Abstract
Heparin is a critically important anticoagulant drug that is prepared from pig intestine. In 2007-2008, there was a crisis in the heparin market when the raw material was adulterated with the toxic polysaccharide, oversulfated chondroitin sulfate, which was associated with 100 deaths in the U.S. alone. As the result of this crisis, our laboratory and others have been actively pursuing alternative sources for this critical drug, including synthetic heparins and bioengineered heparin. In assessing the bioengineering processing costs it has become clear that the use of both enzyme-catalyzed cofactor recycling and enzyme immobilization will be needed for commercialization. In the current study, we examine the use of immobilization of C5-epimerase and 2-O-sulfotransferase involved in the first enzymatic step in the bioengineered heparin process, as well as arylsulfotransferase-IV involved in cofactor recycling in all three enzymatic steps. We report the successful immobilization of all three enzymes and their use in converting N-sulfo, N-acetyl heparosan into N-sulfo, N-acetyl 2-O-sulfo heparin.
Keywords: immobilized enyzmes, 2-O-sulfotransferase, C5-Epimerase, Aryl sulfotransferase IV, heparosan, bioengineered heparin
1. Introduction
Heparin (Hp) is a polysaccharide-based anticoagulant drug that is widely used in extracorporeal therapy (i.e., heart-lung oxygenation, kidney dialysis) and to prevent blood clots during surgical procedures (Linhardt 2003). Hp is prepared in metric ton quantities from animal tissues, such as pig intestines and cow lung, to fill the multi-billion dollar annual demand for this lifesaving drug (Liu H et al., 2009). In 2007-2008, there was a crisis in the heparin market when it was adulterated with a less expensive, structurally similar, but toxic polysaccharide called oversulfated chondroitin sulfate (Guerrini et al., 2008, Kishimoto et al., 2008). This contamination crisis was associated with the death of an estimated hundred Americans and has resulted in stricter regulations and improved methods for the analysis of this complex polysaccharide-based drug (Liu H et al., 2009). Nevertheless, a remaining concern is that the level of regulation of slaughterhouses and meat processing facilities is considerably different than for typical current good manufacturing practices (cGMP) governing pharmaceutical manufacturing facilities (Liu H et al., 2009). As the result of this crisis, our laboratory and others have been actively pursuing alternative sources for this critical drug, including synthetic heparins (Xu et al., 2011; Sinay et al., 1984; Peterson et al., 2009) and bioengineered Hp (Zhang et al., 2008; Kuberan et al., 2003; Lindahl et al., 2005).
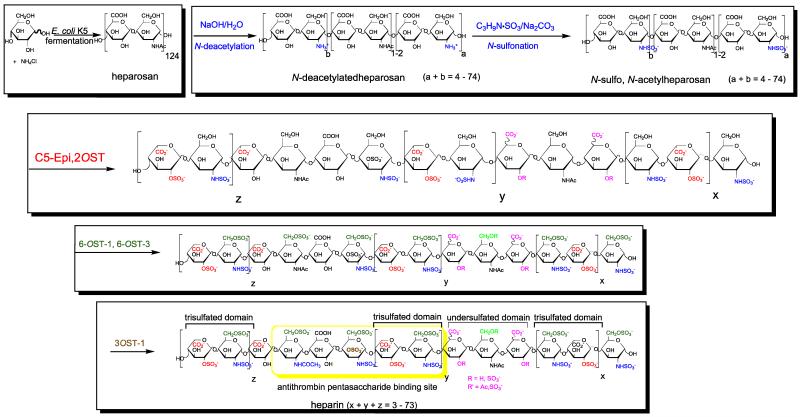
Bioengineered Hp is intended to serve as a generic version of pharmaceutical Hp. In a process being developed in our laboratory, the capsular polysaccharide heparosan is first produced through the fermentation of E. coli K5 strain (Wang et al., 2010). The recovered heparosan is then treated with sodium hydroxide and chemically N-sulfonated to afford either N-sulfo heparosan (NSH) or N-sulfo, N-acetyl heparosan (NSNAH), having an average molecular weight of 8000, for subsequent enzymatic modification using C5-epimerase and O-sulfotransferases (Wang et al., 2011). An initial version of this enzymatic process, relying on free enzymes and cofactor recycling, afforded milligram quantities of a bioengineered Hp (Zhang et al., 2008).
The enzymatic process can be divided into three steps: (1) the conversion of NSNAH into N-sulfo, N-acetyl, 2-O-sulfoheparin (NSNA2SHp) using C5-epimerase (C5-epi) and 2-O-sulfotransferase (2OST) with arylsulfotransferase IV (AST-IV)-catalyzed cofactor recycling; (2) the conversion of NSNA2SHp into non-anticoagulant heparin (NACHp) using 6-O-sulfotransferases-1 and -3 with AST-IV catalyzed cofactor recycling; and the conversion of NACHp into the anticoagulant heparin (Hp) using 3-O-sulfotransferase-1, again with AST-IV catalyzed cofactor recycling. The first step of the enzymatic process is particularly complex since it involves the critical coupling of three enzymatic transformations shown in Scheme 1. In this process step, C5-epi converts glucuronic acid residues in NSNAH into iduronic acid residues in a reversible reaction (Zhang et al., 2008; Chen et al., 2005) that are then locked in place as 2-O-sulfonation of the iduronic acid residues occurs through the action of 2OST with the formation of 3′-phosphoadenosine-5′-phosphate (PAP) from 3′-phosphoadenosine-5′-phosphosulfate (PAPS). AST-IV is then required to regenerate PAPS and prevent the buildup of PAP, which inhibits the action of 2OST (Moon et al., 2012).
Scheme 1.
