This analysis examined clinical outcomes when adding bevacizumab to chemotherapy for patients with metastatic colorectal cancer (mCRC). The use of bevacizumab with chemotherapy resulted in statistically significant increases in overall and progression-free survival for patients with mCRC, and the observed safety profile of bevacizumab was consistent with that reported in individual trials.
Keywords: Angiogenesis inhibitors; Antibodies, monoclonal, humanized; Bevacizumab; Colorectal neoplasms
Abstract
Purpose.
This analysis pooled individual patient data from randomized controlled trials (RCTs) to more thoroughly examine clinical outcomes when adding bevacizumab to chemotherapy for patients with metastatic colorectal cancer (mCRC).
Patients and Methods.
Patient data were pooled from the first-line AVF2107, NO16966, ARTIST, AVF0780, AVF2192, and AGITG MAX RCTs and the second-line E3200 RCT. All analyses were based on the intent-to-treat population. To assess differences in time-to-event variables by treatment (chemotherapy with or without placebo vs. chemotherapy plus bevacizumab), stratified random-effects (overall) and fixed-effects (subgroup comparisons) models were used to estimate pooled hazard ratios (HRs) and 95% confidence intervals (CIs).
Results.
The analysis population comprised 3,763 patients (1,773 chemotherapy with or without placebo; 1,990 chemotherapy plus bevacizumab). The addition of bevacizumab to chemotherapy was associated with statistically significant increases in overall survival (OS; HR, 0.80; 95% CI, 0.71–0.90) and progression-free survival (PFS; HR, 0.57; 95% CI, 0.46–0.71). The effects on OS and PFS across subgroups defined by chemotherapy backbone (oxaliplatin-based, irinotecan-based), extent of disease (liver metastases only, extensive disease), age (<65, ≥65 years), Eastern Cooperative Oncology Group performance status (0, ≥1), and KRAS status (wild-type, mutant) were consistent with the overall analysis. Incidence rates of grade ≥3 hypertension, proteinuria, bleeding, wound-healing complications, gastrointestinal perforations, and thromboembolic events were increased with bevacizumab treatment.
Conclusion.
The use of bevacizumab with chemotherapy resulted in statistically significant increases in OS and PFS for patients with mCRC. The PFS benefit extended across the clinically relevant subgroups examined. The observed safety profile of bevacizumab was consistent with that reported in individual trials.
Implications for Practice:
Several randomized trials of bevacizumab have been conducted to address specific questions regarding its use for patients with metastatic colorectal cancer (mCRC); however, because of their sample size limitations, subgroup analyses are frequently of limited power. By pooling individual patient data from seven randomized trials, more comprehensive analyses of the efficacy and safety of bevacizumab were made possible because of the large number of included patients. In addition, outcomes in clinically relevant subgroups were examined, and the data from these subgroups were consistent with those reported in the overall analyses. The results of this pooled analysis help further the clinician's understanding of the overall risks and benefits associated with adding bevacizumab to chemotherapy for patients with mCRC.
Introduction
Bevacizumab (Avastin; Genentech, Inc, South San Francisco, CA) is a humanized, monoclonal antibody that acts as an antiangiogenic agent by the inhibition of vascular endothelial growth factor A (VEGF-A). In the United States and Europe, bevacizumab was first approved for the treatment of metastatic colorectal cancer (mCRC) in combination with fluorouracil-based chemotherapy, primarily on the basis of results of a phase III randomized, placebo-controlled trial in the first-line treatment setting [1]. The data from this trial, AVF2107, showed that bevacizumab prolonged both overall survival (OS) and progression-free survival (PFS) when added to irinotecan, bolus fluorouracil, and leucovorin. Subsequently, data from additional randomized controlled trials (RCTs) have demonstrated the efficacy and safety profiles of bevacizumab among a wider variety of patients with mCRC, in combination with multiple backbone chemotherapy regimens, and in different treatment settings [2–7].
Meta-analyses of published RCTs have attempted to derive a measure of the overall clinical benefit associated with the addition of bevacizumab to chemotherapy in patients with mCRC. These analyses have consistently shown that bevacizumab plus chemotherapy results in statistically significant reductions in the risk of disease progression and death relative to chemotherapy alone [8–15]. Although meta-analyses reported to date have been useful in characterizing the risk-benefit profile of bevacizumab, they have had limitations. These limitations include the breadth of the trials evaluated and the lack of access to individual patient data, which together necessitate the reporting of combined overall hazard ratios (HRs) and preclude the conduct of subgroup analyses and consistent analyses of certain adverse event (AE) information across trials.
In this paper, we describe the results of a pooled analysis of data from RCTs of bevacizumab in mCRC. The analysis used individual patient data, enabling a more comprehensive analysis of the efficacy and safety of bevacizumab than previously reported. Importantly, pooling of patient data across RCTs allowed for the assessment of the efficacy of bevacizumab in clinically relevant subgroups.
Methods
Individual patient data were pooled from seven RCTs of bevacizumab in the first-line (AVF2107, NO16966, ARTIST, AVF0780, AVF2192, AGITG MAX) and second-line (E3200) treatment of mCRC (Table 1) [1–6, 16]. Previously published phase II and III studies were selected based on (1) their design as RCTs evaluating chemotherapy with or without bevacizumab for mCRC; (2) the use of identical definitions and procedures for collecting patient baseline characteristics, along with primary and secondary efficacy endpoints and safety assessments; and (3) the ability to access individual patient information within study databases. At the time of the analysis, all published, multicenter phase II and III RCTs of first- or second-line mCRC with bevacizumab as the experimental agent were included. Only the principal arms in these studies were analyzed: chemotherapy with or without placebo as the control arm and chemotherapy plus bevacizumab as the experimental arm.
Table 1.
