Abstract
Clinical trials continue to produce conflicting results on the effectiveness of fish oils for the primary and secondary prevention of coronary heart disease. Despite many large, well-performed studies, questions still remain, made even more complex by the addition of early revascularization and statins in our coronary heart disease armamentarium. This is complicated by the reality that fish oil production has a measureable impact on reducing fish populations, which in turn has a negative impact on creating a sustainable product. We review the current data for fish oil usage in the primary and secondary prevention of coronary heart disease with an eye toward future studies, and the effects fish oil production has on the environment and efforts that are currently under way to mitigate these effects.
For the past 70 years, clinicians have been interested in the possible benefits of fish oils for the prevention of coronary heart disease (CHD). Historical cohort evidence suggests that a diet high in fish oils, composed of ω3 polyunsaturated fatty acids (ω3-PUFAs), can prevent CHD; however, the randomized placebo-controlled trials have produced mixed results.1 In this review, we provide an overview of the current data for fish oil’s efficacy in the primary and secondary prevention of CHD with a discussion of the effects that production of fish oil has on the environment.
FISH OIL STRUCTURE, BIOLOGY, AND DOSING
Neither fish nor humans can produce adequate amounts of ω3-PUFAs; therefore, both must ingest them from the environment. Fatty acids are chains of 4 to 28 carbons ending in a carboxylic acid. Fatty acids are saturated when each carbon is attached to the maximum number of possible hydrogen atoms, whereas unsaturated fatty acids contain at least 1 carbon–carbon double bond. Polyunsaturated fatty acids contain at least 2 double bonds, the prefix ω3 indicating that the first double bond is located 3 bonds in from the initial methyl group. There are 3 major ω3-PUFAs: α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). Found in vegetable sources including linseed oil, soybean oil, and flaxseed oil, ALA can be desaturated to EPA and further converted to DHA. However, the conversion rate is poor, and in humans only 5% of the ALA can be converted to DHA. Fish primarily receive DHA and EPA by ingesting marine algae2 and the amount of EPA and DHA found in a particular fish varies depending on the environment of the fish and the types of algae consumed. Oily fish, including salmon, herring, and mackerel, contain the highest levels of combined DHA and EPA.3
The mechanisms of action of fish oils is beyond the scope of this paper, but they have been shown to have a broad range of biological effects including decreasing platelet aggregation,4 stabilizing plaques and reducing atherosclerosis,5 decreasing triglycerides,6 and electrically stabilizing cardiac myocytes.7 Currently, it is unknown which of the primary ω3-PUFAs—DHA, EPA, or, to a lesser extent, ALA—is most important for the prevention of CHD, or what is the correct dosing and ratio of these fatty acids to include in modern fish-oil preparations. Given this uncertainty, there is much heterogeneity in trial data with regard to dosing and optimal ratios of ω3-PUFAs in fish oil supplements.
We focused on the trials that used EPA and DHA as opposed to ALA, as there is more evidence for the former compared with the latter. Many trials use a “standard preparation” of variable multiples of 1-gram capsules of fish oil that each contains approximately 465 milligrams of EPA and 375 milligrams of DHA. Although these capsules only contain about 840 milligrams combined EPA and DHA, they are marketed as 1-gram capsules. In this article, all doses are given in terms of actual EPA and DHA content. Over-the-counter fish-oil preparations have highly variable quantities of EPA and DHA, commonly 300 milligrams or 500 milligrams per capsule, and thus, on average, contain much less EPA and DHA than prescription-dose fish-oil capsules.
FISH OILS FOR THE PRIMARY PREVENTION OF CORONARY HEART DISEASE
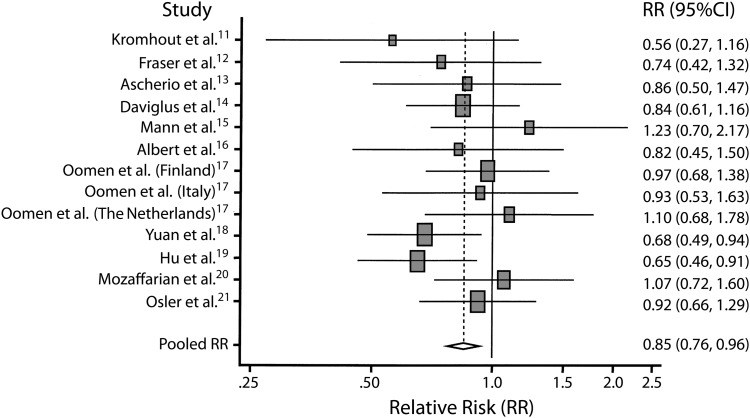
The landmark proposition by Bang et al. that Greenland Eskimos’ diet of whale, seal, and fish was responsible for their lower rate of CHD compared with age-matched Dane counterparts, despite the Greenland Eskimos having a diet lower in fruits and vegetables and higher in saturated fats and cholesterol,8,9 has set off our modern-day trials of fish oil usage in the human population for prevention of CHD. The proposition that fish oil has a protective effect against CHD has been confirmed by many epidemiological cohort studies, including a meta-analysis of more than 222 350 patients from multiple countries, including England, Finland, Denmark, the Netherlands, Italy, China, and the United States10–22 when they compared fish consumption in the highest to lowest quartiles of cohort data (Figure 1). However, some of the largest studies had no significant test of trend or were statistically significant only in certain high-risk populations. It has also been shown that patients who eat fish oil tend to have healthier lifestyles with fewer cardiovascular disease (CVD) risk factors in general, thus confounding their dietary preventive effect.23
FIGURE 1—
Pooled estimates of coronary heart disease mortality rates for fish consumption once per week vs less than once per month.
Note. RR = relative risk. Squares indicate adjusted RR in each study. Size of the square is proportional to the percentage weight of each study in the meta-analysis; horizontal line represents 95% confidence interval. Studies are ordered by year of publication. The shaded diamond indicates meta-analysis data demonstrating that the risk of cardiac heart disease mortality (e.g., death from arrhythmia, myocardial infarction) in a group of patients that eat fish once a week is 15% less (0.85; 95% confidence interval [CI] = 0.76, 0.96) than in a group of patients who eat fish less than once a month. The unshaded diamond indicates pooled RR and 95% CI.