In assessing the bioengineered process costs it has become clear that the use of both catalyzed cofactor recycling and immobilized enzymes will be necessary for commercialization (Linhardt and Liu, 2012). In this study, we examine the immobilization of C5-epi and 2OST involved in the first enzymatic step, as well as AST-IV involved in cofactor recycling in all three enzymatic steps. We report the successful immobilization of all three of these enzymes and their use in converting NSNAH into NSNA2SHp.
2. Materials and Methods
Materials
PAPS, NSNAH, and N-sulfoheparosan were prepared in our lab as previously described (Zhou et al., 2011; Zhang et al., 2008). Agarose aldehyde resin was purchased from Agarose Bead Technologies. Acrylic amino resin and acrylic epoxy resin were purchased from Polysciences Inc. Potassium 4-nitrophenylsulfate (PNPS), 2-(N-morpholino)ethanesulfonic acid (MES) deuterium oxide (99.996 atom %) and sodium cyanoborohydride were purchased from Sigma Chemicals. Other commercially available chemicals and solvents were of analytical grade. Disposable polypropylene columns and 20 ml scintillation vials were obtained from (VWR, USA). Syringe filters (0.22 μm) and 3.5 kDa MWCO centrifugal filters were purchased from EMD Millipore, USA. NMR microtubes (outside diameter, 5 mm) were from Norell, Landisville, NJ. Heparin lyases were expressed in our laboratory using E. coli strains, provided by Professor Jian Liu, University of North Carolina, College of Pharmacy, Chapel Hill, NC, USA.
Expression, purification, and assay of 2OST, C5-epi and AST-IV
Expression of C5-Epimerase, 2-OST and AST IV was carried out in E. coli as described previously (Chen et al., 2005; Burkart et al., 2000; Sheng et al., 2012). Briefly, recombinant E. coli strains, expressing the proteins of interest, were grown in Luria broth (LB) medium (MP Biomedicals) at 37° C using rotary air shaker (New Brunswick Scientific Innova 44R). C5 epimerase-maltose binding protein (MBP) fusion protein was grown in media supplemented with 15 μg/ml kanamycin, 12.5 μg/ml tetracycline, and 50 μg/ml carbenicillin, and 20 μg/ml chloramphenicol. Similarly, 2-OST-MBP fusion protein was grown in LB media supplemented with 50 μg/ml kanamycin, 12.5 μg/ml tetracycline, 50 μg/ml carbenicillin. AST IV-6x His protein was grown in LB medium supplemented with 50 μg/ml kanamycin. Optical density (OD) of these cultures was measured at 600 nm using a spectrophotometer (UV mini 1240, Shimadzu, Japan). Induction was carried out when OD 600 reached between 0.6-0.8. C5-epimerase induction was carried out using 1 mg/ml L-arabinose and 0.2 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) at 22°C for 18 h, while for 2-OST and AST IV, induction was carried out using 0.2 mM IPTG at 22°C for 18 h. At the end of the induction period cells were harvested by centrifugation at 4° C and 3,220 × g for 30 min (Centrifuge 5810 R, Eppendorf). Cell pellets were re-suspended in respective extraction buffer (25 mM monosodium phosphate, 500 mM sodium chloride, 25 mM imidazole, pH 7.4), and then lysed using a flat end tip sonicator (Sonicator 3000, Misomix, USA). The sonicated sample was centrifuged at 4°C and at 8,000 × g for 40 min and supernatant was collected.
C5-epimerase and 2-OST fusion proteins, which contained an MBP tag, were purified using an amylose (New England Biolabs) column on a GE Akta purifier system. The column was washed with four column volumes (CV) each of distilled water, 0.1 M NaOH, distilled water, 20% v/v EtOH and distilled water. After washing, the column was equilibrated with 5 CV of buffer A (25 mM monosodium phosphate, 500 mM sodium chloride). Cell lysate obtained after sonication was filtered using 0.45 μm syringe filters (VWR, USA) and was loaded onto the column through the super loop. Step elution was carried out using a high maltose buffer B (25 mM monosodium phosphate, 200 mM sodium chloride, 40 mM maltose).
AST IV fusion protein, which contained a 6x His tag was purified using a pre-packed His-Prep FF 16/10 column (GE life sciences) connected to a GE Akta purifier system. After washing as described above, the column was equilibrated with 5 CV of buffer A (25 mM monosodium phosphate, 500 mM NaCl, 25 mM imidazole). Cell lysate obtained after sonication was filtered using 0.45 μm syringe filters (VWR, USA) and was loaded onto the column through a 50 ml super loop. Gradient elution was carried out using a high imidazole buffer B (25 mM Tris, 200 mM NaCl, 250 mM imidazole). Fractions were collected, pooled and concentrated (if required). Sodium dodecylsulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) was used to confirm the purity of each of these recombinant proteins.
Direct measurement of AST IV activity was carried out using a high throughput colorimetric 96-well plate assay. Briefly, 125 μl of 2 mM PNPS (Sigma Aldrich, USA), 25 μl of 50 mM MES buffer, pH 7.0, 25 μl of 1 mg/ml AST IV was added to each well. Reactions were initiated by addition of PAP with 25 μl of 2.50 mM PAP added to each well (final volume ~ 200 μl); 25 ul of 50 mM MES buffer was added to the negative control well instead of AST IV. Absorbance at 400 nm was measured at 30 s intervals for 20 min to follow generation of p-nitrophenol (PNP), which is formed as a product after transfer of sulfo group from PNPS, thereby converting PAP to PAPS. Enzyme specific activity was calculated using the extinction coefficient of PNP with units in nmoles of sulfo group transferred/minute/mg of protein.