Overview of clinical trials included in the analysis
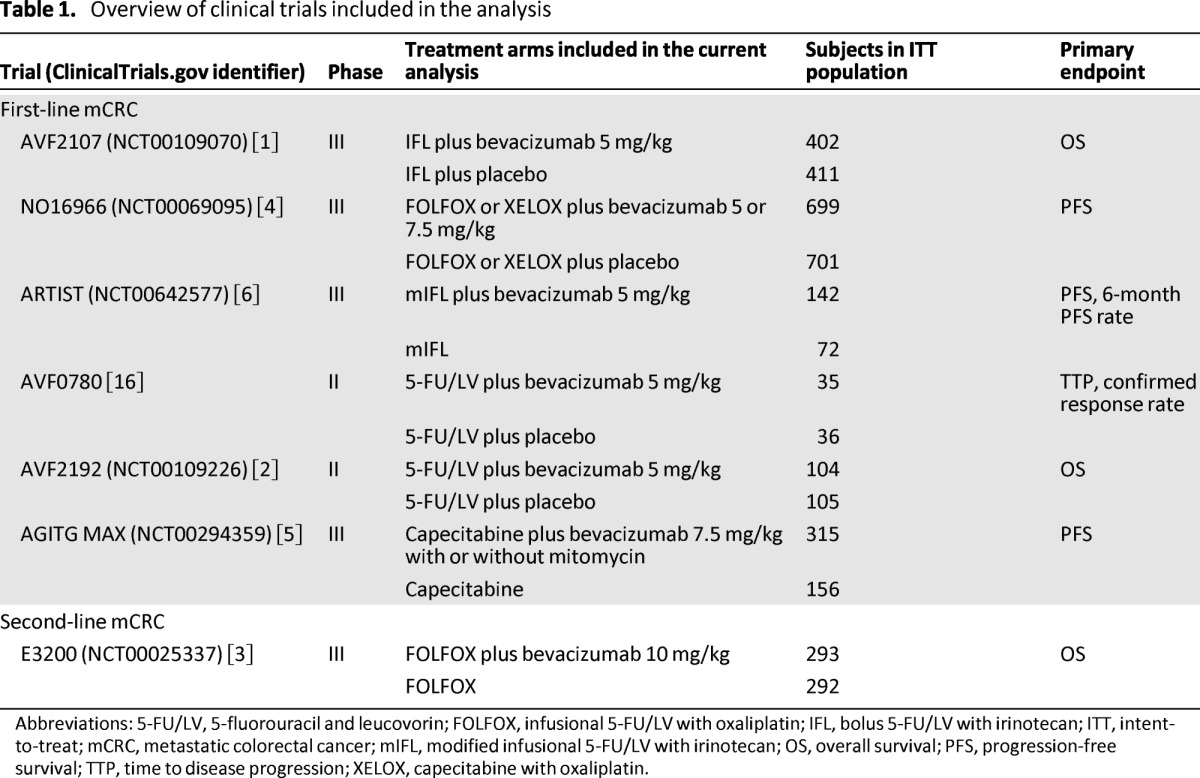
Abbreviations: 5-FU/LV, 5-fluorouracil and leucovorin; FOLFOX, infusional 5-FU/LV with oxaliplatin; IFL, bolus 5-FU/LV with irinotecan; ITT, intent-to-treat; mCRC, metastatic colorectal cancer; mIFL, modified infusional 5-FU/LV with irinotecan; OS, overall survival; PFS, progression-free survival; TTP, time to disease progression; XELOX, capecitabine with oxaliplatin.
The primary outcome of the pooled analysis was OS, defined as the time from randomization to death from any cause. Data for patients for whom death was not recorded were censored at the most recent date they were known to be alive. Secondary efficacy endpoints were PFS and overall response rate (ORR); these were assessed by the investigator in five studies (AVF2107, ARTIST, AVF0780, AGITG MAX, and E3200) and by both the investigator and an independent review facility in two studies (NO16966 and AVF2192). PFS was defined as the time from randomization to investigator-assessed progression or any-cause death, whichever occurred first. PFS results included all progression or death events, regardless of when the last dose of study treatment was administered. Data for patients without documented progression were censored at the most recent date at which no progression was confirmed. The best overall response was defined as the best response from randomization until disease progression, disease recurrence, or death. Responses were defined as partial or complete responses according to the Response Evaluation Criteria in Solid Tumors, version 1.0. Only patients with measurable disease at baseline were included in ORR analyses.
Subgroup analyses were performed in the overall pooled population to evaluate OS and PFS according to chemotherapy backbone (irinotecan-based [two trials] or oxaliplatin-based regimens [two trials]), chemotherapy intensity (monotherapy [three trials] or doublet therapy [four trials]), extent of disease (liver metastases only or metastases in at least one site other than the liver or lung [all trials except ARTIST]), age (<65 years, ≥65 years, or ≥75 years [all trials]), Eastern Cooperative Oncology Group performance status (ECOG PS; 0 or ≥1 [all trials]), and KRAS mutational status (wild-type or mutant [AVF2107, AGITG MAX).
The incidences of grade ≥3 AEs of any type and of special interest to both bevacizumab and chemotherapy were also analyzed. AEs of special interest were selected on the basis of known safety information. Bevacizumab-related AEs included hypertension, proteinuria, bleeding, wound-healing AEs, arterial thromboembolic events, venous thromboembolic events, and any-grade gastrointestinal perforation. Chemotherapy-related AEs included asthenia/fatigue, diarrhea, nausea/vomiting, neuropathy, neutropenia, and stomatitis. AEs were categorized using the Common Terminology Criteria for Adverse Events.
In a separate pooled analysis, the second-line E3200 trial was excluded from the dataset to examine the effects of bevacizumab in the first-line treatment setting.
Statistical Analyses
All analyses were based on the intent-to-treat populations. Estimates of OS and PFS were calculated by Kaplan-Meier methods. Pooled HRs and 95% confidence intervals (CIs) for assessing differences in time-to-event variables were calculated using random- and fixed-effects models. Because of the variation in length of follow-up, chemotherapy regimen, line of therapy, method of drug administration, duration of drug administration, and dose of bevacizumab used, a high level of heterogeneity was expected to be derived from the seven RCTs. Hence, the random-effects model was used to estimate HRs for the overall analysis and for analyses restricted to the six first-line trials. For estimates based on subgroups, however, less variation was observed according to the test of heterogeneity, and a fixed-effects model was used. The pooled HRs were estimated from the stratified analysis model, in which treatment and study were covariance variables. The DerSimonian and Laird random-effects model was used to test heterogeneity among included studies, and a p value <.05 indicated heterogeneity. The fixed-effects model was based on methodology by Parmar et al. [17]. The Cox proportional hazards method was used to estimate HRs and corresponding 95% CIs for OS and PFS in individual studies. Durations of OS and PFS with chemotherapy plus bevacizumab and chemotherapy with or without placebo were compared using two-sided stratified log-rank tests. For the safety analysis, odds ratios (ORs) and corresponding 95% CIs were estimated using a logistic regression model that included treatment effect and study indicator as covariance variables. ORs and corresponding 95% CIs for AEs were estimated in a similar fashion as other safety analyses.
