid hormones levels: T3L 2.50–3.90 pg/mL, T4T 6.09–12.23 ng/dL, and TSH 0.34–5.60 mU/mL, and normal echocardiography parameters: left ventricular diastolic diameter (LVDD) (women 47 mm and men 49 mm), left ventricular ejection fraction (LVEF) (55%), left ventricular end-diastolic volume (LVDV) (women 97 mL and men 102 mL), left ventricular end-systolic volume (LVSD) (women 32 mL and men 34 mL), pulmonary pressure (30 mmHg), and left ventricular mass (women 110 g/m2 and men 134 g/m2).
Most altered echocardiographic parameters were associated with disease duration, with a greater effect in those related to decreased myocardial mass (LVEF, LVDV, and LVSV). Free T3 levels were associated with the LVMI and the LVDD (Table 2).
Table 2.
Association of disease duration or free triiodothyronine (T3) levels on the myocardium.
| β coefficient* | 95% CI* | P value | R 2 | |
|---|---|---|---|---|
| Disease duration | ||||
| Left ventricular diastolic diameter (mm) | 0.056 | 0.043–0.069 | 0.000 | 0.354 |
| Left ventricular systolic diameter (mm) | 0.059 | 0.043–0.073 | 0.000 | 0.326 |
| Left ventricular ejection fraction (%) | −0.176 | −0.219–(−0.133) | 0.000 | 0.343 |
| Shortening fraction (%) | −0.088 | −0.114–(−0.062) | 0.000 | 0.264 |
| Pulmonary pressure (mmHg) | 0.271 | 0.202–0.341 | 0.000 | 0.323 |
| Telediastolic volume (mL) | 0.124 | 0.092–0.157 | 0.000 | 0.313 |
| Telesystolic volume (mL) | 0.103 | 0.061–0.145 | 0.000 | 0.161 |
| Left ventricular mass index (≥62) | −0.022 | −0.035–(−0.007) | 0.004 | 0.294 |
| Left ventricular mass index (≤61) | −0.022 | −0.033–(−0.011) | 0.000 | 0.121 |
| Heart rate (beats/min) | 0.099 | 0.059–0.140 | 0.000 | 0.158 |
| Free T3 | ||||
| Left ventricular diastolic diameter (mm) | 0.023 | 0.0002–0.046 | 0.048 | 0.023 |
| Pulmonary pressure (mmHg) | 0.125 | 0.009–0.240 | 0.034 | 0.028 |
| Left ventricular mass index (46–52) | −0.020 | −0.035–(−0.005) | 0.010 | 0.056 |
*Natural logarithmic units.
3.2. Findings at 6 Months Followup
At the second and third visits, no significant changes were identified. At the 6 months visit, the percentage of patients with complete, partial, or no reversibility of myocardial damage was very similar (31.4%, 31.4%, and 37.2%, resp.); however, analysis by gender showed significant differences; the male group had 50% total recovery, while in the females, complete reversibility was observed in only 26%, and most of them had a partial recovery (40%) (P = 0.000). Disease duration since diagnosis was related to reversibility status; those with complete reversibility had less disease duration (13 months) than those with no regression (24 months) (P < 0.05). When disease duration was analyzed by gender, there was no difference (Table 3).
Table 3.
Myocardial recovery according to gender and disease duration.
| Myocardial reversibility | Gender | P value | ||
|---|---|---|---|---|
| Female (n (%)) | Male (n (%)) | |||
| Complete | 26 (26) | 12 (52) | ||
| Partial | 38 (39) | 0 | 0.0000* | |
| None | 34 (35) | 11 (48) | ||
|
| ||||
| Disease duration (months) | ||||
| 25th percentile | Median | 75th percentile | ||
|
| ||||
| Complete | 8 | 13 | 19 | |
| Partial | 12 | 16.5 | 22 | 0.000** |
| None | 12 | 24 | 28 | |
*Fishers exact test. **Kruskal Wallis.
