Abstract
Background and Aims
Nonalcoholic fatty liver disease (NAFLD) is the number one cause of liver disease in the United States. The prevalence rates in African Americans (AA), while significantly lower than other ethnic groups with similar known risk factors, have been quoted as high as 24 %. We aim to determine if the presence of NAFLD in African Americans is associated with lower triglyceride and/or higher HDL-c levels and if NAFLD risk factors in African Americans differ from other ethnic groups.
Methods
A total of 3,056 participants of the Multi Ethnic Study of Atherosclerosis were included in this study. We utilized the baseline serum, anthropometric and radiographic measurements obtained between 2000 and 2002. NAFLD was defined as liver spleen ratio <1 from CT measurements.
Results
The prevalence of NAFLD was and 11 % in AA. We found that age, education, triglyceride levels, HDL-c levels, waist circumference and HOMA-IR were independent correlates of NAFLD in this population. Among those with NAFLD, AA had significantly lower triglyceride levels than Hispanics [125 mg/dl (95 % CI 107–143) versus 192 mg/dl (95 % CI 169–215), p < 0.001] and Caucasians [185 mg/dl (95 % CI 161–209), p = 0.001]. Serum HDL-c was significantly higher in AA with NAFLD (47 mg/dl; 95 % CI 45–50) when compared to Hispanics (44 mg/dl; 95 % CI 43–66, p = 0.02) and Caucasians (44 mg/dl; 95 % CI 42–46, p = 0.02) with NAFLD.
Conclusions
This study demonstrated that the clinical correlates of NAFLD in African Americans are similar to the correlates of NAFLD in other ethnic groups. Our data also suggests that when evaluating African Americans for NAFLD risk, lower cutoff values should be used to define abnormal triglyceride levels.
Keywords: NAFLD, Prevalence, African Americans, Predictors, Clinical correlates
Introduction
Nonalcoholic fatty liver disease (NAFLD), the spectrum of hepatic disease ranging from bland steatosis, necroinflammation and hepatitis, fibrosis and cirrhosis, is the leading cause of liver disease in the United States [1]. The prevalence of NAFLD in adults in the United States ranges from 15 to 39 % [1–3]. Nonalcoholic steatohepatitis (NASH), a more aggressive manifestation, representing a combination of necroinflammation, hepatitis, perisinusoidal and perivenular fibrosis, and cirrhosis, ranges in prevalence from 2 to 7 % [2, 3]. More important are recent data demonstrating that NAFLD is known to independently increase overall mortality, with malignancy and cardiovascular disease being the most common causes of death [4–8].
Risk factors for NAFLD development have been identified but not entirely validated. Many of the risk factors identified (visceral adiposity, insulin resistance, dyslipidemia) are components of the metabolic syndrome (MetS), of which NAFLD is widely accepted to be the hepatic equivalent [9–12]. Like metabolic syndrome, the prevalence of NAFLD is known to vary widely among different ethnicities [9, 13, 14]. African Americans typically have significantly lower prevalence rates despite equal rates of obesity and insulin resistance. Some authors have suggested that this could be a result of differences in lipid homeostasis, given that African Americans typically have lower triglyceride (TG) levels and higher levels of HDL-c [9, 15].
Patatin-like phospholipase domain containing 3 gene (PNPLA3) has recently been implicated in accounting for up to 72 % of the ethnic differences in NAFLD prevalence [16]. However, it is still unclear what pathophysiological role PNPLA3 plays in human NAFLD development and disease progression. While the prevalence rates of NAFLD are significantly lower in African Americans compared to other racial and ethnic groups, the currently quoted rate of 24 % in the largest population-based study, is still alarmingly high [9]. African Americans are a population that historically have disproportionately higher mortality rates due to cardiovascular disease [17, 18]. Given that NAFLD is an independent marker of cardiovascular risk, identifying NAFLD risk factors in the African American population offers a vital strategy for reduction in mortality as well as long-term cost-benefit savings.
In our proposed studies, we aimed to identify risk factors for NAFLD in African Americans, a population known to have high risk for health disparities as well as poorly defined risk factors for NAFLD when compared to other ethnic groups. Specifically, we aim to determine whether the prevalence of NAFLD in African Americans is associated with lower triglyceride and/or higher HDL-c levels as well as identify other clinical predictors of NAFLD development in African Americans and determine if these differ from other ethnic groups.
