Primary thyroid lymphoma is rare, and there are limited numbers of randomized or prospective trials to guide management, thus controversy remains over optimal treatment. The aim of this paper is to discuss the changes in diagnostic modalities and to focus on the recent alterations in the management of this rare disease, including targeted therapies as well as the more limited role of the endocrine surgeon.
Keywords: Primary thyroid lymphoma, Thyroid, Lymphoma, Thyroid surgery, Thyroid malignancy
Learning Objectives
Explain the diagnostic modalities used to diagnose primary thyroid lymphoma.
Describe the role of the endocrine surgeon in the diagnosis and treatment of thyroid lymphoma.
Cite the recent advances in the treatment of primary thyroid lymphoma.
Abstract
Primary thyroid lymphoma is rare, composing approximately 5% of all thyroid malignancies and less than 3% of all extranodal lymphomas. It typically presents as a rapidly enlarging goiter with associated compressive symptoms. Thyroid ultrasound and fine needle aspiration cytology, using flow cytometry and immunohistochemistry, remain the main modalities used to confirm the presence of lymphoma. The increasing use of an ultrasound-guided core biopsy to achieve an accurate diagnosis has further limited the role of surgery. An open surgical biopsy may still be required not only for definitive diagnosis but also to confirm the subtype of lymphoma. There are limited numbers of randomized or prospective trials to guide management, and controversy remains over optimal treatment. Treatment and prognosis of this disease can be dichotomized into two separate groups: pure mucosa-associated lymphoid tissue (MALT) lymphoma and diffuse large B-cell lymphoma (DLBCL) or mixed subtypes. Early stage (stage IE) intrathyroidal MALT lymphomas typically have an indolent course and may be treated with single-modality surgery, radiotherapy, or a combination of both. DLBCLs are more aggressive, and survival outcomes are highest with multimodal therapy incorporating monoclonal antibodies, chemotherapy, and radiotherapy. The prognosis is generally excellent but can be varied because of the heterogeneous nature of thyroid lymphomas. The aim of this paper is to discuss the changes in diagnostic modalities and to focus on the recent alterations in the management of this rare disease, including targeted therapies as well as the more limited role of the endocrine surgeon.
Implications for Practice:
Primary thyroid lymphoma is a rare malignancy intrinsically associated with Hashimoto's thyroiditis. Presenting with an enlarging goiter and associated pressure symptoms, rapid accurate diagnosis is required. Improvements in fine needle aspiration cytology, its adjuncts, and core biopsy techniques mean that open surgical biopsy is required only if less invasive methods have failed to accurately subtype the lymphoma. Treatment and prognosis are dependent on accurate histological classification. Surgical intervention, although typically incidental in the management of indolent mucosa-associated lymphoid tissue lymphomas, is reserved largely for the emergency preservation of the airway. Multimodal treatment with rituximab, combination chemotherapy, and local radiotherapy provides the highest overall survival rates.
Introduction
Thyroid cancer is the most common endocrine malignancy, accounting for approximately 1% of all malignancies; however, primary thyroid lymphoma (PTL), lymphoma involving the thyroid gland alone, accounts for only 5% of all thyroid malignancies and approximately 3% of all non-Hodgkin's lymphoma. The annual incidence of PTL is one or two cases per million [1–3]. Typically, it presents in the seventh decade of life, with males being affected 5–10 years earlier than females, despite overall female predominance (female:male ratio of approximately 3:1) [4, 5].
The most common presentation of thyroid lymphoma is a rapidly enlarging, painless goiter. Other symptoms such as dyspnea, dysphagia, and hoarseness may arise as a result of the pressure effects of the mass. Rarely, stridor or superior vena cava obstruction can occur. Cervical lymphadenopathy is present in the majority of cases [6, 7]. Classic B-type symptoms such as weight loss and night sweats occur less commonly and have been reported in approximately 20% of patients [8]. The majority of patients (30%–60%) are biochemically euthyroid at presentation [5, 9].
The underlying pathogenesis of PTL remains obscure. Links among autoimmune disease, chronic antigenic stimulation, and PTL have been demonstrated, and the major risk factor for PTL is the presence of Hashimoto's thyroiditis (HT). The risk of PTL is between 40 and 80 times higher in patients with HT, which typically develops 20–30 years after the initial diagnosis [5, 10, 11]. Interestingly, although the incidence of HT in patients with PTL approaches 80%, only 0.6% with HT will go on to develop PTL [12]. The association is postulated to result from the development of intrathyroidal lymphoid tissue in HT. This acquired lymphoid tissue resembles mucosa-associated lymphoid tissue (MALT) and may evolve into non-Hodgkin's lymphoma. It has also been postulated that the stimulation of antigens that are specific to the thyroid microenvironment are necessary for the development of PTL [13]. This theory is supported by the fact that more than half of thyroid lymphoma patients have a previous or concurrent diagnosis of chronic lymphocytic thyroiditis, suggesting that chronic antigenic stimulation may play a role in pathogenesis [14–17].
For treatment and prognosis, PTL is divided into two separate clinicopathological entities: diffuse B-cell lymphoma and mixed subtypes and pure MALT lymphomas. Diffuse B-cell lymphomas have an aggressive clinical course and should be considered for multimodal treatment, whereas MALT lymphomas pursue a more indolent course and may be treated adequately with single therapeutic strategies. No trials have compared single versus multimodal therapies for PTL or its subtypes; therefore, data are largely extrapolated from the treatment of extranodal lymphoma.
The overall prognosis of thyroid lymphoma has been described by the British Thyroid Association guidelines as “generally excellent”; however, prognosis is subtype dependent, and 5-year survival rates can be as low at 45% [18]. The management and prognosis of PTL has changed with the advent of multimodal adjuvant therapy and increasing interest in and research into targeted therapies. Correspondingly, the role of the endocrine surgeon has become more restricted, primarily being limited to achieving a definitive diagnosis where less invasive methods have failed, in the urgent management of the obstructed airway or in the incidental excision of MALT lymphomas. The aim of this paper is to focus on the changes in diagnostic modalities and to discuss, in depth, the recent alterations in management of this rare disease, including targeted therapies and the more limited role of the endocrine surgeon.
Classification and Staging of PTL
PTLs are classified based on pathological subtypes, with each carrying a different prognosis. The two most common subtypes are diffuse large B-cell lymphoma (DLBCL) and MALT lymphoma.
DLBCL
DLBCL accounts for up to 70% of all PTLs [4]. DLBCLs are typically positive for MS4A1 (CD20), with 75% also positive for the BCL6 oncogene and up to 50% positive for the BCL2 oncogene [19]. Historically, it is the most aggressive subtype, with 60% of patients exhibiting metastatic disease at first presentation, and was classically associated with poor prognosis. DLBCL itself has now been divided into two major cell-of-origin phenotypes with differing prognoses: a favorable germinal center B-cell-like lymphoma and a more aggressive activated B-cell-like subgroup with overexpression of the activated B-cell immunophenotype markers IRF4 (MUM1) and FOXP1 [19]. A recent study by Niitsu et al. looking at the use of multimodal therapy for DLBCL described a 5-year overall survival (OS) rate of 90% [19]. Interestingly, the authors identified that within this group of patients, those who overexpressed IRF4 (MUM1) with associated high expression of NM23-H1 had a poorer OS rate.
MALT Lymphoma
MALT lymphomas account for majority of the remaining 30% of PTLs. They are considered to be of similar endodermal origin, whether in Waldeyer's ring, the thyroid, or the gastrointestinal tract. MALT lymphomas are characterized by the presence of lymphoepithelial lesions, lymphocytes “stuffing” glandular lumina, representing colonization of the thyroid follicles by the lymphoma cells [20]. MALT is identified by the presence of immunoglobulin light chains, pan-B-cell antigens, and BCL2 and the absence of CD5, CD10, and CD23 [21–23]. Interestingly, there have also been a number of specific chromosomal translocations associated with MALT lymphoma: t [11, 18](q21;21), t [14, 18](q32;q21), t [3, 14](p14.1;q32), and t [1, 14](q32;q21) [21, 24, 25]. MALT lymphoma of the thyroid follows a relatively benign indolent clinical course and thus is more likely to present at an earlier stage and, historically, to demonstrate a better response to treatment [26]; however, transformation to a higher grade, more aggressive lymphoma has been previously documented [5].
