Summary
The spindle assembly checkpoint (SAC) is the major surveillance system that ensures sister chromatids do not separate until all chromosomes are correctly bi-oriented during mitosis. Components of the checkpoint include Mad1, Mad2, Mad3(BubR1), Bub3 and the kinases Bub1, Mph1(Mps1) and Aurora B [1]. Checkpoint proteins are recruited to kinetochores when individual kinetochores are not bound to spindle microtubules or not under tension [2-5]. Kinetochore association of Mad2 causes it to undergo a conformational change which promotes its association to Mad3 and Cdc20 to form the mitotic checkpoint complex (MCC). The MCC inhibits the anaphase-promoting complex/cyclosome (APC/C) until the checkpoint is satisfied. SAC silencing de-represses Cdc20-APC/C activity. This triggers the poly-ubiquitination of securin and cyclin which promotes the dissolution of sister chromatid cohesion and mitotic progression [6-8]. We, and others, recently showed that association of PP1 to the Spc7/Spc105/KNL1 family of kinetochore proteins is necessary to stabilize microtubule-kinetochore attachments and silence the SAC [9-12]. We now report that phosphorylation of the conserved MELT motifs in Spc7 by Mph1 (Mps1) recruits Bub1 and Bub3 to the kinetochore and that this is required to maintain the SAC signal.
Results
Bub1 and BubR1 checkpoint proteins are thought to bind kinetochores through interaction with the N-terminal region of KNL1 [13, 14]. Crystal structures of complexes between the TPR domains of Bub1 and BubR1 and related, but distinct, motifs in KNL1 (named KI1 and KI2, respectively) have been generated [15, 16]. However, mutations in the TPR domain of Bub1 that abrogate its association to the KI1 domain of KNL1 do not block its association with kinetochores [16]. For this reason the role of KNL1 in the kinetochore association of Bub1 and BubR1 has been brought into question. Mutation of a region of Bub1 (known as the GLEBS domain) that mediates its interaction with Bub3 prevents its association with kinetochores [16]. Whilst this agrees with a previous report, the binding site for Bub3 at kinetochores remains unknown [4]. In fission yeast the Bub1 and Bub3 checkpoint proteins form a complex that binds to kinetochores during prometaphase and metaphase [17, 18]. Kinetochore association of Bub1 and Bub3 is dependent on the presence of both proteins and on Mph1 (Mps1) kinase [18-21]. Indeed, ectopic targeting of Mph1 to the outer kinetochore causes Bub1 to bind kinetochores throughout the cell cycle in checkpoint deficient cells [22]. In this study we examine the mechanism by which Mph1 directs the association of Bub1 and Bub3 to kinetochores.
Mph1 kinase phosphorylates Spc7
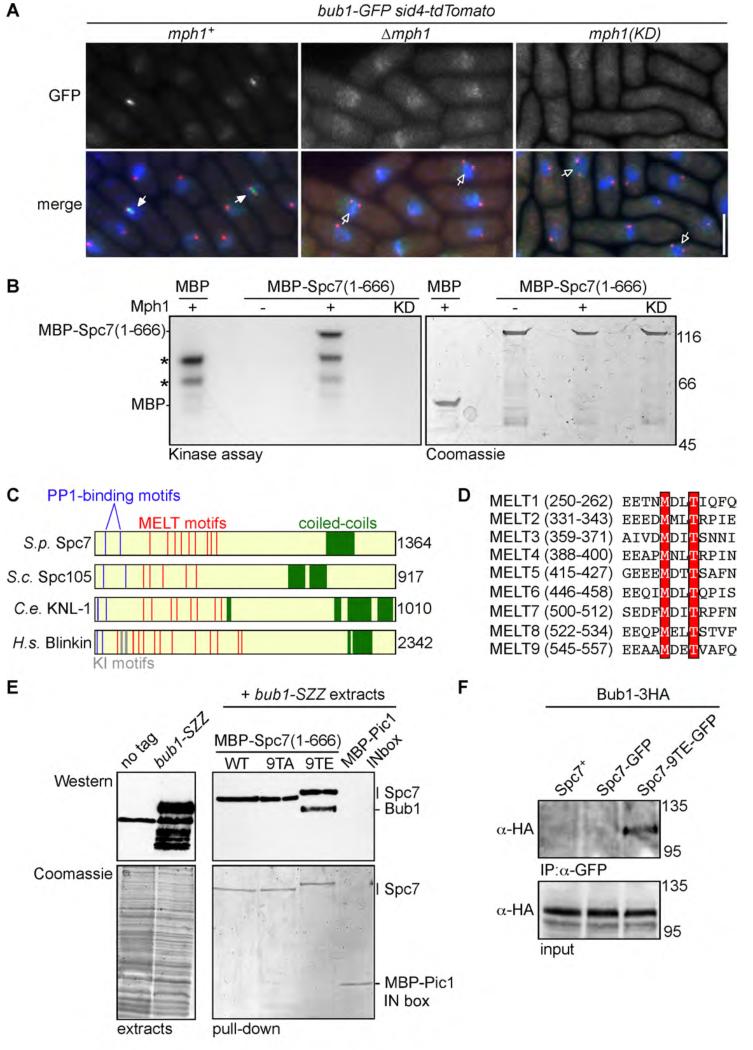
Bub1 fails to form discrete foci during mitosis in either Δmph1 or mph1(D459A) mutants (which are defective for kinase activity [23]), indicating that catalytic activity of Mph1 is required to promote kinetochore association of Bub1 (Figure 1A). To examine whether Mph1 phosphorylates Spc7 to promote kinetochore association of Bub1, we tested if Mph1 phosphorylates Spc7 in vitro. The N-terminal half of Spc7 (residues 1-666) was purified from bacteria and incubated in the presence of wild type or catalytically inactive Mph1. We find that Mph1 phosphorylates Spc7 in vitro on two threonine residues (T453 and T507) which are part of a repetitive motif of unknown function, termed the MELT motif, present in all members of the Spc7/KNL1 family [24] (Figures 1B, 1C, 1D and Supplemental Figure 1A). Phospho-proteomic analysis has revealed that the threonine residues of two MELT motifs in mammalian KNL1 are phosphorylated in vivo [25, 26]. Although mutation of Spc7-T453 or Spc7-T507 alone did not substantially reduce phosphorylation of Spc7 by Mph1 in vitro, mutation of all nine threonine residues of the MELT motifs (Spc7-9TA) reduced Mph1-dependent phosphorylation of Spc7 by 63.5% (Supplemental Figure 1B; data not shown). These results indicate that Mph1 phosphorylates one or more threonine residues in the MELT motifs of Spc7.
Figure 1. Mph1 kinase phosphorylates MELT motifs in Spc7 to promote association of Spc7 with Bub1.