In vitro enzymatic treatment of N-sulfoheparosan was carried out using C5-Epimerase, 2-OST and AST IV leading to generation of IdoA-NS2S as the major dissacharide unit, the presence of which was confirmed using liquid chromatography/mass spectroscopy (LC/MS). PNPS (125 μl of 2 mM), 25 μl of 1 mg/ml N-sulfoheparosan, 25 ul of 1 mg/ml AST IV, 25 μl of 1 mg/ml C5-Epimerase, and 25 μl of 1.5 mg/ml 2-OST were added to wells in the 96-well plate. Reactions were initiated by addition of 25 μl of 3 mM PAPS to each well (final volume ~ 250 μl) and the well plate was incubated overnight at 37°C in an orbital shaker (New Brunswick scientific, USA). The amount of each enzyme was kept constant to minimize batch-to-batch variability with the final volume adjusted using 50 mM MES buffer, pH 7.
Immobilization of 2OST, C5-epi and AST-IV
Prior to immobilization, all the enzymes were buffer exchanged using 3.5 kDa MWCO centrifugal filter to replace the elution buffer with MES buffer (50 mM, pH 7.2) and adjusted to a protein concentration in the range of 1.5-2.0 mg/mL. Immobilization was performed in the following manner. For agarose aldehyde beads, 1 mL of beads, 6 mL of enzyme solution and 70 μL of aqueous sodium cyanoborohydride solution (5 M) were added to a 10 mL disposable polypropylene column. The column was mixed end-over-end at room temperature for 4 h. For acrylic amino beads, 1 g of beads were pre-activated with 4 mL of 2% aqueous glutaraldehyde solution at room temperature for 60 min with end-over-end mixing and washed 2-times with MES buffer. The activated beads were combined with 6 mL enzyme solution and 70 μL aqueous sodium cyanoborohydride solution (5 M), and mixed end-over-end for 4 h in the 10 mL column. For acrylic epoxy beads, 1 g of beads and 6 mL enzyme solution were mixed in a 10 ml column, end-over-end for 20 h at room temperature. After each immobilization, the slurry was filtered and washed 5-times with MES buffer. All flow-through was collected and the protein concentration determined to calculate the coupling efficiency.
Measurement of apparent activities of immobilized 2-OST, C5-epi and AST-IV
NSNAH was used as a substrate to determine the activity of immobilized 2-OST, C5-epi, and AST-IV. Immobilized C5-epi (~25 μL of beads containing 150 μg of protein) was combined with 100 μg of NSNAH substrate and 200 μM PAPS cofactor in 933 μl of 50 mM MES, pH 7.0. After incubation at 37 °C with shaking for 1 h, an additional 67 μL of 3 mM PAPS, immobilized 2-OST (~25 μL of beads containing 150 μg of protein) and immobilized AST-IV (~10 μL of beads containing 150 μg of protein) were added to the reactor. Samples were removed at 24 h to determine the amount of NSNA2SHp product generated to determine the combined immobilized activity of the three enzymes. One unit of apparent specific activity is defined as 1 μmol NSNA2SHp formed/g immobilized protein/h under the assay conditions used.
Batch conversion of NSNAH to NSNA2SHp using immobilized enzymes
The reactions were performed in a batch reactor (20 mL glass vial), which was placed in a rotary shaker maintained at 37 °C and 220 rpm. Approximately 10 μL aliquots of well-stirred reaction mixture were removed periodically for analysis. The reactions were generally performed using 0.1 mg/mL of NSNAH substrate, 150 μg each immobilized enzyme (2-OST, C5-epi and AST-IV), and 5 mM PNPS in 1 mL MES buffer (50 mM, pH 7.0). This mixture was pre-incubated for 1 h prior to adding 200 μM PAPS to initiate the reaction. The reaction conditions were modified as specifically detailed in the text.
Continuous conversion of NSNAH to NSNA2SHp using immobilized enzymes
An immobilized enzyme packed-bed column reactor was designed to convert of NSNAH to NSNA2SHp. The packed-bed reactor was divided into three segments separated by filter paper. At the top was 300 μg of immobilized C5-epi, in the middle was 300 μg of immobilized 2-OST, and at the bottom was 300 μg of immobilized AST-IV. Reaction solution was continuously re-circulated using a peristaltic pump at a flow rate of 0.3 ml/min into the top of the column and out of the bottom of the column and into a 5 mL reservoir. The reaction solution contained 0.1 mg/ml NSNAH substrate, 5 mM PNPS, and 200 μM PAPS in 2 mL of 50 mm, pH 7.0 MES buffer. The reactor was placed in a 37°C constant temperature room and 10 μL aliquots were periodically removed from the reservoir for monitoring the reaction.
Operational stability of immobilized 2-OST, C5-epi and AST-IV
Immobilized enzyme reuse was evaluated by repeated batch experiments using batch reactions. At the end of each batch (72 h), the immobilized enzyme was filtered out using a membrane (pore diameter: 0.22 μm) from the reaction medium and washed with MES buffer (50 mM, pH 7.2). Immobilized 2-OST, C5-epi, and AST-IV were then used in another reaction cycle using fresh substrates.
Storage stability of immobilized 2-OST, C5-epi and AST-IV
Immobilized 2-OST, C5-epi, and AST-IV were stored at 4 °C and the storage stability of the immobilized enzymes was determined by measuring substrate conversion taken from batch reactions at regular time intervals.
Scale-up of batch conversion of NSNAH to NSNA2SHp using immobilized enzymes
The reaction conditions were as follows: 0.5 mg/ml NSNAc heparosan was first treated with C5-Epi in the absence of PNPS for 12 h in 10 ml buffer, followed by addition of 200 uM PAPS, 5 mM PNPS, immobilized 2-OST and AST-IV, and then incubating for up to 72 h with sampling after each 24 h.