Results
Overall Pooled Analysis: Patients and Treatment
The overall pooled population consisted of 3,763 patients: 1,773 received chemotherapy with or without placebo and 1,990 received chemotherapy plus bevacizumab. In total, 58.8% of patients were male, 39.6% were aged ≥65 years, and 45.7% had an ECOG PS ≥1 (2.1% with an ECOG PS of 2). One RCT (ARTIST) did not capture information on the extent of disease at baseline; however, analysis of the six remaining RCTs showed that 36.0% (1,279 of 3,549) of patients in these studies presented with extensive disease. Baseline patient and disease characteristics were well balanced between treatment arms (Table 2). Median time on treatment was 5.7 months (95% CI, 5.5–5.9) with chemotherapy with or without placebo and 7.4 months (95% CI, 7.1–7.8) with chemotherapy plus bevacizumab (excluding patients from ARTIST, for which time-on-treatment data were not available).
Table 2.
Patient and disease characteristics at baseline in the overall pooled population (first- and second-line trials of bevacizumab)
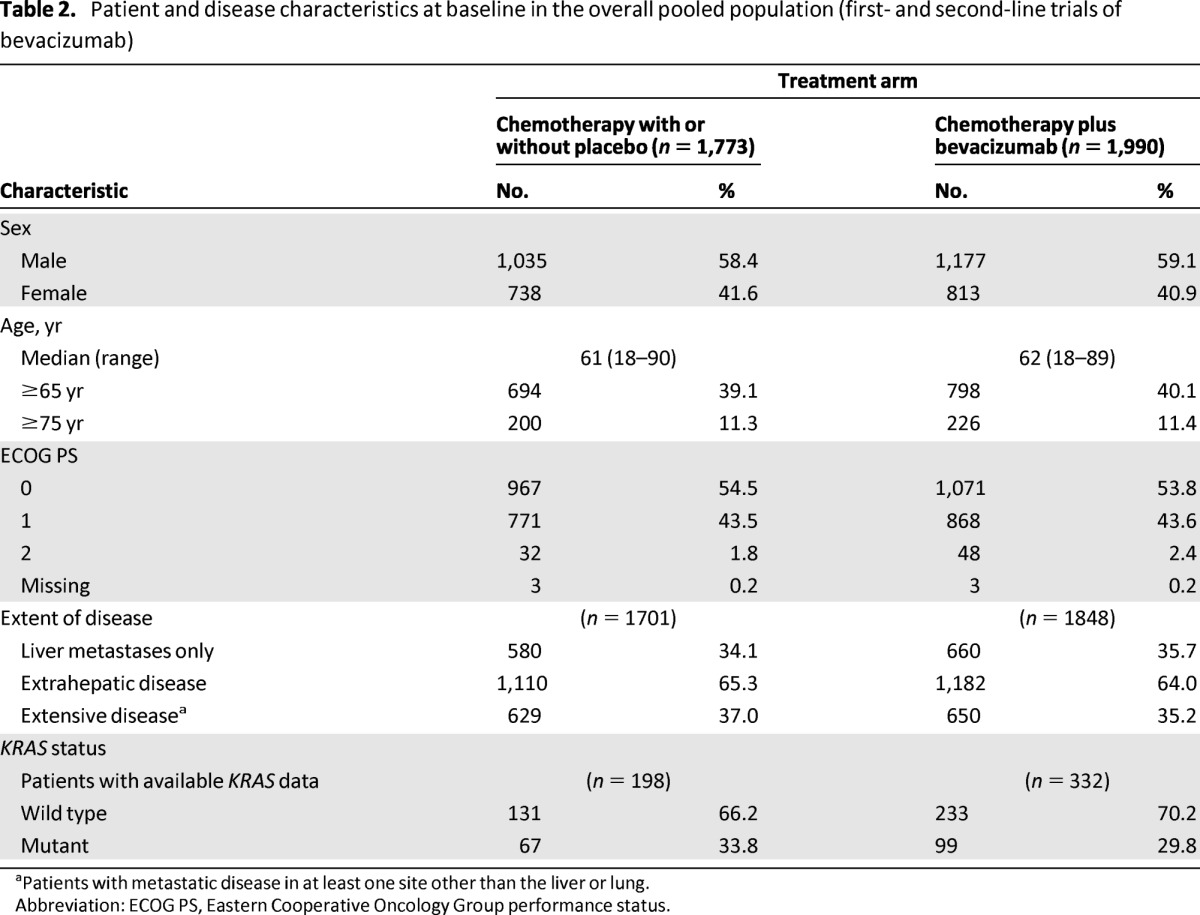
aPatients with metastatic disease in at least one site other than the liver or lung.
Abbreviation: ECOG PS, Eastern Cooperative Oncology Group performance status.