Source. He et al.10
In spite of these methodological issues, the American Heart Association and American College of Cardiology recommended in 2002 that patients without documented CHD “eat a variety of (preferably oily) fish at least twice a week,”24(p2755) which is equivalent to about 400 to 500 milligrams of EPA+DHA per day. In those with documented CHD, they recommend to “consume ∼1g of EPA+DHA per day, preferably from oily fish.”24(p2755) The 2010 Dietary Guidelines for Americans recently published by the US Department of Agriculture are in line with these statements, recommending consumption of “250 milligrams per day [equivalent to ∼8 ounces of fish per week] of long-chain n-3 fatty acids [ω3-PUFAs] … in persons with and without CVD.”25(p413) However, the evidence cited in making this recommendation is heavily weighted toward prospective cohort trial data and secondary prevention randomized placebo-controlled trials. The 4 primary prevention randomized trials reviewed in the guidelines consisted of only 33 to 48 participants, making it difficult to determine the effect fish oil consumption may have on the population at large.
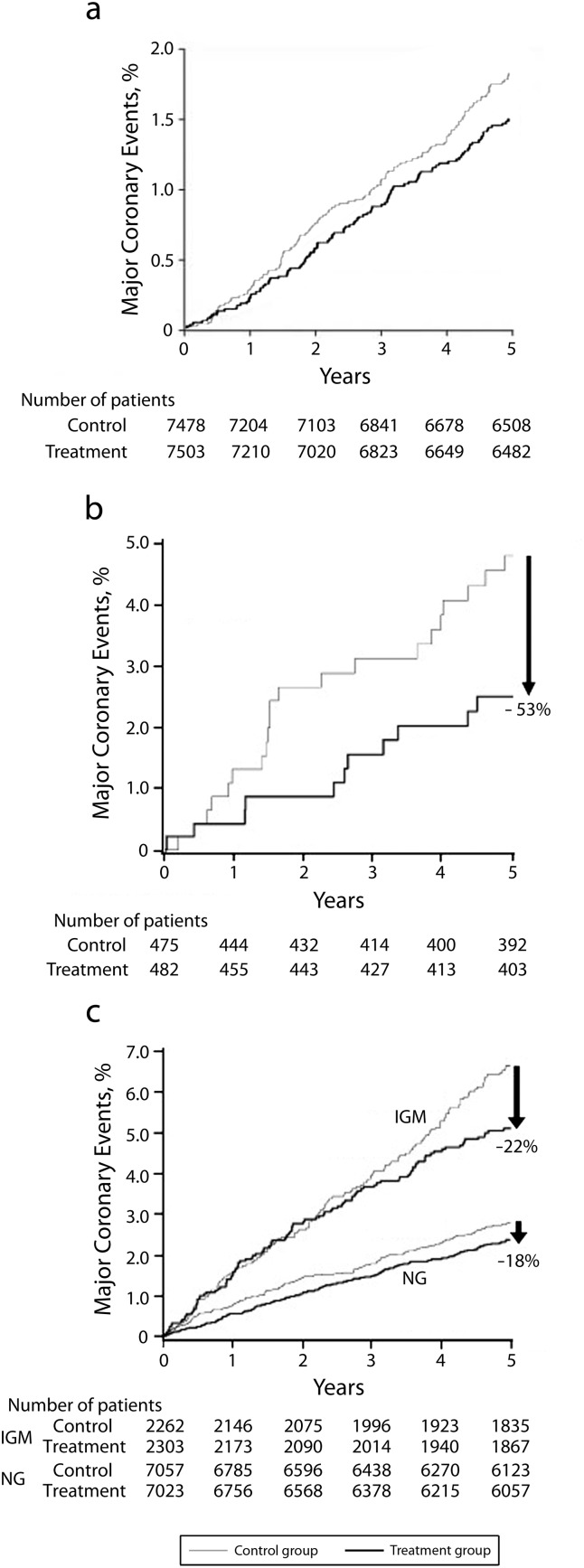
The only major randomized placebo-controlled prospective trial on primary prevention of CHD by fish oil consumption, the Japan EPA Lipid Intervention Study (JELIS) trial26 is at odds with the cohort and small, randomized trials mentioned previously. In the JELIS trial, 14 981 hyperlipidemic (cholesterol > 250 mg/dL) Japanese participants without evidence of CHD were randomized to 1800 milligrams of highly purified EPA plus statin versus statin alone. At 4.6 years the primary prevention arm had a nonsignificant 18% decrease in the combined primary endpoint of any major coronary event (hazard ratio [HR] = 0.82; 95% confidence interval [CI] = 0.62, 1.06; P = .132), defined as sudden cardiac death, fatal and nonfatal myocardial infarction (MI), unstable angina pectoris, angioplasty, stenting, or coronary artery bypass grafting, with nonsignificant decreases in all major CHD outcomes (Figure 2a). If these numbers had been statistically significant, the number needed to treat (NNT) to prevent 1 major coronary event would have been 321 participants. It has been suggested that the potential reasons for nonsignificance were (1) the low event rate in the study population over 5 years prevented the study from reaching statistical significance, and (2) the trial was performed in a Japanese population already known to consume a large amount of ω3-PUFAs in general, thus dampening the possible protective effects of adding additional EPA to their diet.26 The approximate 3.5% CHD event rate over 10 years in the primary prevention arm would place the average participant at low risk by the National Cholesterol Education Program Adult Treatment Panel III criteria, but undoubtedly many such people are consuming fish oils, which at least in this study had no effect on lowering moderately elevated cholesterol levels or CHD events when combined with a statin.
FIGURE 2—
Effects of eicosapentaenoic acid treatment on the incidence of major coronary events for (a) the primary prevention total population, (b) the primary prevention high triglyceride and low high-density lipoprotein subgroup, and (c) the primary and secondary prevention impaired glucose metabolism subgroups: the Japan EPA Intervention Lipid Study.
Note. IGM = impaired glucose metabolism; NG = normoglycemia. Hazard ratios were (a) 0.82 (95% confidence interval [CI] = 0.63, 1.06; P = .132), (b) 0.47 (95% CI = 0.23, 0.98; P = .043), and (c) 0.78 (95% CI = 0.60, 0.998; P = .048) for IGM and 0.82 (95% CI = 0.66, 1.01; P = .062) for NG.