Disaccharide analysis using liquid chromatography/mass spectrometry (LC/MS) to monitor conversion of NSNAH to NSNA2SHp
An NSN2SHp sample (20 mg/mL) was treated with heparin lyase I, II, III (3 m-unit each in 10 μL of 5 mM, pH 7.1 sodium phosphate buffer) and incubated at 35 °C overnight (Zhang et al., 2009). The reaction products were filtered using 10,000 MWCO centrifugal filters and the disaccharides were recovered in the filtrate. The total recovery of disaccharides from the samples generated by heparin lyases digestion followed by a membrane filtration (10,000 MWCO) was in the range from 55% to 65%. The disaccharides were freeze-dried and exactly 100 μL of water was added prior to their analysis. Disaccharide analysis was performed on an HPLC-MS system (Agilent LC/MSD trap MS) (Zhang et al., 2009). Solutions A and B for the HPLC separation contained 37.5 mM NH4HCO3 and 11.25 mM tributylamine in 15% and 70% acetonitrile, respectively. The pH values of these solutions were adjusted to 6.5 with acetic acid. Separation was performed on a C-18 column (Agilent) at a flow rate of 10 μL/min using solution A for 20 min, followed by a linear gradient from 20 to 45 min starting at 0% and ending at 50% solution B. The column effluent entered the source of the electrospray ionization (ESI)-MS for continuous detection by MS. The electrospray interface was set in the negative ionization mode with the skimmer potential of −40.0 V, capillary exit at −40.0 V, and a source temperature at 325 °C to obtain the maximum abundance of the ions in full scan spectra (150–1500 Da, 10 full scans/s). Nitrogen was used as a drying (5 L/min) and nebulizing gas (20 psi).
Nuclear magnetic resonance (NMR) spectroscopy to monitor conversion of NSNAH to NSNA2SHp
NSNAH and NSNA2SHp were analyzed by one- and two-dimensional 1H-NMR spectroscopy. Heteronuclear multiple-quantum coherence (HMQC), and proton–proton correlation spectroscopy (HHCOSY) were used to characterize polysaccharide structure (Zhang et al., 2011). All NMR experiments were performed on Bruker Advance II 600 MHz spectrometer (Bruker Bio Spin, Billerica, MA) with Topspin 2.1.6 software (Bruker). Samples were dissolved in 0.5 mL D2O (99.996 atom%) and freeze-dried repeatedly to remove the exchangeable protons. The samples were re-dissolved in 0.4 mL D2O and transferred to NMR microtubes (outside diameter, 5 mm, Norell (Norell, Landisville, NJ)). The conditions for one-dimensional 1H-NMR spectra were as follows: wobble sweep width of 12.3 kHz, acquisition time of 2.66 s, and relaxation delay of 8.00 s. Temperature was 298 K. The conditions for two-dimensional HMQC spectra were as follows: 32 scans, sweep width of 6.15 kHz, acquisition time of 0.33 s, and relaxation delay of 0.90 s. The conditions for two-dimensional HHCOSY spectra were as follows: 16 scans, sweep width of 7.46 kHz, acquisition time of 0.28 s, and relaxation delay of 1.50 s.
3. Results and discussion
Screening of resin
Three different beads -- agarose aldehyde, acrylic amino and acrylic epoxy -- were evaluated for immobilization of 2-OST, C5-epi and AST-IV (Table 1). Higher coupling efficiency (more than 85% protein immobilization) was achieved for the immobilization of 2-OST and C5-epi on acrylic beads. In contrast, AST-IV showed higher coupling efficiency (~90% protein immobilization) on agarose aldehyde beads. The activities of the three immobilized enzymes were also examined. The agarose amino beads, with an underlying positive charge, bound the negatively charged NSNAH substrate and NSNA2SHp product, thereby interfering with their detection. The agarose aldehyde beads afforded higher immobilized activity than that of acrylic epoxy beads so it was selected for 2-OST, C5-epi and AST-IV immobilization.
Table 1.
Coupling efficiency of 2-OST, C5-epi and AST-IV on three activated resins.
| Resin | Coupling efficiency (%) |
Apparent activity (mmol/g/h) |
||
|---|---|---|---|---|
| 2-OST | C5-epi | AST-IV | ||
| Acrylic amino resin | 86.7 | 92.6 | 66 | -* |
| Acrylic epoxy resin | 90.8 | 88.8 | 87 | 1.7 |
| Agarose aldehyde resin | 31.5 | 43.4 | 89 | 2.6 |
The product and substrate cannot be detected in the reaction system suggesting that they interact with the basic resin.
Effect of substrate N-acetyl groups on enzymatic step on the production of NSNA2SHp
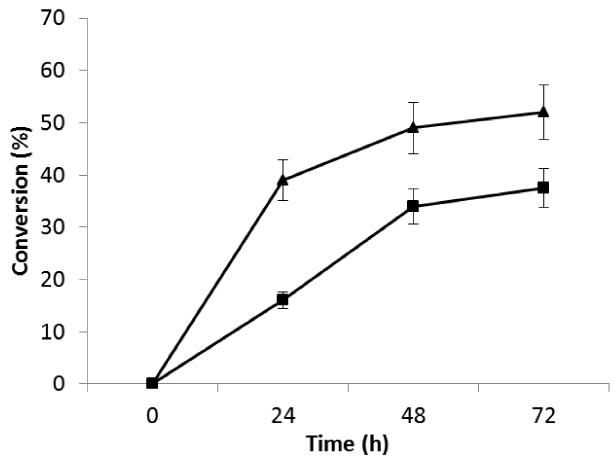
NSNAH affords a bioengineered Hp comparable to porcine intestinal Hp, while NSH affords a bioengineered Hp comparable to bovine lung Hp (Loganathan et al., 1990). Thus, both NSH and NSNAH were evaluated as substrates for immobilized 2-OST, C5-epi and AST-IV. Under batch reaction conditions NSH was a poorer substrate than NSNAH (Figure 1). Since NSNAH affords a bioengineered Hp comparable to the more widely used porcine intestinal Hp, and was better transformed using immobilized 2-OST, C5-epi and AST-IV, it was selected for subsequent studies.
