This article summarizes existing literature exploring reasons for racial disparities in breast cancer mortality, with an emphasis on treatment disparities and opportunities for future research. Recognition that variation in cancer care quality may be correlated with race (and socioeconomic and health system factors) may assist policy makers in identifying strategies to more equally distribute clinical expertise and health infrastructure across multiple user populations.
Keywords: Breast cancer, Disparities, Cancer care quality, Race, Access to care
Abstract
Racial disparities in breast cancer mortality have been widely documented for several decades and persist despite advances in receipt of mammography across racial groups. This persistence leads to questions about the roles of biological, social, and health system determinants of poor outcomes. Cancer outcomes are a function not only of innate biological factors but also of modifiable characteristics of individual behavior and decision making as well as characteristics of patient-health system interaction and the health system itself. Attempts to explain persistent racial disparities have mostly been limited to discussion of differences in insurance coverage, socioeconomic status, tumor stage at diagnosis, comorbidity, and molecular subtype of the tumor. This article summarizes existing literature exploring reasons for racial disparities in breast cancer mortality, with an emphasis on treatment disparities and opportunities for future research. Because breast cancer care requires a high degree of multidisciplinary team collaboration, ensuring that guideline recommended treatment (such as endocrine therapy for hormone receptor positive patients) is received by all racial/ethnic groups is critical and requires coordination across multiple providers and health care settings. Recognition that variation in cancer care quality may be correlated with race (and socioeconomic and health system factors) may assist policy makers in identifying strategies to more equally distribute clinical expertise and health infrastructure across multiple user populations.
Implications for Practice:
Disparities in breast cancer outcomes result not only from racially specific tumor differences, but also modifiable social and health system determinants, such as poor access to care and health education, lack of financial resources, problematic patient-provider interactions, and structural barriers within the health system itself. Breast cancer care requires a high degree of multidisciplinary team collaboration; therefore, ensuring that care is delivered in a coordinated, continuous, culturally and socioeconomically sensitive fashion is critical to ensuring equitable receipt of guideline recommended treatments, which in turn, will help improve outcomes across racial groups. Understanding how biological, social, and health system factors act in concert to influence outcomes may help clinicians, researchers, and policy makers to identify more innovative strategies to address breast cancer disparities going forward.
Introduction
Overview of Breast Cancer Treatment Disparities
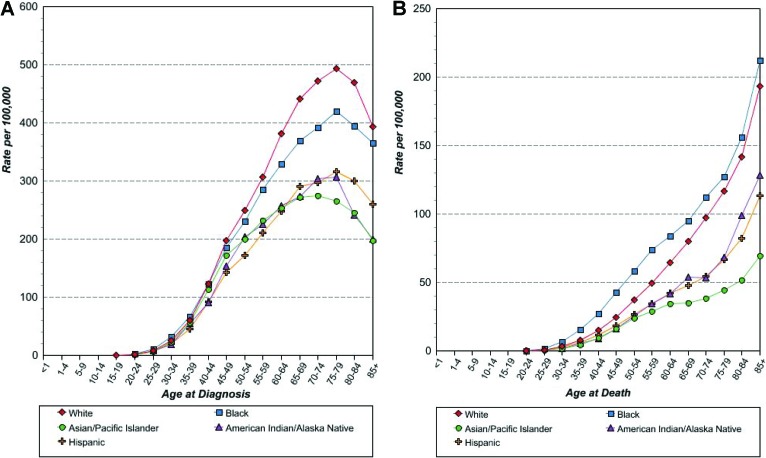
Health disparities in breast cancer treatment and outcomes have been widely documented for several decades. In spite of marked advances in breast cancer survival, disparities persist with respect to timeliness of diagnosis, receipt of treatment, and long-term health outcomes. These differences have been particularly stark between white and black patients [1–14]. Although breast cancer is diagnosed more often in white women, breast cancer mortality is higher among black women [15]. Racial trends in breast cancer incidence and mortality also vary depending on age (Fig. 1) [16]. Specifically, younger black women (<50 years old) have a higher incidence of breast cancer than younger white women, but around the time of menopause, this trend reverses, and older white women have higher breast cancer incidence [17, 18]. Epidemiology studies suggest that disparities in outcomes by race and ethnicity have not improved over time, despite the fact that the use of screening mammography is now nearly equivalent nationally among black and white women of all ages [19, 20]. Consequently, in the Healthy People 2010 report, the United States Department of Health and Human Services made it clear that elimination of health disparities was a national priority [21].
Figure 1.
Breast cancer incidence and mortality by age at diagnosis and race/ethnicity. (A): Age-specific SEER female breast cancer incidence rates by race/ethnicity, all ages, 1992–2010. (B): Age-specific US female breast cancer mortality rates by race/ethnicity, all ages, 1992–2010 [16].
Although developing interventions to ensure high-quality cancer treatment and optimal outcomes in the general population is of critical importance, we should be especially concerned about persistent disparities in treatment and outcomes within vulnerable subpopulations. In many cases, vulnerable subpopulations have not benefited equally from novel interventions to improve cancer outcomes [22, 23]. Over time, if the quality of cancer care improves, on average, in the general population but poor-quality care persists among minority groups, inequities in treatment that lead to disparate health outcomes may have been essentially ignored, resulting in widening gaps in outcomes between groups. Understanding the reasons for persistent disparities in care for vulnerable populations may indicate opportunities to improve outcomes by targeting modifiable factors that disproportionately affect vulnerable patients. In addition, basic tenets of medical ethics compel health care providers to pay attention to such inequities.
Factors Contributing to Breast Cancer Disparities
The picture of health disparities in breast cancer is multifaceted and complex. Although black women, for example, are diagnosed at a more advanced disease stage [24], survival differences persist between blacks and whites diagnosed at similar stages of illness, suggesting that breast cancer in black women is fundamentally different from that in white women. Other support for biological disparity comes from studies finding that although differences in patient stage largely disappear when mammography receipt is controlled, black women still have significantly more high-grade breast cancers [25]. Moreover, black women are at substantially higher risk of developing “triple-negative” (hormone receptor- and HER2-negative) breast cancer [26]. After controlling for patient and biologic tumor characteristics at diagnosis, such as comorbidities and hormone receptor and HER2 status, a clear stage-specific survival gap remains [27]. This gap has been essentially stable over the past two decades, despite significant gains in overall breast cancer outcomes. This gap suggests that nonbiologic factors and factors independent of stage of disease at presentation, such as treatment differences, may also explain a significant portion of observed outcome disparities.