Other Subtypes of PTL
Follicular lymphoma is rare, accounting for only 3%–5% of all PTLs [9, 15, 16, 27, 28]. It is a neoplasm of germinal center B cells that, in the majority of cases, shows strong aberrant expression of BCL2 [29, 30]. Follicular lymphoma, although usually presenting with nodal or disseminated disease, has a 5-year OS rate of 87% [31, 32]. Bacon et al. identified two distinct groups of thyroid follicular lymphoma [33]. The first group, although carrying IGH/BCL2 t [14, 18] and/or overexpressing BCL2, was of lower grade but was more likely to have extrathyroidal disease at presentation. These patients had an aggressive clinical course, with five of the seven patients followed having progression or recurrence, and four of those died of PTL. The second group lacked IGH/BCL2 and BCL2 expression and was of higher grade but was less likely to present with extrathyroidal disease. These patients had a more indolent clinical course, with all eight patients in this group alive and disease free at the end of the study.
Classic Hodgkin's lymphoma of the thyroid, which is characterized by the presence of Reed-Sternberg cells mixed with a population of non-neoplastic reactive cells, accounts for 2% of PTL. Small lymphocytic lymphoma is also an uncommon subtype of PTL and affects 3% of PTL patients [4, 34]. Patients with a diagnosis of Hodgkin's lymphoma of the thyroid tend to be younger than patients diagnosed with other subtypes [4]. It carries a female preponderance and is usually associated with a good prognosis [35].
Thyroid lymphomas of T-cell origin are extremely rare, with only approximately 15 reported cases in the literature [36]. Less than half of the cases reported were associated with pre-existing thyroiditis [37]. T-cell lymphomas in general are associated with a shorter survival time than B-cell lymphomas [38], although a spontaneous regression with no specific treatment has been documented in one case report [37].
Staging
The Ann Arbor criteria, similar to staging of lymphoma at other sites, is used for the staging of PTL [39]. Stage IE applies to disease localized within the thyroid; stage IIE applies to disease confined to the thyroid and regional lymph node basins; stage IIIE applies to disease that involves the thyroid, the lymph node basins on both sides of the diaphragm, and/or the spleen; and stage IVE is used to describe disseminated disease. Approximately 56% of PTLs are stage IE at the time of presentation, with 32% at stage IIE, 2% at stage IIIE, and 11% at stage IVE [4].
Diagnosis
Biochemistry
The definitive diagnosis of PTL is obtained by histological analysis of tissue. Evidence suggests, however, that serum antimicrosomal and antithyroglobulin antibodies are raised in up to 95% of patients with a diagnosis of thyroid lymphoma; therefore, they may lend weight to the diagnosis of thyroid lymphoma [40, 41]. Because of the association between PTL and HT, up to 80% of patients with PTL test positive for circulating antibodies to thyroid peroxidase [42]. It is important to note that this test is not specific for thyroid lymphoma and is not used as a diagnostic tool, but it highlights the association between HT and PTL. Serum lactate dehydrogenase levels are raised in almost one-third of non-Hodgkin's lymphomas and are associated with a higher grade of disease [43]. Levels of β2 microglobulin have also been shown to be raised in patients with non-Hodgkin's lymphoma and have been used to detect recurrence [44].
Imaging
Ultrasonography is the imaging modality of choice and can typically show one of three patterns: nodular, diffuse, or mixed. When presenting as a solitary mass, the radiological appearance can resemble that of anaplastic thyroid carcinoma but can be distinguished by its homogenous appearance as well as the lack of calcification, necrosis, and cystic degeneration within the nodule [45]. When diffuse lymphoma is identified, it appears as a heterogeneous hypoechoic parenchyma with the presence of structures resembling septae [46]. Ultrasonography is particularly helpful in differentiating between other rapidly enlarging lesions such as anaplastic thyroid carcinoma, subacute thyroiditis, or hemorrhage into a cyst or adenoma.
Radionuclide scanning is extremely nonspecific in the setting of PTL and is not routinely included in the work-up of these patients. Findings may include thyroid enlargement and/or heterogeneity with the presence of cold nodules [42].
Cross-sectional imaging is rarely used in the diagnosis of PTL; however, it is indicated in the presence of symptoms such as stridor, hoarseness, dysphagia, or fixation on physical examination [47]. In these cases, it can be used to assess involvement of surrounding structures, to assist in accurate surgical planning, and to diagnose cervical and mediastinal nodal disease [48]. Magnetic resonance imaging may be more sensitive than computed tomography in the detection of extrathyroidal involvement [49].
Fluorine-18-fluorodeoxyglucose positron emission tomography (FDG-PET) can be useful in staging and restaging or in assessing response to treatment in PTL [50]. Strong evidence supports the use of FDG-PET for lymphoma in general because FDG-PET has superior diagnostic accuracy in lymphoma when compared with computed tomography and magnetic resonance imaging [51]. Although there are no specific studies assessing its usefulness in PTL, several case reports have documented its use in this rare disease [52, 53]. PTL typically demonstrates avid uptake of fluorodeoxyglucose within the thyroid and any associated positive cervical lymphadenopathy [54].
Fine Needle Aspiration Cytology
Fine needle aspiration cytology (FNAC) is the initial technique of choice for pathological assessment of a thyroid lesion. Because PTL is a small cell tumor with few pathogenomic features, it may present diagnostic challenges for the pathologist [31]. Although no randomized clinical trials have assessed the accuracy of thyroid FNAC for the diagnosis of PTL, multiple small retrospective studies have demonstrated an increasing sensitivity and specificity with newer adjuncts to FNAC, such as flow cytometry, immunperoxidase studies, and polymerase chain reaction (PCR) (Table 1).
Table 1.
Pathological diagnosis of primary thyroid lymphoma: fine needle aspiration cytology versus open surgical biopsy
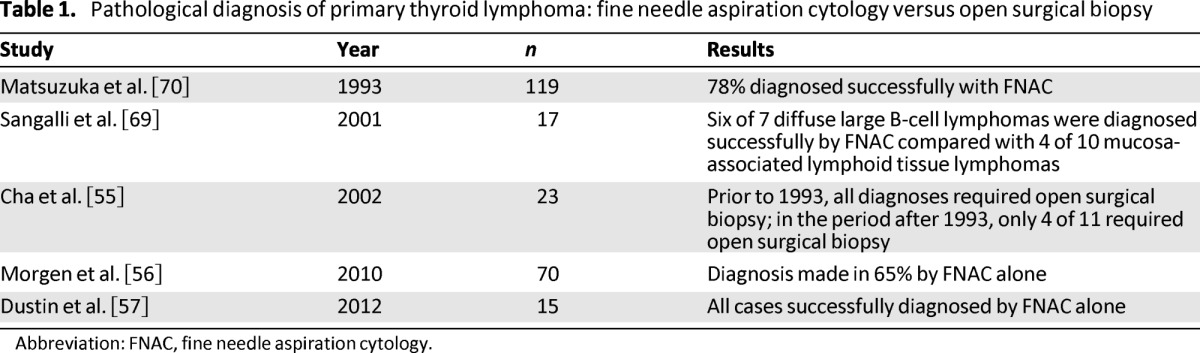
Abbreviation: FNAC, fine needle aspiration cytology.
A study by Cha et al. from John Hopkins Hospital highlights the improvement in the sensitivity of FNAC in recent decades [55]. The authors assessed 23 patients with PTL between the years of 1985 and 2000, and of those diagnosed after 1993 (n = 11), 63% were diagnosed with FNAC alone and thus did not require an open surgical biopsy. In contrast, all of the patients diagnosed earlier (n = 12) required open surgical biopsy. This improvement was attributed to the introduction of immunophenotyping on FNAC samples that were suspicious for PTL. Similarly, in a larger study by Morgen et al., with 70 patients in total, a diagnosis of thyroid lymphoma was made in 65% using FNAC alone [56]. More encouraging was a recent study by Dustin et al., with all cases of thyroid lymphoma (n = 15) successfully diagnosed with FNAC, showing sensitivity of 100% and positive predictive value of 100% [57]. Multiple authors have suggested that the diagnosis of DLBCL is easier because of the presence of large monotonous atypical cells. In comparison, the diagnosis of MALT is harder because of the presence of large numbers of heterogeneous cells and the associated presence of HT.
Focusing on IHC, the realization that PTLs are positive for leucocyte common antigen, MS4A1 (CD20), and λ light chain and negative for cytokeratins on immunohistochemical staining has already improved the sensitivity and specificity of FNAC.