Figure 1.
Substrate comparison studies on NSH ( ) and NSNAH (
) and NSNAH ( ) using immobilized 2-OST, C5-epi and AST-IV. Error bars associated with analytical method are shown.
) using immobilized 2-OST, C5-epi and AST-IV. Error bars associated with analytical method are shown.
Effect of various reaction conditions on production of NSNA2SHp
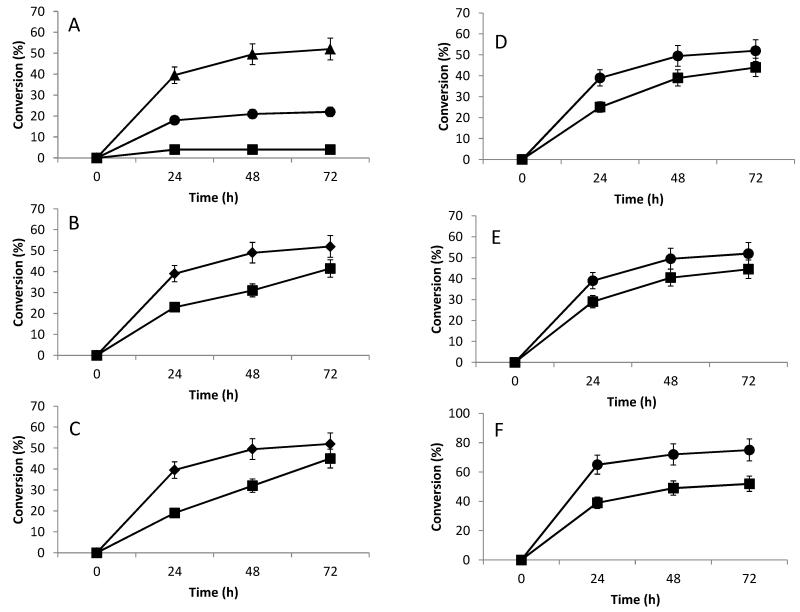
The effect of NSNAH concentration was next examined over the range of 0.1 to 10 mg/mL, while maintaining constant other reaction conditions. The conversion of various substrate concentrations was determined after 24, 48 and 72 h (Figure 2A). As expected, conversion decreased as the initial substrate concentration increased, potentially the result of modest substrate inhibition.
Figure 2.
Optimization of conditions for conversion of NSNAH to NSNA2SHp using immobilized 2-OST, C5-epi and AST-IV. A. Effect of different substrate concentrations on conversion of NSNAH to NSNA2SHp: 0.1 mg/ml ( ); 1.0 mg/ml (
); 1.0 mg/ml ( ); 10 mg/ml (
); 10 mg/ml ( ). B. Effect of amount of PAPS on conversion of NSNAH to NSNA2SHp: 40 μM (
). B. Effect of amount of PAPS on conversion of NSNAH to NSNA2SHp: 40 μM ( ); 200 μM (
); 200 μM ( ). C. Effect of different methods of reaction on conversion of NSNAH to NSNA2SHp: over 72 h and separate treatment of C5-epi (
). C. Effect of different methods of reaction on conversion of NSNAH to NSNA2SHp: over 72 h and separate treatment of C5-epi ( ); normal treatment (
); normal treatment ( ). D. Effect of separate epimerization reaction on conversion of NSNAH to NSNA2SHp: three-step treatment (
). D. Effect of separate epimerization reaction on conversion of NSNAH to NSNA2SHp: three-step treatment ( ); normal treatment (
); normal treatment ( ). E. Effect of types of different reactors on the conversion of NSNAH to NSNA2SHp: column reactor (
). E. Effect of types of different reactors on the conversion of NSNAH to NSNA2SHp: column reactor ( ); batch reactor (
); batch reactor ( ). F. Effect of amount of enzymes on conversion of NSNAH to NSNA2SHp: 150 μg/ml (
). F. Effect of amount of enzymes on conversion of NSNAH to NSNA2SHp: 150 μg/ml ( ); 1500 μg/ml (
); 1500 μg/ml ( ). Error bars associated with analytical method are shown.
). Error bars associated with analytical method are shown.
PAPS, a reaction cofactor and sulfo group donor, is also a substrate in the reaction. Since PAPS is relatively expensive it requires continuous regeneration using AST-IV and PNPS (Burkart et al., 2000; Zhou X et al., 2011). The amount of PAPS used was minimized to further reduce costs to produce NSNA2SHp. The results are shown in Figure 2B. Decreasing PAPs from 200 μM to 40 μM conversion decreased product yield by ~20%. In subsequent experiments we fixed the PAPS concentration at 40μM.
A three-step treatment was used to produce NSNA2SHp. The reaction processes examined included a 1:1 ratio of C5-Epi and 2-OST for 0-24 h, a 0.1:1 ratio of C5-epi and 2-OST for 24-48 h, and a 0.01:1 ratio of C5-epi and 2-OST for 48-72 h. The remaining reaction conditions remained unchanged. These experiments showed that it was not possible to improve the conversion (Figure 2C).
The C5-epi catalyzed reaction was then separated to examine its impact on the 2-OST and AST-IV catalyzed sulfonation reactions. The C5-epi reaction was first performed on NSNAH for 6 h after which C5-epi was removed. The reaction solution was then treated with 2-OST and AST-IV in the presence of PAPS and PNPS for 18 h before removing 2-OST and AST-IV. Even after repeating this process three-times the conversion of NSNAH to NSNA2SHp could not be improved compared to standard reaction conditions (Figure 2D).