Organizational, structural, socioeconomic, and sociopolitical dynamics of the American health system likely contribute to racial and ethnic health disparities and may not be amenable to rapid change. In epidemiological terms, racial and ethnic differences in breast cancer-related morbidity and mortality can be thought of as being produced by multiple complementary causes [9, 14], none of which is sufficiently explanatory alone. Possible explanations for enduring racial and ethnic disparities include biological differences in tumor behavior and morphology [6, 26, 28–30], differences in therapeutic response [31], patient-level psychosocial or behavioral factors [32], socioeconomic status and access to care [9, 33, 34], and treatment differences [8, 11, 35]. Determining which of these multifaceted factors can be adjusted to improve outcomes is vital and requires deeper understanding of the complex relationships between biological, behavioral, and social determinants of health. Genetic susceptibility to more aggressive cancers, for example, cannot be changed by public health efforts; however, understanding racial differences in tumor biology may point to environment-gene interactions that could benefit from targeted intervention. In addition, sociodemographic factors that tend to vary by race, such as access to insurance coverage, income, and educational attainment, may be slow to change over time; however, if access to care and socioeconomic status are held constant, there is no good reason why a black woman should be offered lower quality treatment than a white woman with the same clinical disease.
Biological Factors
As previously noted, black women diagnosed with breast cancer tend to have more aggressive tumors and worse prognosis than white women [17, 24, 26, 27, 36–39]. Younger black women are also more likely to have triple-negative breast cancers and less likely to have estrogen receptor (ER)-positive cancers [26, 29, 37, 40]. Understanding the characterization and clinical behavior of cancers with different biological features has become increasingly important in recent years [24, 26]. Indeed, many clinicians and researchers would argue that the various biological subtypes of breast cancer are so strikingly different from one another that they represent entirely different diseases and must be treated as such [26, 36, 37, 41, 42]. Given the diversity in tumor biology in breast cancer and the importance of differential signatures by race, understanding the biological uniqueness of tumors presenting in black women is essential [37, 43, 44].
Histological features of breast cancers also vary by race and ethnicity [6, 28, 30, 45]. Chen and colleagues adjusted for age, stage, socioeconomic status, body mass index, reproductive history, insurance status, and location and found that black breast cancer patients had greater nuclear atypia and more necrosis compared with white women [40]. Although it had long been recognized that black women were more likely to have hormone receptor-negative breast cancer, recent studies have shed additional light on this phenomenon. A series of immunohistochemical studies on tumors obtained within the population-based Carolina Breast Cancer Study (CBCS) found that a significantly lower proportion of black women had the good-prognosis, hormone receptor-positive, HER2-negative breast cancer; conversely, a higher proportion of black women, particularly younger black women, had the poor-prognosis, triple-negative breast cancer. Triple-negative disease is so called because it lacks all three of the known targetable breast cancer proteins, the ERs and progesterone receptors, and the signaling pathway member HER2.
Some protective factors, like early age of menarche, obesity, and breastfeeding, were far stronger for basal-like breast cancer than for luminal hormone receptor-positive, HER2-negative breast cancer. Other factors actually appeared to have opposite effects: Multiparity and young age at first full-term pregnancy, long acknowledged to be protective factors, appear to be protective only for luminal breast cancer and rather to be risk factors for basal-like breast cancer.
Higher incidence of triple-negative breast cancer has also been observed among West African breast cancer patients [46]. Intriguingly, the CBCS analysis and others have suggested that traditional risk factors varied by subtype [36, 47]. Some protective factors, like early age of menarche, obesity, and breastfeeding, were far stronger for basal-like breast cancer than for luminal hormone receptor-positive, HER2-negative breast cancer. Other factors actually appeared to have opposite effects: Multiparity and young age at first full-term pregnancy, long acknowledged to be protective factors, appear to be protective only for luminal breast cancer and rather to be risk factors for basal-like breast cancer [36]. These studies imply that lifestyle exposures may be responsible for variations in outcomes by race in certain breast cancer subtypes; meanwhile, recent genome-wide association studies implicate novel breast cancer risk variants in women of African ancestry [48].
In a review of the literature on tumor aggressiveness in black women, Morris and Mitchell report that in addition to these known differences in pathologically defined subtypes and BRCA mutations, black women also have more overexpression of cell-cycle regulators, such as cyclin E, p16, and p53, and polymorphisms in nucleotide excision repair genes [45]. This large body of evidence lends considerable support to the hypothesis that breast cancer in black women is biologically different. It is clear, however, that biological factors cannot explain all of the racial disparity in morbidity and mortality [5, 36, 27, 49].
Social Factors and Screening Behaviors
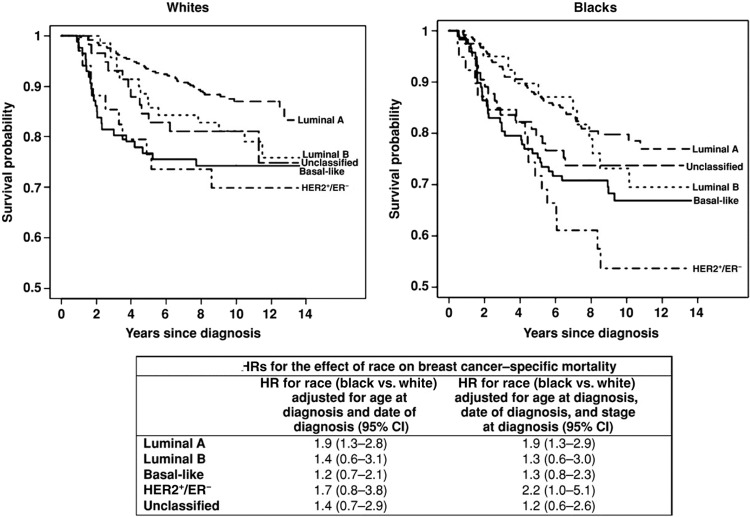
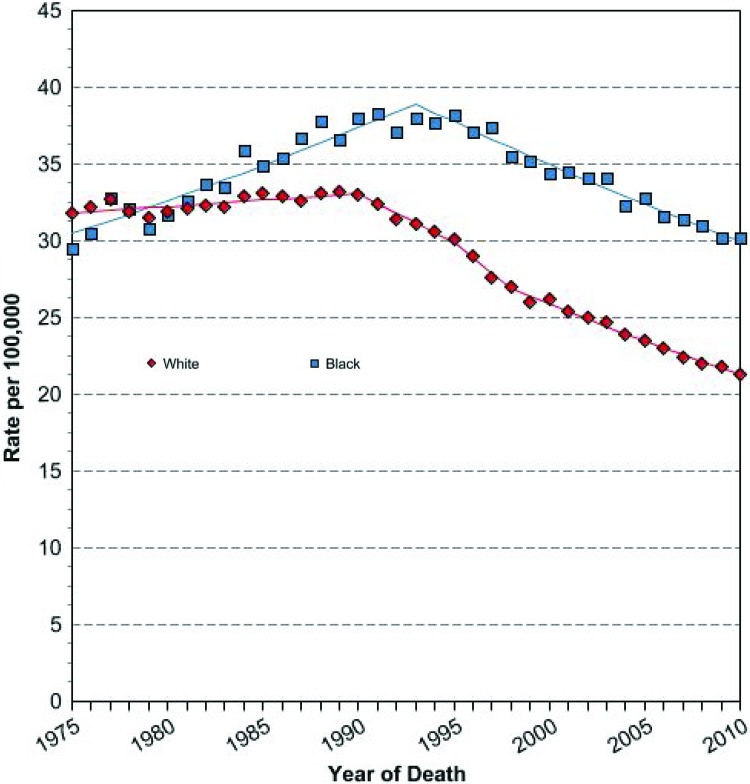
Although biologic differences contribute to breast cancer disparities, it is also widely agreed that social and behavioral factors play a large role in the racial differences observed in breast cancer mortality. In the CBCS, after controlling for biologic differences in tumor subtype, racial disparities in outcomes not only persisted but were particularly prominent among the good-prognosis, hormone receptor positive, HER2-negative tumors, suggesting that social and behavioral factors that vary by race may have been driving outcome differences (Fig. 2) [27]. Persistent racial disparity has been linked to underuse of screening mammography and lack of diagnostic follow-up after an abnormal mammogram result [6, 49]. Use of screening mammography, however, has been nearly equivalent among racial and ethnic groups for a decade [49, 50], and even when controlling for stage at diagnosis and insurance status, differences in mortality persist; although secular trends in disease-specific mortality have shown improvements in the past 30 years, the relative difference between mortality rates for black women and non-Hispanic women has not changed (Fig. 3) [16]. Rather, the gap appears to be widening.