To address this issue specifically, Takano et al. described a method of using reverse transcriptase PCR to detect the monoclonality of IGH mRNA in fine-needle aspirates, allowing differentiation between lymphoma and HT in 44% of cases [58]. In a later study, the same group demonstrated that monoclonality was detected in 76% of thyroid lymphoma cases and was not found in benign tissue at all [59]. More recently, Takano et al. published a novel method to detect B-cell monoclonality using a combination of vectorette PCR and the digestion of restriction enzymes in aspiration biopsy nucleic acid diagnosis. The overall sensitivity in this small trial study was 50% [60].
Recent decades have seen vast amounts of research examining methods that could increase the sensitivity of fine needle aspirates for the detection of thyroid lymphoma, such as immunohistochemistry (IHC) and flow cytometry. Focusing on IHC, the realization that PTLs are positive for leucocyte common antigen, MS4A1 (CD20), and λ light chain and negative for cytokeratins on immunohistochemical staining has already improved the sensitivity and specificity of FNAC [61]. A study by Ito et al. identified increased expression of CDC25A and CDC25B, which have oncogenic potential, in 47% and 67% of thyroid lymphomas, respectively, using IHC [62]. A similar study by Sugawara et al. demonstrated increased expression of survivin, a protein intricately associated with the inhibition of apoptosis, in PTL tissue when compared with benign tissue using both IHC and PCR [63].
Generally for lymphoma, which may be extrapolated to PTL, Swart et al. found that the addition of flow cytometry to FNAC analysis produced sensitivity of 97% and specificity of 87% for the detection of B-cell lymphoma [64]; however, 13.7% of the original cohort was excluded as a result of an insufficient number of cells to perform flow cytometry, cytology, or both. Inadequate sampling unfortunately remains a limiting factor for performing flow cytometry. Advantages include quicker turnaround time and the facility to examine a single cell for multiple antigens [65].
Core Biopsy
A core biopsy yields more tissue than FNAC and maintains the architecture of the tissue. It can facilitate the distinction among HT, PTL, and anaplastic carcinoma; this is not always possible with FNAC. Recent studies have shown that a core biopsy can yield sufficient tissue for diagnosis and subtyping in up to 95% of lymphomas in general, but few data regard PTL specifically [66]. A small retrospective study was performed by Ravinski et al. demonstrating that performance of a core biopsy can improve the diagnostic accuracy when compared with FNAC and flow cytometry (82% vs. 93%) [67].
Guidance of the biopsy using imaging such as ultrasonography can minimize the risk of trauma to adjacent structures and can avoid taking cores of necrotic tissue within the mass. Novoa et al. carried out a meta-analysis of the use of ultrasound-guided core biopsy in head and neck malignancies [68]. The sensitivity for the detection of malignancy in the thyroid was 68%, but a subgroup analysis of thyroid lymphoma was not performed. They confirmed that the procedure is safe and minimally invasive, with postprocedure hemorrhage occurring in only 1%. The authors concluded that this technique is useful in patients in whom a diagnosis has not been reached using fine needle aspiration and in whom surgery would not be indicated.
Surgical Open Biopsy
Despite these advances in the diagnostic accuracy of FNAC and core biopsy, a limited role remains for an open surgical biopsy to allow for definitive subtyping that may direct future treatment. The diagnosis of MALT lymphomas specifically can be particularly challenging with less invasive modalities. No prospective studies or randomized trials address the utility of an open surgical biopsy; therefore, the evidence for it is limited to retrospective reviews and expert opinion. Sangalli et al. reported that an open surgical biopsy was required to diagnose PTL in 41% of patients (n = 17), with a higher proportion of MALT lymphomas requiring an open biopsy [69]. In contrast, Cha et al. showed in their study of 23 patients that 63% of those diagnosed after 1993 (n = 11) were diagnosed with FNAC alone and thus did not require an open biopsy [55]. An older paper by Matsuzuka et al. contradicts this by stating that an open biopsy is required for subtyping of disease despite the fact that a diagnosis was reached in 79% of patients with FNAC [70]. Currently, surgical open biopsy is recommended only when less invasive techniques fail to achieve a definitive diagnosis of PTL or identification of the exact subtype so that treatment can be tailored to individual patients.
Treatment
The optimal treatment of PTL remains controversial because of the limited evidence harvested from many small retrospective studies, with significant methodological flaws, in the absence of large prospective trials (Table 2). The treatment for thyroid lymphoma is broadly divided according to lymphoma subtype. As with other lymphomas, PTL is sensitive to both chemotherapy and radiotherapy. The gold standard for management of DLBCL is multimodal because of the typically aggressive clinical course and uses a combination of the monoclonal antibody rituximab, chemotherapy (a combination of cyclophosphamide, doxorubicin, vincristine, and prednisone [CHOP]), and radiotherapy. Conversely, MALT lymphomas may be amenable to single-modality treatment because of the indolent nature of the disease. Treatment may consist of surgery alone, which typically is performed when the MALT lymphoma is detected incidentally; radiotherapy alone; or a combination of both. In general, surgery has a very limited role in the management of PTL but may be indicated in the urgent management of the airway.
Table 2.
Studies comparing treatment modalities for thyroid lymphoma
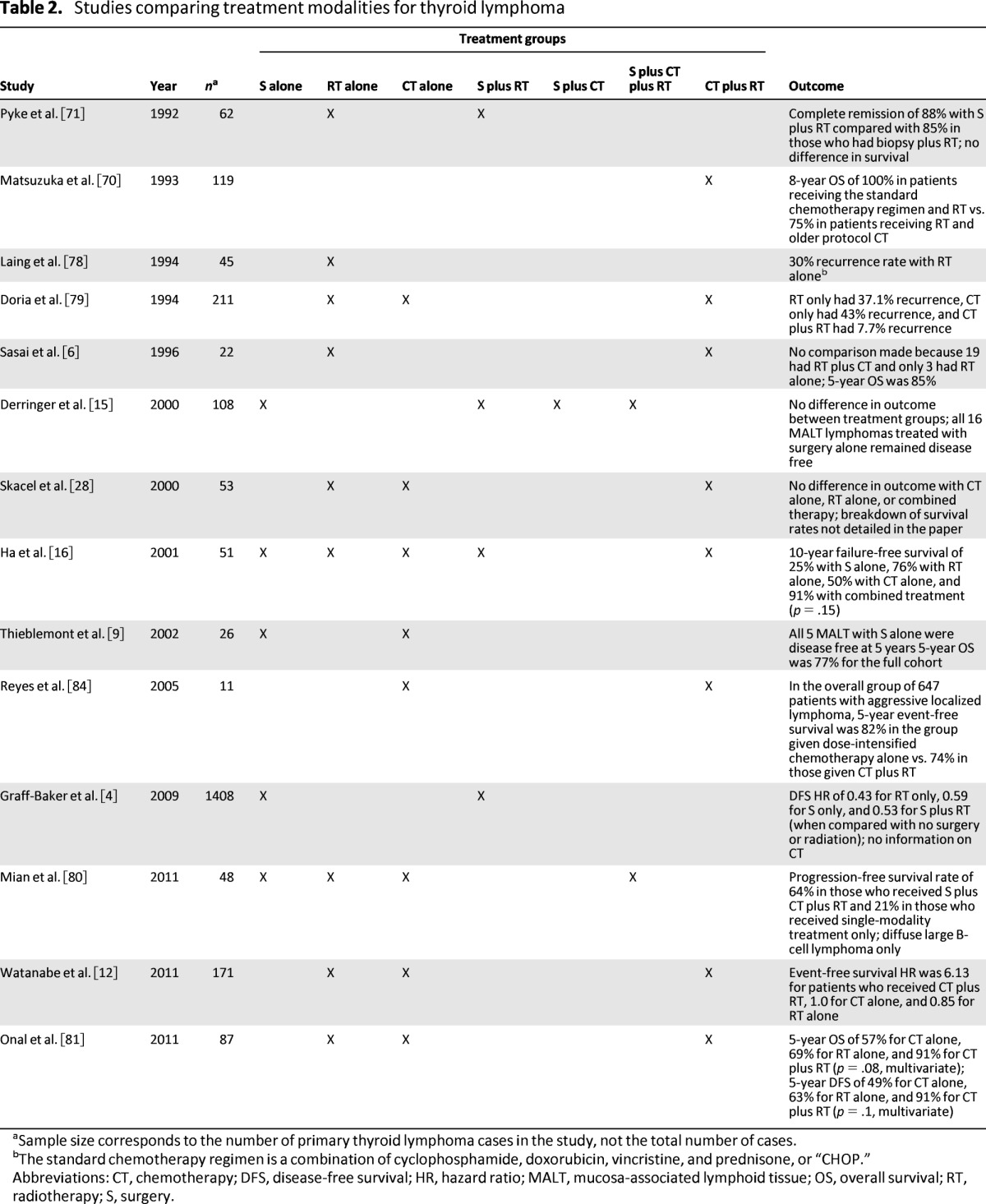
aSample size corresponds to the number of primary thyroid lymphoma cases in the study, not the total number of cases.
bThe standard chemotherapy regimen is a combination of cyclophosphamide, doxorubicin, vincristine, and prednisone, or “CHOP.”