Survival Outcomes and Response Rate
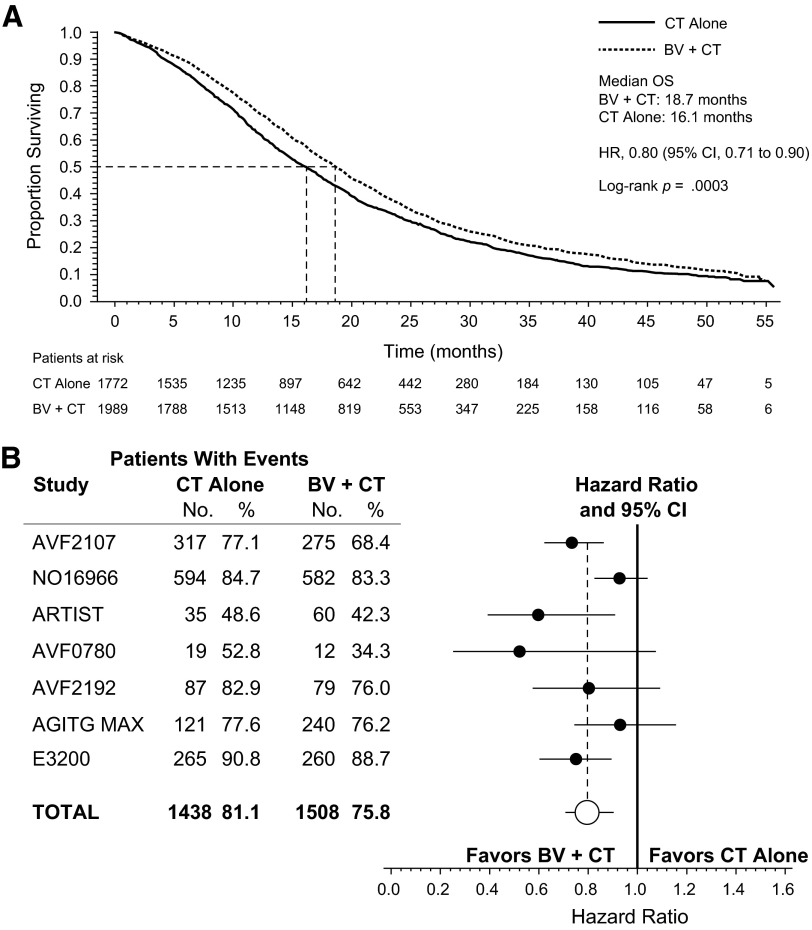
The analysis of the overall pooled population showed that adding bevacizumab to chemotherapy was associated with an increase in OS (Fig. 1A). The median OS for patients receiving chemotherapy plus bevacizumab was 18.7 months, and it was 16.1 months for patients receiving chemotherapy with or without placebo (HR, 0.80; 95% CI, 0.71–0.90; p = .0003). In the pooled OS analysis, there was evidence of statistically significant heterogeneity among the studies (p = .0445). This was largely influenced by data from study NO16966, which provided 37% of the overall pooled population (Table 1). HRs for OS in the individual RCTs and the overall pooled analysis are depicted in Figure 1B.
Figure 1.
Overall survival (OS) in the overall pooled population and in individual studies (first- and second-line trials of bevacizumab). (A): Kaplan-Meier estimate of OS for the overall pooled population. (B): Forest plot of OS by study.
Abbreviations: BV, bevacizumab; CI, confidence interval; CT, chemotherapy; HR, hazard ratio; OS, overall survival.
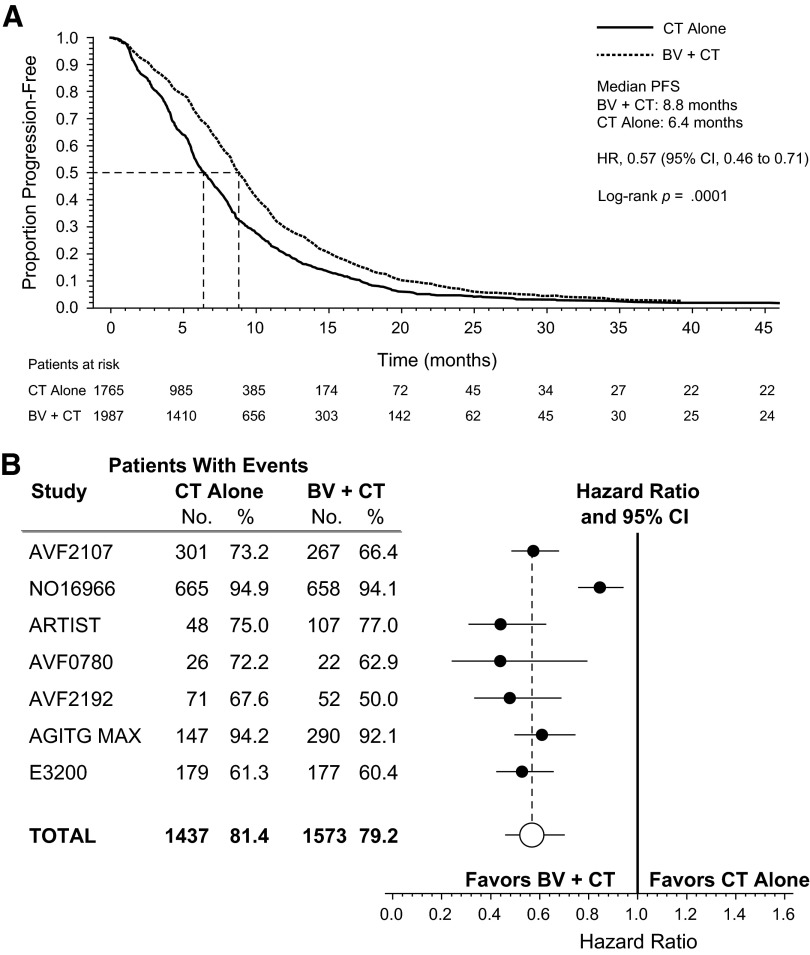
PFS was also improved (HR, 0.57; 95% CI, 0.46–0.71; p < .0001) with the addition of bevacizumab to chemotherapy (Fig. 2A). The median PFS was 8.8 months for patients receiving chemotherapy plus bevacizumab and 6.4 months for patients receiving chemotherapy with or without placebo. Heterogeneity was observed in the pooled PFS analysis (p < .0001), again largely influenced by study NO16966. Figure 2B illustrates the HRs for PFS in the individual RCTs.
Figure 2.
Progression-free survival (PFS) in the overall pooled population and in individual studies (first- and second-line trials of bevacizumab). (A): Kaplan-Meier estimate of PFS for the pooled population. (B): Forest plot of PFS by study.
Abbreviations: BV, bevacizumab; CI, confidence interval; CT, chemotherapy; HR, hazard ratio; PFS, progression-free survival.
In the overall pooled population, the addition of bevacizumab to chemotherapy was associated with an increased best overall response rate (39% [95% CI, 37%–42%]) vs. 33% [95% CI, 31%–36%] for chemotherapy with or without placebo), as assessed by investigators and/or independent review committees.