Methods
Study Population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective study designed to evaluate the mechanisms underlying the development and progression of subclinical cardiovascular disease (CVD) in asymptomatic individuals. The study design has been described previously [19]. Briefly, 6,814 Caucasian, African American, Hispanic, and Asian men and women aged 45–84 who were clinically free from CVD were recruited from six communities (Los Angeles County, CA; northern Manhattan, New York, NY; Baltimore, MD; Chicago, IL; St. Paul, MN; and Forsyth County, NC) in the United States. Participants with active cancer, cognitive impairment, weight greater than 300 pounds (136 kg) and those who were pregnant were excluded.
Methods
We utilized the baseline serum, anthropometric and radiographic measurements obtained between 2000 and 2002. Information on socio-demographic factors (age, sex, and education), lifestyle factors (alcohol consumption and smoking status) and self-reported medical history (hypertension, diabetes, liver disease and cirrhosis) were collected at the baseline examination using standardized questionnaires. A central laboratory (University of Vermont, Burlington) measured levels of total and HDL-c cholesterol, triglycerides, plasma glucose, and high-sensitivity C-reactive protein in blood samples obtained after a 12-h fast. Waist circumference was measured horizontally at the level of the umbilicus. Body mass index was calculated as weight in kilograms divided by height in meters squared. Homeostatic model assessment was used to measure insulin resistance (HOMA-IR) calculated as fasting glucose (mg/dl) × fasting insulin (μU/mL)/405 [20].
Non contrast cardiac chest computed tomography (CT) scans were obtained on all the participants in the MESA study. A majority of these studies had visible liver and spleen tissue available for evaluation. A cardiac CT is a specialized form of computed tomography that differs from standard CT techniques in that it additionally uses ECG-monitoring during the scan acquisition (ECG-gating) to create high-resolution images of the heart and coronary vessels [21]. The existence of fatty liver was assessed by the range of attenuation coefficient (in HU), measured in four chosen areas of the liver. Two measurements of spleen were also conducted. Measurements included the minimum, maximum and mean HU for a 2-cm round/ellipse region of interest (ROI) in the right and left lobes of the liver, as well as in the spleen. If sufficient tissue was not available for a 2-cm measure, a 1-cm measurement was obtained. If less than 1 cm of tissue was evident on the cardiac scan, the study was deemed not sufficient for measure. We calculated an overall mean hepatic attenuation using the means obtained from the right and left lobes of the liver. This hepatic mean HU as well as the mean HU of the spleen was used to calculate a liver/spleen (L/S) ratio.
NAFLD was defined as LS ratio <1, which has an area under the receiver operating curve (AUROC) of >0.96 when measuring hepatic steatosis >30 % on non-contrast CT images [22]. Exclusion criteria for potential participants included the absence of both a visible liver and spleen on cardiac CT, unavailable HDL-c and triglyceride values, “significant alcohol consumption” (defined as >20 g per day in men and >10 g per day in women), a self-reported history of liver disease and cirrhosis, and use of medications associated with NAFLD such as oral steroids and amiodarone. Chinese Americans were also excluded from analysis due to relatively smaller numbers.
Statistical Analysis
Results of summary outcome measures were reported as mean (SD) and proportions. Differences between groups were tested using chi-square analyses for categorical data and two sample Student's t test for continuous variables. Univariate and multivariable analysis were used to determine predictors of NAFLD using logistic regression. In our analytical models we included a core set of covariates which included age (as a continuous measure), gender and education (measured dichotomously; completed high school, yes or no). We included the variables that were significant with a p value of 0.20 or smaller on univariate analysis into our multivariate model of the entire population. These selected variables were then used in multivariate analysis stratified by race. In addition we forced age and gender into all models. All analyses were performed using Stata version 11.0 (StataCorp LP, College Station, TX, USA).