Abbreviations: CT, chemotherapy; DFS, disease-free survival; HR, hazard ratio; MALT, mucosa-associated lymphoid tissue; OS, overall survival; RT, radiotherapy; S, surgery.
Local Therapy: Surgery Alone, Radiotherapy Alone, or a Combination of Surgery and Radiotherapy
Surgery
The role of surgical intervention in the treatment of PTL remains controversial, and no evidence from large academic centers supports its use. The potential morbidity of the procedure should be borne in mind, given that surgery does not appear to improve OS, when deciding on a treatment strategy. The largest study examining this issue was performed by Pyke et al. from the Mayo Clinic, with 62 patients with PTL [71]. The combination of debulking surgery and external beam radiotherapy (EBRT) did not demonstrate a survival benefit over a surgical biopsy and radiotherapy (88% vs. 85%) in stage IE or IIE disease. The authors concluded that the addition of extensive surgery to radiotherapy was of no clinical benefit. A study by Ha et al., with a small number of patients, similarly demonstrated no survival benefit with the addition of surgery to radiotherapy when accounting for other prognostic factors [16].
The role of surgical intervention in the treatment of PTL remains controversial, and no evidence from large academic centers supports its use. The potential morbidity of the procedure should be borne in mind, given that surgery does not appear to improve OS, when deciding on a treatment strategy.
There may be a role for surgical intervention in MALT lymphomas, although its sole use remains debatable. Typically, MALT lymphomas are detected incidentally when the thyroid is removed for another indication and, if confined to the thyroid (stage IE), may not require further adjuvant treatment. A study by Derringer et al. included 16 patients with MALT lymphoma who were treated with surgery alone and showed survival rates of 100% at 7 years [15]. Similarly, Thieblemont et al. included five patients with intrathyroidal MALT lymphoma who were treated successfully with surgery alone and who were 100% disease free after 5 years [9]. Surgery in MALT lymphomas is contraindicated in lymphoma higher than stage IE, in bulky tumors greater than 10 cm, and in mixed tumors. Given that MALT lymphomas do not compose the majority of PTL, surgery is not a mainstay of treatment.
Surgical intervention may be required for palliation in the setting of critical airway obstruction. Intervention in this setting carries high morbidity and should be approached with caution. A small series published by Sippel et al. confirmed the utility of surgery for effective palliation in patients with an obstructive airway [72]. More recent evidence challenges the role of surgery in this setting because patients may be better served with corticosteroids and chemoradiotherapy, which may give rapid relief of pressure symptoms without necessitating surgical intervention. A case report by Myatt et al. described a rapid response to high-dose corticosteroids in a case of critical airway obstruction secondary to thyroid lymphoma [73]. No trials have investigated steroids as an alternative to surgical intervention in the emergency setting. The temporary use of tracheal stents has been proposed as an alternative and, in combination with EBRT, may also provide rapid relief of symptoms [74–77].
Radiotherapy
Like surgery, radiotherapy alone should be considered only for localized stage IE MALT lymphomas. Evidence in this specific group of patients shows that radiotherapy alone, typically to the neck and upper mediastinum, can achieve excellent local control, and the treatment morbidity is considerably less than that of radiotherapy for squamous cell carcinomas [14, 78]. When appropriately selected for radiation alone, patients can achieve local control rates of between 70% and 100%. Currently, there are two regimens of radiotherapy that can be administered to patients with PTL, and there is controversy as to which type is superior. “Involved field radiotherapy” includes the thyroid bed and cervical lymph nodes only, whereas “extended field radiotherapy” also includes the mediastinal nodes and sometimes the axillary lymph nodes. A retrospective review carried out at the Royal Marsden Hospital looked at the efficacy of these two different radiotherapy regimens for the treatment of PTL [17]. The patients who received involved field radiotherapy were significantly more likely to have a local recurrence than those who had extended field radiotherapy (52% vs. 27%). Consequently, it is clear that early stage MALT lymphomas may be treated with radiotherapy alone. In the presence of DLBCL or mixed subtypes, stages higher than IE, or extensive bulky local disease, multimodality treatment is strongly recommended.
Systemic Treatment and Combined Chemoradiotherapy
Chemotherapy
Chemotherapy as a single modality for the treatment of PTL has been demonstrated to be inferior to combination chemoradiotherapy and thus is not recommended [12, 16, 79–81]. Most PTL subtypes, excluding MALT, are treated with combination chemotherapy and radiotherapy. The standard chemotherapy regimen is CHOP [79]. The efficacy of this regimen has been demonstrated in large-scale studies of non-Hodgkin's lymphoma, although its use in PTL is documented only in small retrospective studies [70, 82, 83]. Typically, patients with PTL respond rapidly to combination chemotherapy. A more recent study by Reyes et al. looked at a chemotherapy regimen using a combination of dose-intensified doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone [84]. The authors documented the superiority of this regimen of chemotherapy alone when compared with the standard CHOP regimen plus radiotherapy in patients younger than 61 years of age with localized lymphoma. This finding should be considered with caution when treating patients with PTL because only 11 patients in the study cohort had PTL.
Combined Chemoradiotherapy
An expanding body of evidence demonstrates that combined treatment modalities, such as chemotherapy and localized radiotherapy, should be used in both early localized disease as well as more advanced presentations, excluding the more indolent MALT lymphomas [85, 86]. The aim of combination chemotherapy is to reduce the incidence of distant recurrence while EBRT reduces the risk of localized recurrence, thereby offering superior long-term disease control when compared to single-modality treatment. Typically, radiotherapy is administered after the third cycle of CHOP chemotherapy [85]. The standard was set in 1993 when Matsuzuka et al. demonstrated 100% 8-year survival for 16 patients who were treated with a single course of chemotherapy (CHOP), followed by 60 Gy of local radiotherapy and concluding with a further five cycles of CHOP chemotherapy [70]. Since then, these findings have been supported by a systematic review performed by Doria et al., who found recurrence rates of a 7.7% following chemoradiation therapy, 37.1% following radiotherapy alone, and 43% following chemotherapy alone [79]. A more recent retrospective study by Onal et al. confirmed the survival advantage of multimodality treatment in a cohort of 87 patients with PTL [81]. OS was 91% for patients receiving combined-modality therapy, whereas it was 57% for those who received chemotherapy alone (p = .01) and 69% for those who received radiotherapy alone (p = .03). These findings were also confirmed by Watanabe et al., who demonstrated an event-free survival hazard ratio of 6.13 with combination treatments versus 1.0 for chemotherapy alone and 0.85 for radiotherapy alone [12].
The literature is not unanimous regarding the use of combined therapy, and some studies have failed to detect superiority of multimodal therapy over chemotherapy alone. A methodologically flawed retrospective study by Skacel et al. detected no survival difference between patients treated by chemotherapy alone, by radiotherapy alone, or by combinations of chemotherapy, radiotherapy, and surgery in patients with either MALT or DLBCL [28]. It is important to note that this was a small (n = 53) retrospective study spanning 18 years and several institutions and including six different treatment approaches. The mean follow-up was only 46 months. Consequently, the treatment modalities cannot be accurately compared in this study.
These data show that multimodality treatment is important for patients with PTL, particularly those with more aggressive subgroups and extensive locoregional or distant disease. For patients with MALT lymphomas, the benefit may be outweighed by the risks. This highlights the importance of accurately defining the histological subtype before deciding on a definitive treatment plan.