Survival Outcomes by Subgroup
Statistically significant improvements or trends for improvement in OS and PFS were seen in all clinical subgroups that were evaluated (Table 3). The use of bevacizumab with chemotherapy was associated with increases in OS and PFS in patients receiving either irinotecan- or oxaliplatin-based regimens. Although there were modest numerical differences in the HRs for death and progression when combining bevacizumab with an irinotecan- versus oxaliplatin-based regimen, OS and PFS benefits were seen in both subgroups. Bevacizumab-associated benefits were also observed when patient data were grouped by chemotherapy intensity: monotherapy (numerical trend for improvement for OS; statistically significant improvement for PFS) or doublet therapy (statistically significant for OS and PFS). The magnitude of benefit with bevacizumab was similar in the monotherapy and doublet therapy subgroups.
Table 3.
Subgroup analyses in the overall pooled population (first- and second-line trials of bevacizumab) and first-line pooled population
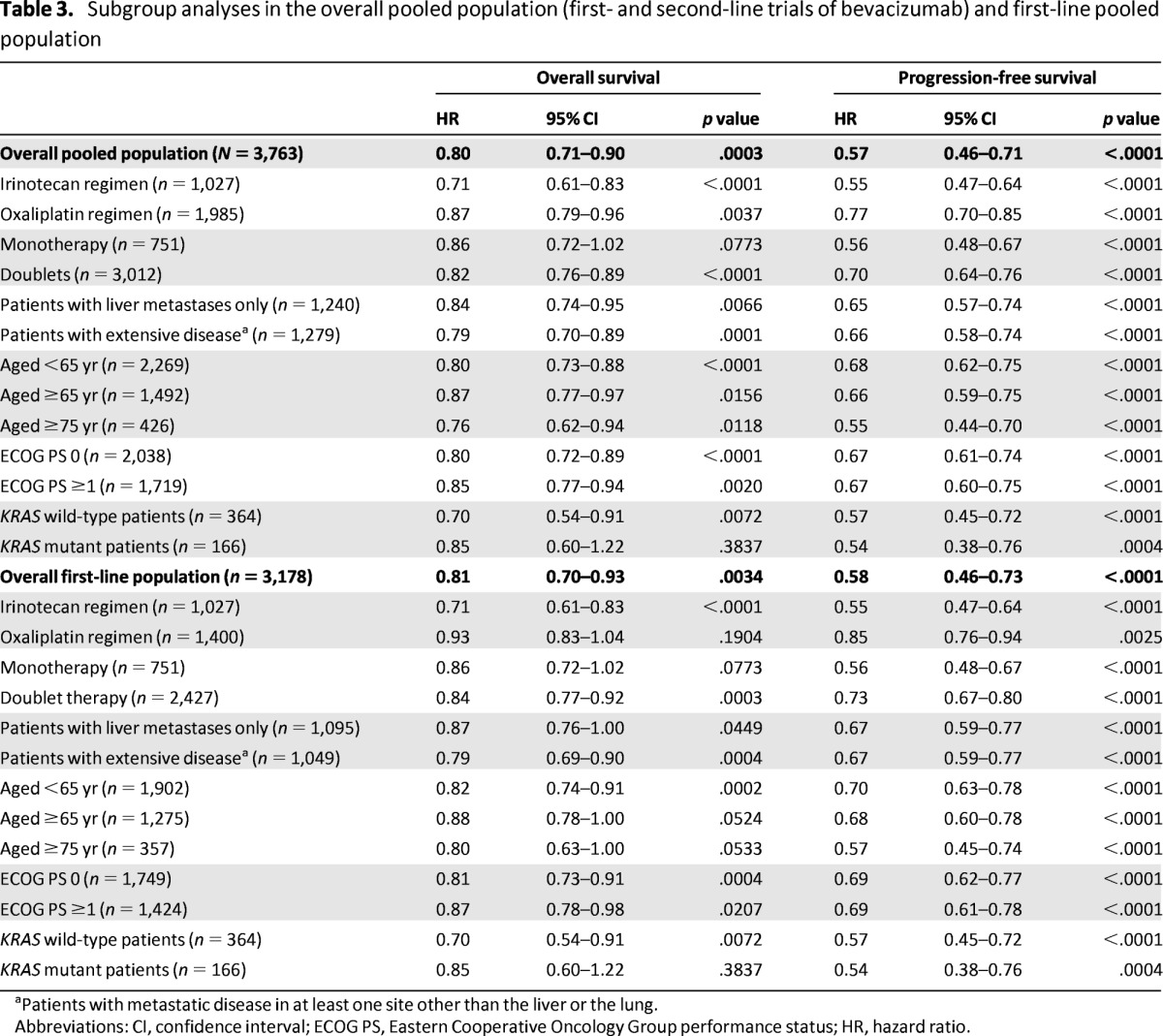
aPatients with metastatic disease in at least one site other than the liver or the lung.
Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio.
OS and PFS were prolonged with bevacizumab across the age subgroups (<65 years, ≥65 years, and ≥75 years) and the ECOG PS subgroups (0, ≥1) that were evaluated. Data on KRAS mutational status were available from two of the RCTs (AVF2107, AGITG MAX), totaling 530 patients (14.1% of the overall pooled population). When these patient data were pooled, the addition of bevacizumab to chemotherapy was shown to increase PFS regardless of KRAS status. Analyses also showed more favorable OS in bevacizumab-treated patients with either KRAS wild-type or mutant tumors, although the OS benefit did not reach statistical significance in the KRAS mutant subgroup. These data should be considered exploratory because of the limited number of patients for which KRAS status was available. Last, statistically significant survival benefits with bevacizumab were seen in patients with liver metastases only and in patients with metastatic disease in at least one site beyond the liver and the lung.
Safety
Safety analyses of the overall pooled population showed that 78.1% and 68.3% (OR, 1.88; 95% CI, 1.61–2.18) of patients experienced grade ≥3 AEs with chemotherapy plus bevacizumab and chemotherapy with or without placebo, respectively (Table 4); fatal AEs occurred in 4.0% and 3.0% of patients, respectively (OR, 1.45; 95% CI, 1.01–2.06). Pooled incidence rates of grade ≥3 AEs of special interest were consistent with those reported in the individual studies.