Source. Yokoyama et al.26-28
The many subanalyses of JELIS deserve mention, given that JELIS is the only large randomized placebo-controlled trial of ω3-PUFAs for the primary prevention of CHD. In one subanalysis of primary care participants in JELIS,27 it was shown that participants who had triglycerides greater than 150 milligrams per deciliter and high-density lipoprotein less than 40 milligrams per deciliter (n = 957) and consumed ω3-PUFAs had a significant decrease in major coronary events (HR = 0.47; 95% CI = 0.23, 0.98; P = .043) compared with controls (Figure 2b) suggesting a potential beneficial effect of ω3-PUFAs for primary prevention of major coronary events in patients taking a statin who have hypertriglyceridemia and low high-density lipoprotein. This preventive effect was similar to the preventive benefits of fenofibrate 160 milligrams versus placebo when added to statins in the Action to Control Cardiovascular Risk in Diabetes-Lipids trial in diabetic participants. There was a decrease in the primary endpoint of nonfatal MI, stroke, or cardiovascular death only in those with triglycerides greater than or equal to 204 milligrams per deciliter and high-density lipoprotein cholesterol less than or equal to 34 milligrams per deciliter, suggesting that the primary preventive effects of fish oils added to statins may only be seen in severely dyslipidemic participants.29
In another subanalysis28 of the entire cohort of JELIS participants, including participants both with and without CHD at baseline, those patients who had impaired glucose metabolism, defined as fasting glucose 110 milligrams per deciliter or higher, physician-diagnosed diabetes, or those on diabetic medications had significantly reduced major coronary events compared with their non–impaired glucose metabolism counterparts (HR = 0.78; 95% CI = 0.60, 0.998; P = .048; Figure 2c). This finding was further explored in the recently published Outcome Reduction With Initial Glargine Intervention (ORIGIN) trial.30 In the ORIGIN trial, 12 536 patients at high risk for cardiovascular events, defined as presence of impaired glucose tolerance, impaired glucose metabolism, diabetes, or history of MI, stroke, or revascularization were randomized to 840 milligrams EPA+DHA versus placebo. At 6.2 years there was no significant difference between the groups in either the primary outcome of death from any cardiovascular cause (HR = 0.98; 95% CI = 0.87, 1.10; P = .72) or any of the secondary outcomes of MI, stroke, death from cardiovascular cause, death from any cause, or death from arrhythmia. Given that the ORIGIN trial, like the impaired glucose metabolism patient subanalysis of JELIS, includes both primary and secondary prevention patients, it is difficult to definitively conclude that high-risk patients in a purely primary prevention population stand to benefit from fish oil intake. However, the fact that it identifies and does not find a benefit for patients deemed at highest risk for CVD strongly suggests that with modern therapies, there is likely little evidence for the use of fish oil in purely primary prevention populations.
Given these variable findings, 3 large primary prevention, double-blinded, placebo-controlled randomized trials have been funded and are ongoing. The Rischio and Prevenzione (Risk and Prevention [R&P]) trial started in 2004 is randomizing 12 513 patients at high risk for CVD but without evidence of MI to 840 milligrams EPA+DHA versus placebo (NTC00317707). A Study of Cardiovascular Events iN Diabetes (ASCEND) trial, started in 2005 and run by the University of Oxford, is a 2-way stratified trial of 10 000 diabetic participants aged 40 years or older without vascular disease randomized to both or either 840 milligrams EPA+DHA and 100 milligrams aspirin versus placebo (NCT00135226). The Vitamin D and Ω-3 (VITAL) trial, started in 2010 and run by Brigham and Women’s Hospital, is another 2-way stratified trial of 20 000 older participants (men older than 60 years, women older than 65 years) randomized to either or both 840 milligrams EPA+DHA and vitamin D3 2000 international units versus placebo (NCT01653678). All of these trials will examine primary prevention of CVD and the third also includes primary prevention of cancer.
FISH OILS FOR THE SECONDARY PREVENTION OF CORONARY HEART DISEASE
Secondary prevention has been the major focus of most large prospective trials on fish oil (Tables 1 and 2). The first major trial to examine the effects of fish oil intake on CHD prevention was the Diet and Reinfarction (DART) trial,31 which randomized 2033 post-MI participants to receive dietary advice on fish-oil consumption versus no dietary advice. Participants advised to increase fish-oil consumption to 2500 milligrams EPA weekly had a significantly decreased mortality reduction at 2 years of 29% (HR = 0.71; CI = 0.54, 0.93; P < .05); this was especially pronounced in the group that consumed fish-oil capsules as their form of fish-oil intake. Several years later, a follow-up study, DART-235 randomized 3114 men with angina to dietary fish or capsular fish oil intake versus nonspecific dietary advice and showed that the fish-oil intake group had an increased risk of cardiac mortality (HR = 1.26; 95% CI = 1.00, 1.58; P = .047). This study has been criticized for its poor execution (there were very few baseline characteristics and compliance was only checked in 2% of the study population),39 but the difference in study outcomes seen within the same population exemplifies the confusion surrounding fish oil.
TABLE 1—
Summary of Major Randomized Placebo-Controlled Secondary Prevention Trials Examining the Effect of Fish or ω3-PUFA Intake on Various Cardiovascular Endpoints
| Study and Outcomes | Patient Population | ω3-PUFA Intake | Control |
| DART31 | 2033 nondiabetic male participants < 70 y randomized at a mean of 41d after MI | Fatty fish intake of 2 portions/wk (350 mg EPA+DHA/d) | No dietary advice |
| Singh32 | 240 participants randomized within 24 h after MI | 1200 mg EPA+DHA | Non-oil placebo |
| GISSI-Prevenzione33 | 11 324 participants randomized at a mean of 16 d after MI (all within 3 mo) to ω3-PUFA, ω3-PUFA + vitamin E, vitamin E, or none | 840 mg EPA+DHA | Usual care (statins not standard of care at time of trial) |
| Nilsen34 | 300 participants randomized 4–8 d post-MI | 3360 mg EPA+DHA | Corn oil |
| DART-235 | 3114 male participants with clinical angina < 70 y | Fatty fish intake of 2/wk (350 mg EPA+DHA/d), fruit intake, or fish intake + fruit intake | Nonspecific dietary advice |
| JELIS26 | 3664 participants with known CHD ≤ 75 y | 1800 mg EPA | None |
| GISSI-HF36 | 6975 participants with NYHA Class II–IV CHF | 840 mg EPA+DHA | Non-oil placebo |
| OMEGA37 | 3851 participants randomized 3–14 d post-MI | 840 mg EPA+DHA | Olive oil |
| α-OMEGA38 | 4837 participants 60–80 y with MI within 10 y | 400 mg EPA+DHA | Unsupplemented margarine |
Note. CHD = coronary heart disease; CHF = congestive heart failure; DART = Diet and Reinfarction Trial; DHA = docosahexaenoic acid; EPA = eicosapentaenoic acid; GISSI-HF = Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (Italian Group for the Study of Myocardial Infarction Survival) trial; HF = heart failure; JELIS = Japan EPA Intervention Lipid Study; MI = myocardial infarction; NYHA = New York Heart Association; ω3-PUFA = ω3 polyunsaturated fatty acid.