Figure 2.
Race-stratified Kaplan-Meier plots and race effect estimates for breast cancer-specific mortality by immunohistochemical subtype in the Carolina Breast Cancer Study, 1993–2006 [27]. Abbreviations: CI, confidence interval; ER, estrogen receptor; HR, hazard ratio.
Figure 3.
Age-adjusted U.S. female breast cancer-specific mortality rates by race, 1975–2005 [16].
Social factors, including poverty and financial insecurity, lack of transportation, poor access to care, poor health literacy, low educational attainment, and lack of health insurance contribute substantially to differences in breast cancer outcomes [5, 9, 12, 51–56]. Lower income and uninsured cancer patients are especially sensitive to the high costs of cancer care and may be more likely to be from minority groups [57]. Regardless of insurance status, the hidden costs of cancer care, including transportation and the inability to work, may be especially burdensome to the minority cancer patient [58]. The addition of novel biologic therapies such as trastuzumab to standard treatment for breast cancer has increased costs to such a point that even insured patients may face staggering copayments and other shared costs [59, 60]. In one national survey of cancer patients and families conducted by the Kaiser Family Foundation, USA Today, and the Harvard School of Public Health (2006), nearly one in four privately insured patients had exhausted all or most of their personal savings to pay for cancer-related care, and 13% of cancer patients reported being contacted by a collection agency demanding payment for cancer treatment [61]. As treatment options become more sophisticated and prognosis continues to improve, costs associated with treatment, survivorship, and surveillance will become increasingly important issues to consider in understanding racial variation in outcomes.
Several other patient-level social and behavioral factors may help explain why different patients receive different treatments and experience worse health outcomes. These include health literacy and personal preferences [62], cognitive and social network correlates [32], trust in the health care system, and health care-seeking behavior [35, 63].
Finally, competing comorbidities, which differ by race, also affect breast cancer screening, diagnosis, and outcomes [14]. Higher comorbidity burden among blacks could affect both overall survival and disease-specific survival, if care for comorbidities competes with cancer care priorities. In addition, if functional status or mental health status is compromised by comorbid conditions, these may also inhibit—rather than promote—health-seeking behaviors for cancer diagnoses [14].
Health System Factors
Although much research has been published in the areas of biological tumor variation and social and behavioral factors as they relate to race and ethnicity-related breast cancer disparities, relatively little has been published about how characteristics of the health system itself, including provider- and facility-level factors, affect racial variation in treatment and outcomes. Structural and organizational differences in health services available to women can explain some of the racial and ethnic disparity in breast cancer treatment and outcomes [64–66].
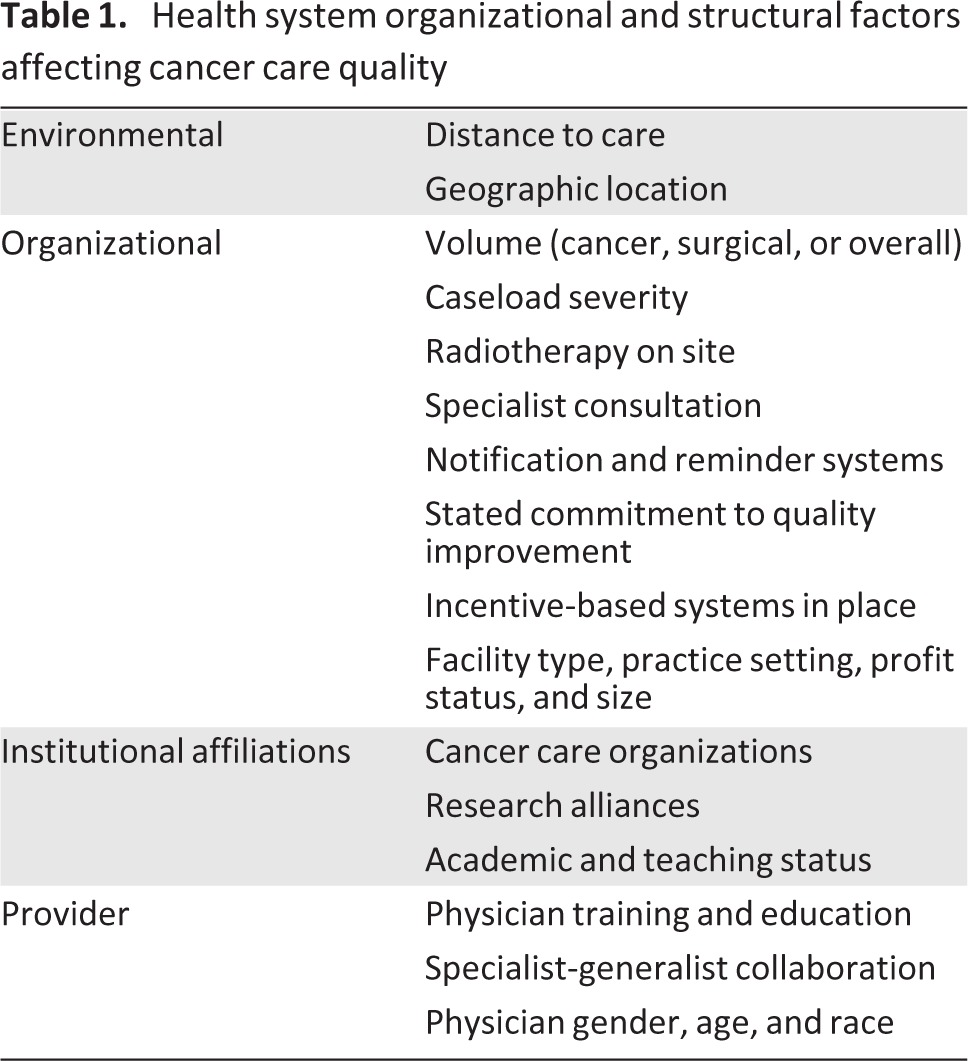
Some of the principal structural and organizational factors known to affect the quality of cancer care are summarized in Table 1. These physician- and facility-level factors have varying degrees of influence on quality of cancer care received. Several may be more or less problematic for particular patient subpopulations. For example, distance to care may pose a greater barrier to care for older women, given the transportation and mobility difficulties many elderly women face [67]. In addition, health-seeking behavior and use of health services with certain structural and organizational characteristics likely vary by subgroups. For example, some ethnic groups may prefer to access health care facilities that recognize and address language barriers by hiring translators; those health facilities that can afford to offer such patient services are likely different in terms of organization and size from those facilities that do not offer bilingual services. In addition, membership in racial and ethnic groups may correlate with community residence, and type and location of health facilities may be related to sociodemographic makeup of the local user population. Consequently, innovative care processes may be distributed unequally. If distribution and diffusion of evidence-based innovations are disproportionately benefiting certain women compared with others, differential quality of care may be observed across sociodemographic groups.