Targeted Therapies: Rituximab
The introduction of rituximab, a chimeric monoclonal antibody that acts against CD20, which is found on the surface of B cells, represents the most significant advance in the treatment of lymphoma since the introduction of chemotherapy [87]. The addition of rituximab to combination chemotherapy has been shown to improve OS and recurrence-free survival in DLBCL, and its use has subsequently been extended to more indolent and follicular lymphomas [87, 88]. A randomized controlled trial published by Hochester et al. confirmed that maintenance therapy with rituximab after standard chemotherapy significantly improved progression-free survival in patients with advanced-stage indolent lymphoma [89]. Interestingly, the response to rituximab may be subgroup specific. Visco et al. found that patients with overexpression of the BCL2 protein had a poorer response to rituximab in combination with CHOP chemotherapy [90]. A similar study by Winter et al. found that patients with DLBCL who overexpressed the protein BCL6 were less likely to benefit from the addition of rituximab to CHOP chemotherapy [91]. In contrast, Mounier et al. showed that treatment with rituximab could potentially overcome chemotherapy resistance in patients overexpressing BCL2 [92].
Rituximab has been licensed for use in the treatment of DLBCL, but there has been little research into its use specifically for the treatment of PTL [93, 94]. Many regimens for the treatment of DLBCL of the thyroid include rituximab in combination with chemotherapy or chemotherapy followed by maintenance rituximab [95].
Emerging Therapies
The identification of mutations and upregulation of cell-signaling pathways has revolutionized cancer treatment in recent years. Although molecular testing for mutations in the BRAF proto-oncogene is now used routinely to aid diagnosis and guide treatment of papillary thyroid carcinoma, protein kinase inhibition has not yet been applied in the management of thyroid lymphoma. A recent study by Aggarwal et al. looked at the expression of BRAF mutations in a cohort of 25 patients with DLBCL [96]. BRAF mutations were identified in 24% of these patients, with a further 8% having NRAS mutations. These findings suggest that the mitogen-associated protein kinase pathway is involved in the development and growth of these aggressive tumors and may be a potential therapeutic target for treatment.
Similarly, there has been increasing interest in the use of the vascular endothelial growth factor inhibitor bevacizumab. The SO515 trial was a phase II trial that looked at the addition of bevacizumab to rituximab plus standard chemotherapy for the treatment of 64 patients with DLBCL [97]. The early results were disappointing (median follow-up: 3.5 years), with no disease-free survival advantage and a high number of cardiac and gastrointestinal toxicities. These results suggest that targeting vascular endothelial growth factor may not a viable strategy in the treatment of PTL, and it has not been routinely incorporated into the treatment paradigm.
Prognosis
The overall prognosis of PTL has been generated from the Surveillance Epidemiology and End Results database [4]. Follow-up of 32 years was obtained for 1,408 patients with PTL, of which 56% were stage IE at diagnosis. The median OS was 9.3 years, and the 5-year survival was 66%. The disease-specific survival of PTL was related to histological subtype. The database estimates 5-year disease-free survival of 96% for MALT lymphoma, 75% for DLBCL, 87% for follicular PTL, and 86% for small lymphocytic lymphoma.
The prognosis of DLBCL can be estimated by using the International Prognostic Index, which uses only clinical parameters [98]. There are two indexes: one for all patients, called the “international index,” and one that is age adjusted, called the “age-adjusted international index.” The international index score is based on age, tumor stage, serum lactate dehydrogenase concentration, performance status, and number of extranodal disease sites. The age-adjusted international index score is based on tumor stage, lactate dehydrogenase level, and performance status. These indexes stratify patients into four risk groups with specific 5-year survival rates, which, when compared with the Ann Arbor staging system, appear to be more accurate in predicting survival.
One of the main challenges in the search for prognostic indicators for PTL is the small numbers of patients diagnosed with this disease. Rosenwald et al. identified a subgroup of patients with DLBCL, the germinal center B-cell-like lymphoma with BCL2 translocation and c-Rel amplification, who have a more favorable prognosis when compared with patients without this profile [99]. Further studies, including that by Offit et al., also identified rearrangement of BCL6 as a predictor of favorable clinical outcome [100–103]. Conversely, Katna et al. studied 37 patients with thyroid lymphoma and carried out a tissue-microarray analysis using stored samples [8]. Tumors were assessed for expression of CD10, the activated B-cell immunophenotyping markers MUM1 and FOXP1, as well as BCL2 and BCL6 oncogenes. The authors demonstrated that overexpression of FOXP1 was associated with poor response to treatment and increased mortality; however, given the small sample size, this finding failed to reach statistical significance. More detailed research with larger cohorts of patients needs to be performed prior to establishing these markers as prognostic.
Conclusion
PTL is a rare malignancy intrinsically associated with HT. Presenting with an enlarging goiter and associated pressure symptoms, rapid accurate diagnosis is required. Improvements in FNAC, its adjuncts, and core biopsy techniques mean that open surgical biopsy is required only if less invasive methods failed to accurately subtype the lymphoma. Treatment and prognosis are dependent on accurate histological classification. Surgical intervention, although typically incidental in the management of indolent MALT lymphomas, is reserved largely for the emergency preservation of the airway. Multimodal treatment with rituximab, combination chemotherapy, and local radiotherapy provides the highest OS rates. The management algorithms documented in this paper are largely extrapolated from the treatment of extranodal lymphoma. More specific research with larger cohorts of patients with PTL is required to confirm the roles of these algorithms for management.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Author Contributions
Conception/Design: Siun Walsh, Ruth S. Prichard
Manuscript writing: Siun Walsh, Aoife J. Lowery, Denis Evoy, Enda W. McDermott, Ruth S. Prichard
Final approval of manuscript: Enda W. McDermott, Ruth S. Prichard
Disclosures
The authors indicated no financial relationships.
Section editors: Stan Sidhu: None; Herbert Chen: None
Reviewer “A”: None
Reviewer “B”: None
Reviewer “C”: XOMA (C/A)
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Ansell SM, Grant CS, Habermann TM. Primary thyroid lymphoma. Semin Oncol. 1999;26:316–323. [PubMed] [Google Scholar]
- 2.Green LD, Mack L, Pasieka JL. Anaplastic thyroid cancer and primary thyroid lymphoma: A review of these rare thyroid malignancies. J Surg Oncol. 2006;94:725–736. doi: 10.1002/jso.20691. [DOI] [PubMed] [Google Scholar]
- 3.Fujita A, Tomita N, Fujita H, et al. Features of primary extranodal lymphoma in Kanagawa, a human T-cell leukemia virus type 1 nonendemic area in Japan. Med Oncol. 2009;26:49–54. doi: 10.1007/s12032-008-9080-0. [DOI] [PubMed] [Google Scholar]