Table 4.
Safety outcomes in the overall pooled population (first- and second-line trials of bevacizumab)
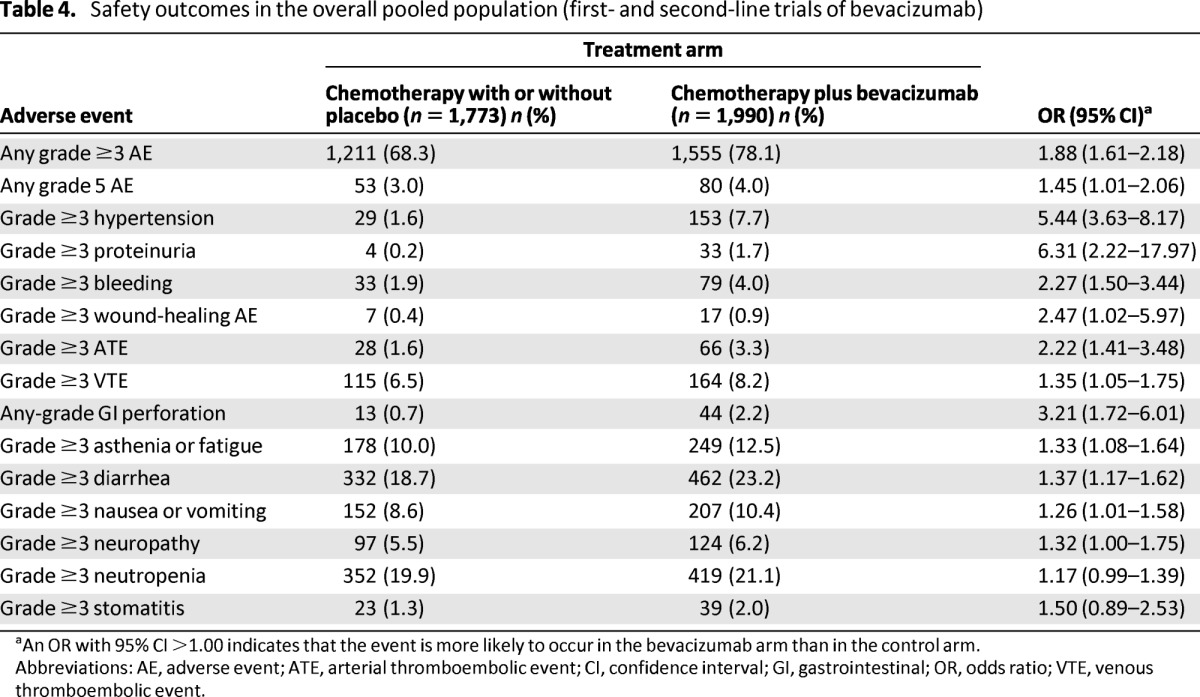
aAn OR with 95% CI >1.00 indicates that the event is more likely to occur in the bevacizumab arm than in the control arm.
Abbreviations: AE, adverse event; ATE, arterial thromboembolic event; CI, confidence interval; GI, gastrointestinal; OR, odds ratio; VTE, venous thromboembolic event.
As expected, the use of chemotherapy plus bevacizumab was associated with a higher incidence of bevacizumab-associated AEs than chemotherapy with or without placebo (e.g., grade ≥3 hypertension [7.7% vs. 1.6%], grade ≥3 proteinuria [1.7% vs. 0.2%], and any-grade gastrointestinal perforation [2.2% vs. 0.7%]) (Table 4). Analyses of chemotherapy-related AEs of interest also showed a slightly higher incidence of grade ≥3 events in the chemotherapy plus bevacizumab arm.
First-Line Pooled Analysis
The first-line pooled population consisted of 3,178 patients, of whom 1,481 received chemotherapy with or without placebo and 1,697 were treated with chemotherapy plus bevacizumab. Baseline and disease characteristics were again well balanced between groups (data not shown). Overall, 58.5% of patients in the first-line pooled population were male, 40.1% were aged ≥65 years, and 44.9% had an ECOG PS ≥1.
In the first-line pooled population, chemotherapy plus bevacizumab prolonged OS and PFS relative to chemotherapy with or without placebo. Median OS was 19.8 months with chemotherapy plus bevacizumab and 17.6 months with chemotherapy with or without placebo (HR, 0.81; 95% CI, 0.70–0.93; p = .0034) (Table 3). PFS was also increased with chemotherapy plus bevacizumab relative to chemotherapy with or without placebo, with median values of 9.1 months and 6.9 months, respectively (HR, 0.58; 95% CI, 0.46–0.73; p < .0001).
Additional analyses in the first-line treatment setting showed a consistent trend for an improved OS benefit with bevacizumab across patient subgroups. Treatment-related differences in OS were statistically significant in some subgroups, including patients receiving irinotecan-based chemotherapy, patients receiving doublet therapy, patients aged <65 years, patients with an ECOG PS ≥1, patients with KRAS wild-type tumors, and patients with extensive disease at baseline (Table 3). PFS was statistically significantly prolonged by the use of first-line bevacizumab in all examined subgroups (Table 3).
Discussion
Since the initial approval of bevacizumab, treatment patterns for patients with mCRC have evolved, and subsequent RCTs evaluating bevacizumab have reported varying degrees of survival benefit in different treatment settings [15]. Although meta-analyses of published RCT data have increased the understanding of bevacizumab-associated survival benefits in mCRC, they have generally been limited in scope, owing to their reliance on published information. The current analysis of pooled patient data from seven RCTs allowed robust assessment of the efficacy and safety of bevacizumab in mCRC across both first- and second-line treatment settings. Because the analysis accessed individual patient data, subgroup analyses could also be conducted.
Complementary to the results from individual studies, this pooled analysis provides an estimate for the effect of bevacizumab on PFS and OS. The HRs of 0.80 for OS and 0.57 for PFS seen in this analysis were statistically significant and clinically meaningful; however, better patient selection strategies and more effective treatments are still needed.