TABLE 2—
Results From Major Randomized Placebo-Controlled Secondary Prevention Trials Examining the Effect of Fish or ω3-PUFA Intake on Various Cardiovascular Endpoints
| Results (All Adjusted) |
||
| Study and Outcomes | RR (95% CI) | P |
| DART31 | ||
| Total mortality | 0.71 (0.54, 0.93) | <.05 |
| CHD mortality | 0.67 (NA) | <.01 |
| Nonfatal MI | 1.48 (NA) | >.05 |
| CHD deaths + nonfatal MI | 0.84 (0.66, 1.07) | >.05 |
| Singh32 | ||
| Cardiac mortality | 0.52 (0.22, 1.21) | |
| Sudden death | 0.24 (0.03, 2.0) | |
| Nonfatal MI | 0.51 (0.23, 1.28) | |
| All cardiac events | 0.70 (0.29, 0.90) | |
| GISSI-Prevenzione33 | ||
| Total mortality | 0.86 (0.76, 0.98); 0.80 (0.67, 0.94)a | |
| CVD mortality | 0.83 (0.71, 0.97); 0.70 (0.56, 0.87)a | |
| Cardiac mortality | 0.78 (0.65, 0.92); 0.65 (0.51, 0.82)a | |
| CHD mortality | 0.80 (0.67, 0.96); 0.65 (0.51, 0.84)a | |
| Sudden cardiac death | 0.74 (0.58, 0.93); 0.55 (0.40, 0.76)a | |
| Stroke | 1.21 (0.91, 1.63); 1.30 (0.87, 1.96)a | |
| Nonfatal CV event | 0.98 (0.83, 1.15); 0.96 (0.76, 1.21)a | |
| CHD deaths + nonfatal MI | 0.87 (0.76, 0.99); 0.75 (0.62, 0.90)a | |
| Nilsen34 | ||
| Total mortality | 1.02 (0.44, 2.36) | |
| Cardiac mortality | 1.02 (0.38, 2.71) | |
| Nonfatal CV event | 1.30 (0.81, 2.08) | |
| DART-235 | ||
| Total mortality | 1.15 (0.96, 1.36)b | .13 |
| Cardiac mortality | 1.26 (1.00, 1.58)b | .047 |
| Sudden death | 1.54 (1.06, 2.23)b | .025 |
| JELIS26 | ||
| CHD mortality | 0.87 (0.46, 1.64) | .67 |
| Sudden cardiac death | 1.02 (0.47, 2.19) | .97 |
| Nonfatal MI | 0.70 (0.42, 1.14) | .15 |
| CHD death + nonfatal MI | 0.75 (0.47, 1.19) | .22 |
| Major coronary event | 0.81 (0.66, 1.00) | .048 |
| GISSI-HF36 | ||
| Total mortality | 0.91 (0.83, 0.998) | .041 |
| CVD mortality | 0.90 (0.81, 0.99) | .045 |
| Sudden cardiac death | 0.93 (0.79, 1.08) | .333 |
| Stroke | 1.16 (0.89, 1.51) | .271 |
| CHF admission | 0.94 (0.86, 1.02) | .147 |
| CHD deaths + nonfatal MI | 0.82 (0.63, 1.06) | .121 |
| OMEGA37 | ||
| Total mortality | 1.24 (NA) | .18 |
| Cardiac mortality | 1.29 (NA) | .16 |
| CHD event | 1.09 (NA) | .63 |
| Stroke | 2.00 (NA) | .07 |
| Sudden cardiac death | 0.98 (NA) | .84 |
| Total mortality + CHD event + stroke | 1.18 (NA) | .1 |
| α-OMEGA38 | ||
| Total mortality | 1.01 (0.82, 1.24) | .92 |
| CVD mortality | 0.98 (0.72, 1.33) | .89 |
| CHD mortality | 0.95 (0.68, 1.32) | .75 |
| Sudden cardiac death | 0.90 (0.65, 1.26) | .55 |
| CHD deaths + nonfatal MI | 1.01 (0.87, 1.17) | .93 |
Note. CHD = coronary heart disease; CHF = congestive heart failure; CI = confidence interval; CV = cardiovascular; CVD = cardiovascular disease; DART = Diet and Reinfarction Trial; GISSI = Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (Italian Group for the Study of Myocardial Infarction Survival) trial; HF = heart failure; JELIS = Japan EPA Intervention Lipid Study; MI = myocardial infarction; NA = not available; ω3-PUFA = ω3 polyunsaturated fatty acid; RR = relative risk.
2-way analysis; 4-way analysis.
2-way analysis of results for fish-intake groups.
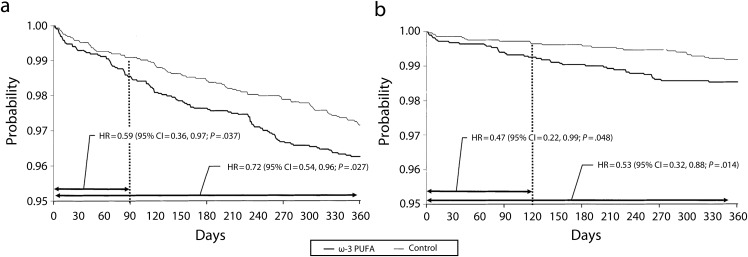
The initial conclusions reached by DART were first tested by Singh32 in 1997 in a small trial of 240 post-MI participants randomized to 1200 milligrams EPA+DHA versus placebo. Treated participants had nonsignificant differences in individual cardiac endpoints but a significant reduction in aggregate cardiovascular outcomes, defined as sudden cardiac death, cardiac death, and nonfatal reinfarction (relative risk [RR] = 0.70; 95% CI = 0.29, 0.90; P < .05). These results were confirmed in the largest secondary prevention trial, the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (Italian Group for the Study of Myocardial Infarction Survival [GISSI-Prevenzione]) trial,33 which randomized 11 323 post-MI participants to 840 milligrams EPA+DHA versus usual care with a combined primary endpoint of death, nonfatal MI, and stroke. At 3.5 years, the treatment arm had a nonsignificant 10% decrease in the combined endpoint with a statistically significant 14% reduction in total mortality (HR = 0.86; 95% CI = 0.76, 0.98; P < .05; NNT = 78; Figure 3a).
FIGURE 3—
Total mortality and sudden death at 1 year: Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (Italian Group for the Study of Myocardial Infarction Survival–Prevenzione trial).
Note. ω3-PUFA = ω3 polyunsaturated fatty acid; CI = confidence interval; HR = hazard ratio.