Results
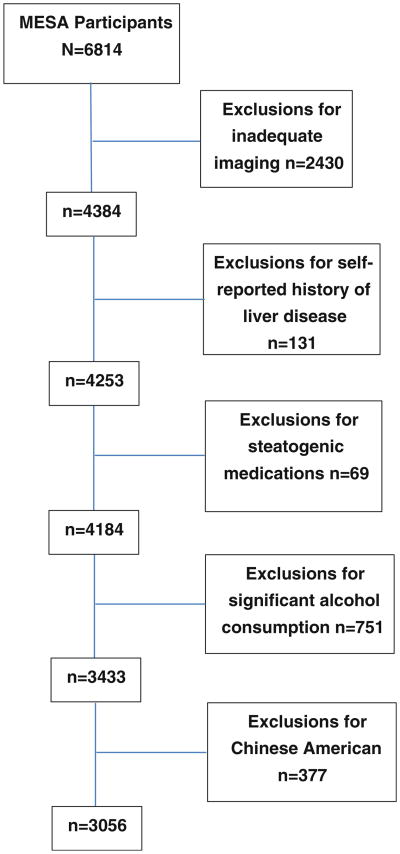
After applying our exclusion criteria there were a total of 3,056 participants available for analysis (Fig. 1). The mean age of the participants was 63 ± 10 and 55 % were female. Among the participants 41 % (n = 1,244) were Caucasian, 32 % (n = 992) African Americans, and 27 % Hispanic (n = 820). The mean waist circumference was 99.8 ± 14 cm, the mean body mass index (BMI; kg/m2) was 29 ± 5 with 36 % of the population meeting criteria for obesity (BMI ≥ 30). The mean HDL-c level was 50 ± 15 mg/dl, and TG level was 131 ± 87 mg/dl. Diabetes was reported in 12 % of the population. The median HOMA-IR value and interquartile range was 1.3 (0.8–2.1). A total of 521 (17 %) participants had NAFLD at baseline as defined by LS ratio <1 (Table 1).
Fig. 1. Study participants.
Table 1. Clinical correlates of NAFLD for univariate analysis.
| Characteristic | No NAFLD (n = 2,535) | NAFLD (n = 521) | p value |
|---|---|---|---|
| Age (years) | 63.6 ± 10.6 | 60.9 ± 9.7 | <0.001 |
| Men | 1,144 (45) | 227 (43) | 0.37 |
| BMI, kg/m2 | 28.4 ± 5.1 | 31.8 ± 5.4 | <0.001 |
| BMI ≥ 30 | 812 (32) | 301 (58) | <0.001 |
| Waist (cm) | 98.3 ± 13.5 | 107.2 ± 13.1 | <0.001 |
| History of ever smoking | 1,247 (49) | 227 (44) | 0.02 |
| Education (%) (<high school) | 435 (17) | 143 (28) | <0.001 |
| Hypertension | 1,031 (41) | 247 (47) | 0.02 |
| Total cholesterol, mg/dl | 193.7 ± 35.1 | 194.6 ± 39.6 | 0.60 |
| LDL-c, mg/dl | 118.0 ± 31.2 | 115.9. ± 31.4 | 0.17 |
| HDL-c, mg/dl | 51.4 ± 14.8 | 44.7 ± 12.1 | <0.001 |
| Triglycerides, mg/dl | 121.6 ± 70.7 | 175.4 ± 134.8 | <0.001 |
| Diabetes | 284 (11) | 90 (17) | <0.001 |
| C-reactive protein, mg/dla | 1.9 (0.89–4.2) | 3.5 (1.6–7.0) | <0.001 |
| HOMA-IRa | 1.1 (0.7–1.8) | 2.4 (1.5–3.7) | <0.001 |
NAFLD nonalcoholic fatty liver disease, BMI body mass index
Reported as mean ± SD or number (%)
Median and interquartile range reported
Characteristics of Participants with NAFLD
Compared to those without, participants with NAFLD were significantly younger, more obese, had a higher waist circumference lower HDL-c levels, higher TG levels, and a greater prevalence of hypertension, insulin resistance, and diabetes. The prevalence of NAFLD within each ethnic group was; 15 % for Caucasians, 28 % of Hispanics, and 11 % in African Americans. The baseline characteristics of the participants stratified by race and the presence of NAFLD are listed in Table 2.