(A) Log phase cultures of bub1-GFP sid4-tdTomato cells either wild type (mph1+), lacking Mph1 (Δmph1) or defective in Mph1 kinase activity (mph1(KD)) were fixed and imaged. Representative images are shown. Cells with mitotic spindles less than 2.5μm exhibiting localised Bub1-GFP (closed arrowheads) or lacking Bub1-GFP localisation (open arrowheads) are highlighted. Bar, 5μm. (B) Mph1 phosphorylates Spc7 in vitro. Mph1 kinase or catalytically inactive Mph1(KD) was incubated with MBP or MBP-Spc7(1-666) fusion protein. Kinase assay (left panel) and coomassie stained gel of input proteins (right panel) are shown. Asterisks indicate Mph1 autophosphorylation. (C) Domain architecture of fission yeast Spc7 and its homologues in budding yeast (S.c. Spc105), worm (C.e. KNL1), and human (H.s. Blinkin). PP1-binding sites (blue), KI motifs (grey), MELT motifs (red) and the coiled-coil kinetochore-binding domain (green) are shown. (D) Protein alignments of the nine MELT motifs in Spc7. Invariant methionine and threonine residues are highlighted in red. (E) Spc7-9TE interacts with Bub1 in vitro. MBP-Spc7 (WT), MBP-Spc7-9TA (9TA), MBP-Spc7-9TE (9TE) or MBP-Pic1-INbox fusion proteins were incubated in extracts of untagged wild type (no tag) or bub1-SZZ cells. Interacting proteins precipitated on amylose beads, separated by SDS-PAGE and subjected to western blot with anti-PAP antibody. Note that the anti-PAP antibody cross-reacts with the MBP-Spc7 proteins. (F) Spc7-9TE interacts with Bub1 in vivo. Extracts from log phase bub1-3HA spc7+, bub1-3HA spc7-GFP or bub1-3HA spc7-9TE-GFP cells were prepared. Proteins were immuno-precipitated with anti-GFP antibodies, separated by SDS-PAGE and subjected to western blot with anti-HA antibodies.
Phosphorylation of Spc7 promotes its association to Bub1
To examine whether phosphorylation of Spc7 MELT motifs by Mph1 influences its association to Bub1, we constructed spc7-9TA and spc7-9TE mutants, in which the threonine residues of all nine Spc7 MELT motifs were mutated to either alanine or glutamic acid. Bacterially expressed Spc7, Spc7-9TA and Spc7-9TE fusions proteins were incubated in extracts of bub1-SZZ cells. We find that Spc7-9TE, but neither wild type Spc7 nor Spc7-9TA, fusion proteins efficiently precipitate Bub1 from cell extracts (Figure 1E). Additionally, we constructed spc7-9MA and spc7-9MA,9TA mutants, in which the methionine residues of all nine MELT motifs were mutated to alanine. When expressed from the endogenous promoter, all these mutant Spc7 proteins localise to the kinetochore, are expressed at comparable levels to wild type cells and rescue viability in the absence of endogenous Spc7 (Supplemental Figures 1C & 1D). We also find that the Spc7-9TE-GFP, but not wild type Spc7-GFP, protein coimmunoprecipitates with Bub1 from extracts of log phase cells (Figure 1F). Together these data suggest Mph1 phosphorylates Spc7 on MELT motifs to stimulate its association with Bub1 both in vitro and in vivo.
Phosphorylation of Spc7 recruits the Bub1-Bub3 complex to kinetochores
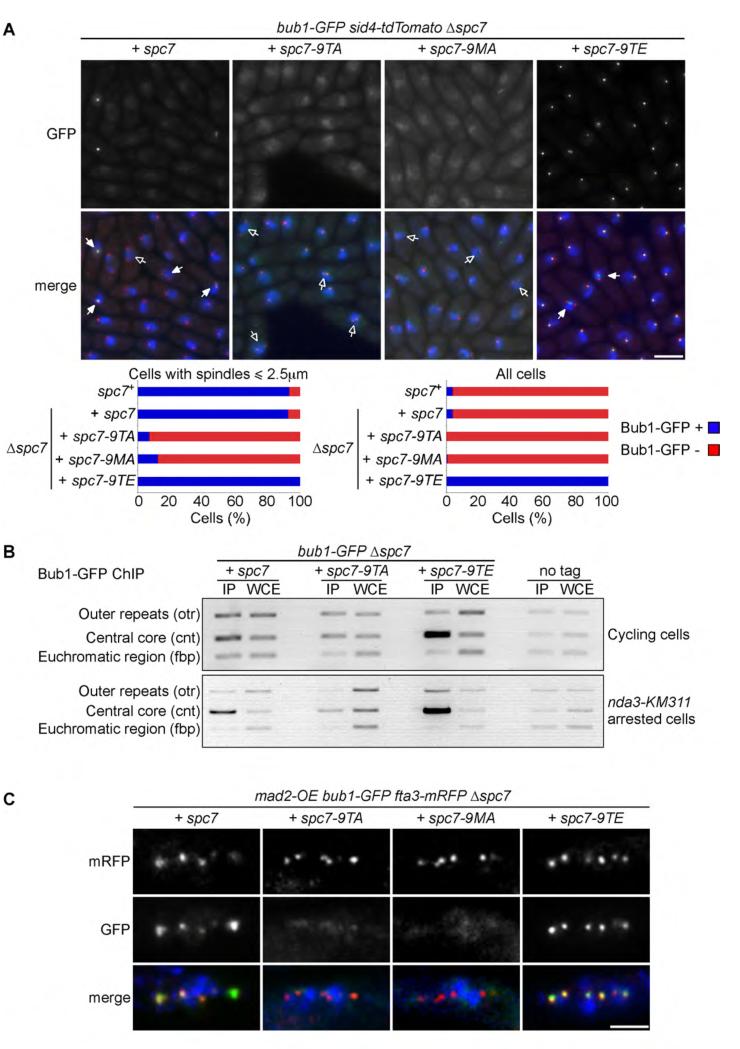
These results persuaded us to examine the influence of Spc7 phosphorylation on cell cycle-dependent localisation of Bub1. We find that Bub1 localises between separated spindle poles in control and spc7-9TE cells during early mitosis, but is undetectable in either spc7-9TA or spc7-9MA mutants during mitosis or at any other stage of the cell cycle (Figure 2A). Strikingly, Bub1 co-localises with spindle poles throughout the cell cycle in spc7-9TE, but not in wild type, cells (Figures 2A). To verify this we performed chromatin immunoprecipitation (ChIP) in cycling and metaphase arrested cultures. Whereas Bub1 binds to centromeric DNA only during mitosis in wild type cells, Bub1 binds strongly to centromeric DNA even in cycling spc7-9TE cells (Figure 2B). Notably, association of Bub1 to centromeric DNA is substantially reduced, but not completely abolished, in metaphase arrested spc7-9TA cells (Figure 2B). To confirm this localisation we monitored Bub1-GFP localisation in fta3-mRFP cells following over-expression of Mad2, which arrests cells in metaphase by hyperactivation of the SAC [27]. Fta3 is a component of the Mis6 complex that binds the kinetochore throughout the cell cycle [28]. Although Bub1 co-localises with kinetochores in wild type and spc7-9TE cells, we were unable to detect Bub1 at kinetochores in metaphase arrested spc7-9TA or spc7-9MA cells (Figure 2C). These data suggest that Mph1 phosphorylates the MELT motifs in Spc7 to recruit Bub1 to kinetochores during early mitosis.