Table 1.
Health system organizational and structural factors affecting cancer care quality
Finally, patient sociodemographics could be associated with the types and the quality of providers and health facilities that are available to the local user population. Black cancer patients may have worse access to well-trained providers and more often be treated by physicians who lack measurable skills, board certification, and technical resources [68]. In one study, physicians of black cancer patients more often reported that they were unable to provide the best care to their patients, although the reasons are somewhat unclear [68].
Health services characteristics are rarely considered in empirical analyses to confound or modify the effect of race on breast cancer treatment and outcomes. Analyses that have included health system variables as covariates in addition to race and ethnicity, such as one study using data from the National Cancer Database [69], have generally highlighted the importance of a few structural and organizational variables as potential confounders only. In this study, after controlling for hospital teaching status, regional supply of radiation oncologists, surgical volume, and ratio of specialists to generalists in predicting receipt of breast-conserving surgery over time, race was statistically nonsignificant. In addition, black women who failed to receive timely screening mammography reported that they lacked physician referral or recommendation for the test [70].
The interactive effects of structural and organizational variables and race and ethnicity have been explored to a greater degree in the literature of other cancers. In prostate cancer, black men have poorer access to health care and less continuity of care for screening and, once diagnosed, are more likely to be treated by lower volume surgeons [63, 71]. In rectal cancer, black patients are more likely to be treated by low-volume physicians and less likely to receive adjuvant therapy [72]. In a study of advanced lung cancer patients, black patients, particularly of lower socioeconomic status, were less likely to see an oncology specialist and subsequently less likely to receive clinically recommended chemotherapy, after controlling for age, sex, year of diagnosis, region, hospital teaching status, and comorbidities [73].
To date, the breast cancer literature has rarely considered the role of multiple health service organization measures explicitly on racial and ethnic disparities in treatment and outcomes. The exceptions are two Surveillance Epidemiology and End Results (SEER)-Medicare studies that showed that health system factors, including distance to care, may modify the relationship between race and ethnicity and receipt of guideline-recommended breast cancer treatment, including radiation therapy and chemotherapy [65, 66]. Research suggests that pinpointing system-level factors that may contribute to persistent disparities can help policy makers focus efforts to equalize health care access and quality across diverse user populations.
Treatment Differences
Differences in treatment, working in parallel with race-based biological differences, are believed to account for a large portion of the variation in breast cancer outcomes, in particular, the persistent state-specific survival gap observed between white and black women [5]. If certain subpopulations receive less-than-standard treatment (i.e., treatment appropriate for the features of the cancer), differences in outcomes are likely to result. Black women, more often than other women with the same stage disease, fail to receive timely diagnosis and recommended treatment for breast cancer [13, 74].
Multiple studies have documented racial disparities in almost every aspect of breast cancer treatment. Black and Hispanic women fail to receive definitive local therapy for curable breast cancers more often than whites, after adjustment for age and tumor characteristics [8]. Similar patterns are seen for adjuvant chemotherapy and radiotherapy, with black and Hispanic women failing to receive appropriate adjuvant therapy more often than whites, even after controlling for access to a medical oncologist [3]. Black women are four to five times more likely to experience treatment delays longer than 60 days and significantly less likely to receive cancer-directed surgery, radiation therapy after lumpectomy, and hormonal therapy for hormone receptor-positive tumors, after controlling for tumor characteristics [11]. Such findings of treatment disparities appear to be consistent across geographical areas, having been documented in studies in Detroit, Michigan [1]; Atlanta, Georgia [11]; and in national SEER samples [8, 66]. In a SEER-Medicare study using a composite measure of adequate breast cancer that included appropriate radiation therapy following breast-conserving surgery, documentation of ER status, and surveillance mammography, black and Hispanic women were significantly less likely to receive adequate care, and the disparity actually increased over time [10]. Even in the controlled setting of a large randomized clinical trial, black women were significantly more likely to have early treatment discontinuation or delay than the white participants [75].
Studies examining treatment disparities have several limitations that complicate attempts to determine the root causes of differences in treatment. These include limited follow-up periods in national datasets such as SEER, underreporting of adjuvant therapies, and limited information regarding patient socioeconomic status and other social factors. Black women with breast cancer also have more comorbidities than their white counterparts [1], a factor that may influence treatment decision making, and not all studies have been able to control for this difference.
Because the racial gap in breast cancer survival is known to be largest among estrogen-sensitive subtypes in whom prolonged treatment over years is key to improving outcomes, and because nonadherence has been demonstrated to affect survival outcomes, differences in utilization of antiestrogen therapies may well explain some portion of the disparity in outcomes for these patients.