Significant changes were observed only in the LVEF, LVDD, LVSD, LVEDV, and LVESD between the second and 6 months follow-up visits in patients with complete and partial reversibility, but not in those with no reversibility (Table 4). Heart rate and ejection fraction showed significant variations between these two visits. In patients with complete reversibility, heart rate decreased 42% (from 118.5 to 67.5 bpm). A similar situation was observed in patients with partial reversibility (heart rate decreased 38%, from 113 to 73 bpm); in patients with no reversibility, the heart rate only decreased 23% (from 127 to 98 bpm) (upper panel in Figure 1). The ejection fraction returned to normal in all patients with complete reversibility, while in those with partial reversibility, it increased 29.4%, and in patients with no reversibility, the percentage of change was only 2.9% (lower panel in Figure 1). The remaining echocardiographic parameters showed significant changes only in patients with complete and partial reversibility.
Table 4.
Echocardiographic parameters at enrollment and at the 6 months followup.
| Parameters | Referral | 6 months | ||||
|---|---|---|---|---|---|---|
| 25th percentile | Median | 75th percentile | 25th percentile | Median | 75th percentile | |
| Complete reversibility (n = 38) | ||||||
| LVEF* | 45 | 48 | 50 | 55 | 58 | 60 |
| LVDD* | 50 | 52 | 53 | 43 | 45 | 46 |
| LVSD* | 30 | 31 | 32 | 27 | 27 | 28 |
| LVEDV* | 80 | 84 | 101 | 76 | 80 | 90 |
| LVESV* | 30 | 32 | 39 | 27 | 30 | 31 |
| Partial reversibility (n = 34) | ||||||
| LVEF* | 35 | 43 | 49 | 55 | 56 | 61 |
| LVDD* | 52 | 54 | 56 | 45 | 47 | 48 |
| LVSD* | 31 | 32 | 35 | 27 | 27 | 28 |
| LVEDV* | 87 | 100.5 | 108 | 80 | 94 | 99 |
| LVESV* | 34 | 40.5 | 44 | 30 | 35 | 38 |
| No reversibility (n = 49) | ||||||
| LVEF | 33 | 37 | 49 | 32 | 40 | 50 |
| LVDD | 54 | 55 | 56 | 49 | 54 | 57 |
| LVSD | 33 | 34 | 35 | 30 | 33 | 36 |
| LVEDV | 98 | 103 | 107 | 90 | 110 | 114 |
| LVESV | 35 | 39 | 41 | 30 | 40 | 45 |
*MANOVA ANOVA P < 0.05.
Note: LVEF: left ventricular ejection fraction (%), LVDD: left ventricular diastolic diameter (mm), LVSD: left ventricular systolic diameter (mm), LVEDV: left ventricular end-diastolic volume (mL), *LVESV: left ventricular end-systolic volume (mL).
Figure 1.
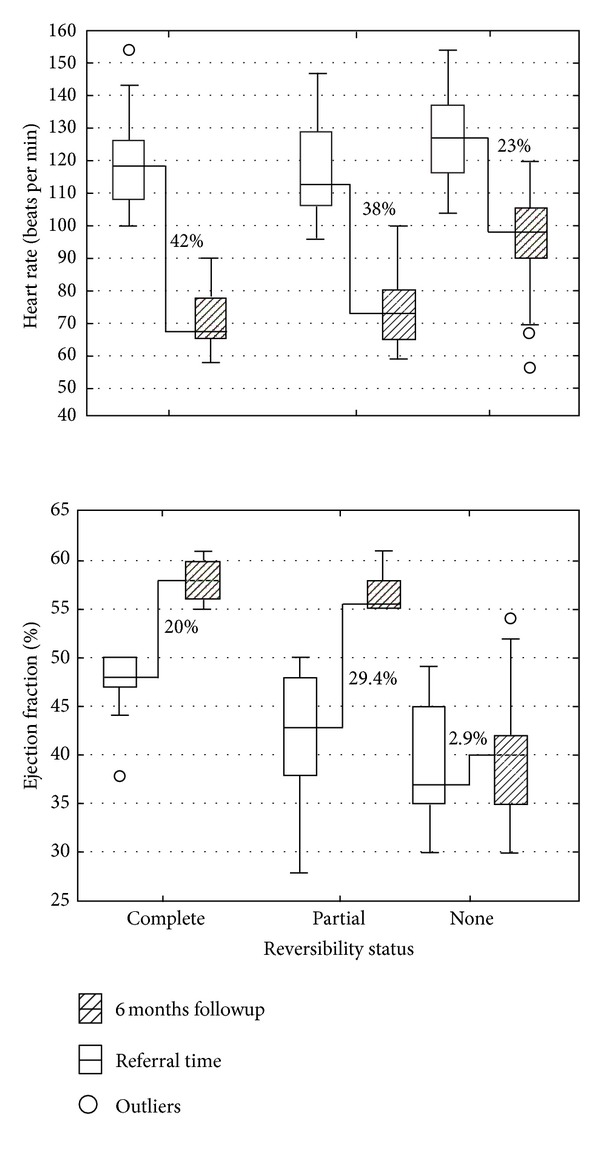
Percentage of change at 6 months followup.