Figure 2. Phosphorylation of the MELT motifs in Spc7 recruits Bub1 to kinetochores.
(A) Log phase cultures of Δspc7 spc7+, Δspc7 spc7-9TA, Δspc7 spc7-9MA and Δspc7 spc7-9TE expressing bub1-GFP sid4-tdTomato were fixed. The percentage of pre-anaphase mitotic cells (n=3; left panels) or all cells (n=3; left panels) with Bub1 foci (closed arrowheads, blue bars) or lacking foci (open arrowheads, red bars) was assessed. Bar, 5μm. (B) Log phase or metaphase arrested cultures of Δspc7 spc7+, Δspc7 spc7-9TA and Δspc7 spc7-9TE cells expressing nda3-KM311 bub1-GFP or nda3-KM311 cells (no tag) were fixed and subjected to ChIP analysis with anti-GFP antibodies. The immunoprecipitated DNA was amplified by PCR using primers specific to the central core (cnt), which is the site of kinetochore assembly, and control primers that amplify a noncentromeric, euchromatic negative control (fbp) or centromeric outer repeats (otr). Representative PCRs are shown for each tagged protein, as well as the untagged negative control. (C) Cultures of Δspc7 spc7+, Δspc7 spc7-9TA, Δspc7 spc7-9MA and Δspc7 spc7-9TE expressing bub1-GFP fta3-mRFP pREP3x-mad2 were incubated in medium lacking thiamine for 18 hours to induce Mad2 over-expression. Cells were fixed and imaged. Representative images are shown. Bar, 2μm.
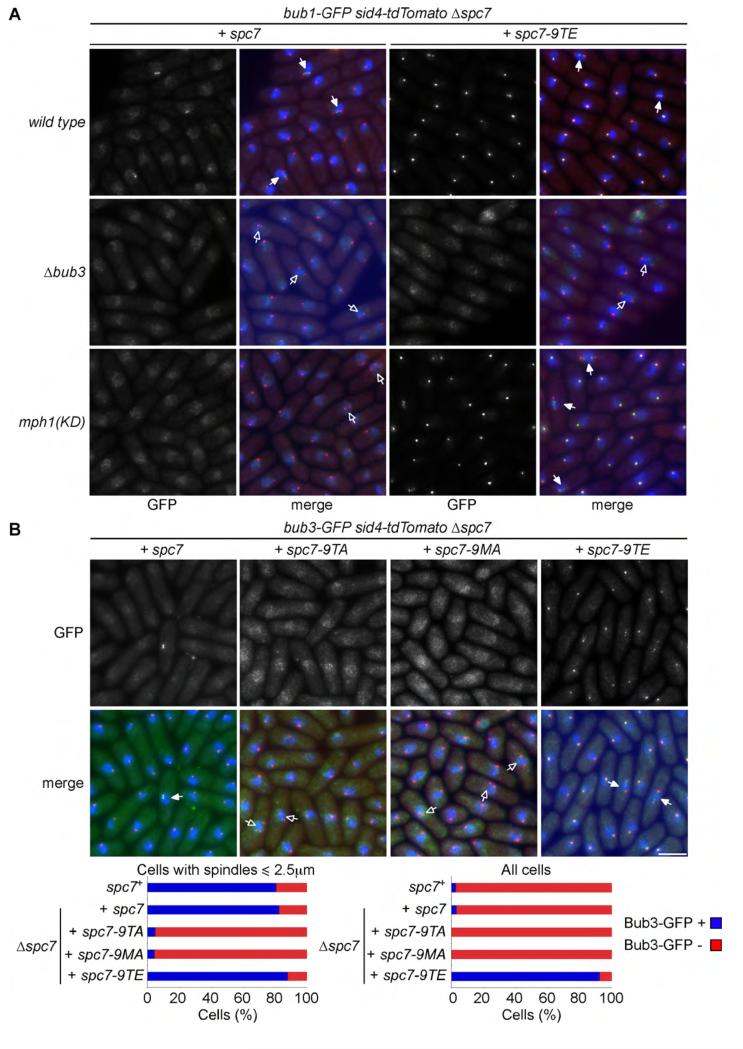
We next examined the dependency of Bub1 localisation on Mph1 and Bub3. Bub1 colocalises with kinetochores throughout the cell cycle in mph1-D459A spc7-9TE cells but not in Δbub3 spc7-9TE cells. The phospho-mimetic spc7-9TE mutant therefore bypasses the requirement for Mph1 kinase activity to load Bub1 to kinetochores, but Bub1 loading still requires the presence of Bub3 (Figure 3A). Consistent with this, interaction of bacterially expressed Spc7-9TE with Bub1 in cell extracts requires Bub3, but not Mad3 (Supplemental Figure 2A). Like Bub1, Bub3 localises to the kinetochore throughout the cell cycle in spc7-9TE cells, but fails to accumulate at kinetochores in mitotic spc7-9TA or spc7-9MA cells (Figure 3B). Moreover, association of Bub3 with kinetochores is dependent on Mph1 activity and ectopic association of Bub3 to kinetochores in spc7-9TE cells bypasses the requirement for Mph1 (Supplemental Figure 2B & 2C). However, Bub3 loading still requires Bub1 in these cells (Supplemental Figure 2C). These data suggest that Mph1 phosphorylation of the MELT motifs in Spc7 recruits the Bub1-Bub3 complex to kinetochores. Notably, ectopic association of Bub1 and Bub3 to kinetochores in spc7-9TE cells does not by itself cause cell cycle arrest. By contrast, when Mph1 is ectopically tethered to Ndc80, Mph1 promotes both association of Bub1 to kinetochores and SAC arrest [22]. One possibility is that, in this situation, Mph1 phosphorylates other components of the SAC, such as Mad1, Mad2 and Mad3, to directly activate the SAC, or destabilises microtubule-kinetochore interactions to indirectly activate the SAC [23, 29].
Figure 3. Mph1-dependent recruitment of Bub1 to kinetochores requires Bub3.