Effect of a packed-bed reactor on the production of NSNA2SHp
The continuous packed-bed reactor was next examined to produce NSNA2SHp. Conversion in the column reactor was less than that from the batch reactor (Figure 2E). There are two likely reasons for these results; potential poor mass transfer in the relatively static packed-bed reactor, or physical separation of C5-epi catalysis from 2OST catalysis reduces the effectiveness of the latter. Understanding the impact of both of these possibilities will require additional studies.
Effect of amount of immobilized enzymes on the production of NSNA2SHp
In pharmaceutical Hp, the content of IdoA2S is >80%, but only about 50% IdoA2S was produced herein for NSNA2SHp. The amount of immobilized enzymes was, therefore, increased 10-fold, which resulted in a conversion that was close to the required 80% level (Figure 2F).
Operational and storage stability of immobilized enzymes
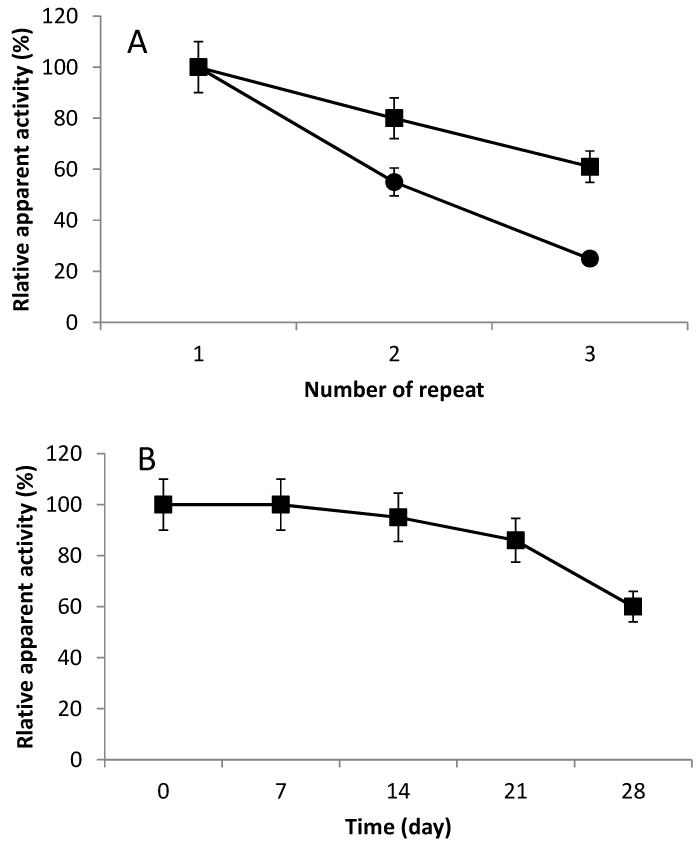
Although the activity of immobilized enzymes using acrylic epoxy beads is lower than agarose aldehyde beads, acrylic epoxy beads might still be selected if the immobilized enzymes can be easily reused. Thus, the operational stabilities of the immobilized enzymes were investigated and the results are shown in Figure 3A. The enzymes immobilized on agarose aldehyde beads retained approximately 60% of their initial activity after three uses, while the enzymes immobilized on acrylic epoxy beads only retained approximately 20% after three uses. Therefore, from the standpoint of operational stability the agarose aldehyde resin is the best carrier of those tested for the enzymes.
Figure 3.
Stability studies on immobilized enzymes. A. Operational stability of immobilized enzymes: agarose aldehyde resin ( ); acrylic epoxy resin (
); acrylic epoxy resin ( ). B. Storage stability of immobilized enzymes. Error bars associated with analytical method are shown.
). B. Storage stability of immobilized enzymes. Error bars associated with analytical method are shown.
Investigation of the storage stability for immobilized enzymes on agarose aldehyde beads revealed that the residual apparent activity was reduced to about 50% after 28 days (Figure 3B) while the free enzymes could only be stored for 10 days at 4°C before near complete loss of activity. As expected, the stability of enzymes was improved by immobilization.
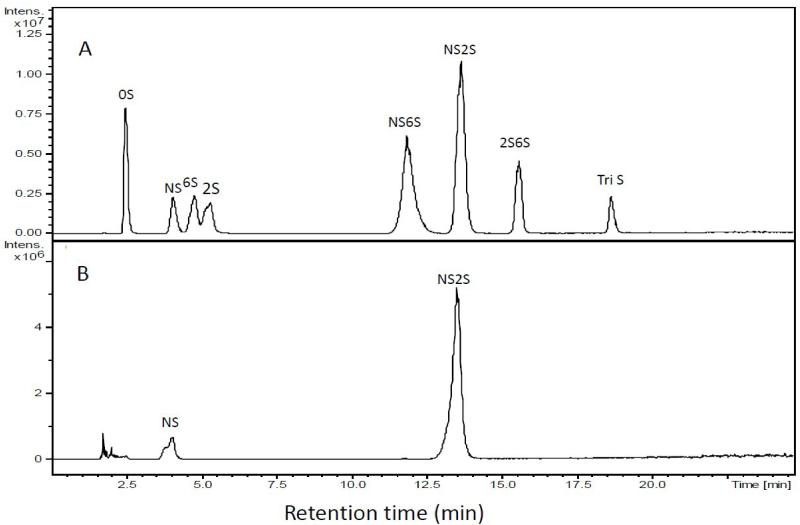
Analysis of reaction product NSN2SHp
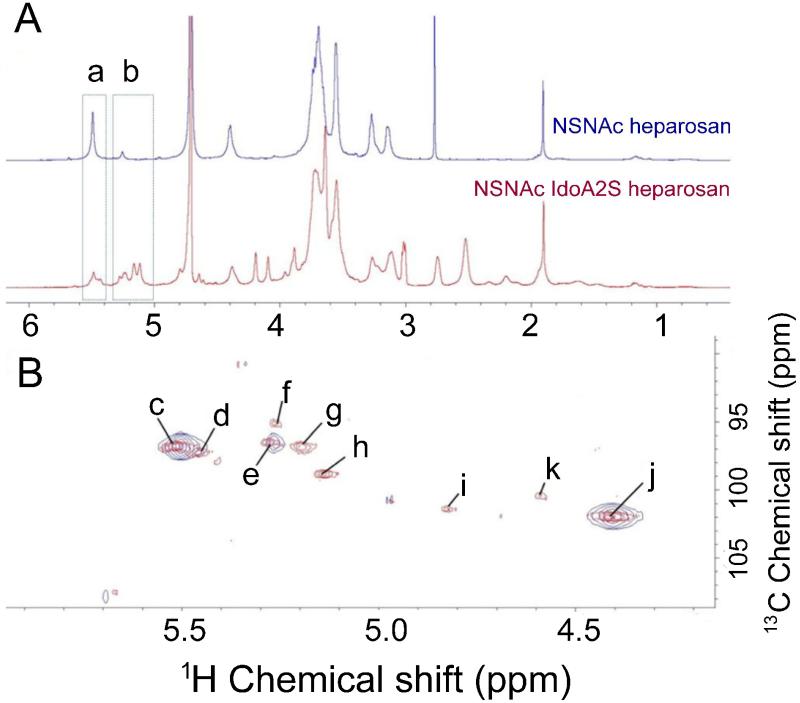