Table 2. Demographic and clinical characteristics of study population by liver/spleen ratio.
| Characteristic | African American | Caucasian | Hispanic | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| No NAFLD (n = 886) | NAFLD (n = 106) | p value | No NAFLD (n = 1,055 | NAFLD (n = 189) | p value | No NAFLD (n = 567) | NAFLD (n = 208) | p value | |
| Age | 63.5 ± 10.1 | 63.4.6 ± 9.3 | 0.86 | 63.9 ± 10.8 | 61.5 ± 9.2 | 0.003 | 62.9 ± 10.8 | 59.3 ± 9.9 | <0.001 |
| Men | 382 (43) | 36 (34) | 0.07 | 496 (47) | 95 (50) | 0.41 | 266 (45) | 93 (41) | 0.3 |
| BMI, kg/m2 | 29.5 ± 5.6 | 32.8 ± 5.8 | <0.001 | 27.4 ± 4.8 | 31.4 ± 5.1 | <0.001 | 28.5 ± 4.4 | 31.7 ± 5.3 | <0.001 |
| Obese | 370 (41) | 72 (67) | <0.001 | 265 (25) | 108 (57) | <0.001 | 186 (31) | 123 (54) | <0.001 |
| Waist (cm) | 99.8 ± 13.8 | 109.0 ± 14.4 | <0.001 | 96.9 ± 13.8 | 108.6 ± 12.8 | <0.001 | 98.5 ± 12.2 | 105.3 ± 12.4 | <0.001 |
| History of smoking | 453 (52) | 47 (44) | 0.17 | 538 (51) | 90 (48) | 0.43 | 256 (43) | 90 (40) | 0.4 |
| Education (<high school) | 111 (13) | 9 (8) | 0.22 | 55 (5) | 12 (6) | 0.51 | 325 (55) | 104 (46) | 0.03 |
| Hypertension | 498 (56) | 66 (62) | 0.20 | 329 (31) | 93 (49) | <0.001 | 204 (34) | 88 (39) | 0.22 |
| Total cholesterol, mg/dl | 190.2 ± 35.9 | 189.5 ± 34.8 | 0.85 | 194.2 ± 34.0 | 193.8 ± 43.4 | 0.87 | 197.8 ± 35.3 | 197.6 ± 38.0 | 0.94 |
| LDL-c, mg/dl | 117.2 ± 32.1 | 117.0 ± 32.9 | 0.97 | 117.4 ± 29.5 | 114.2 ± 31.8 | 0.18 | 120.3 ± 32.4 | 116.8 ± 30.3 | 0.16 |
| HDL-c, mg/dl | 53.0 ± 15.3 | 47.4 ± 12.8 | <0.001 | 51.5 ± 14.7 | 43.9 ± 12.8 | <0.001 | 48.9 ± 13.8 | 44.1 ± 10.9 | <0.001 |
| Triglycerides, mg/dl | 99.8 ± 54.3 | 125.0 ± 64.9 | <0.001 | 126.3 ± 70.8 | 191.9 ± 191.5 | <0.001 | 145.5 ± 82.0 | 185.1 ± 88.1 | <0.001 |
| Diabetes | 132 (15) | 23 (22) | 0.07 | 65 (6) | 20 (11) | 0.08 | 87 (15) | 47 (21) | 0.03 |
| C-reactive protein mg/dla | 2.3 (1.1–5.0) | 3.7 (1.7–8.4) | 0.05 | 1.5 (0.7–3.5) | 3.3 (1.5–6.2) | <0.001 | 2.1 (1.0–4.6) | 3.4 (1.7–6.8) | 0.005 |
| HOMAa | 1.2 (0.8–2.0) | 2.5 (1.5–3.8) | <0.001 | 1.0 (0.6–1.5) | 2.3 (1.4–3.5) | <0.001 | 1.3 (0.8–2.0) | 2.5 (1.6–3.9) | <0.001 |
NAFLD nonalcoholic fatty liver disease, BMI body mass index
Reported as mean ± SD or number (%)
Median and interquartile range reported
African Americans with NAFLD were significantly more likely to have HTN when compared to Caucasians and Hispanics with NAFLD (AA: 62 % vs Hispanics: 39 %, p < 0.001 vs Caucasians: 49 %, p = 0.03). AA had significantly lower TG levels than Hispanics [125 mg/dl (95 % CI 112–137) vs 185 mg/dl (95 % CI 174–197), p < 0.001] and Caucasians [191 mg/dl (95 % CI 164–219), p < 0.001]. Serum HDL-c was significantly higher in AA with NAFLD (47 mg/dl; 95 % CI 45–50) when compared to Hispanics (44 mg/dl; 95 % CI 43–66, p = 0.02) and Caucasians (44 mg/dl; 95 % CI 42–46, p = 0.02) with NAFLD.