Multivariate analysis (CART modeling) showed that disease duration was the main predictive variable in the recovery of dilated cardiomyopathy, followed by pharmacological treatment, and the least important were T3 serum levels.
Those patients that had a disease duration shorter or equal to 10.38 months recovered only with thyroid treatment within six months (left side of Figure 2); however, when disease duration was longer, they required beta-blocker (β-blocker) treatment to achieve complete recovery. Those with a long disease duration (>10.38 months), not receiving β-blocker treatment and with elevated levels of serum T3, did not recover or had a partial recovery (right side of Figure 2).
Figure 2.
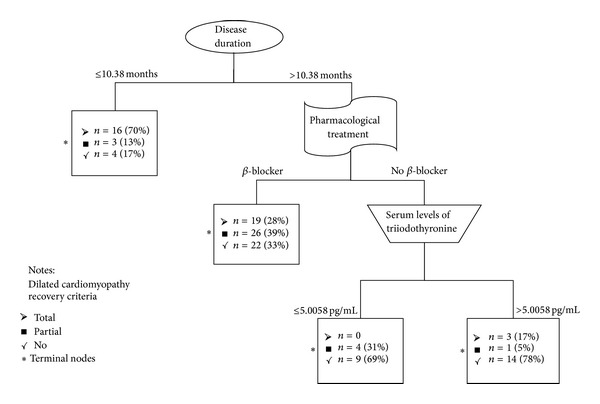
Predictive variables related to reversibility of dilated cardiomyopathy.
4. Discussion
Myocardial damage is directly related to the action of thyroid hormones, increased early in hyperthyroidism producing heart failure, that with time becomes chronic [2, 9–11]. However, most studies agree that once the effect of thyroid hormones is eliminated, myocardial damage may be reversible in patients with less than 6 months disease duration [12–15]. In a recent study, Smit et al. [16] found that even long-term subclinical hyperthyroidism has profound effects on cardiac diastolic function that may disappear when euthyroidism is restored.
Although the exact mechanism remains unclear, multiple factors are likely to participate in the development of dilated cardiomyopathy and left ventricular systolic dysfunction in patients with hyperthyroidism. Patients with long-standing hyperthyroidism may occasionally have poor cardiac contractility, low cardiac output, and symptoms and signs of congestive heart failure associated with dilated cardiomyopathy (DCM) because of a decreased LVEF, suggesting that the heart undergoes some pathological remodeling [17].
Thyrotoxic heart disease clinical manifestation is characterized by its rapid course and recovery after thyroid dysfunction normalization [1]. In the present study, those patients with longer disease duration had worse myocardial damage represented by left ventricular systolic dysfunction, an increased left ventricular end diastolic size, and a LVEF less than 0.35 and were from the no reversibility group. These results also showed that myocardial recovery was more often observed in men. Most women with dilation of left ventricle persisted with a decreased systolic function 6 months after euthyroid state was achieved despite the treatment used.
Chronic exposure to thyroid hormone may trigger heart failure because of a high cardiac output associated with systolic left ventricular dysfunction with subsequent cardiovascular morbidity by volume overload due to reduced myocardial contractility, which activates sympathetic activity on the heart.
In this study, the duration of thyroid dysfunction suggests that physiological compensatory and pathological hypertrophy may coexist and lead to a concentric cardiac hypertrophy, in which thyroid hormones may play very different roles from those in eccentric cardiac hypertrophy [13].