Source. Marchioli et al.40
Multiple subanalyses40 have shown that this decreased cardiovascular and total mortality was largely secondary to a 45% decrease in sudden cardiac death, and that this effect was most pronounced and had the largest impact within 4 months of the MI. Almost all of the mortality benefit occurred within the first year after the MI (Figure 3b). This outcome led many observers to conclude that the cardiovascular beneficial effects of fish oil after MI are not caused by the hypolipidemic effects per se, but rather by the antiarrhythmic effects.
The antiarrhythmic effects of fish oils are likely secondary to alteration of transmembrane ionic channels induced by the ω3-PUFAs themselves. It has been shown that myocytes bathed in EPA have decreased resting membrane potential, delayed firing after repetitive electrical stimuli, and decreased spontaneous firing, all of which may reduce arrhythmias induced by an ischemic state.7 Clinical trials exploring this antiarrhythmic effect are beyond the scope of this review, but, in short, 3 large trials41-43 of participants with implantable cardiac defibrillators randomized to fish oil versus placebo with a primary endpoint of rate of fatal arrhythmias have shown mixed results confirmed by a meta-analysis demonstrating significant heterogeneity in the studies.44 Similarly, 3 trials involving fish oil for the prevention of adverse outcomes of atrial fibrillation also exhibit mixed results.45-47 Most recently in late 2010, a study of 663 participants with paroxysmal or persistent atrial fibrillation in normal sinus rhythm randomized to 3360 milligrams EPA+DHA versus placebo demonstrated no reduction in recurrence of atrial fibrillation (HR = 1.22; 95% CI = 0.98, 1.52; P = .08).48
Other subanalyses of GISSI-Prevenzione49 have demonstrated that participants with an ejection fraction less than or equal to 50% had much higher rates of mortality and sudden cardiac death than participants with an ejection fraction greater than 50% (12.3% vs 6.0%; 3.4% vs 1.4%). In the participants with lower ejection fractions, fish oils provided no differential mortality benefit but did tend to preferentially reduce sudden cardiac death compared with those with higher ejection fractions (RR = 0.42 vs 0.89; P = .07). This has been further studied in the GISSI-Heart Failure (HF) study36 (not to be confused with the GISSI-Prevenzione study), which randomized 6975 participants with class II–IV heart failure (irrespective of etiology) to 840 milligrams EPA+DHA, rosuvastatin 10 milligrams, both, or double placebo for 3.9 years. Secondary outcomes showed a small but significant decrease in all-cause mortality from 29% to 27% (HR = 0.91; 95% CI = 0.833, 0.998; P = .041; NNT = 56) in the fish oil–treated group. Interestingly, the GISSI-HF trial was not able to replicate the initial GISSI–Prevenzione fish oil effect of reducing sudden cardiac death, which was thought to be its main mechanism of mortality prevention. It is now thought that fish oil’s benefits on the failing heart are secondary to its ability to inhibit cardiac remodeling,50,51 which has also just been demonstrated clinically52 and may soon prove to be an effective therapy for patients with congestive heart failure.
Although much important information has been gained from GISSI-Prevenzione, much has changed in modern post-MI care since the original publication. Early revascularization and statins have become more commonplace and the apparent benefit of fish oil for the secondary prevention of CHD has become less clear. In 2001, Nilsen et al. were the first to demonstrate this lack of efficacy for fish oils for modern post-MI patients when they randomized 300 recently post-MI participants to high-dose fish oil supplementation consisting of 3360 milligrams EPA+DHA for 1.5 years with a primary endpoint of lipid profile and all cardiac events (defined as cardiac death, resuscitation, recurrent MI, and unstable angina).34 Despite an improved lipid profile, there was no difference in mortality or cardiac events.
In the JELIS trial27 mentioned previously, a secondary prevention arm comprising 3664 participants with hyperlipidemia (total cholesterol > 250 mg/dL) and known CHD (but not specifically with a recent MI) were randomized to 1800 milligrams EPA plus statin versus statin alone with a primary endpoint of any major coronary event. These secondary prevention participants had a significant 19% decrease in major coronary events (RR = 0.81; 95% CI = 0.66, 1.00; P = .048; NNT = 50) becoming apparent at 3 years in this 4.6-year trial, but no significant changes in any individual cardiac endpoints, including sudden cardiac death.
More recently, in 2009, the results of the German OMEGA trial became known. In the OMEGA trial,53,54,37 3851 recently post-MI participants were randomized to 840 milligrams EPA+DHA versus placebo for 12 months with a primary outcome of sudden cardiac death. Not only was there no significant difference between the 2 groups (RR = 0.98; P = .84), but in the secondary outcomes measured including all major mortality and cardiovascular endpoints, there were also no significant differences observed with a trend toward increased risks in the fish oil–treated group. Most recently, in 2010, the Dutch α-OMEGA trial (not to be confused with the OMEGA trial)38 showed that in 4837 participants with known CHD randomized to either 400 milligrams EPA+DHA versus placebo for 3.3 years with a primary endpoint of fatal and nonfatal cardiovascular events there was a nonsignificant increase of events in the fish oil–treated group.
The JELIS, OMEGA, and α-OMEGA participants were aggressively treated with all modern post-MI therapies including aspirin, β-blockers, clopidogrel, angiotensin-converting enzyme inhibitors, statins, and early revascularization, which were not all in complete usage at the time of GISSI–Prevenzione. Because early revascularization and statins decrease ischemic time and fatal arrhythmias,55 these studies suggest that any mortality and sudden cardiac death decreases seen in GISSI–Prevenzione caused by fatal arrhythmias that were previously inhibited by fish oil are now negated by early revascularization and statins in modern post-MI care. This is demonstrated in the data by comparing the 3-year CVD rate of GISSI–Prevenzione versus α-OMEGA (5.6% vs 3.3%), suggestive of the benefit of modern post-MI care. Given that the effect of fish oil on prevention of sudden cardiac death was seen within 4 months of the MI in GISSI–Prevenzione, presumably the JELIS, OMEGA, and α-OMEGA trials would have been able to detect this difference in trial lengths of 4.6, 1, and 3.3 years, respectively. Unexplained by this data, however, is why the JELIS participants that only had a history of CHD but not a recent MI received a benefit from fish oil. Three explanations are likely: (1) the higher EPA dose of 1800 milligrams in JELIS compared with the lower doses of 400 milligrams and 840 milligrams EPA+DHA in the α-OMEGA and OMEGA trials may have been a factor, (2) the longer study time in JELIS may have made clinically relevant endpoints easier to attain, and (3) the benefits that OMEGA participants received from immediate modern post-MI care may have negated any benefits that would have been received from fish oil for chronic CHD, thus making it difficult to show a beneficial effect in recently post-MI participants.