(A) Log phase cultures of bub1-GFP sid4-tdTomato Δspc7 spc7+ and bub1-GFP sid4-tdTomato Δspc7 spc7-9TE cells either wild type (wild type), lacking Bub3 (Δbub3) or defective in Mph1 kinase activity (mph1(KD)) were fixed and imaged. Cells with mitotic spindles less than 2.5μm exhibiting localised Bub1-GFP (closed arrowheads) or lacking Bub1-GFP localisation (open arrowheads) are highlighted. Bar, 5μm. (B) Log phase cultures of Δspc7 spc7+, Δspc7 spc7-9TA, Δspc7 spc7-9MA and Δspc7 spc7-9TE expressing bub3-GFP sid4-tdTomato were fixed. The percentage of pre-anaphase mitotic cells (n=3; left panels) or all cells (n=3; left panels) with Bub1 foci (closed arrowheads, blue bars) or lacking foci (open arrowheads, red bars) was assessed. Bar, 5μm.
Phosphorylation of Spc7 is required for normal recruitment of Mad1 and Mad2 to kinetochores
We previously found that association of Mad1 and Mad2 to kinetochores is substantially reduced in cells lacking Bub3 [30]. This persuaded us to examine whether phosphorylation of Spc7 MELT motifs influences the localisation of other SAC proteins. In wild type cells Mad1 and Mad2 form bright foci that co-localise with kinetochores in approximately 3% of cells. We find that localisation of Mad1 and Mad2 to kinetochores is substantially diminished, but not completely abolished, in mitotic spc7-9TA and spc7-9MA cells (Supplemental Figure 3A and 3B). Importantly, neither Mad1 nor Mad2 is ectopically recruited to kinetochores during interphase in spc7-9TE cells, indicating ectopic recruitment of Bub1 and Bub3 to kinetochores is not sufficient to recruit Mad1 and Mad2, consistent with previous observations [22] (Supplemental Figure 3A and 3B).
Spc7-MELT mutants have severe chromosome segregation defects
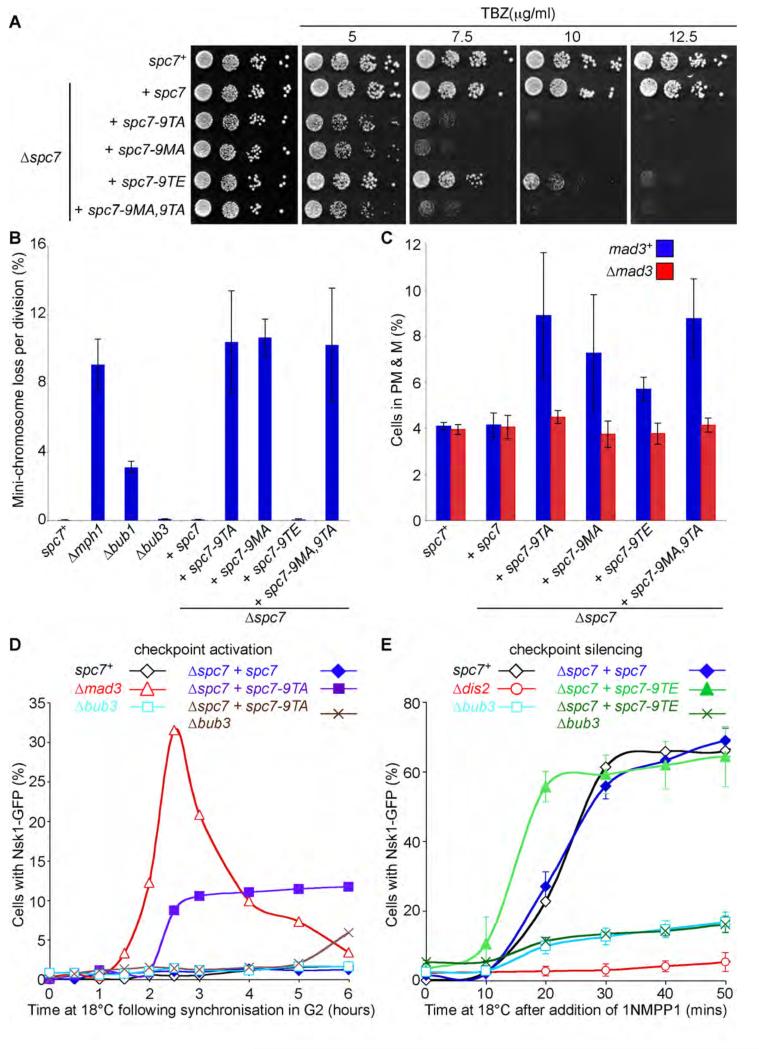
We next examined the effect of spc7-MELT mutants on chromosome segregation during mitosis. We find spc7-9TA, spc7-9MA and spc7-9MA,9TA mutants are acutely sensitive to thiabendazole (TBZ), a microtubule depolymerising agent, and profoundly defective in maintenance of an artificial mini-chromosome, similar to that observed in the absence of Mph1 [31] (Figures 4A & 4B). By comparison, spc7-9TE mutants are slightly sensitive to TBZ and have no appreciable defect in chromosome segregation compared to wild type cells (Figure 4A & 4B). Congruously, we find that spc7-9TA, spc7-9MA and spc7-9MA,9TA but not spc7-9TE, mutants are lethal in cells lacking Dis2 (Type 1 phosphatase), Klp5 (kinesin-8) or Dis1 (XMAP215), all of which are required for accurate chromosome segregation (Supplemental Table 1, [32-34]). Notably, both spc7-9TA and spc7-9TE mutants are synthetically lethal with Δdam1 mutants, indicating that the spc7-9TE mutant is not completely wild type (Supplemental Table 1). Cells lacking Dam1, Dis2, Klp5 or Dis1 exhibit delayed anaphase onset and numerous synthetic lethal interactions with components of the SAC [34-38]. By contrast, none of the spc7-MELT mutants showed any synthetic interactions with cells lacking Bub1, Bub3, Mph1, Mad2 or Mad3 (Supplemental Table 1). Moreover, the synthetic lethality between spc7-MELT mutants and cells lacking Dam1, Dis2, Klp5 or Dis1 is not reversed by simultaneously deleting Mad3, indicating that these synthetic lethalities are probably due to a defect in chromosome segregation rather than hyper-activation of the SAC (Supplemental Table 1).
Figure 4. Phosphorylation of Spc7 MELT motifs is required for accurate chromosome segregation and maintenance of the spindle checkpoint.