When compared to Hispanics with NAFLD, AA with NAFLD were significantly older [63 (95 % CI 62–65) vs 59 (95 % CI 58–61), p < 0.001]. AA waist circumference was significantly larger [109 (95 % CI 106–112) versus 105 (95 % CI 104–107), p = 0.02]. There were more AA high school graduates (96 versus 46 %, p < 0.001). Finally, although there were no significant differences in BMI, AA had significantly more individuals who were obese (66 versus 54 %, p = 0.05) when compared to Hispanics. When compared to Caucasians, AA had more women with NAFLD (66 versus 50 %, p = 0.007) and more diabetics (22 versus 11 %, p = 0.009). AA also had significantly higher BMI than Caucasians [33 (95 % CI 32–34) versus 31 (95 % CI 31–32, p = 0.04)].
When we compared AA with NAFLD to those AA without it, we found that those subjects with NAFLD were more likely to have a higher waist circumference (cm), higher BMI (and obesity rates), hypertension, type 2 diabetes (T2DM), lower HDL-c, higher serum TG levels, higher CRP, HOMA-IR levels and lower education levels (Table 2).
Multivariable Predictors of NAFLD
We found that age, TG levels, HDL-c levels, waist circumference, education, and HOMA-IR were significant independent correlates of NAFLD in this population. Results of multivariate analysis are presented in Table 3.
Table 3. Multivariate predictors of NAFLD.
| Patient | Total population N = 3,056 | African Americans N = 992 | White N = 1,244 | Hispanic N = 820 | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Characteristic | OR (95 % CI) | p value | OR (95 % CI) | p value | OR (95 % CI) | p value | OR (95 % CI) | p value |
| Gender | 0.77 (0.60–0.98) | 0.06 | 0.61 (0.37–1.02) | 0.06 | 0.75 (0.50–1.13) | 0.17 | 0.87 (0.59–1.28) | 0.48 |
| Age | 0.98 (0.96–0.98) | <0.001 | 1.00 (0.98–1.02) | 0.98 | 0.98 (0.96–0.99) | 0.01 | 0.96 (0.95–0.98) | <0.001 |
| BMI (kg/m2) | 1.02 (0.98–1.06) | 0.40 | 1.00 (0.99–1.07) | 1.00 | 0.98 (0.96–0.99) | 0.56 | 1.11 (1.03–1.19) | 0.004 |
| HTN | 1.08 (0.92–1.27) | 0.35 | 1.16 (0.88–1.54) | 0.28 | 1.11 (0.88–1.42) | 0.38 | 1.33 (0.93–1.92) | 0.12 |
| Education (high school graduate) | 0.65 (0.50–0.85) | 0.001 | 1.62 (0.76–3.45) | 0.21 | 0.85 (0.40–1.80) | 0.67 | 0.63 (0.44–0.89) | 0.009 |
| HDL-c (mg/dl) | 0.98 (0.97–0.99) | <0.001 | 0.98 (0.96–1.00) | 0.04 | 0.98 (0.97–1.00) | 0.08 | 0.98 (0.97–1.00) | 0.08 |
| Triglyceride (mg/dl) | 1.004 (1.003–1.006) | <0.001 | 1.004 (1.000–1.007) | 0.02 | 1.002 (1.000–1.004) | 0.01 | 1.004 (1.001–1.006) | <0.001 |
| CRP (mg/dl) | 1.01 (1.00–1.03) | 0.07 | 1.008 (0.98–1.04) | 0.54 | 1.03 (1.00–1.06) | 0.04 | 1.01 (0.98–1.04) | 0.50 |
| Waist (cm) | 1.03 (1.01–1.04) | <0.001 | 1.03 (1.004–1.06) | 0.03 | 1.04 (1.01–1.07) | 0.004 | 1.00 (0.97–1.03) | 0.88 |
| HOMA-IR | 1.12 (1.07–1.18) | <0.001 | 1.10 (1.03–11.17) | 0.005 | 1.36 (1.20–1.56) | <0.001 | 1.04 (0.98–1.12) | 0.17 |
NAFLD nonalcoholic fatty liver disease, BMI body mass index
There were differences in the clinical correlates of NAFLD within each race. When we analyzed just African Americans in multivariate analysis, we found that TG and HDL levels along with waist circumference and HOMA-IR values were the only significant correlates (Table 3). We performed a test for interaction to see if the effect of TG and HDL level on NAFLD was different in African Americans. We did not find a significant interaction effect between triglycerides and AA race (p = 0.77) or HDL-c and AA race (p = 0.14) and NAFLD presence.