Previous studies [12, 15] suggest that if hyperthyroidism is recognized early and treated, dilated cardiomyopathy could be prevented; results in this study showed a negative prognosis once patients had developed dilated cardiomyopathy with impaired left ventricular diastolic function. Therefore, it seems that chronic exposure to thyroid hormones may have more long-lasting hemodynamic changes [18, 19].
The no reversibility may not only be related to high levels of thyroid hormones, but could also be the result of an alteration of the sympathetic nervous system with increased catecholamines. This combination could result in a tachycardiomyopathy [20, 21].
Antithyroid therapy and β-blockers for heart rate control, prevention of heart remodeling, and avoidance of hemodynamic overload will lead to the resolution of heart failure.
5. Conclusions
Patient's recovery is mainly dependent on the hyperthyroidism duration previous to treatment. Factors which may play a role in no recovery are no β-blocker administration and high T3 serum levels. Therefore, the use of β-blocker is an important factor for complete recovery.
6. Study Limitations
The real disease duration is unknown; it depends on the patient's recollection at the time of history taking on the first endocrinology visit. Patients included in this study were only those the endocrinologist considered to have a cardiac abnormality. The follow-up was limited to 6 months; therefore, complete reversibility in those with a partial recovery may be achieved later and could not been determined in this study.
Conflict of Interests
The authors declare that there is no conflict of interests.
Authors' Contribution
Lucia Oliveros-Ruiz, Maite Vallejo, and Manuel Cárdenas participated in the design and conduct of the study, data collection and analysis, data interpretation, and paper writing. Fernando Diez Canseco L and J. Antonio G. Hermosillo participated in the paper writing. All authors read and approved the final version of the paper.
Acknowledgment
The authors thank Ms. Betty Low Chin, RN, BS, and English teacher, for help in rewriting the paper.
References
- 1.Cordova-Alvelais L, Verdejo-Paris J, Cárdenas-Loaeza M. Serrano P. Cardiopatia tirotoxica. Archivos de Cardiología de México. 1979;49:1046–1054. [PubMed] [Google Scholar]
- 2.Wang Y-Y, Jiao B, Guo W-G, Che H-L, Yu Z-B. Excessive thyroxine enhances susceptibility to apoptosis and decreases contractility of cardiomyocytes. Molecular and Cellular Endocrinology. 2010;320(1-2):67–75. doi: 10.1016/j.mce.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 3.Cesarman E, Rockey J. Cardiopatía hipertiroidea. Archivos del Instituto de Cardiología de México. 1965;I35:166–185. [PubMed] [Google Scholar]
- 4.Forfar JC, Matthews DM, Toft AD. Delayed recovery of left ventricular function after antithyroid treatment. Further evidence for reversible abnormalities of contractility in hyperthyroidism. British Heart Journal. 1984;52(2):215–222. doi: 10.1136/hrt.52.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mercé J, Ferrás S, Oltra C, Sanz E, Vendrell J, Simón I. Cardiovascular abnormalities in hyperthyroidism: a prospective Doppler Echocardiographic study. American Journal of Medicine. 2005;118:126–131. doi: 10.1016/j.amjmed.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Sánchez-Carbayo M, Mauri M, Alfayate R, Miralles C, Soria F. Analytical and clinical evaluation of TSH and thyroid hormones by electrochemiluminescent immunoassays. Clinical Biochemistry. 1999;32(6):395–403. doi: 10.1016/s0009-9120(99)00032-6. [DOI] [PubMed] [Google Scholar]
- 7.Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography) Circulation. 2003;108(9):1146–1162. doi: 10.1161/01.CIR.0000073597.57414.A9. [DOI] [PubMed] [Google Scholar]