(A) spc7-MELT mutants are sensitive to thiabendazole (TBZ). Serial dilutions of wild type (spc7+), Δspc7 spc7, Δspc7 spc7-9TA, Δspc7 spc7-9MA, Δspc7 spc7-9TE, and Δspc7 spc7-9MA,9TA cells were plated onto YEA plates containing indicated TBZ concentrations and incubated for 3 days at 30°C. (B) Loss of the Ch16(ade6-M216) mini-chromosome was measured using a colony-sectoring assay. Error bars represent the standard deviation of three independent experiments. (C) Anaphase onset is profoundly delayed in spc7-9TA, 9MA and 9MA,9TA mutants but only slightly delayed in spc7-9TE, mutants. Log phase cultures of wild type (spc7+), Δspc7 spc7, Δspc7 spc7-9TA, Δspc7 spc7-9MA, Δspc7 spc7-9TE, and Δspc7 spc7-9MA,9TA cells, expressing cdc13-GFP in the presence (blue bars) or absence (red bars) of Mad3 were fixed and the percentage of cells with Cdc13 on spindles and separated spindle pole bodies was assessed. Error bars represent the standard deviation from three independent experiments. (D) Phosphorylation of Spc7 MELT motifs is required for maintenance of the spindle checkpoint. Log phase cultures of wild type (spc7+), Δmad3, Δbub3, Δspc7 spc7, Δspc7 spc7-9TA and Δspc7 spc7-9TA Δbub3 cells expressing nda3-KM311 ark1-as3 nsk1-GFP were synchronised in early G2 by lactose gradient centrifugation and incubated at 18°C for the times indicated. The cells were fixed and the percentage of cells with spindle pole associated Nsk1 was assessed. (E) Ectopic recruitment of Bub1 and Bub3 to Spc7 aids spindle checkpoint silencing. Log phase cultures of wild type (spc7+), Δdis2, Δbub3, Δspc7 spc7, Δspc7 spc7-9TE and Δspc7 spc7-9TE Δbub3 cells expressing nda3-KM311 ark1-as3 nsk1-GFP were synchronised in prometaphase by incubating at 18°C for 6 hours and 5μM 1NMPP1 was added. At the times indicated the cells were fixed and the percentage of cells with spindle pole associated Nsk1 was assessed. Error bars represent the standard deviation from three independent experiments.
Spc7-MELT mutants are defective in maintenance of the spindle checkpoint
To examine the effect of spc7-MELT mutants on the timing of anaphase onset we first measured the percentage of cells with spindle and spindle pole associated Cdc13 (Cyclin B) in log phase populations. Both spc7-9TA and spc7-9MA mutants spend longer in prometaphase and metaphase than spc7-9TE or control cells. This is likely to be due to activation of the SAC caused by defects in kinetochore-microtubule attachment. Consistent with this hypothesis, we found that the delay in anaphase onset observed in these mutants is dependent on Mad3 (Figure 4C). To examine the efficiency of SAC signalling in spc7-MELT mutants, we assayed their SAC arrest in cells that carry the cold-sensitive nda3-KM311 allele of the gene encoding β-tubulin [39]. Unfortunately we were unable to generate spc7-9TA nda3-KM311 cdc13-GFP strains, so instead we utilised Nsk1-GFP as an anaphase marker. Nsk1 is a recently described substrate of Cdk1 kinase that binds spindle poles only during anaphase B when Cyclin B is degraded and Nsk1 is dephosphorylated [35, 40]. Nsk1 localisation is unaffected in spc7-MELT mutants in log phase cultures (Supplemental Figure 4A). To quantify the efficiency of SAC signalling, cells were synchronised in early G2 using lactose gradients, then shifted to 18°C for 6 hours. Nsk1 localisation was analysed at 30minute intervals. The nda3-KM311 spc7+ cells maintain a SAC arrest for 6 hours: no cells are observed with Nsk1-GFP at spindle poles. Using Δmad3 as a checkpoint null mutant control we saw a wave of cells with Nsk1-GFP at spindle poles. These peaked at 2.5 hours (~30% of the culture) and then the numbers dropped as cells enter the next cell cycle and Nsk1-GFP is no longer at spindle poles. By analysing the area under this curve it can be seen that, during the 6 hour time course, almost all of the Δmad3 cells (>90%) have exited mitosis, as expected for a checkpoint mutant (Figure 4D). Nsk1-GFP does not appear at spindle poles at early time points in spc7-9TA mutants, indicating phosphorylation of the MELT motifs is not required for the initial SAC response when microtubules are completely absent (2 hour time point, Figure 4D). However, at later time points, Nsk1 appears at spindle poles in ~10% of spc7-9TA cells (3-6 hour time points, Figure 4D) indicating that phosphorylation of the MELT motifs is required to maintain the SAC signal. Counting the area under this curve shows that >60% of these cells have failed to maintain the SAC arrest through the 6 hours time course. SAC proficiency is similarly affected in spc7-9MA cells, and to a lesser extent in spc7-9TE cells (Supplemental Figure 4B). This checkpoint defect is stronger than that observed in Δbub3 fission yeast cells, where neither Bub1 nor Mad3 can be recruited to kinetochores yet the SAC remains robust (Figure 4D [30, 41]). This creates an apparent paradox: why should mutation of the kinetochore binding site for Bub1-Bub3 cause more of a SAC defect than completely abolishing Bub1 recruitment to kinetochores (in Δbub3)? Surprisingly, when spc7-9TA was combined with Δbub3 the SAC response was much improved, although this did not suppress the chromosome mis-segregation defects of spc7-9TA (Figure 4D; data not shown). This demonstrates that the SAC defect observed in spc7-9TA is not due to reduced Bub1-Bub3 recruitment at kinetochores, but suggests that when the Bub1-Bub3 complex is not bound to Spc7 it acts as a dominant negative factor that prevents maintenance of the SAC signal. The precise explanation of this effect requires further analysis.
Enhanced recruitment of Bub1-Bub3 to Spc7 aids spindle checkpoint silencing
We previously showed that Δbub3 mutants, where Bub1 and Mad3 are entirely absent from kinetochores, have SAC silencing defects [30], so we were keen to analyse SAC silencing in spc7-MELT mutants. Unfortunately, we were unable to construct the necessary spc7-9TA or spc7-9MA strains, but we have analysed the spc7-9TE silencing phenotype. To do this we monitored Nsk1 association to spindle poles following chemical inactivation of Ark1 (Aurora B) in mitotically arrested nda3-KM311 ark1-as3 nsk1-GFP cells. In this assay, the SAC is inactivated by addition of 1NMPP1, which selectively inhibits analogue-sensitive Ark1 [42] thus promoting silencing (or override) of the SAC signal. In this situation PP1Dis2 is essential for dissociation of Mad2 and Mad3 from the APC/C and for activation of the APC/C complex, which triggers Cyclin B destruction and appearance of Nsk1 on spindle poles (Figure 4E [35, 43]). We find that, upon addition of 1NMPP1, Nsk1 appears more rapidly on spindle poles in spc7-9TE cells than in wild type cells (Figure 4E). Likewise, we also observe more rapid destruction of Cdc13 (Cyclin B) in metaphase arrested nda3-KM311 ark1-as3 cdc13-GFP spc7-9TE cells upon addition of 1NMPP1 than in control cells (Supplemental Figure 4C). Importantly, the rapid appearance of Nsk1 on spindle poles in spc7-9TE cells is completely abolished in the absence of Bub3 (Figure 4E). These results suggest that enhanced recruitment of Bub1 and Bub3 to the kinetochore in spc7-9TE cells promotes silencing of the SAC to such an extent that it becomes more efficient than in wild type cells.