Racial disparities also exist in access to cutting-edge breast cancer treatments, both in the clinical trial setting and as innovations move into standard clinical practice. Black women are more reluctant to enroll in clinical trials and thus may have poorer access to trial-based innovations in cancer care [22, 23, 76–78]. In addition, few breast cancer randomized trials analyze outcomes based on race and ethnicity because of insufficient sample size, indicating a failure to report data that may inform disparities-focused interventions [79]. Consequently, certain groups may be denied research-related innovations, and this trend may continue as novel treatments move into clinical treatment settings. Black women, for example, less often receive sentinel lymph node biopsy (SLNB), a morbidity-sparing approach to axillary lymph node staging [7, 80]. Despite overall higher levels of uptake of SLNB between 1998 and 2005, racial and ethnic gaps in receipt of SLNB remained largely the same over time [7]. Black women are also less likely to receive any type of lymph node surgery for axillary staging [81].
Additional review articles overwhelmingly echo the findings of the specific empirical studies highlighted above [13, 14]. Shavers and Brown reported on several other treatment disparities between racial and ethnic groups, including differences in receipt of biomarker testing, follow-up after diagnosis and initial treatment, and surveillance mammography [13]. In addition, black and Hispanic women suffer more often from inadequate pain management and serious side effects of treatment [5, 14, 82]. Other authors concluded that disparities in treatment were the result of patient- or tumor-related, provider-related, and health system-related differences but that these factors were rarely considered explicitly in empirical studies as acting together [14, 35].
Significant gaps remain in our understanding of treatment disparities in breast cancer. Among the most prominent is the use of endocrine therapy in hormone receptor-positive patients, who compose the majority of breast cancer survivors. Although the problem of early discontinuation and nonadherence to endocrine therapy has been documented in a variety of patient populations [83–93], little is known about differences in endocrine therapy adherence among women of different races. Studies have shown conflicting relationships between race and endocrine therapy utilization among hormone receptor-positive subtypes [85, 87, 88, 94]. Because the racial gap in breast cancer survival is known to be largest among estrogen-sensitive subtypes in whom prolonged treatment over years is key to improving outcomes [27], and because nonadherence has been demonstrated to affect survival outcomes [95], differences in utilization of antiestrogen therapies may well explain some portion of the disparity in outcomes for these patients.
Opportunities and Recommendations for Future Research
Improving health-seeking behavior and trust in the health care system are difficult targets for research interventions, given the complex historical experiences of American minority groups [9, 96]. Similarly, advances in understanding of molecular differences in breast cancer by race that will enable individualized therapy are still on the horizon. As a starting point, ensuring equal access to provider- and facility-level health care resources should be a priority.
Although biologic and behavioral differences may influence outcomes to some extent, evidence suggests that when women across racial and ethnic groups receive equal treatment, equal outcomes follow [97, 98]. Black patients are at no greater risk for chemotherapy-related hematologic toxicity than white patients [99], and clinical trial results suggest that patterns of response to local and systemic therapy are similar for black and white women with clinically equivalent disease [75, 100–102]. In light of this evidence, it is critically important that the health system itself is designed in such a way that all women have access to life-prolonging cancer treatments, regardless of race, age, or socioeconomic status.
Conclusion
Cancer outcomes are a function not only of innate biological factors but also of modifiable characteristics of individual behavior and decision making as well as characteristics of the patient-health system interaction and the health system itself. Attempts to explain persistent racial and ethnic disparities have mostly been limited to discussion of differences in insurance coverage, socioeconomic status, stage at diagnosis, comorbidity, and molecular subtype of the tumor. Because breast cancer care requires a high degree of multidisciplinary team collaboration, ensuring that critical guideline-recommended treatment is received (e.g., endocrine therapy for ER-positive and progesterone receptor-positive patients) is critical and requires coordination across multiple providers and health care settings. Recognizing that variation in quality of cancer care received may be correlated with sociodemographic and health system characteristics may assist policy makers in identifying strategies to more equally distribute clinical expertise and health infrastructure across multiple user populations.
Acknowledgments
Stephanie B. Wheeler's effort working on this article was supported by an Agency for Healthcare Research and Quality Comparative Effectiveness Research Career Development Award, 1-K-12 HS019468-01 (Weinberger).
Author Contributions
Conception/Design: Stephanie B. Wheeler, Katherine E. Reeder-Hayes, Lisa A. Carey
Manuscript writing: Stephanie B. Wheeler, Katherine E. Reeder-Hayes, Lisa A. Carey
Final approval of manuscript: Stephanie B. Wheeler, Katherine E. Reeder-Hayes, Lisa A. Carey
Disclosures
The authors indicated no financial relationships.
References
- 1.Banerjee M, George J, Yee C, et al. Disentangling the effects of race on breast cancer treatment. Cancer. 2007;110:2169–2177. doi: 10.1002/cncr.23026. [DOI] [PubMed] [Google Scholar]
- 2.Bickell NA, Mendez J, Guth AA. The quality of early-stage breast cancer treatment: What can we do to improve? Surg Oncol Clin N Am. 2005;14:103–117. vi. doi: 10.1016/j.soc.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357–1362. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 4.Bickell NA, LePar F, Wang JJ, et al. Lost opportunities: Physicians' reasons and disparities in breast cancer treatment. J Clin Oncol. 2007;25:2516–2521. doi: 10.1200/JCO.2006.09.5539. [DOI] [PubMed] [Google Scholar]
- 5.Bigby J, Holmes MD. Disparities across the breast cancer continuum. Cancer Causes Control. 2005;16:35–44. doi: 10.1007/s10552-004-1263-1. [DOI] [PubMed] [Google Scholar]
- 6.Bowen RL, Stebbing J, Jones LJ. A review of the ethnic differences in breast cancer. Pharmacogenomics. 2006;7:935–942. doi: 10.2217/14622416.7.6.935. [DOI] [PubMed] [Google Scholar]
- 7.Chen AY, Halpern MT, Schrag NM, et al. Disparities and trends in sentinel lymph node biopsy among early-stage breast cancer patients (1998–2005) J Natl Cancer Inst. 2008;100:462–474. doi: 10.1093/jnci/djn057. [DOI] [PubMed] [Google Scholar]
- 8.Freedman RA, He Y, Winer EP, et al. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27:713–719. doi: 10.1200/JCO.2008.17.9234. [DOI] [PubMed] [Google Scholar]
- 9.Gerend MA, Pai M. Social determinants of black-white disparities in breast cancer mortality: A review. Cancer Epidemiol Biomarkers Prev. 2008;17:2913–2923. doi: 10.1158/1055-9965.EPI-07-0633. [DOI] [PubMed] [Google Scholar]
- 10.Haggstrom DA, Quale C, Smith-Bindman R. Differences in the quality of breast cancer care among vulnerable populations. Cancer. 2005;104:2347–2358. doi: 10.1002/cncr.21443. [DOI] [PubMed] [Google Scholar]
- 11.Lund MJ, Brawley OP, Ward KC, et al. Parity and disparity in first course treatment of invasive breast cancer. Breast Cancer Res Treat. 2008;109:545–557. doi: 10.1007/s10549-007-9675-8. [DOI] [PubMed] [Google Scholar]
- 12.Masi CM, Olopade OI. Racial and ethnic disparities in breast cancer: A multilevel perspective. Med Clin North Am. 2005;89:753–770. doi: 10.1016/j.mcna.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 14.Tammemagi CM. Racial/ethnic disparities in breast and gynecologic cancer treatment and outcomes. Curr Opin Obstet Gynecol. 2007;19:31–36. doi: 10.1097/GCO.0b013e3280117cf8. [DOI] [PubMed] [Google Scholar]
- 15.Bach PB, Schrag D, Brawley OW, et al. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287:2106–2113. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 16.Fast Stats: An interactive tool for access to SEER cancer statistics. Surveillance Research Program, National Cancer Institute. [Accessed August 4, 2013]. Available at http://seer.cancer.gov/faststats.