- 8.Breiman L, Frieman JH, Olshen RA, Stone CJ. Classification and Regression Trees. Belmont, Cali, USA: Wadsworth International Group; 1984. [Google Scholar]
- 9.Siu C-W, Yeung C-Y, Lau C-P, Kung AWC, Tse H-F. Incidence, clinical characteristics and outcome of congestive heart failure as the initial presentation in patients with primary hyperthyroidism. Heart. 2007;93(4):483–487. doi: 10.1136/hrt.2006.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iqbal A, Schirmer H, Lunde P, Figenschau Y, Rasmussen K, Jorde R. Thyroid stimulating hormone and left ventricular function. Journal of Clinical Endocrinology and Metabolism. 2007;92(9):3504–3510. doi: 10.1210/jc.2007-0727. [DOI] [PubMed] [Google Scholar]
- 11.Wen Shen Y, Boon-Hor C. Hyperthyroidism-induced veft ventricular diastolic dysfunction; implication in hyperthyrodism-related heart failure. Journal of Clinical Endocrinology and Metabolism. 2011;74:636–643. doi: 10.1111/j.1365-2265.2011.03981.x. [DOI] [PubMed] [Google Scholar]
- 12.Sgarbi JA, Villaça FG, Garbeline B, Villar HE, Romaldini JH. The effects of early antithyroid therapy for endogenous subclinical hyperthyroidism in clinical and heart abnormalities. Journal of Clinical Endocrinology and Metabolism. 2003;88(4):1672–1677. doi: 10.1210/jc.2002-021046. [DOI] [PubMed] [Google Scholar]
- 13.Dörr M, Wolff B, Robinson DM, et al. The association of thyroid function with cardiac mass and left ventricular hypertrophy. Journal of Clinical Endocrinology and Metabolism. 2005;90(2):673–677. doi: 10.1210/jc.2004-1554. [DOI] [PubMed] [Google Scholar]
- 14.Nagarakanti R, Whellan D, Rubin S, Mather PJ. Reversible cardiomyopathies. Cardiology in Review. 2007;15(4):178–183. doi: 10.1097/CRD.0b013e31804c98b1. [DOI] [PubMed] [Google Scholar]
- 15.Kaminski G, Michalkiewicz D, Makowski K, et al. Prospective echocardiographic evaluation of patients with endogenous subclinical hyperthyroidism and after restoring euthyroidism. Clinical Endocrinology. 2011;74(4):501–507. doi: 10.1111/j.1365-2265.2010.03957.x. [DOI] [PubMed] [Google Scholar]
- 16.Smit JWA, Eustatia-Rutten CFA, Corssmit EPM, et al. Reversible diastolic dysfunction after long-term exogenous subclinical hyperthyroidism: a randomized, placebo-controlled study. Journal of Clinical Endocrinology and Metabolism. 2005;90(11):6041–6047. doi: 10.1210/jc.2005-0620. [DOI] [PubMed] [Google Scholar]
- 17.Basset A, Blanc J, Messas E, Hagège A, Elghozi J-L. Renin-angiotensin system contribution to cardiac hypertrophy in experimental hyperthyroidism: an echocardiographic study. Journal of Cardiovascular Pharmacology. 2001;37(2):163–172. doi: 10.1097/00005344-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Pearce EN, Yang Q, Benjamin EJ, Aragam J, Vasan RS. Thyroid function and left ventricular structure and function in the framingham heart study. Thyroid. 2010;20(4):369–373. doi: 10.1089/thy.2009.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yue W-S, Chong B-H, Zhang X-H, et al. Hyperthyroidism-induced left ventricular diastolic dysfunction: implication in hyperthyroidism-related heart failure. Clinical Endocrinology. 2011;74(5):636–643. doi: 10.1111/j.1365-2265.2011.03981.x. [DOI] [PubMed] [Google Scholar]
- 20.Frustaci A, Loperfido F, Gentiloni N, Caldarulo M, Morgante E, Russo MA. Catecholamine-induced cardiomyopathy in multiple endocrine neoplasia: a histologic, ultrastructural, and biochemical study. Chest. 1991;99(2):382–385. doi: 10.1378/chest.99.2.382. [DOI] [PubMed] [Google Scholar]
- 21.Lee SJ, Kang JG, Ryu OK, et al. The relationship of thyroid hormone status with myocardial function in stress cardiomyopathy. European Journal of Endocrinology. 2009;160(5):799–806. doi: 10.1530/EJE-08-0808. [DOI] [PubMed] [Google Scholar]