A large-scale (5 mg) reaction was carried out for 72 h. After purification, the product was assayed by RPIP-UPLC-MS. The extracted ion chromatogram (EIC) of disaccharide standards was first performed (Figure 4A). Examination of NSN2SHp product showed an NS2S content of 53% (Figure 4B) calculated from a linear equation derived from a calibration curve of eight disaccharide standards. The structure of the product was next assessed by NMR spectroscopy. The anomeric region of 1H spectrum of NSNAH showed a major change compared to NSN2SHp, although complete assignment of the 1D spectra was not possible due to peak overlap (Figure 4C). The anomeric region of HMQC spectrum, the anomeric proton of N-sulfo-glucosamine (ANS), N-sulfo-glucosamine (ANAc), glucuronic acid (G), iduronic acid (I) and 2-sulfo-iduronic acid (I2S) residues could be clearly observed (Figure 4D). According to this HMQC spectrum, the relative integral of the anomeric proton of glucuronic acid is taken as 1.000, which results in an integral of the anomeric proton of iduronic acid of 0.815. This indicates that the I2S generation reaches about 45.7%.
Figure 4.
Compositional analysis of NSNA2SHp product. A. EIC of disaccharide analysis of an equi-molar mixture of eight disaccharide standards used for peak identification and injection of different amounts afford a standard curve for quantification. B. RPIP-UPLC-MS disaccharide analysis of NSNA2SHp.
Conclusions
This study demonstrates the covalent immobilization of the three enzymes involved in the first enzymatic step required for the chemoenzymatic synthesis of bioengineered heparin. The immobilization of these enzymes prose a particular challenge as they
Highlights.
Immobilized three enzymes required for chemoenzymatic synthesis.
Facilitated PAPS cofactor recycling using immobilized enzyme.
Simultaneously, epimerize, sulfonate and regenerate cofactors.
Optimized enzymatic step in the synthesis of bioengineered heparin.
Produced critical intermediate for bioengineered heparin.
Figure 5.
NMR analysis of NSNA2SHp product. A. 1H-NMR spectrum of NSNAH (red) and NSNA2SHp (blue) at 298K, a, H1 ANS region; b, H1 ANAc and I2S region. B. HMQC spectrum at 298K, NSNAH (red) and NSNA2SHp (blue) c, ANS-(G) C1H1; d, ANS-(G) C1H1; e, ANAc-(G) C1H1; f, ANAc-(I) C1H1; g, ANAc-(I2S) C1H1; h, I2S C1H1; i, I C1H1; j, G C1H1; and k, impurity peak.
Acknowledgement
This work was supported by grants from the Chinese National Natural Science Foundation (No.21006051) and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions
Funding
This work was supported by grants from the Chinese National Natural Science Foundation (No. 21006051) and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions and by grants from the United States National Institutes of Health (HL096972, HL62244 and HL094463).