- 17.Vogel VG. Epidemiology, genetics, and risk evaluation of postmenopausal women at risk of breast cancer. Menopause. 2008;15(suppl):782–789. doi: 10.1097/gme.0b013e3181788d88. [DOI] [PubMed] [Google Scholar]
- 18.Colditz GA, Baer HJ, Tamimi RM. Chapter 51: Breast cancer. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. 3rd ed. New York, NY: Oxford University Press; 2006. pp. 995–1012. [Google Scholar]
- 19.American Cancer Society. Cancer Facts & Figures for African Americans: 2009–2010. [Accessed August 4, 2013]. Available at http://www.cancer.org/acs/groups/content/@nho/documents/document/cffaa20092010pdf.pdf.
- 20.Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–1427. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 21.Feenstra C. Healthy people 2010. Mich Nurse. 2000;73:14–15. [PubMed] [Google Scholar]
- 22.Movsas B, Moughan J, Owen J, et al. Who enrolls onto clinical oncology trials? A radiation Patterns Of Care Study analysis. Int J Radiat Oncol Biol Phys. 2007;68:1145–1150. doi: 10.1016/j.ijrobp.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 23.Gross CP, Filardo G, Mayne ST, et al. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103:483–491. doi: 10.1002/cncr.20792. [DOI] [PubMed] [Google Scholar]
- 24.Anders C, Carey LA. Understanding and treating triple-negative breast cancer. Oncology (Williston Park) 2008;22:1233–1239. discussion 1239–1240, 1243. [PMC free article] [PubMed] [Google Scholar]
- 25.Smith-Bindman R, Miglioretti DL, Lurie N, et al. Does utilization of screening mammography explain racial and ethnic differences in breast cancer? Ann Intern Med. 2006;144:541–553. doi: 10.7326/0003-4819-144-8-200604180-00004. [DOI] [PubMed] [Google Scholar]
- 26.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien KM, Cole SR, Tse CK, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16:6100–6110. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demicheli R, Retsky MW, Hrushesky WJ, et al. Racial disparities in breast cancer outcome: Insights into host-tumor interactions. Cancer. 2007;110:1880–1888. doi: 10.1002/cncr.22998. [DOI] [PubMed] [Google Scholar]
- 29.Lund MJ, Trivers KF, Porter PL, et al. Race and triple negative threats to breast cancer survival: A population-based study in Atlanta. GA Breast Cancer Res Treat. 2009;113:357–370. doi: 10.1007/s10549-008-9926-3. [DOI] [PubMed] [Google Scholar]
- 30.Porter PL, Lund MJ, Lin MG, et al. Racial differences in the expression of cell cycle-regulatory proteins in breast carcinoma. Cancer. 2004;100:2533–2542. doi: 10.1002/cncr.20279. [DOI] [PubMed] [Google Scholar]
- 31.Caudle AS, Gonzalez-Angulo AM, Hunt KK, et al. Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:1821–1828. doi: 10.1200/JCO.2009.25.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magai C, Consedine NS, Adjei BA, et al. Psychosocial influences on suboptimal adjuvant breast cancer treatment adherence among African American women: Implications for education and intervention. Health Educ Behav. 2008;35:835–854. doi: 10.1177/1090198107303281. [DOI] [PubMed] [Google Scholar]
- 33.Maloney N, Koch M, Erb D, et al. Impact of race on breast cancer in lower socioeconomic status women. Breast J. 2006;12:58–62. doi: 10.1111/j.1075-122X.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- 34.Mobley LR, Kuo TM, Clayton LJ, et al. Mammography facilities are accessible, so why is utilization so low? Cancer Causes Control. 2009;20:1017–1028. doi: 10.1007/s10552-009-9295-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blackman DJ, Masi CM. Racial and ethnic disparities in breast cancer mortality: Are we doing enough to address the root causes? J Clin Oncol. 2006;24:2170–2178. doi: 10.1200/JCO.2005.05.4734. [DOI] [PubMed] [Google Scholar]
- 36.Millikan RC, Newman B, Tse CK, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peppercorn J, Perou CM, Carey LA. Molecular subtypes in breast cancer evaluation and management: Divide and conquer. Cancer Invest. 2008;26:1–10. doi: 10.1080/07357900701784238. [DOI] [PubMed] [Google Scholar]
- 38.Schneider BP, Winer EP, Foulkes WD, et al. Triple-negative breast cancer: Risk factors to potential targets. Clin Cancer Res. 2008;14:8010–8018. doi: 10.1158/1078-0432.CCR-08-1208. [DOI] [PubMed] [Google Scholar]
- 39.Wildiers H, Brain EG. Adjuvant chemotherapy in elderly patients with breast cancer: Where are we? Curr Opin Oncol. 2005;17:566–572. doi: 10.1097/01.cco.0000180433.93872.d3. [DOI] [PubMed] [Google Scholar]
- 40.Chen VW, Correa P, Kurman RJ, et al. Histological characteristics of breast carcinoma in blacks and whites. Cancer Epidemiol Biomarkers Prev. 1994;3:127–135. [PubMed] [Google Scholar]
- 41.Fejerman L, Ziv E. Population differences in breast cancer severity. Pharmacogenomics. 2008;9:323–333. doi: 10.2217/14622416.9.3.323. [DOI] [PubMed] [Google Scholar]
- 42.Kang SP, Martel M, Harris LN. Triple negative breast cancer: Current understanding of biology and treatment options. Curr Opin Obstet Gynecol. 2008;20:40–46. doi: 10.1097/GCO.0b013e3282f40de9. [DOI] [PubMed] [Google Scholar]
- 43.Andre F, Pusztai L. Molecular classification of breast cancer: Implications for selection of adjuvant chemotherapy. Nat Clin Pract Oncol. 2006;3:621–632. doi: 10.1038/ncponc0636. [DOI] [PubMed] [Google Scholar]
- 44.Munoz M, Estevez LG, Alvarez I, et al. Evaluation of international treatment guidelines and prognostic tests for the treatment of early breast cancer. Cancer Treat Rev. 2008;34:701–709. doi: 10.1016/j.ctrv.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Morris GJ, Mitchell EP. Higher incidence of aggressive breast cancers in African-American women: A review. J Natl Med Assoc. 2008;100:698–702. doi: 10.1016/s0027-9684(15)31344-4. [DOI] [PubMed] [Google Scholar]
- 46.Huo D, Ikpatt F, Khramtsov A, et al. Population differences in breast cancer: Survey in indigenous African women reveals over-representation of triple-negative breast cancer. J Clin Oncol. 2009;27:4515–4521. doi: 10.1200/JCO.2008.19.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang XR, Sherman ME, Rimm DL, et al. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers Prev. 2007;16:439–443. doi: 10.1158/1055-9965.EPI-06-0806. [DOI] [PubMed] [Google Scholar]