In summary, these new studies suggest that the use of fish oils for the prevention of CHD in post-MI patients who have benefited from revascularization and modern post-MI care is ineffective. However, for secondary prevention of CHD in patients with a history of chronic CHD, not in the setting of a recent MI, fish oil may be effective but at much higher doses than currently recommended.
FISH OILS AND THE ENVIRONMENT
At the same time that interest in the beneficial health effects of fish oil has been increasing within the scientific community and the general public, concern has grown over the status of the world’s fish populations. In the 1950s, as fishing techniques became more sophisticated, worldwide fish catches began increasing rapidly. However, global catches leveled off in the 1990s and appear to have been on the decline since 1995.56,57 These trends are not caused by decreased fishing efforts per se, but rather by a startling reduction in global fish populations.
In 2005, it was estimated that 25% of the world’s fish stocks were underexploited or moderately exploited, 50% were fully exploited and close to the maximum sustainable limits, and 25% were overexploited or completely depleted.58 These troubling trends are caused both by overfishing and by degradation of marine habitats.56 Aquaculture (fish farming) has been unable to make up for the decreasing availability of wild fish stock. In fact, because most fish farms raise carnivorous fish fed on diets of smaller fish in a ratio of 3-to-1 fish-fed to fish-produced, fish farming likely exacerbates the problem.59 It has been estimated that if the amounts of low-dose EPA+DHA suggested by various government organizations are followed, the world fish population may eventually collapse by midcentury.60–63
These predictions may worsen now that the newest clinical trials have not shown a benefit for low-dose fish oil supplementation in either primary or secondary prevention. The approximately 6-fold higher doses of 1800 milligrams EPA per day required by JELIS for the secondary prevention of CHD will likely be an enormous burden on the world’s fish population. Complex equations with multiple variables are needed to estimate this burden. As a simplistic illustration of the effect on fish populations, assuming that 1800 milligrams of combined EPA+DHA per day is equivalent in efficacy to the 1800 milligrams EPA per day it required in JELIS to prevent 1 major coronary event, it would take, at a minimum, 50 patients eating 3 ounces (85 g) of farmed salmon per day (farmed salmon fed with marine feed being the highest EPA+DHA-containing fish)25,64,65 for 4.6 years, which, at an average weight of 4 kilograms per farmed salmon,66 would be equal to roughly 1750 fish. Patients could also choose to consume the equivalent amount of fish oil—which requires the same amount of fish to create, if not more given the current EPA+DHA content of fish used in modern fish oil preparations.67 Given that each kilogram of farmed fish requires harvesting 2.5 to 5 kilograms of raw fish feed,59,60 the numbers become quite staggering. Fish intake that is not of high EPA content, such as halibut, cod, and haddock, can bring the amount of fish required to prevent 1 coronary event to greater than 20 000.
Even larger numbers are reached when one considers fish oil for the unproven primary prevention of CHD, which, if it had been statistically significant in JELIS, would have required a NNT of 321 as opposed to the NNT of 50 for secondary prevention. This scenario conservatively represents ingestion of more than 10 000 fish of high EPA content to prevent 1 event in a primary prevention population, and reaches more than 100 000 fish when eating fish of low EPA content. The planet’s fish population is unlikely to survive this kind of consumption and will deprive many parts of the world an important source of protein, as well as a source of dietary pleasure.
Harvesting marine algae for EPA and DHA and yeast for EPA alone have been suggested as a potential solution to overfishing and as a sustainable alternative to fish farming. Although marine algae contain a relatively low level of total lipids, their levels of EPA and DHA are higher than those in terrestrial plants.68 The feasibility of cultivating marine algae and yeast on a large scale is currently being explored and may prove to be a viable, environmentally sound, mercury-free, option for ω3-PUFA production.
CONCLUSIONS
Although there is moderate evidence that high-dose fish oils given for many years can benefit populations having previously experienced an ischemic coronary event, newer trials are demonstrating a marked lack of benefit for patients given low-dose fish oil or in those who have received immediate modern post-MI care. This is in stark contrast to the results of previous trials on fish oil and is likely secondary to the beneficial effects of statins and early revascularization for post-MI patients.
In addition, the evidence for fish oil as a primary prevention treatment against CHD is currently unconvincing. Although the JELIS data are suggestive that there may be a role for high-dose fish oil in primary prevention for those with severe dyslipidemia, the ORIGIN trial strongly suggests that those at highest risk do not receive any benefit when taking modern therapies. The R&P, ASCEND, and VITAL trials exploring these discrepancies are still under way and this question will remain unanswered at least until the results of those trials are known.
Because our current dietary guidelines from the American Heart Association and American College of Cardiology were written in 2002, before the routine usage of statins and early revascularization in clinical trials using fish oil, it is prudent to re-examine our dietary and medical guidelines. Newer trials, including the α-OMEGA, OMEGA, ORIGIN, and JELIS trials, are yielding important data that question the benefits of fish oil, irrespective of dose, for the primary prevention of CHD, as well as standard-dose fish oil for the secondary prevention of CHD in patients who have benefited from modern post-MI care. Given these new data, combined with the harmful environmental impacts of fish oil production on the world’s fish population, clinicians should not recommend fish oil intake or fish consumption solely for the primary or secondary prevention of CHD until the results of current trials are known.
Human Participant Protection
No protocol approval was needed for this study because no human participants were involved.