Subgroup analyses consistently confirmed a PFS benefit with bevacizumab in clinically relevant groups. Of particular interest were the analyses according to chemotherapy backbone. There has been some suggestion that bevacizumab may be more effective with irinotecan-based regimens than with oxaliplatin-based regimens [4, 15]. The current analysis showed that chemotherapy plus bevacizumab reduced the hazards for death and progression compared with chemotherapy with or without placebo, regardless of whether patients received an irinotecan- or an oxaliplatin-containing regimen, although the HRs favored irinotecan-containing regimens. This may be explained by high rates of early bevacizumab discontinuation reported in trials of first-line FOLFOX (infusional 5-fluorouracil and leucovorin with oxaliplatin) and bevacizumab, including the NO16966 study [4, 18]. Other RCTs, as well as an observational cohort study, have shown similar clinical outcomes with oxaliplatin-based and irinotecan-based regimens that included bevacizumab in the treatment of colorectal cancer [7, 19–22].
Elderly patients are generally underrepresented in RCTs; therefore, analyses in this patient subgroup are often limited by insufficient patient numbers and a lack of statistical power. In this analysis, 1462 patients aged ≥65 years and 426 patients aged ≥75 years were included, and a PFS benefit was seen for elderly patients receiving bevacizumab. The benefit in OS with bevacizumab was also statistically significant across all age and ECOG PS subgroups. A previously published pooled analysis of studies AVF2107, AVF2192, NO16966, and E3200 also reported that adding bevacizumab to chemotherapy prolonged OS and PFS in patients grouped by age and that AE rates were generally similar between older and younger bevacizumab-treated patients, with the exception of a higher rate of thromboembolic events (driven primarily by an increase in arterial thromboembolic events) in older patients [10]. A retrospective analysis of patients aged ≥75 years from the AGITG MAX trial reported similar findings [23].
The current analysis also examined the effect of bevacizumab exclusively in the first-line treatment setting, where bevacizumab has been studied most extensively. Analyses of the first-line pooled population showed that the addition of bevacizumab to first-line chemotherapy led to statistically significant improvements in OS and PFS. Benefits for PFS extended across subgroups defined by chemotherapy backbone, intensity of chemotherapy, age, extent of disease at baseline, and KRAS mutation status. Although no statistically significant benefit for OS was observed among patients treated with oxaliplatin-containing chemotherapy in the first-line setting, this is also a possible consequence of the premature discontinuation of bevacizumab therapy seen in the NO16966 trial [4]. This outcome reflects the ongoing challenge of combining a biologic agent that inhibits disease progression with a chemotherapy regimen that is frequently interrupted or discontinued before progression.
The safety profile of bevacizumab is well characterized in RCTs (including those assessed in the current analysis) and data from clinical practice [21, 24–26]. The AE incidences observed were consistent with previously published data, which showed that bevacizumab is associated with increases in the frequency of AEs that are seen with essentially all VEGF-A inhibitors, such as hypertension, proteinuria, and thromboembolic events.
Limitations of the analysis primarily involve its retrospective nature; the heterogeneity in terms of treatment and line of therapy between the individual studies; and the lack of data availability for certain variables across all studies, including KRAS/BRAF tumor status (missing in NO16966, AVF0780, AVF2192, E3200, and ARTIST), tumor assessments by independent review committees (missing in AVF2107, NO16966, E3200, ARTIST, and AGITG MAX), and AEs (limited information in AGITG MAX).
Conclusion
Comprehensive assessments of the risk-benefit profiles of approved agents are critical to determining the value and comparative effectiveness of currently available therapies and regimens. This pooled analysis of patient data from seven phase II and III RCTs demonstrated that the use of bevacizumab with chemotherapy was associated with statistically significant benefits in survival outcomes, relative to chemotherapy alone, in the treatment of mCRC. The benefits for PFS and OS were observed across a number of patient subgroups. In addition, the analysis confirmed the safety profile of bevacizumab reported in the individual trials from which the data were pooled.
Acknowledgments
The conduct of the analysis as well as support for third-party medical writing assistance was funded by F. Hoffmann-La Roche, Ltd (Basel, Switzerland). We thank Uwe Heberle and Christina Gelhorn of Accovion GmbH for their assistance with statistical analyses. Third-party editorial, copyediting, and production assistance for this manuscript, provided by Christopher M. Brown, Ph.D. of CodonMedical, was funded by F. Hoffmann-La Roche, Ltd.
Author Contributions
Conception/Design: Herbert I. Hurwitz, Niall C. Tebbutt, Bruce J. Giantonio, Daniel Waterkamp, Josep Tabernero
Provision of study material or patients: Herbert I. Hurwitz, Niall C. Tebbutt, Fairooz Kabbinavar, Bruce J. Giantonio, Zhong-Zhen Guan
Collection and/or assembly of data: Niall C. Tebbutt, Bruce J. Giantonio, Zhong-Zhen Guan, Daniel Waterkamp, Josep Tabernero
Data analysis and interpretation: Herbert I. Hurwitz, Niall C. Tebbutt, Fairooz Kabbinavar, Bruce J. Giantonio, Zhong-Zhen Guan, Lada Mitchell, Daniel Waterkamp, Josep Tabernero
Manuscript writing: Herbert I. Hurwitz, Niall C. Tebbutt, Fairooz Kabbinavar, Bruce J. Giantonio, Daniel Waterkamp, Josep Tabernero
Final approval of manuscript: Herbert I. Hurwitz, Niall C. Tebbutt, Fairooz Kabbinavar, Bruce J. Giantonio, Zhong-Zhen Guan, Lada Mitchell, Daniel Waterkamp, Josep Tabernero
Disclosures