ABBREVIATIONS
- Hp
Heparin
- cGMP
current good manufacturing practices
- NSH
N-sulfo heparosan
- NSNAH
N-sulfo, N-acetyl heparosan
- NSNA2SHp
N-sulfo, N-acetyl, 2-O-sulfoheparin
- C5-epi
C5-epimerase
- 2OST
2-O-sulfotransferase
- AST-IV
arylsulfo transferase IV
- NACHp
non-anticoagulant heparin
- PAP
3′-phosphoadenosine-5′-phosphate
- PAPS
3′-phosphoadenosine-5′-phosphosulfate
- PNPS
4-nitrophenyl sulfate
- PNPS
liquid chromatography/mass spectrometry
- NMR
Nuclear magnetic resonance
- ESI
electrospray ionization
- HMQC
Heteronuclear multiple-quantum coherence
- HHCOSY
proton–proton correlation spectroscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burkart MD, Izumi M, Chapman E, Lin C, Wong C. Regeneration of PAPS for the enzymatic synthesis of sulfated oligosaccharides. Journal of Organic Chemistry. 2000;65:5565–5574. doi: 10.1021/jo000266o. [DOI] [PubMed] [Google Scholar]
- Chen J, Avci FY, Munoz EM, McDowell LM, Chen M, Pedersen LC, Zhang L, Linhardt RJ, Liu J. Enzymatically redesigning of biologically active heparan sulfate. Journal of Biological Chemistry. 2005;280:42817–42825. doi: 10.1074/jbc.M504338200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Li G, Yang B, Onishi A, Li L, Sun P, Zhang F, Linhardt RJ. Structural characterization of pharmaceutical heparins prepared from different animal tissues. Journal of Pharmaceutical Sciences. 2013;102:1447–1457. doi: 10.1002/jps.23501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini M, Beccati D, Shriver Z, Naggi AM, Bisio A, Capila I, Lansing J, Guglieri S, Fraser B, Al-Hakim A, Gunay S, Viswanathan K, Zhang Z, Robinson L, Venkataraman G, Buhse L, Nasr M, Woodcock J, Langer R, Linhardt RJ, Casu B, Torri G, Sasisekharan R. Oversulfated chondroitin sulfateis a major contaminant in heparin associated with adverse clinical events. Nature Biotechnology. 2008;26:669–775. doi: 10.1038/nbt1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto TK, Viswanathan K, Ganguly T, Elankumaran S, Smith S, et al. Contaminated heparinassociated with adverse clinical events and activation of the contact system. New England Journal of Medicine. 2008;358:2457–2467. doi: 10.1056/NEJMoa0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuberan B, Lech MZ, Beeler DL, Wu ZL, Rosenberg RD. Enzymatic synthesis of antithrombin III-binding heparan sulfate pentasaccharide. Nature Biotechnology. 2003;21:1343–1346. doi: 10.1038/nbt885. [DOI] [PubMed] [Google Scholar]
- Lindahl U, Li JP, Kusche-Gullberg M, Salmivirta M, Alaranta S, Veromaa T, Emeis J, Roberts I, Taylor C, Oreste P, et al. Generation of “neoheparin” from E. coli K5 capsular polysaccharide. Journal of Medicinal Chemistry. 2005;48:349–352. doi: 10.1021/jm049812m. [DOI] [PubMed] [Google Scholar]
- Linhardt RJ. Heparin: Structure and activity. Journal of Medicinal Chemistry. 2003;46:2551–2554. doi: 10.1021/jm030176m. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhang Z, Linhardt RJ. Lessons learned from the contamination of heparin. Natural Product Reports. 2009;26:313–321. doi: 10.1039/b819896a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhardt RJ, Liu J. Synthetic heparin. Current Opinions in Pharmacology. 2012;12:217–219. doi: 10.1016/j.coph.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loganathan D, Wang HM, Mallis LM, Linhardt RJ. Structural variation in the antithrombin III binding site region and its occurrence in heparin from different sources. Biochemistry. 1990;29:4362–4368. doi: 10.1021/bi00470a015. [DOI] [PubMed] [Google Scholar]
- Moon AF, Xu Y, Woody SM, Krahn JM, Linhardt RJ, Liu J, Pedersen LC. Dissecting the substrate recognition of 3-O-sulfotransferase for the biosynthesis of anticoagulant heparin. Proceedings of the National Academy of Sciences USA. 2012;109:5265–5270. doi: 10.1073/pnas.1117923109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson S, Frick A, Liu J. Design of biologically active heparan sulfate and heparin using an enzyme-based approach. Natural Product Reports. 2009;26:610–627. doi: 10.1039/b803795g. [DOI] [PubMed] [Google Scholar]
- Sheng J, Xu Y, Dulaney SB, Huang X, Liu J. Uncovering biphasic catalytic mode of C5-Epimerase in heparan sulfate biosynthesis. Journal of Biological Chemistry. 2012;287:20996–21002. doi: 10.1074/jbc.M112.359885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinay P, Jacquinet J-C, Petitou M, Duchaussoy P, Lederman I, Choay J, Torri G. Total synthesis of a heparin pentasaccharide fragment having high affinity for antithrombin III. Carbohydrate Research. 1984;132:C5–C9. doi: 10.1016/0008-6215(87)80268-9. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ly M, Zhang F, Zhong W, Suen A, Hickey AM, Dordick JS, Linhardt RJ. E. coli K5 Accepted Manuscript fermentation and the preparation of heparosan, a bioengineered heparin precursor. Biotechnology and Bioengineering. 2010;107:968–977. doi: 10.1002/bit.22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yang B, Zhang Z, Ly M, Takieddin M, Mousa S, Liu J, Dordick JS, Linhardt RJ. Control of the heparosan N-deacetylation leads to an improved bioengineerelod heparin. Applied Microbiology and Biotechnology. 2011;91:91–99. doi: 10.1007/s00253-011-3231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Masuko S, Takieddin M, Xu H, Liu R, Jing J, Mousa S, Linhardt RJ, Liu J. Chemoenzymatic synthesis of structurally homogeneous ultra-low molecular weight heparins. Science. 2011;334:498–501. doi: 10.1126/science.1207478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Xie J, Liu H, Liu J, Linhardt RJ. Quantitification of heparan sulfate and heparin disaccharides using ion pairing, reverse-phase, micro-flow, high performance liquid chromatography coupled with electrospray ionization trap mass spectrometry. Analytical Chemistry. 2009;81:4349–4355. doi: 10.1021/ac9001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, McCallum SA, Xie J, Nieto L, Corzana F, Jiménez-Barbero J, Chen M, Liu J, Linhardt RJ. Solution structures of chemoenzymatically synthesized heparin and its precursors. Journal of the American Chemical Society. 2008;130:12998–13007. doi: 10.1021/ja8026345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Chandarajoti K, Pham TQ, Liu R, Liu J. Expression of heparan sulfate sulfotransferases in Kluyveromyces lactis and preparation of 3-phosphoadenosine-5-phosphosulfate. Glycobiology. 2011;21:771–780. doi: 10.1093/glycob/cwr001. [DOI] [PMC free article] [PubMed] [Google Scholar]