- 48.Chen F, Chen GK, Stram DO, et al. A genome-wide association study of breast cancer in women of African ancestry. Hum Genet. 2013;132:39–48. doi: 10.1007/s00439-012-1214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bickell NA. Race, ethnicity, and disparities in breast cancer: Victories and challenges. Womens Health Issues. 2002;12:238–251. doi: 10.1016/s1049-3867(02)00145-7. [DOI] [PubMed] [Google Scholar]
- 50.Breen N, Wagener DK, Brown ML, et al. Progress in cancer screening over a decade: Results of cancer screening from the 1987, 1992, and 1998 National Health Interview Surveys. J Natl Cancer Inst. 2001;93:1704–1713. doi: 10.1093/jnci/93.22.1704. [DOI] [PubMed] [Google Scholar]
- 51.Baquet CR, Commiskey P. Socioeconomic factors and breast carcinoma in multicultural women. Cancer. 2000;88(suppl):1256–1264. doi: 10.1002/(sici)1097-0142(20000301)88:5+<1256::aid-cncr13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 52.Du XL, Fang S, Vernon SW, et al. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer. 2007;110:660–669. doi: 10.1002/cncr.22826. [DOI] [PubMed] [Google Scholar]
- 53.Du XL, Fang S, Meyer TE. Impact of treatment and socioeconomic status on racial disparities in survival among older women with breast cancer. Am J Clin Oncol. 2008;31:125–132. doi: 10.1097/COC.0b013e3181587890. [DOI] [PubMed] [Google Scholar]
- 54.Ren XS, Amick BC, Williams DR. Racial/ethnic disparities in health: The interplay between discrimination and socioeconomic status. Ethn Dis. 1999;9:151–165. [PubMed] [Google Scholar]
- 55.Canto MT, Anderson WF, Brawley O. Geographic variation in breast cancer mortality for white and black women: 1986–1995. CA Cancer J Clin. 2001;51:367–370. doi: 10.3322/canjclin.51.6.367. [DOI] [PubMed] [Google Scholar]
- 56.Schleinitz MD, DePalo D, Blume J, et al. Can differences in breast cancer utilities explain disparities in breast cancer care? J Gen Intern Med. 2006;21:1253–1260. doi: 10.1111/j.1525-1497.2006.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ashing-Giwa KT, Padilla G, Tejero J, et al. Understanding the breast cancer experience of women: A qualitative study of African American, Asian American, Latina and Caucasian cancer survivors Psychooncology. 2004;13:408–428. doi: 10.1002/pon.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wagner L, Lacey MD. The hidden costs of cancer care: An overview with implications and referral resources for oncology nurses. Clin J Oncol Nurs. 2004;8:279–287. doi: 10.1188/04.CJON.279-287. [DOI] [PubMed] [Google Scholar]
- 59.Warren JL, Yabroff KR, Meekins A, et al. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100:888–897. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee DW, Stang PE, Goldberg GA, et al. Resource use and cost of diagnostic workup of women with suspected breast cancer. Breast J. 2009;15:85–92. doi: 10.1111/j.1524-4741.2008.00675.x. [DOI] [PubMed] [Google Scholar]
- 61.USA Today/Kaiser Family Foundation/Harvard School of Public Health National Survey of Households Affected by Cancer [Publication No. 7591] [Accessed August 4, 2013]. Available at http://kaiserfamilyfoundation.files.wordpress.com/2013/01/7591.pdf.
- 62.Polacek GN, Ramos MC, Ferrer RL. Breast cancer disparities and decision-making among U.S. women. Patient Educ Couns. 2007;65:158–165. doi: 10.1016/j.pec.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 63.Talcott JA, Spain P, Clark JA, et al. Hidden barriers between knowledge and behavior: The North Carolina prostate cancer screening and treatment experience. Cancer. 2007;109:1599–1606. doi: 10.1002/cncr.22583. [DOI] [PubMed] [Google Scholar]
- 64.Carpenter WR, Reeder-Hayes K, Bainbridge J, et al. The role of organizational affiliations and research networks in the diffusion of breast cancer treatment innovation. Med Care. 2011;49:172–179. doi: 10.1097/MLR.0b013e3182028ff2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wheeler SB, Carpenter WR, Peppercorn J, et al. Predictors of timing of adjuvant chemotherapy in older women with hormone receptor-negative, stages II-III breast cancer. Breast Cancer Res Treat. 2012;131:207–216. doi: 10.1007/s10549-011-1717-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wheeler SB, Carpenter WR, Peppercorn J, et al. Structural/organizational characteristics of health services partly explain racial variation in timeliness of radiation therapy among elderly breast cancer patients. Breast Cancer Res Treat. 2012;133:333–345. doi: 10.1007/s10549-012-1955-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Punglia RS, Weeks JC, Neville BA, et al. Effect of distance to radiation treatment facility on use of radiation therapy after mastectomy in elderly women. Int J Radiat Oncol Biol Phys. 2006;66:56–63. doi: 10.1016/j.ijrobp.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 68.Bach PB, Pham HH, Schrag D, et al. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351:575–584. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 69.Jerome-D'Emilia B, Begun JW. Diffusion of breast conserving surgery in medical communities. Soc Sci Med. 2005;60:143–151. doi: 10.1016/j.socscimed.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 70.Vernon SW, Vogel VG, Halabi S, et al. Breast cancer screening behaviors and attitudes in three racial/ethnic groups. Cancer. 1992;69:165–174. doi: 10.1002/1097-0142(19920101)69:1<165::aid-cncr2820690128>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 71.Gooden K, Howard D, Carpenter W, et al. The effect of hospital and surgeon volume on racial differences in recurrence-free survival after radical prostatectomy. Med Care. 2008;46:1170–1176. doi: 10.1097/MLR.0b013e31817d696d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morris AM, Wei Y, Birkmeyer NJ, et al. Racial disparities in late survival after rectal cancer surgery. J Am Coll Surg. 2006;203:787–794. doi: 10.1016/j.jamcollsurg.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 73.Earle CC, Neumann PJ, Gelber RD, et al. Impact of referral patterns on the use of chemotherapy for lung cancer. J Clin Oncol. 2002;20:1786–1792. doi: 10.1200/JCO.2002.07.142. [DOI] [PubMed] [Google Scholar]