Discussion
At present it is unclear whether Bub1 or Bub3 interact directly with phosphorylated MELT motifs in Spc7/KNL1. Bub3 contains seven WD40 repeats that are arranged in a radial pattern to form a β-propeller, a three-dimensional structure that mediates protein–protein interactions [44]. Some proteins containing WD40 domains, such as Cdc4, only interact with phosphorylated target proteins. Indeed Sic1, an inhibitor of Cyclin B/Cdk1 in budding yeast, is only recognized and ubiquitinylated by SCFCdc4 when phosphorylated on multiple sites by G1 and S phase Cyclin/Cdk1 complexes [45]. In the same manner Bub3 may only interact with Spc7/KNL1 that has been multiply phosphorylated to recruit Bub1. If this is the case we predict that the methionine of the MELT motif is also essential for Bub3 binding as spc7-9TA, spc7-9MA and spc7-9TA,9MA mutants have indistinguishable phenotypes. However, we observe weak binding of Bub1 to centromeric DNA in metaphase arrested spc7-9TA cells, indicating that Bub1 may form additional contacts with Spc7 that contribute to the overall stability of the Spc7-Bub1-Bub3 complex. This may be analogous to the interaction observed between the TPR domains of Bub1 and the KI1 domain of KNL1, although the KI motifs do not appear to be conserved in fission yeast Spc7 [13, 14, 16]. Alternatively, as Mph1 can phosphorylate other sites in Spc7-9TA in vitro, Mph1 may promote Bub1 binding through a mechanism that does not involve phosphorylation of the MELT motifs.
It is important to note that spc7-9TA, spc7-9MA and spc7-9TA,9MA mutants mis-segregate chromosomes at high frequency, whereas cells lacking Bub3 are only marginally more defective than wild type [41] (Figure 4B). Thus, phosphorylation of the MELT motifs is necessary for accurate chromosome segregation independently of, or in addition to, recruitment of Bub1 and Bub3. Potentially, MELT motifs could bind an unidentified factor required for chromosome segregation. Alternatively, phosphorylation of the MELT motifs may influence the dynamic architecture of the outer kinetochore KMN complex during mitosis, and thus reduce the ability of kinetochores to interact with microtubules. For example MELT phosphorylation may be required to position PP1Dis2 bound to the N-terminus of Spc7 near its substrates so that it can stabilize microtubule-kinetochore interactions. Two studies have recently demonstrated that intra-kinetochore stretch is necessary and sufficient to satisfy the SAC, although it is unknown how this stretching is sensed [46, 47]. Since spc7-9TA and spc7-9MA mutants are profoundly defective in chromosome segregation and fail to accumulate SAC components at kinetochores, it is tempting to speculate that the repetitive MELT motifs in Spc7/KNL1 may act as a quantitative sensor of intra-kinetochore stretch. Further experiments are needed to address this and other possibilities.
Although Bub3 is not critical for SAC arrest in S.pombe [30, 41, 48], disruption of the binding sites for the Bub1-Bub3 complex on Spc7 causes a defect in maintenance of the SAC signal. Conversely, ectopic recruitment of the Bub1-Bub3 complex to Spc7 in spc7-9TE cells enhances SAC silencing, providing strong confirmation of our previous observations [30]. One possibility is that association of the Bub1-Bub3 complex to the MELT motifs in Spc7/KNL1 provides a docking site for the MCC to be dephosphorylated and inactivated by PP1, which binds the N-terminus of Spc7/KNL1 [11, 12]. Although this model is attractive, it is probably too simplistic as at least one other pool of PP1, bound to Klp5/Klp6 complex (kinesin-8), is also required for efficient SAC silencing in fission yeast [11]. Clearly, identifying the PP1 targets required for SAC silencing and analysing the function of the conserved MELT motifs in KNL1 for SAC signalling in vertebrate cells are important goals for the future.
Supplementary Material
Supplemental Figure 1 (relating to Figure 1). Phosphorylation of Spc7 by Mph1 does not influence Spc7 localisation or expression
Supplemental Figure 2 (relating to Figure 3). Phosphorylation of Spc7 stimulates the binding and recruitment of the Bub1-Bub3 complex to kinetochores
Supplemental Figure 3 (relating to Figure 3). Phosphorylation of the Spc7 MELT motifs is required for recruitment of Mad1 and Mad2 during mitosis
Supplemental Figure 4 (relating to Figure 4). Ectopic recruitment of Bub1 and Bub3 to Spc7 aids spindle checkpoint silencing.
Supplemental Table 1. Genetic interactions of spc7-MELT mutants
Supplemental Table 2. Strains used in this study
Supplemental Table 3. Oligonucleotides used in this study
Highlights.
* Mph1 kinase phosphorylates Spc7
* Phosphorylation of the MELT motifs in Spc7 recruits Bub1 and Bub3 to kinetochores
* Recruitment of Bub1 and Bub3 to Spc7 maintains the spindle checkpoint signal
Acknowledgements
We thank Iain Hagan, Silke Hauf, Xiang-wei He, Jean-Paul Javerzat, Tomohiro Matsumoto, Paul Russell, Takashi Toda and Mitsuhiro Yanagida for strains. We thank Andrew McAinsh and Prakash Arumugam for critical reading of the manuscript, and Jimi-Carlo Bukowski-Wills for help with analysing and presenting the MS/MS data. This work was supported by a programme grant from the Medical Research Council to JBAM [G0601118], a Wellcome Trust programme grant to KGH [083610] and the Wellcome Trust Centre for Cell Biology core grant [077707]. AMS is supported by a SULSA-funded PhD studentship, LAS is funded by a MRC doctoral studentship.