- 4.Graff-Baker A, Roman SA, Thomas DC, et al. Prognosis of primary thyroid lymphoma: Demographic, clinical, and pathologic predictors of survival in 1,408 cases. Surgery. 2009;146:1105–1115. doi: 10.1016/j.surg.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen RK, Pedersen NT. Primary non-Hodgkin's lymphoma of the thyroid gland: A population based study. Histopathology. 1996;28:25–32. doi: 10.1046/j.1365-2559.1996.268311.x. [DOI] [PubMed] [Google Scholar]
- 6.Sasai K, Yamabe H, Haga H, et al. Non-Hodgkin's lymphoma of the thyroid. A clinical study of twenty-two cases Acta Oncol. 1996;35:457–462. doi: 10.3109/02841869609109922. [DOI] [PubMed] [Google Scholar]
- 7.Ruggiero FP, Frauenhoffer E, Stack BC., Jr Thyroid lymphoma: A single institution's experience. Otolaryngol Head Neck Surg. 2005;133:888–896. doi: 10.1016/j.otohns.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 8.Katna R, Shet T, Sengar M, et al. Clinicopathologic study and outcome analysis of thyroid lymphomas: Experience from a tertiary cancer center. Head Neck. 2013;35:165–171. doi: 10.1002/hed.22928. [DOI] [PubMed] [Google Scholar]
- 9.Thieblemont C, Mayer A, Dumontet C, et al. Primary thyroid lymphoma is a heterogeneous disease. J Clin Endocrinol Metab. 2002;87:105–111. doi: 10.1210/jcem.87.1.8156. [DOI] [PubMed] [Google Scholar]
- 10.Holm LE, Blomgren H, Lowhagen T. Cancer risks in patients with chronic lymphocytic thyroiditis. N Engl J Med. 1985;312:601–604. doi: 10.1056/NEJM198503073121001. [DOI] [PubMed] [Google Scholar]
- 11.Pasieka JL. Hashimoto's disease and thyroid lymphoma: Role of the surgeon. World J Surg. 2000;24:966–970. doi: 10.1007/s002680010159. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe N, Noh JY, Narimatsu H, et al. Clinicopathological features of 171 cases of primary thyroid lymphoma: A long-term study involving 24553 patients with Hashimoto's disease. Br J Haematol. 2011;153:236–243. doi: 10.1111/j.1365-2141.2011.08606.x. [DOI] [PubMed] [Google Scholar]
- 13.Rossi D. Thyroid lymphoma: Beyond antigen stimulation. Leuk Res. 2009;33:607–609. doi: 10.1016/j.leukres.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Tsang RW, Gospodarowicz MK, Sutcliffe SB, et al. Non-Hodgkin's lymphoma of the thyroid gland: Prognostic factors and treatment outcome. The Princess Margaret Hospital Lymphoma Group Int J Radiat Oncol Biol Phys. 1993;27:599–604. doi: 10.1016/0360-3016(93)90385-9. [DOI] [PubMed] [Google Scholar]
- 15.Derringer GA, Thompson LD, Frommelt RA, et al. Malignant lymphoma of the thyroid gland: A clinicopathologic study of 108 cases. Am J Surg Pathol. 2000;24:623–639. doi: 10.1097/00000478-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Ha CS, Shadle KM, Medeiros LJ, et al. Localized non-Hodgkin lymphoma involving the thyroid gland. Cancer. 2001;91:629–635. [PubMed] [Google Scholar]
- 17.Harrington KJ, Michalaki VJ, Vini L, et al. Management of non-Hodgkin's lymphoma of the thyroid: The Royal Marsden Hospital experience. Br J Radiol. 2005;78:405–410. doi: 10.1259/bjr/31803121. [DOI] [PubMed] [Google Scholar]
- 18.Alzouebi M, Goepel JR, Horsman JM, et al. Primary thyroid lymphoma: The 40 year experience of a UK lymphoma treatment centre. Int J Oncol. 2012;40:2075–2080. doi: 10.3892/ijo.2012.1387. [DOI] [PubMed] [Google Scholar]
- 19.Niitsu N, Okamoto M, Nakamura N, et al. Clinicopathologic correlations of stage IE/IIE primary thyroid diffuse large B-cell lymphoma. Ann Oncol. 2007;18:1203–1208. doi: 10.1093/annonc/mdm094. [DOI] [PubMed] [Google Scholar]
- 20.Rawal A, Finn WG, Schnitzer B, et al. Site-specific morphologic differences in extranodal marginal zone B-cell lymphomas. Arch Pathol Lab Med. 2007;131:1673–1678. doi: 10.5858/2007-131-1673-SMDIEM. [DOI] [PubMed] [Google Scholar]
- 21.Vega F, Lin P, Medeiros LJ. Extranodal lymphomas of the head and neck. Ann Diagn Pathol. 2005;9:340–350. doi: 10.1016/j.anndiagpath.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Isaacson PG. Mucosa-associated lymphoid tissue lymphoma. Semin Hematol. 1999;36:139–147. [PubMed] [Google Scholar]
- 23.Suh C, Huh J, Roh JL. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue arising in the extracranial head and neck region: A high rate of dissemination and disease recurrence. Oral Oncol. 2008;44:949–955. doi: 10.1016/j.oraloncology.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Ott G, Katzenberger T, Greiner A, et al. The t(11;18)(q21;q21) chromosome translocation is a frequent and specific aberration in low-grade but not high-grade malignant non-Hodgkin's lymphomas of the mucosa-associated lymphoid tissue (MALT-) type. Cancer Res. 1997;57:3944–3948. [PubMed] [Google Scholar]
- 25.Kerrigan DP, Irons J, Chen IM. bcl-2 gene rearrangement in salivary gland lymphoma. Am J Surg Pathol. 1990;14:1133–1138. doi: 10.1097/00000478-199012000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Hwang YC, Kim TY, Kim WB, et al. Clinical characteristics of primary thyroid lymphoma in Koreans. Endocr J. 2009;56:399–405. doi: 10.1507/endocrj.k08e-355. [DOI] [PubMed] [Google Scholar]
- 27.Lam KY, Lo CY, Kwong DL, et al. Malignant lymphoma of the thyroid. A 30-year clinicopathologic experience and an evaluation of the presence of Epstein-Barr virus. Am J Clin Pathol. 1999;112:263–270. doi: 10.1093/ajcp/112.2.263. [DOI] [PubMed] [Google Scholar]
- 28.Skacel M, Ross CW, Hsi ED. A reassessment of primary thyroid lymphoma: High-grade MALT-type lymphoma as a distinct subtype of diffuse large B-cell lymphoma. Histopathology. 2000;37:10–18. doi: 10.1046/j.1365-2559.2000.00941.x. [DOI] [PubMed] [Google Scholar]
- 29.Horsman DE, Gascoyne RD, Coupland RW, et al. Comparison of cytogenetic analysis, southern analysis, and polymerase chain reaction for the detection of t(14; 18) in follicular lymphoma. Am J Clin Pathol. 1995;103:472–478. doi: 10.1093/ajcp/103.4.472. [DOI] [PubMed] [Google Scholar]
- 30.Lai R, Arber DA, Chang KL, et al. Frequency of bcl-2 expression in non-Hodgkin's lymphoma: A study of 778 cases with comparison of marginal zone lymphoma and monocytoid B-cell hyperplasia. Mod Pathol. 1998;11:864–869. [PubMed] [Google Scholar]
- 31.Jaffe ES, Banks PM, Nathwani B, et al. Recommendations for the reporting of lymphoid neoplasms: A report from the Association of Directors of Anatomic and Surgical Pathology. Mod Pathol. 2004;17:131–135. doi: 10.1038/sj.modpathol.3800028. [DOI] [PubMed] [Google Scholar]
- 32.Rohatiner AZ, Lister TA. The clinical course of follicular lymphoma. Best Pract Res Clin Haematol. 2005;18:1–10. doi: 10.1016/j.beha.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Bacon CM, Diss TC, Ye H, et al. Follicular lymphoma of the thyroid gland. Am J Surg Pathol. 2009;33:22–34. doi: 10.1097/PAS.0b013e31817d7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vailati A, Marena C, Aristia L, et al. Primary Hodgkin's disease of the thyroid: Report of a case and a review of the literature. Haematologica. 1991;76:69–71. [PubMed] [Google Scholar]
- 35.Wang SA, Rahemtullah A, Faquin WC, et al. Hodgkin's lymphoma of the thyroid: A clinicopathologic study of five cases and review of the literature. Mod Pathol. 2005;18:1577–1584. doi: 10.1038/modpathol.3800501. [DOI] [PubMed] [Google Scholar]
- 36.Yokoyama J, Ito S, Ohba S, et al. Problems of primary T-cell lymphoma of the thyroid gland–a case report. World J Surg Oncol. 2012;10:58. doi: 10.1186/1477-7819-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okamoto A, Namura K, Uchiyama H, et al. Cytotoxic T-cell non-Hodgkin's lymphoma of the thyroid gland. Am J Hematol. 2005;80:77–78. doi: 10.1002/ajh.20393. [DOI] [PubMed] [Google Scholar]