- 74.Bradley CJ, Given CW, Roberts C. Disparities in cancer diagnosis and survival. Cancer. 2001;91:178–188. doi: 10.1002/1097-0142(20010101)91:1<178::aid-cncr23>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 75.Hershman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: Retrospective analysis of Southwest Oncology studies S8814/S8897. J Clin Oncol. 2009;27:2157–2162. doi: 10.1200/JCO.2008.19.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Newman LA, Martin IK. Disparities in breast cancer. Curr Probl Cancer. 2007;31:134–156. doi: 10.1016/j.currproblcancer.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 77.Sateren WB, Trimble EL, Abrams J, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20:2109–2117. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- 78.Tejeda HA, Green SB, Trimble EL, et al. Representation of African-Americans, Hispanics, and whites in National Cancer Institute cancer treatment trials. J Natl Cancer Inst. 1996;88:812–816. doi: 10.1093/jnci/88.12.812. [DOI] [PubMed] [Google Scholar]
- 79.Mitchell KW, Carey LA, Peppercorn J. Reporting of race and ethnicity in breast cancer research: Room for improvement. Breast Cancer Res Treat. 2009;118:511–517. doi: 10.1007/s10549-009-0411-4. [DOI] [PubMed] [Google Scholar]
- 80.Reeder-Hayes KE, Bainbridge J, Meyer AM, et al. Race and age disparities in receipt of sentinel lymph node biopsy for early-stage breast cancer. Breast Cancer Res Treat. 2011;128:863–871. doi: 10.1007/s10549-011-1398-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Halpern MT, Chen AY, Marlow NS, et al. Disparities in receipt of lymph node biopsy among early-stage female breast cancer patients. Ann Surg Oncol. 2009;16:562–570. doi: 10.1245/s10434-008-0205-7. [DOI] [PubMed] [Google Scholar]
- 82.Payne R, Medina E, Hampton JW. Quality of life concerns in patients with breast cancer: Evidence for disparity of outcomes and experiences in pain management and palliative care among African-American women. Cancer. 2003;97(suppl):311–317. doi: 10.1002/cncr.11017. [DOI] [PubMed] [Google Scholar]
- 83.Demissie S, Silliman RA, Lash TL. Adjuvant tamoxifen: Predictors of use, side effects, and discontinuation in older women. J Clin Oncol. 2001;19:322–328. doi: 10.1200/JCO.2001.19.2.322. [DOI] [PubMed] [Google Scholar]
- 84.Owusu C, Buist DS, Field TS, et al. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2008;26:549–555. doi: 10.1200/JCO.2006.10.1022. [DOI] [PubMed] [Google Scholar]
- 85.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barron TI, Connolly R, Bennett K, et al. Early discontinuation of tamoxifen: A lesson for oncologists. Cancer. 2007;109:832–839. doi: 10.1002/cncr.22485. [DOI] [PubMed] [Google Scholar]
- 87.Kimmick G, Anderson R, Camacho F, et al. Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol. 2009;27:3445–3451. doi: 10.1200/JCO.2008.19.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Partridge AH, Wang PS, Winer EP, et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 89.Partridge AH. Non-adherence to endocrine therapy for breast cancer. Ann Oncol. 2006;17:183–184. doi: 10.1093/annonc/mdj141. [DOI] [PubMed] [Google Scholar]
- 90.Partridge AH, LaFountain A, Mayer E, et al. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556–562. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 91.Ziller V, Kalder M, Albert US, et al. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann Oncol. 2009;20:431–436. doi: 10.1093/annonc/mdn646. [DOI] [PubMed] [Google Scholar]
- 92.Lash TL, Fox MP, Westrup JL, et al. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 2006;99:215–220. doi: 10.1007/s10549-006-9193-0. [DOI] [PubMed] [Google Scholar]
- 93.Nekhlyudov L, Li L, Ross-Degnan D, et al. Five-year patterns of adjuvant hormonal therapy use, persistence, and adherence among insured women with early-stage breast cancer. Breast Cancer Res Treat. 2011;130:681–689. doi: 10.1007/s10549-011-1703-z. [DOI] [PubMed] [Google Scholar]
- 94.Kohler RE, Wheeler SB, Reeder-Hayes KE, et al. Endocrine therapy use among Medicaid-insured breast cancer survivors with hormone receptor-positive tumors. J Clin Oncol. 2012;30(suppl):6017a. doi: 10.1007/s11764-014-0365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shavers VL, Shavers BS. Racism and health inequity among Americans. J Natl Med Assoc. 2006;98:386–396. [PMC free article] [PubMed] [Google Scholar]
- 97.Dignam JJ, Redmond CK, Fisher B, et al. Prognosis among African-American women and white women with lymph node negative breast carcinoma: Findings from two randomized clinical trials of the National Surgical Adjuvant Breast and Bowel Project (NSABP) Cancer. 1997;80:80–90. doi: 10.1002/(sici)1097-0142(19970701)80:1<80::aid-cncr11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 98.Yood MU, Johnson CC, Blount A, et al. Race and differences in breast cancer survival in a managed care population. J Natl Cancer Inst. 1999;91:1487–1491. doi: 10.1093/jnci/91.17.1487. [DOI] [PubMed] [Google Scholar]
- 99.Smith K, Wray L, Klein-Cabral M, et al. Ethnic disparities in adjuvant chemotherapy for breast cancer are not caused by excess toxicity in black patients. Clin Breast Cancer. 2005;6:260–266. doi: 10.3816/CBC.2005.n.029. [DOI] [PubMed] [Google Scholar]
- 100.Newman LA, Theriault R, Clendinnin N, et al. Treatment choices and response rates in African-American women with breast carcinoma. Cancer. 2003;97(suppl):246–252. doi: 10.1002/cncr.11015. [DOI] [PubMed] [Google Scholar]
- 101.Dawood S, Broglio K, Kau SW, et al. Triple receptor-negative breast cancer: The effect of race on response to primary systemic treatment and survival outcomes. J Clin Oncol. 2009;27:220–226. doi: 10.1200/JCO.2008.17.9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tichy JR, D A, Anders CK, Carey LA. Race, Response to Chemotherapy, and Outcome within Clinical Breast Cancer Subtypes. Cancer Res. 2011;71(suppl 3) doi: 10.1007/s10549-015-3350-2. nr P2–14-01a. [DOI] [PMC free article] [PubMed] [Google Scholar]