References
- 1.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 2.Chen RH, Shevchenko A, Mann M, Murray AW. Spindle checkpoint protein Xmad1 recruits Xmad2 to unattached kinetochores. J Cell Biol. 1998;143:283–295. doi: 10.1083/jcb.143.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen RH, Waters JC, Salmon ED, Murray AW. Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science. 1996;274:242–246. doi: 10.1126/science.274.5285.242. [DOI] [PubMed] [Google Scholar]
- 4.Taylor SS, Ha E, McKeon F. The human homologue of Bub3 is required for kinetochore localization of Bub1 and a Mad3/Bub1-related protein kinase. J Cell Biol. 1998;142:1–11. doi: 10.1083/jcb.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor SS, McKeon F. Kinetochore localization of murine Bub1 is required for normal mitotic timing and checkpoint response to spindle damage. Cell. 1997;89:727–735. doi: 10.1016/s0092-8674(00)80255-x. [DOI] [PubMed] [Google Scholar]
- 6.Hwang LH, Lau LF, Smith DL, Mistrot CA, Hardwick KG, Hwang ES, Amon A, Murray AW. Budding yeast Cdc20: a target of the spindle checkpoint. Science. 1998;279:1041–1044. doi: 10.1126/science.279.5353.1041. [DOI] [PubMed] [Google Scholar]
- 7.Funabiki H, Yamano H, Kumada K, Nagao K, Hunt T, Yanagida M. Cut2 proteolysis required for sister-chromatid seperation in fission yeast. Nature. 1996;381:438–441. doi: 10.1038/381438a0. [DOI] [PubMed] [Google Scholar]
- 8.King RW, Peters JM, Tugendreich S, Rolfe M, Hieter P, Kirschner MW. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell. 1995;81:279–288. doi: 10.1016/0092-8674(95)90338-0. [DOI] [PubMed] [Google Scholar]
- 9.Espeut J, Cheerambathur DK, Krenning L, Oegema K, Desai A. Microtubule binding by KNL-1 contributes to spindle checkpoint silencing at the kinetochore. J Cell Biol. 2012;196:469–482. doi: 10.1083/jcb.201111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu D, Vleugel M, Backer CB, Hori T, Fukagawa T, Cheeseman IM, Lampson MA. Regulated targeting of protein phosphatase 1 to the outer kinetochore by KNL1 opposes Aurora B kinase. J Cell Biol. 2010;188:809–820. doi: 10.1083/jcb.201001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meadows JC, Shepperd LA, Vanoosthuyse V, Lancaster TC, Sochaj AM, Buttrick GJ, Hardwick KG, Millar JB. Spindle checkpoint silencing requires association of PP1 to both Spc7 and kinesin-8 motors. Dev Cell. 2011;20:739–750. doi: 10.1016/j.devcel.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg JS, Cross FR, Funabiki H. KNL1/Spc105 recruits PP1 to silence the spindle assembly checkpoint. Curr Biol. 2011;21:942–947. doi: 10.1016/j.cub.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiyomitsu T, Murakami H, Yanagida M. Protein interaction domain mapping of human kinetochore protein Blinkin reveals a consensus motif for binding of spindle assembly checkpoint proteins Bub1 and BubR1. Mol Cell Biol. 2011 doi: 10.1128/MCB.00815-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiyomitsu T, Obuse C, Yanagida M. Human Blinkin/AF15q14 is required for chromosome alignment and the mitotic checkpoint through direct interaction with Bub1 and BubR1. Dev Cell. 2007;13:663–676. doi: 10.1016/j.devcel.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Bolanos-Garcia VM, Lischetti T, Matak-Vinkovic D, Cota E, Simpson PJ, Chirgadze DY, Spring DR, Robinson CV, Nilsson J, Blundell TL. Structure of a Blinkin-BUBR1 complex reveals an interaction crucial for kinetochore mitotic checkpoint regulation via an unanticipated binding Site. Structure. 2011;19:1691–1700. doi: 10.1016/j.str.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krenn V, Wehenkel A, Li X, Santaguida S, Musacchio A. Structural analysis reveals features of the spindle checkpoint kinase Bub1-kinetochore subunit Knl1 interaction. J Cell Biol. 2012;196:451–467. doi: 10.1083/jcb.201110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernard P, Hardwick K, Javerzat JP. Fission yeast bub1 is a mitotic centromere protein essential for the spindle checkpoint and the preservation of correct ploidy through mitosis. J Cell Biol. 1998;143:1775–1787. doi: 10.1083/jcb.143.7.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanoosthuyse V, Valsdottir R, Javerzat JP, Hardwick KG. Kinetochore targeting of fission yeast Mad and Bub proteins is essential for spindle checkpoint function but not for all chromosome segregation roles of Bub1p. Mol Cell Biol. 2004;24:9786–9801. doi: 10.1128/MCB.24.22.9786-9801.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maciejowski J, George KA, Terret ME, Zhang C, Shokat KM, Jallepalli PV. Mps1 directs the assembly of Cdc20 inhibitory complexes during interphase and mitosis to control M phase timing and spindle checkpoint signaling. J Cell Biol. 2010;190:89–100. doi: 10.1083/jcb.201001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin-Lluesma S, Stucke VM, Nigg EA. Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science. 2002;297:2267–2270. doi: 10.1126/science.1075596. [DOI] [PubMed] [Google Scholar]
- 21.Vigneron S, Prieto S, Bernis C, Labbe JC, Castro A, Lorca T. Kinetochore localization of spindle checkpoint proteins: who controls whom? Mol Biol Cell. 2004;15:4584–4596. doi: 10.1091/mbc.E04-01-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito D, Saito Y, Matsumoto T. Centromere-tethered Mps1 pombe homolog (Mph1) kinase is a sufficient marker for recruitment of the spindle checkpoint protein Bub1, but not Mad1. Proc Natl Acad Sci U S A. 2011;109:209–214. doi: 10.1073/pnas.1114647109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zich J, Sochaj AM, Syred HM, Milne L, Cook AG, Ohkura H, Rappsilber J, Hardwick KG. Kinase activity of fission yeast Mph1 is required for Mad2 and Mad3 to stably bind the anaphase promoting complex. Curr Biol. 2012;22:296–301. doi: 10.1016/j.cub.2011.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheeseman IM, Niessen S, Anderson S, Hyndman F, Yates JR, 3rd, Oegema K, Desai A. A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension. Genes Dev. 2004;18:2255–2268. doi: 10.1101/gad.1234104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dephoure N, Zhou C, Villen J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci U S A. 2008;105:10762–10767. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hegemann B, Hutchins JR, Hudecz O, Novatchkova M, Rameseder J, Sykora MM, Liu S, Mazanek M, Lenart P, Heriche JK, et al. Systematic phosphorylation analysis of human mitotic protein complexes. Sci Signal. 2011;4:rs12. doi: 10.1126/scisignal.2001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He X, Patterson TE, Sazer S. The Schizosaccharomyces pombe spindle checkpoint protein mad2p blocks anaphase and genetically interacts with the anaphase-promoting complex. Proc Natl Acad Sci U S A. 1997;94:7965–7970. doi: 10.1073/pnas.94.15.7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, McLeod I, Anderson S, Yates JR, 3rd, He X. Molecular analysis of kinetochore architecture in fission yeast. EMBO J. 2005;24:2919–2930. doi: 10.1038/sj.emboj.7600762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardwick KG, Weiss E, Luca FC, Winey M, Murray AW. Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption. Science. 1996;273:953–956. doi: 10.1126/science.273.5277.953. [DOI] [PubMed] [Google Scholar]