- 38.Shimoyama M, Oyama A, Tajima K, et al. Differences in clinicopathological characteristics and major prognostic factors between B-lymphoma and peripheral T-lymphoma excluding adult T-cell leukemia/lymphoma. Leuk Lymphoma. 1993;10:335–342. doi: 10.3109/10428199309148557. [DOI] [PubMed] [Google Scholar]
- 39.Graff-Baker A, Sosa JA, Roman SA. Primary thyroid lymphoma: A review of recent developments in diagnosis and histology-driven treatment. Curr Opin Oncol. 2010;22:17–22. doi: 10.1097/CCO.0b013e3283330848. [DOI] [PubMed] [Google Scholar]
- 40.Yahaya N, Din SW, Ghazali MZ, et al. Primary thyroid lymphoma with elevated free thyroxine level. Singapore Med J. 2011;52:e173–e176. [PubMed] [Google Scholar]
- 41.Gupta N, Nijhawan R, Srinivasan R, et al. Fine needle aspiration cytology of primary thyroid lymphoma: A report of ten cases. Cytojournal. 2005;2:21. doi: 10.1186/1742-6413-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakorafas GH, Kokkoris P, Farley DR. Primary thyroid lymphoma (correction of lympoma): Diagnostic and therapeutic dilemmas. Surg Oncol. 2010;19:e124–e129. doi: 10.1016/j.suronc.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Fasola G, Fanin R, Gherlinzoni F, et al. Serum LDH concentration in non-Hodgkin's lymphomas. Relationship to histologic type, tumor mass, and presentation features Acta Haematol. 1984;72:231–238. doi: 10.1159/000206395. [DOI] [PubMed] [Google Scholar]
- 44.Cooper EH, Bunning R, Illingworth S, et al. Serial measurement of beta2 microglobulin, acute phase reactant proteins and the ESR in non-Hodgkin's lymphomas and chronic lymphocytic leukaemia. Biomedicine. 1978;29:154–158. [PubMed] [Google Scholar]
- 45.Aiken AH. Imaging of thyroid cancer. Semin Ultrasound CT MR. 2012;33:138–149. doi: 10.1053/j.sult.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Nam M, Shin JH, Han BK, et al. Thyroid lymphoma: Correlation of radiologic and pathologic features. J Ultrasound Med. 2012;31:589–594. doi: 10.7863/jum.2012.31.4.589. [DOI] [PubMed] [Google Scholar]
- 47.Loevner LA, Kaplan SL, Cunnane ME, et al. Cross-sectional imaging of the thyroid gland. Neuroimaging Clin N Am. 2008;18:445–461. vii. doi: 10.1016/j.nic.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Kim HC, Han MH, Kim KH, et al. Primary thyroid lymphoma: CT findings. Eur J Radiol. 2003;46:233–239. doi: 10.1016/s0720-048x(02)00134-1. [DOI] [PubMed] [Google Scholar]
- 49.Takashima S, Nomura N, Noguchi Y, et al. Primary thyroid lymphoma: Evaluation with US, CT, and MRI. J Comput Assist Tomogr. 1995;19:282–288. [PubMed] [Google Scholar]
- 50.Treglia G, Del Ciello A, Di Franco D. Recurrent lymphoma in the thyroid gland detected by fluorine-18-fluorodeoxyglucose PET/CT. Endocrine. 2013;43:242–243. doi: 10.1007/s12020-012-9782-0. [DOI] [PubMed] [Google Scholar]
- 51.Baba S, Abe K, Isoda T, et al. Impact of FDG-PET/CT in the management of lymphoma. Ann Nucl Med. 2011;25:701–716. doi: 10.1007/s12149-011-0549-0. [DOI] [PubMed] [Google Scholar]
- 52.Arabi M, Dvorak R, Smith LB, et al. Fluorodeoxyglucose positron emission tomography in primary thyroid lymphoma with coexisting lymphocytic thyroiditis. Thyroid. 2011;21:1153–1156. doi: 10.1089/thy.2011.0064. [DOI] [PubMed] [Google Scholar]
- 53.Mehta A, Muthukrishnan A. Stage IE non-Hodgkin's thyroid lymphoma on (18)F-FDG-PET/CT. Hell J Nucl Med. 2011;14:186–187. [PubMed] [Google Scholar]
- 54.Chander S, Zingas AP, Bloom DA, et al. Positron emission tomography in primary thyroid lymphoma. Clin Nucl Med. 2004;29:572–573. doi: 10.1097/01.rlu.0000134987.48416.05. [DOI] [PubMed] [Google Scholar]
- 55.Cha C, Chen H, Westra WH, et al. Primary thyroid lymphoma: Can the diagnosis be made solely by fine-needle aspiration? Ann Surg Oncol. 2002;9:298–302. doi: 10.1007/BF02573069. [DOI] [PubMed] [Google Scholar]
- 56.Morgen EK, Geddie W, Boerner S, et al. The role of fine-needle aspiration in the diagnosis of thyroid lymphoma: A retrospective study of nine cases and review of published series. J Clin Pathol. 2010;63:129–133. doi: 10.1136/jcp.2009.071423. [DOI] [PubMed] [Google Scholar]
- 57.Dustin SM, Jo VY, Hanley KZ, et al. High sensitivity and positive predictive value of fine-needle aspiration for uncommon thyroid malignancies. Diagn Cytopathol. 2012;40:416–421. doi: 10.1002/dc.21802. [DOI] [PubMed] [Google Scholar]
- 58.Takano T, Miyauchi A, Matsuzuka F, et al. Diagnosis of thyroid malignant lymphoma by reverse transcription-polymerase chain reaction detecting the monoclonality of immunoglobulin heavy chain messenger ribonucleic acid. J Clin Endocrinol Metab. 2000;85:671–675. doi: 10.1210/jcem.85.2.6390. [DOI] [PubMed] [Google Scholar]
- 59.Takano T, Miyauchi A, Matsuzuka F, et al. Detection of monoclonality of the immunoglobulin heavy chain gene in thyroid malignant lymphoma by vectorette polymerase chain reaction. J Clin Endocrinol Metab. 2005;90:720–723. doi: 10.1210/jc.2004-0951. [DOI] [PubMed] [Google Scholar]
- 60.Takano T, Asahi S, Matsuzuka F, et al. Aspiration biopsy-nucleic acid diagnosis of thyroid malignant lymphoma by vectorette PCR: Experience of eight cases. Leuk Res. 2008;32:151–154. doi: 10.1016/j.leukres.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 61.Daneshbod Y, Omidvari S, Daneshbod K, et al. Diffuse large B cell lymphoma of thyroid as a masquerader of anaplastic carcinoma of thyroid, diagnosed by FNA: A case report. Cytojournal. 2006;3:23. doi: 10.1186/1742-6413-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ito Y, Yoshida H, Matsuzuka F, et al. Cdc25A and cdc25B expression in malignant lymphoma of the thyroid: Correlation with histological subtypes and cell proliferation. Int J Mol Med. 2004;13:431–435. [PubMed] [Google Scholar]
- 63.Sugawara M, Matsuzuka F, Fukata S, et al. Excessive survivin expression in thyroid lymphomas. Hum Pathol. 2002;33:524–527. doi: 10.1053/hupa.2002.124783. [DOI] [PubMed] [Google Scholar]
- 64.Swart GJ, Wright C, Brundyn K, et al. Fine needle aspiration biopsy and flow cytometry in the diagnosis of lymphoma. Transfus Apher Sci. 2007;37:71–79. doi: 10.1016/j.transci.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 65.Swart GJ, Wright CA. The utilization of fine needle aspiration biopsy (FNAB) and flow cytometry (FC) in the diagnosis and classification of non-Hodgkin B-cell and T-cell lymphomas. Transfus Apher Sci. 2010;42:199–207. doi: 10.1016/j.transci.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 66.Demharter J, Muller P, Wagner T, et al. Percutaneous core-needle biopsy of enlarged lymph nodes in the diagnosis and subclassification of malignant lymphomas. Eur Radiol. 2001;11:276–283. doi: 10.1007/s003300000540. [DOI] [PubMed] [Google Scholar]
- 67.Ravinsky E, Morales C. Diagnosis of lymphoma by image-guided needle biopsies: Fine needle aspiration biopsy, core biopsy or both? Acta Cytol. 2005;49:51–57. doi: 10.1159/000326095. [DOI] [PubMed] [Google Scholar]
- 68.Novoa E, Gurtler N, Arnoux A, et al. Role of ultrasound-guided core-needle biopsy in the assessment of head and neck lesions: A meta-analysis and systematic review of the literature. Head Neck. 2012;34:1497–1503. doi: 10.1002/hed.21821. [DOI] [PubMed] [Google Scholar]
- 69.Sangalli G, Serio G, Zampatti C, et al. Fine needle aspiration cytology of primary lymphoma of the thyroid: A report of 17 cases. Cytopathology. 2001;12:257–263. doi: 10.1046/j.1365-2303.2001.00338.x. [DOI] [PubMed] [Google Scholar]
- 70.Matsuzuka F, Miyauchi A, Katayama S, et al. Clinical aspects of primary thyroid lymphoma: Diagnosis and treatment based on our experience of 119 cases. Thyroid. 1993;3:93–99. doi: 10.1089/thy.1993.3.93. [DOI] [PubMed] [Google Scholar]