Discussion
While African American's have the lowest prevalence rates of NAFLD in the United States compared to other ethnic groups, assuming a prevalence rate of 24 % obtained by Browning et al. [9] (the largest population based study of NAFLD prevalence), approximately 9 million African Americans are currently affected. This is significantly higher than the entire population of patients suffering from chronic hepatitis C virus infections in the United States (approximately 4 million) [23]. It is therefore important to determine the risk factors for NAFLD development specific to this population given that ethnic differences in pathophysiology of this disease have been established [13, 16, 24].
We found that after controlling for the major clinical risk factors, African Americans still had half the odds of having NAFLD when compared to Hispanics and Caucasians, suggesting there is some yet-to-be-measured protective element. Compared to the many clinical correlates of NAFLD seen when we evaluated the entire cohort, only insulin resistance, waist circumference, TG and HDL-c levels correlated to NAFLD in African Americans. Of note, the mean TG levels in African Americans with NAFLD (125 mg/dl; 95 % CI 112.5–137.6) is significantly lower than the mean found in the other ethnic groups and is considered normal according to the National Cholesterol Education Program (NCEP) which defines normal serum triglyceride levels in adults as <150 mg/dl [25]. Guerrero et al. [13] found serum triglyceride levels and hepatic triglyceride content to be lowest among the African Americans despite having the highest insulin levels and HOMA-IR values when compared to Caucasians and Hispanics. They concluded that the African American metabolic response to obesity and insulin resistance differed from that of Hispanics and Caucasians given that they appeared to be more resistant to the accumulation of triglyceride in the liver as well as hypertriglyceridemia associated with insulin resistance. We make the suggestion that perhaps lower TG thresholds should be used to identify African Americans at risk for NAFLD development.
This study does have limitations. First, our measure of NAFLD is not the gold standard and is only able to identify hepatic steatosis greater that 30 % with accuracy [26]. However, there is still no accurate means of predicting those individuals at risk for developing NASH and no accurate noninvasive methods of determining which individuals have simple steatosis and which have NASH. As a result, the majority of NAFLD in the general population is identified and studied through imaging studies [27]. Second, 36 % of the participants were excluded, although the vast majority due to non-visible liver or spleen on CT scan and missing lipid values. This data was likely missing at random and should not contribute a selection bias. The baseline laboratory measurements and CT scans were performed 10–12 years ago and so likely underestimate mean BMI and HOMA-IR as well as the prevalence of diabetes and therefore the prevalence of NAFLD. Our findings however are consistent with the prevalence rates seen in other large population-based studies [28]. Finally, the cross-sectional design of this study prevents us from assessing temporal relationships and therefore making causal inferences.