- 30.Vanoosthuyse V, Meadows JC, van der Sar SJ, Millar JB, Hardwick KG. Bub3p facilitates spindle checkpoint silencing in fission yeast. Mol Biol Cell. 2009;20:5096–5105. doi: 10.1091/mbc.E09-09-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He X, Jones MH, Winey M, Sazer S. Mph1, a member of the Mps1-like family of dual specificity protein kinases, is required for the spindle checkpoint in S. pombe. J Cell Sci. 1998;111(Pt 12):1635–1647. doi: 10.1242/jcs.111.12.1635. [DOI] [PubMed] [Google Scholar]
- 32.Nabeshima K, Kurooka H, Takeuchi M, Kinoshita K, Nakaseko Y, Yanagida M. p93dis1, which is required for sister chromatid separation, is a novel microtubule and spindle pole body-associating protein phosphorylated at the Cdc2 target sites. Genes Dev. 1995;9:1572–1585. doi: 10.1101/gad.9.13.1572. [DOI] [PubMed] [Google Scholar]
- 33.Ohkura H, Kinoshita N, Miyatani S, Toda T, Yanagida M. The fission yeast dis2+ gene required for chromosome disjoining encodes one of two putative type 1 protein phosphatases. Cell. 1989;57:997–1007. doi: 10.1016/0092-8674(89)90338-3. [DOI] [PubMed] [Google Scholar]
- 34.West RR, Malmstrom T, McIntosh JR. Kinesins klp5(+) and klp6(+) are required for normal chromosome movement in mitosis. J Cell Sci. 2002;115:931–940. doi: 10.1242/jcs.115.5.931. [DOI] [PubMed] [Google Scholar]
- 35.Buttrick GJ, Meadows JC, Lancaster TC, Vanoosthuyse V, Shepperd LA, Hoe KL, Kim DU, Park HO, Hardwick KG, Millar JB. Nsk1 ensures accurate chromosome segregation by promoting association of kinetochores to spindle poles during anaphase B. Mol Biol Cell. 2011;22:4486–4502. doi: 10.1091/mbc.E11-07-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia MA, Koonrugsa N, Toda T. Spindle-kinetochore attachment requires the combined action of Kin I-like Klp5/6 and Alp14/Dis1-MAPs in fission yeast. EMBO J. 2002;21:6015–6024. doi: 10.1093/emboj/cdf611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi Y, Saitoh S, Ogiyama Y, Soejima S, Takahashi K. The fission yeast DASH complex is essential for satisfying the spindle assembly checkpoint induced by defects in the inner-kinetochore proteins. Genes Cells. 2007;12:311–328. doi: 10.1111/j.1365-2443.2007.01053.x. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-Perez I, Renwick SJ, Crawley K, Karig I, Buck V, Meadows JC, Franco-Sanchez A, Fleig U, Toda T, Millar JB. The DASH complex and Klp5/Klp6 kinesin coordinate bipolar chromosome attachment in fission yeast. EMBO J. 2005;24:2931–2943. doi: 10.1038/sj.emboj.7600761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiraoka Y, Toda T, Yanagida M. The NDA3 gene of fission yeast encodes beta-tubulin: a cold-sensitive nda3 mutation reversibly blocks spindle formation and chromosome movement in mitosis. Cell. 1984;39:349–358. doi: 10.1016/0092-8674(84)90013-8. [DOI] [PubMed] [Google Scholar]
- 40.Chen JS, Lu LX, Ohi MD, Creamer KM, English C, Partridge JF, Ohi R, Gould KL. Cdk1 phosphorylation of the kinetochore protein Nsk1 prevents error-prone chromosome segregation. J Cell Biol. 2011;195:583–593. doi: 10.1083/jcb.201105074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tange Y, Niwa O. Schizosaccharomyces pombe Bub3 is dispensable for mitotic arrest following perturbed spindle formation. Genetics. 2008;179:785–792. doi: 10.1534/genetics.107.081695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hauf S, Biswas A, Langegger M, Kawashima SA, Tsukahara T, Watanabe Y. Aurora controls sister kinetochore mono-orientation and homolog bi-orientation in meiosis-I. EMBO J. 2007;26:4475–4486. doi: 10.1038/sj.emboj.7601880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanoosthuyse V, Hardwick KG. A novel protein phosphatase 1-dependent spindle checkpoint silencing mechanism. Curr Biol. 2009;19:1176–1181. doi: 10.1016/j.cub.2009.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larsen NA, Harrison SC. Crystal structure of the spindle assembly checkpoint protein Bub3. J Mol Biol. 2004;344:885–892. doi: 10.1016/j.jmb.2004.09.094. [DOI] [PubMed] [Google Scholar]
- 45.Orlicky S, Tang X, Willems A, Tyers M, Sicheri F. Structural basis for phosphodependent substrate selection and orientation by the SCFCdc4 ubiquitin ligase. Cell. 2003;112:243–256. doi: 10.1016/s0092-8674(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 46.Maresca TJ, Salmon ED. Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity. J Cell Biol. 2009;184:373–381. doi: 10.1083/jcb.200808130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uchida KS, Takagaki K, Kumada K, Hirayama Y, Noda T, Hirota T. Kinetochore stretching inactivates the spindle assembly checkpoint. J Cell Biol. 2009;184:383–390. doi: 10.1083/jcb.200811028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Windecker H, Langegger M, Heinrich S, Hauf S. Bub1 and Bub3 promote the conversion from monopolar to bipolar chromosome attachment independently of shugoshin. EMBO Rep. 2009;10:1022–1028. doi: 10.1038/embor.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 (relating to Figure 1). Phosphorylation of Spc7 by Mph1 does not influence Spc7 localisation or expression
Supplemental Figure 2 (relating to Figure 3). Phosphorylation of Spc7 stimulates the binding and recruitment of the Bub1-Bub3 complex to kinetochores
Supplemental Figure 3 (relating to Figure 3). Phosphorylation of the Spc7 MELT motifs is required for recruitment of Mad1 and Mad2 during mitosis
Supplemental Figure 4 (relating to Figure 4). Ectopic recruitment of Bub1 and Bub3 to Spc7 aids spindle checkpoint silencing.
Supplemental Table 1. Genetic interactions of spc7-MELT mutants
Supplemental Table 2. Strains used in this study
Supplemental Table 3. Oligonucleotides used in this study