Herbert I. Hurwitz: Genentech, Roche, Sanofi, GSK (C/A); Genentech, Roche, Sanofi, Regeneron (H); Niall C. Tebbutt: Roche (C/A); Roche (RF); Fairooz Kabbinavar: Roche (H); Lada Mitchell: GGL Statistics GMA Biometrics, F. Hoffmann La Roche (E); Daniel Waterkamp: Roche (E); Josep Tabernero: Amgen, Boehringer, BMS, Genentech, Imclone, Lilly, Merck KGaA, Millennium, Onyx, Pfizer, Roche, Sanofi, Celgene (C/A); Amgen, Nerck KGaG, Novartis, Roche, Sanofi (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 2.Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: Results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–3705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 3.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 4.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 5.Tebbutt NC, Wilson K, Gebski VJ, et al. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: Results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin Oncol. 2010;28:3191–3198. doi: 10.1200/JCO.2009.27.7723. [DOI] [PubMed] [Google Scholar]
- 6.Guan ZZ, Xu JM, Luo RC, et al. Efficacy and safety of bevacizumab plus chemotherapy in Chinese patients with metastatic colorectal cancer: A randomized phase III ARTIST trial. Chin J Cancer. 2011;30:682–689. doi: 10.5732/cjc.011.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol. 2013;14:29–37. doi: 10.1016/S1470-2045(12)70477-1. [DOI] [PubMed] [Google Scholar]
- 8.Wagner AD, Arnold D, Grothey AA, et al. Anti-angiogenic therapies for metastatic colorectal cancer. Cochrane Database Syst Rev. 2009:CD005392. doi: 10.1002/14651858.CD005392.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Cao Y, Tan A, Gao F, et al. A meta-analysis of randomized controlled trials comparing chemotherapy plus bevacizumab with chemotherapy alone in metastatic colorectal cancer. Int J Colorectal Dis. 2009;24:677–685. doi: 10.1007/s00384-009-0655-9. [DOI] [PubMed] [Google Scholar]
- 10.Cassidy J, Saltz LB, Giantonio BJ, et al. Effect of bevacizumab in older patients with metastatic colorectal cancer: Pooled analysis of four randomized studies. J Cancer Res Clin Oncol. 2010;136:737–743. doi: 10.1007/s00432-009-0712-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welch S, Spithoff K, Rumble RB, et al. Bevacizumab combined with chemotherapy for patients with advanced colorectal cancer: A systematic review. Ann Oncol. 2010;21:1152–1162. doi: 10.1093/annonc/mdp533. [DOI] [PubMed] [Google Scholar]
- 12.Loupakis F, Bria E, Vaccaro V, et al. Magnitude of benefit of the addition of bevacizumab to first-line chemotherapy for metastatic colorectal cancer: Meta-analysis of randomized clinical trials. J Exp Clin Cancer Res. 2010;29:58. doi: 10.1186/1756-9966-29-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galfrascoli E, Piva S, Cinquini M, et al. Risk/benefit profile of bevacizumab in metastatic colon cancer: A systematic review and meta-analysis. Dig Liver Dis. 2011;43:286–294. doi: 10.1016/j.dld.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Chi P. Optimizing the efficacy of first-line chemotherapy plus bevacizumab in metastatic colorectal cancer: Analysis of multiple methods. BioDrugs. 2011;25:43–50. doi: 10.2165/11584680-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Macedo LT, da Costa Lima AB, Sasse AD. Addition of bevacizumab to first-line chemotherapy in advanced colorectal cancer: A systematic review and meta-analysis, with emphasis on chemotherapy subgroups. BMC Cancer. 2012;12:89. doi: 10.1186/1471-2407-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 17.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Berlin J, Bendell J, Hart LL, et al. A phase 2, randomized, double-blind, placebo-controlled study of hedgehog pathway inhibitor (HPI) GDC-0449 in patients with previously untreated metastatic colorectal cancer (mCRC) Ann Oncol. 2010;21(suppl):LBA21a. [Google Scholar]
- 19.Reinacher-Schick AC, Kubicka S, Freier W, et al. Activity of the combination of bevacizumab (Bev) with capecitabine/irinotecan (Capiri/Bev) or capecitabine/oxaliplatin (CapOx/Bev) in advanced colorectal cancer (ACRC): A randomized phase II study of the AIO Colorectal Study Group (AIO trial 0604) J Clin Oncol. 2008;26(suppl):4030a. [Google Scholar]
- 20.Bekaii-Saab TS, Grothey A, Bendell JC, et al. Effectiveness and safety of second-line (2L) irinotecan- and oxaliplatin-based regimens after first-line (1L) bevacizumab (BV)-containing treatment (tx) for metastatic colorectal cancer (mCRC): Results from the ARIES observational cohort study. J Clin Oncol. 2012;30(suppl):535a. [Google Scholar]
- 21.Bendell JC, Bekaii-Saab TS, Cohn AL, et al. Treatment patterns and clinical outcomes in patients with metastatic colorectal cancer initially treated with FOLFOX-bevacizumab or FOLFIRI-bevacizumab: Results from ARIES, a bevacizumab observational study. The Oncologist. 2012;17:1486–1495. doi: 10.1634/theoncologist.2012-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Österlund P, Alonso-Orduna V, Schlichting C, et al. Bevacizumab (BEV) + chemotherapy (CT) beyond first progression in patients (pts) with metastatic colorectal cancer (mCRC) previously treated with first-line BEV+CT (ML18147): Efficacy and safety analyses by oxaliplatin vs irinotecan-based CT. Ann Oncol. 2012;23(suppl):571Pa. [Google Scholar]
- 23.Price TJ, Zannino D, Wilson K, et al. Bevacizumab is equally effective and no more toxic in elderly patients with advanced colorectal cancer: A subgroup analysis from the AGITG MAX trial: An international randomised controlled trial of capecitabine, bevacizumab, and mitomycin C. Ann Oncol. 2012;23:1531–1536. doi: 10.1093/annonc/mdr488. [DOI] [PubMed] [Google Scholar]
- 24.Kozloff M, Yood MU, Berlin J, et al. Clinical outcomes associated with bevacizumab-containing treatment of metastatic colorectal cancer: The BRiTE observational cohort study. The Oncologist. 2009;14:862–870. doi: 10.1634/theoncologist.2009-0071. [DOI] [PubMed] [Google Scholar]
- 25.Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: The BEAT study Ann Oncol. 2009;20:1842–1847. doi: 10.1093/annonc/mdp233. [DOI] [PubMed] [Google Scholar]
- 26.Arnold D, Kindler M, Petersen V, et al. Bevacizumab plus chemotherapy as first-line treatment for patients with metastatic colorectal cancer: First results from a large community-based observational cohort study in Germany. Presented at: the 2010 Gastrointestinal Symposium; January 22–24, 2010; Orlando, Florida, USA. [Google Scholar]