References
- 1.Wang C, Harris WS, Chung Met al. N-3 fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006;84(1):5–17 [DOI] [PubMed] [Google Scholar]
- 2.Bajpai P, Bajpai PK. Eicosapentaenoic acid (EPA) production from microorganisms: a review. J Biotechnol. 1993;30(2):161–183 [DOI] [PubMed] [Google Scholar]
- 3.Huynh MD, Kitts DD. Evaluating nutritional quality of Pacific fish species from fatty acid signatures. Food Chem. 2009;114(3):912–918 [Google Scholar]
- 4.Din JN, Harding SA, Valerio CJet al. Dietary intervention with oil rich fish reduces platelet-monocyte aggregation in man. Atherosclerosis. 2008;197(1):290–296 [DOI] [PubMed] [Google Scholar]
- 5.Thies F, Garry JM, Yaqoob Pet al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003;361(9356):477–485 [DOI] [PubMed] [Google Scholar]
- 6.Pan M, Cederbaum AI, Zhang YL, Ginsberg HN, Williams KJ, Fisher EA. Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production. J Clin Invest. 2004;113(9):1277–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang JX, Xiao YF, Leaf A. Free, long-chain, polyunsaturated fatty acids reduce membrane electrical excitability in neonatal rat cardiac myocytes. Proc Natl Acad Sci USA. 1995;92(9):3997–4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinclair HM. The diet of Canadian Indians and Eskimos. Proc Nutr Soc. 1953;12(1):69–82 [Google Scholar]
- 9.Bang HO, Dyerberg J, Nielson AB. Plasma lipid and lipoprotein pattern in Greenlandic west-coast Eskimos. Lancet. 1971;1(7710):1143–1145 [DOI] [PubMed] [Google Scholar]
- 10.He K, Song YQ, Daviglus MLet al. Accumulated evidence on fish consumption and coronary heart disease mortality—a meta-analysis of cohort studies. Circulation. 2004;109(22):2705–2711 [DOI] [PubMed] [Google Scholar]
- 11.Kromhout D, Bosschieter EB, de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med. 1985;312(19):1205–1209 [DOI] [PubMed] [Google Scholar]
- 12.Fraser GE, Sabate J, Beeson WL, Strahan TM. A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist Health Study. Arch Intern Med. 1992;152(7):1416–1424 [PubMed] [Google Scholar]
- 13.Ascherio A, Rimm EB, Stampfer MJ, Giovannucci EL, Willett WC. Dietary intake of marine n-3 fatty acids, fish intake, and the risk of coronary disease among men. N Engl J Med. 1995;332(15):977–982 [DOI] [PubMed] [Google Scholar]
- 14.Daviglus ML, Stamler J, Orencia AJet al. Fish consumption and the 30-year risk of fatal myocardial infarction. N Engl J Med. 1997;336(15):1046–1053 [DOI] [PubMed] [Google Scholar]
- 15.Mann JI, Appleby PN, Key TJ, Thorogood M. Dietary determinants of ischaemic heart disease in health conscious individuals. Heart. 1997;78(5):450–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albert CM, Hennekens CH, O’Donnell CJet al. Fish consumption and risk of sudden cardiac death. JAMA. 1998;279(1):23–28 [DOI] [PubMed] [Google Scholar]
- 17.Oomen CM, Feskens EJ, Rasanen Let al. Fish consumption and coronary heart disease mortality in Finland, Italy, and The Netherlands. Am J Epidemiol. 2000;151(10):999–1006 [DOI] [PubMed] [Google Scholar]
- 18.Yuan JM, Ross RK, Gao YT, Yu MC. Fish and shellfish consumption in relation to death from myocardial infarction among men in Shanghai, China. Am J Epidemiol. 2001;154(9):809–816 [DOI] [PubMed] [Google Scholar]
- 19.Hu FB, Bronner L, Willett WCet al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287(14):1815–1821 [DOI] [PubMed] [Google Scholar]
- 20.Mozaffarian D, Lemaitre RN, Kuller LHet al. Cardiac benefits of fish consumption may depend on the type of fish meal consumed: the cardiovascular health study. Circulation. 2003;107(10):1372–1377 [DOI] [PubMed] [Google Scholar]
- 21.Osler M, Andreasen AH, Hoidrup S. No inverse association between fish consumption and risk of death from all-causes, and incidence of coronary heart disease in middle-aged, Danish adults. J Clin Epidemiol. 2003;56(3):274–279 [DOI] [PubMed] [Google Scholar]
- 22.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296(15):1885–1899 [DOI] [PubMed] [Google Scholar]
- 23.Iso H, Rexrode KM, Stampfer MJet al. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA. 2001;285(3):304–312 [DOI] [PubMed] [Google Scholar]
- 24.Kris-Etherton PM, Harris WS, Appel LJ, American Heart Association Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106(21):2747–2757 [DOI] [PubMed] [Google Scholar]
- 25. US Department of Agriculture and US Department of Health and Human Services. Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2010. Available at: http://www.cnpp.usda.gov/DGAs2010-DGACReport.htm. Accessed November 2, 2010.
- 26.Yokoyama M, Origasa H, Matsuzaki Met al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090–1098 [DOI] [PubMed] [Google Scholar]
- 27.Saito Y, Yokoyama M, Origasa Het al. Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub-analysis of primary prevention cases from the Japan EPA lipid intervention study (JELIS). Atherosclerosis. 2008;200(1):135–140 [DOI] [PubMed] [Google Scholar]
- 28.Oikawa S, Yokoyama M, Origasa Het al. Suppressive effect of EPA on the incidence of coronary events in hypercholesterolemia with impaired glucose metabolism: sub-analysis of the Japan EPA lipid intervention study (JELIS). Atherosclerosis. 2009;206(2):535–539 [DOI] [PubMed] [Google Scholar]
- 29.The ACCORD Study Group Ginsberg HN, Elam MBet al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563–1574 Available at: http://content.nejm.org/cgi/content/abstract/362/17/1563. Accessed December 5, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ORIGIN Trial Investigators Bosch J, Gerstein HCet al. n–3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367(4):309–318 [DOI] [PubMed] [Google Scholar]
- 31.Burr ML, Fehily AM, Gilbert JFet al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet. 1989;2(8666):757–761 [DOI] [PubMed] [Google Scholar]
- 32.Singh RB, Niaz MA, Sharma JP, Kumar R, Rastogi V, Moshiri M. Randomized, double-blind, placebo-controlled trial of fish oil and mustard oil in patients with suspected acute myocardial infarction: the Indian experiment of infarct survival–4. Cardiovasc Drugs Ther. 1997;11(3):485–491 [DOI] [PubMed] [Google Scholar]
- 33. [No authors listed.] Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI–Prevenzione trial. Lancet. 1999;354(9177):447–455. [PubMed]
- 34.Nilsen DWT, Albrektsen G, Landmark K, Moen S, Aarsland T, Woie L. Effects of a high-dose concentrate of n-3 fatty acids or corn oil introduced early after an acute myocardial infarction on serum triacylglycerol and HDL cholesterol. Am J Clin Nutr. 2001;74(1):50–56 [DOI] [PubMed] [Google Scholar]