- 71.Pyke CM, Grant CS, Habermann TM, et al. Non-Hodgkin's lymphoma of the thyroid: Is more than biopsy necessary? World J Surg. 1992;16:604–609. doi: 10.1007/BF02067333. discussion 609–610. [DOI] [PubMed] [Google Scholar]
- 72.Sippel RS, Gauger PG, Angelos P, et al. Palliative thyroidectomy for malignant lymphoma of the thyroid. Ann Surg Oncol. 2002;9:907–911. doi: 10.1007/BF02557529. [DOI] [PubMed] [Google Scholar]
- 73.Myatt HM. Acute airway obstruction due to primary thyroid lymphoma. Rev Laryngol Otol Rhinol (Bord) 1996;117:237–239. [PubMed] [Google Scholar]
- 74.Hopkins C, Stearns M, Watkinson AF. Palliative tracheal stenting in invasive papillary thyroid carcinoma. J Laryngol Otol. 2001;115:935–937. doi: 10.1258/0022215011909413. [DOI] [PubMed] [Google Scholar]
- 75.Noppen M, Poppe K, D'Haese J, et al. Interventional bronchoscopy for treatment of tracheal obstruction secondary to benign or malignant thyroid disease. Chest. 2004;125:723–730. doi: 10.1378/chest.125.2.723. [DOI] [PubMed] [Google Scholar]
- 76.Tsutsui H, Kubota M, Yamada M, et al. Airway stenting for the treatment of laryngotracheal stenosis secondary to thyroid cancer. Respirology. 2008;13:632–638. doi: 10.1111/j.1440-1843.2008.01309.x. [DOI] [PubMed] [Google Scholar]
- 77.Lee J, Won JH, Kim HC, et al. Emergency dilation by self-expandable tracheal stent for upper airway obstruction in a patient with a giant primary thyroid lymphoma. Thyroid. 2009;19:193–195. doi: 10.1089/thy.2008.0166. [DOI] [PubMed] [Google Scholar]
- 78.Laing RW, Hoskin P, Hudson BV, et al. The significance of MALT histology in thyroid lymphoma: A review of patients from the BNLI and Royal Marsden Hospital. Clin Oncol (R Coll Radiol) 1994;6:300–304. doi: 10.1016/s0936-6555(05)80271-7. [DOI] [PubMed] [Google Scholar]
- 79.Doria R, Jekel JF, Cooper DL. Thyroid lymphoma. The case for combined modality therapy. Cancer. 1994;73:200–206. doi: 10.1002/1097-0142(19940101)73:1<200::aid-cncr2820730135>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 80.Mian M, Gaidano G, Conconi A, et al. High response rate and improvement of long-term survival with combined treatment modalities in patients with poor-risk primary thyroid diffuse large B-cell lymphoma: An International Extranodal Lymphoma Study Group and Intergruppo Italiano Linfomi study. Leuk Lymphoma. 2011;52:823–832. doi: 10.3109/10428194.2011.555888. [DOI] [PubMed] [Google Scholar]
- 81.Onal C, Li YX, Miller RC, et al. Treatment results and prognostic factors in primary thyroid lymphoma patients: A rare cancer network study. Ann Oncol. 2011;22:156–164. doi: 10.1093/annonc/mdq310. [DOI] [PubMed] [Google Scholar]
- 82.McKelvey EM, Gottlieb JA, Wilson HE, et al. Hydroxyldaunomycin (Adriamycin) combination chemotherapy in malignant lymphoma. Cancer. 1976;38:1484–1493. doi: 10.1002/1097-0142(197610)38:4<1484::aid-cncr2820380407>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 83.Fisher RI. Treatment of aggressive non-Hodgkin' lymphomas. Lessons from the past 10 years. Cancer. 1994;74(suppl):2657–2661. doi: 10.1002/1097-0142(19941101)74:9+<2657::aid-cncr2820741812>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 84.Reyes F, Lepage E, Ganem G, et al. ACVBP versus CHOP plus radiotherapy for localized aggressive lymphoma. N Engl J Med. 2005;352:1197–1205. doi: 10.1056/NEJMoa042040. [DOI] [PubMed] [Google Scholar]
- 85.Miller TP, Dahlberg S, Cassady JR, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin's lymphoma. N Engl J Med. 1998;339:21–26. doi: 10.1056/NEJM199807023390104. [DOI] [PubMed] [Google Scholar]
- 86.Horning SJ, Weller E, Kim K, et al. Chemotherapy with or without radiotherapy in limited-stage diffuse aggressive non-Hodgkin's lymphoma: Eastern Cooperative Oncology Group study 1484. J Clin Oncol. 2004;22:3032–3038. doi: 10.1200/JCO.2004.06.088. [DOI] [PubMed] [Google Scholar]
- 87.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 88.Gao G, Liang X, Jiang J, et al. A systematic review and meta-analysis of immunochemotherapy with rituximab for B-cell non-Hodgkin's lymphoma. Acta Oncol. 2010;49:3–12. doi: 10.3109/02841860903150502. [DOI] [PubMed] [Google Scholar]
- 89.Hochster H, Weller E, Gascoyne RD, et al. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: Results of the randomized phase III ECOG1496 Study. J Clin Oncol. 2009;27:1607–1614. doi: 10.1200/JCO.2008.17.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Visco C, Tzankov A, Xu-Monette ZY, et al. Patients with diffuse large B cell lymphoma of germinal center origin with BCL2 translocations have poor outcome, irrespective of MYC status: A report from an International DLBCL rituximab-CHOP Consortium Program Study. Haematologica. 2013;98:255–263. doi: 10.3324/haematol.2012.066209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Winter JN, Weller EA, Horning SJ, et al. Prognostic significance of Bcl-6 protein expression in DLBCL treated with CHOP or R-CHOP: A prospective correlative study. Blood. 2006;107:4207–4213. doi: 10.1182/blood-2005-10-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mounier N, Briere J, Gisselbrecht C, et al. Rituximab plus CHOP (R-CHOP) overcomes bcl-2–associated resistance to chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL) Blood. 2003;101:4279–4284. doi: 10.1182/blood-2002-11-3442. [DOI] [PubMed] [Google Scholar]
- 93.Dai CW, Zhang GS, Pei MF, et al. Thyroid diffuse large B cell lymphoma (DLBCL) following thyroid medullary cancer: Long-term complete remission with R-CHOP therapy. Ann Hematol. 2009;88:701–702. doi: 10.1007/s00277-008-0639-9. [DOI] [PubMed] [Google Scholar]
- 94.Jonak C, Troch M, Mullauer L, et al. Rituximab plus dose-reduced cyclophosphamide, mitoxantrone, vincristine, and prednisolone are effective in elderly patients with diffuse large B-cell lymphoma of the thyroid. Thyroid. 2010;20:425–427. doi: 10.1089/thy.2009.0440. [DOI] [PubMed] [Google Scholar]
- 95.Karlin L, Coiffier B. Improving survival and preventing recurrence of diffuse large B-cell lymphoma in younger patients: Current strategies and future directions. Onco Targets Ther. 2013;6:289–296. doi: 10.2147/OTT.S42574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aggarwal N, Swerdlow SH, Kelly LM, et al. Thyroid carcinoma-associated genetic mutations also occur in thyroid lymphomas. Mod Pathol. 2012;25:1203–1211. doi: 10.1038/modpathol.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stopeck AT, Unger JM, Rimsza LM, et al. A phase 2 trial of standard-dose cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) and rituximab plus bevacizumab for patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: SWOG 0515. Blood. 2012;120:1210–1217. doi: 10.1182/blood-2012-04-423079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 99.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 100.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 101.Kramer MH, Hermans J, Parker J, et al. Clinical significance of bcl2 and p53 protein expression in diffuse large B-cell lymphoma: A population-based study. J Clin Oncol. 1996;14:2131–2138. doi: 10.1200/JCO.1996.14.7.2131. [DOI] [PubMed] [Google Scholar]
- 102.Hermine O, Haioun C, Lepage E, et al. Prognostic significance of bcl-2 protein expression in aggressive non-Hodgkin's lymphoma. Groupe d'Etude des Lymphomes de l'Adulte (GELA) Blood. 1996;87:265–272. [PubMed] [Google Scholar]
- 103.Offit K, Lo Coco F, Louie DC, et al. Rearrangement of the bcl-6 gene as a prognostic marker in diffuse large-cell lymphoma. N Engl J Med. 1994;331:74–80. doi: 10.1056/NEJM199407143310202. [DOI] [PubMed] [Google Scholar]