In conclusion, this study demonstrated that the clinical correlates of NAFLD presence in African Americans are similar to the correlates of NAFLD in other ethnic groups [10–12]; however, many of the risk factors for NAFLD seen in other ethnic groups do not appear to influence NAFLD presence in AA. Our data suggests that physicians should consider using lower cutoffs for defining abnormal TG levels (than the NCEP defined 150 mg/dl) to identify African Americans at potential risk for NAFLD development. Finally, our findings add to the growing data showing a decreased prevalence of NAFLD in African Americans compared to Caucasians and Hispanics, despite controlling for most known risk factors suggesting an unmeasured protective element, one perhaps influenced by PNPLA3. Until the role of PNPLA3 in the pathogenesis of NAFLD is clarified, and a screening test becomes readily available, we will have to rely on surrogate clinical markers to help us screen for NAFLD in this population. Clearly further studies need to be performed in this area.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health grants R01 HL071739 and by contracts N01-HC-95159 through N01-HC-95165 and N01 HC 95169.
Footnotes
Conflict of interest None.
Contributor Information
Temitope Foster, Email: tyfoste@emory.edu, Division of Digestive Disease, Emory University, 49 Jesse Hill Jr. Dr. S.E., Rm 437, Druid Hills, Atlanta, GA 30324, USA.
Frank A. Anania, Division of Digestive Disease, Emory University, 49 Jesse Hill Jr. Dr. S.E., Rm 437, Druid Hills, Atlanta, GA 30324, USA
Dong Li, Los Angeles BioMedical Research Institute, Harbor–UCLA, Medical Center, Torrance, CA, USA.
Ronit Katz, University of Washington, Seattle, WA, USA.
Matthew Budoff, Los Angeles BioMedical Research Institute, Harbor–UCLA, Medical Center, Torrance, CA, USA.
References
- 1.Kim WR, Brown RS, Jr, Terrault NA, El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36:227–242. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- 2.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 3.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 4.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Soderberg C, Stal P, Askling J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 6.Dunn W, Xu R, Wingard DL, et al. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol. 2008;103:2263–2271. doi: 10.1111/j.1572-0241.2008.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in nonalcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia. 2008;51:1947–1953. doi: 10.1007/s00125-008-1135-4. [DOI] [PubMed] [Google Scholar]
- 8.Jepsen P, Vilstrup H, Mellemkjaer L, et al. Prognosis of patients with a diagnosis of fatty liver—a registry-based cohort study. Hepatogastroenterology. 2003;50:2101–2104. [PubMed] [Google Scholar]
- 9.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 10.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 11.Fracanzani AL, Valenti L, Bugianesi E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48:792–798. doi: 10.1002/hep.22429. [DOI] [PubMed] [Google Scholar]
- 12.Donati G, Stagni B, Piscaglia F, et al. Increased prevalence of fatty liver in arterial hypertensive patients with normal liver enzymes: role of insulin resistance. Gut. 2004;53:1020–1023. doi: 10.1136/gut.2003.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009;49:791–801. doi: 10.1002/hep.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagenknecht LE, Scherzinger AL, Stamm ER, et al. Correlates and heritability of nonalcoholic fatty liver disease in a minority cohort. Obesity. 2009;17:1240–1246. doi: 10.1038/oby.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008;196:696–703. doi: 10.1016/j.atherosclerosis.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Romeo S, Huang-Doran I, Baroni MG, Kotronen A. Unravelling the pathogenesis of fatty liver disease: patatin-like phospholipase domain-containing 3 protein. Curr Opin Lipidol. 2010;21:247–252. doi: 10.1097/mol.0b013e328338ca61. [DOI] [PubMed] [Google Scholar]
- 17.Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17:143–152. [PubMed] [Google Scholar]
- 18.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 19.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 20.Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 21.Schoepf UJ, Becker CR, Ohnesorge BM, Yucel EK. CT of coronary artery disease. Radiology. 2004;232:18–37. doi: 10.1148/radiol.2321030636. [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki M, Takada Y, Hayashi M, et al. Noninvasive evaluation of graft steatosis in living donor liver transplantation. Transplantation. 2004;78:1501–1505. doi: 10.1097/01.tp.0000140499.23683.0d. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 24.Bambha K, Belt P, Abraham M, et al. Ethnicity and nonalcoholic fatty liver disease. Hepatology (Baltimore, MD) 2012;55:769–780. doi: 10.1002/hep.24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 26.Park SH, Kim PN, Kim KW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105–112. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 27.Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- 28.Speliotes EK, Massaro JM, Hoffmann U, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51:1979–1987. doi: 10.1002/hep.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]