- 35.Burr ML, Ashfield-Watt PAL, Dunstan FDJet al. Lack of benefit of dietary advice to men with angina: results of a controlled trial. Eur J Clin Nutr. 2003;57(2):193–200 . [DOI] [PubMed] [Google Scholar]
- 36.GISSI-HF Investigators Tavazzi L, Maggioni APet al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9645):1223–1230 [DOI] [PubMed] [Google Scholar]
- 37.Senges J. Omega-3 fatty acids on top of modern therapy after acute Mmyocardial infarction (OMEGA). Paper presented at the American College of Cardiology meeting; March 28–31, 2009; Orlando, FL: Available at: http://www.cardiosource.org/news-media/meeting-coverage/acc/acc-2009.aspx. Accessed January 27, 2013 [Google Scholar]
- 38.Kromhout D, Giltay EJ, Geleijnse JM, Alpha Omega Trial Group n–3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;18;363(21):2015–2026 [DOI] [PubMed] [Google Scholar]
- 39.von Schacky C, Harris WS. Cardiovascular benefits of omega-3 fatty acids. Cardiovasc Res. 2007;73(2):310–315 [DOI] [PubMed] [Google Scholar]
- 40.Marchioli R, Barzi F, Bomba Eet al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio Della Sopravvivenza Nell’infarto Miocardico (GISSI)–Prevenzione. Circulation. 2002;105(16):1897–1903 [DOI] [PubMed] [Google Scholar]
- 41.Raitt MH, Connor WE, Morris Cet al. Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators—a randomized controlled trial. JAMA. 2005;293(23):2884–2891 [DOI] [PubMed] [Google Scholar]
- 42.Leaf A, Albert CM, Josephson Met al. Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake. Circulation. 2005;112(18):2762–2768 [DOI] [PubMed] [Google Scholar]
- 43.Brouwer IA, Zock PL, Camm AJet al. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators—the study on omega-3 fatty acids and ventricular arrhythmia (SOFA) randomized trial. JAMA. 2006;295(22):2613–2619 [DOI] [PubMed] [Google Scholar]
- 44.Brouwer IA, Raitt MH, Dullemeijer Cet al. Effect of fish oil on ventricular tachyarrhythmia in three studies in patients with implanA cardioverter defibrillators. Eur Heart J. 2009;30(7):820–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brouwer IA, Heeringa J, Geleijnse JM, Zock PL, Witteman JC. Intake of very long-chain n-3 fatty acids from fish and incidence of atrial fibrillation. The Rotterdam Study. Am Heart J. 2006;151(4):857–862 [DOI] [PubMed] [Google Scholar]
- 46.Mozaffarian D, Psaty BM, Rimm EBet al. Fish intake and risk of incident atrial fibrillation. Circulation. 2004;110(4):368–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calò L, Bianconi L, Colivicchi Fet al. N-3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial. J Am Coll Cardiol. 2005;45(10):1723–1728 [DOI] [PubMed] [Google Scholar]
- 48.Kowey PR, Reiffel JA, Ellenbogen KA, Naccarelli GV, Pratt CM. Efficacy and safety of prescription omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: a randomized controlled trial. JAMA. 2010;304(21):2363–2372 Available at: http://jama.jamanetwork.com/article.aspx?articleid=186998. Accessed November 14, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Macchia A, Levantesi G, Franzosi MGet al. Left ventricular systolic dysfunction, total mortality, and sudden death in patients with myocardial infarction treated with n-3 polyunsaturated fatty acids. Eur J Heart Fail. 2005;7(5):904–909 [DOI] [PubMed] [Google Scholar]
- 50.Duda MK, Tintinu A, O’Shea KM, Barrows B, Harris WS, Stanley W. Abstract 2371: Dietary supplementation with eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) prevents activation of inflammation and development of left ventricular dysfunction induced by chronic pressure overload. Circulation. 2008;118(18):S711 [Google Scholar]
- 51.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96(9):939–949 [DOI] [PubMed] [Google Scholar]
- 52.Nodari S. Effects of n-3 polyunsaturated fatty acids (PUFAs) on left ventricular function and functional capacity in patients with dilated cardiomyopathy. 2011;57(7):870–879 [DOI] [PubMed] [Google Scholar]
- 53.Rauch B, Schiele R, Schneider Set al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122(21):2152–2159 [DOI] [PubMed] [Google Scholar]
- 54.Rauch B, Schiele R, Schneider Set al. Highly purified omega-3 fatty acids for secondary prevention of sudden cardiac death after myocardial infarction—aims and methods of the OMEGA-study. Cardiovasc Drugs Ther. 2006;20(5):365–375 [DOI] [PubMed] [Google Scholar]
- 55.Vyas AK, Guo H, Moss AJet al. Reduction in ventricular tachyarrhythmias with statins in the multicenter automatic defibrillator implantation trial (MADIT)-II. J Am Coll Cardiol. 2006;47(4):769–773 [DOI] [PubMed] [Google Scholar]
- 56.Christensen V, Aiken KA, Villanueva MC. Threats to the ocean: on the role of ecosystem approaches to fisheries. Soc Sci Inf Sci Soc. 2007;46(1):67–86 [Google Scholar]
- 57.Watson R, Pauly D. Systematic distortions in world fisheries catch trends. Nature. 2001;414(6863):534–536 [DOI] [PubMed] [Google Scholar]
- 58.The State of World Fisheries and Aquaculture 2006. Rome, Italy: Fisheries and Aquaculture Department, Food and Agriculture Organization of the United Nations; 2007 [Google Scholar]
- 59.Naylor RL, Goldburg RJ, Primavera JHet al. Effect of aquaculture on world fish supplies. Nature. 2000;405(6790):1017–1024 [DOI] [PubMed] [Google Scholar]
- 60.Jenkins DJ, Sievenpiper JL, Pauly D, Sumaila UR, Kendall CW, Mowat FM. Are dietary recommendations for the use of fish oils sustainable? CMAJ. 2009;180(6):633–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pauly D, Alder J, Bennett E, Christensen V, Tyedmers P, Watson R. The future for fisheries. Science. 2003;302(5649):1359–1361 [DOI] [PubMed] [Google Scholar]
- 62.Costello C, Gaines SD, Lynham J. Can catch shares prevent fisheries collapse? Science. 2008;321(5896):1678–1681 [DOI] [PubMed] [Google Scholar]
- 63.Worm B, Barbier EB, Beaumont Net al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314(5800):787–790 [DOI] [PubMed] [Google Scholar]
- 64.US Department of Agriculture, Agricultural Research Service Nutrient data laboratory. Available at: http://www.ars.usda.gov/nutrientdata. Accessed November 11, 2010
- 65.Institute of Medicine Seafood Choices. Washington, DC: National Academies Press; 2006 [Google Scholar]
- 66.Ford R. Norwegian salmon and trout farming. Mar Fish Rev. 1984;46(3):44–47 [Google Scholar]
- 67. Omacor capsules new drug application 21-654. Center for Drug Evaluation and Research approval package for application 21-654. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/21-654_Omacor_Chemr.pdf. Accessed November 11, 2010.
- 68.Kumari P, Kumar M, Gupta V, Reddy CRK, Jha B. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010;120(3):749–757 [Google Scholar]