Abstract
While our previous work suggests that the midazolam-induced memory impairment results from the inhibition of new association formation, little is known about the neural correlates underlying these effects beyond the effects of GABA agonists on the brain. We used arterial spin-labeling perfusion MRI to measure cerebral blood flow changes associated with the effects of midazolam on ability to learn arbitrary word-pairs. Using a double-blind, within-subject cross-over design, subjects studied word-pairs for a later cued-recall test while they were scanned. Lists of different word-pairs were studied both before and after an injection of either saline or midazolam. As expected, recall was severely impaired under midazolam. The contrast of MRI signal before and after midazolam administration revealed a decrease in CBF in the left dorsolateral prefrontal cortex (DLPFC), left cingulate gyrus and left posterior cingulate gyrus/precuneus. These effects were observed even after controlling for any effect of injection. A strong correlation between the midazolam-induced changes in neural activity and memory performance was found in the left DLPFC. These findings provide converging evidence that this region plays a critical role in the formation of new associations and that low functioning of this region is associated with anterograde amnesia.
Keywords: associative memory, arterial spin labeling, dorsolateral prefrontal cortex
Introduction
Benzodiazepines are GABA (gamma aminobutyric acid) agonists that have been used safely in research on memory (Hirshman et al., 2001; Mintzer et al., 2001). GABA is the primary inhibitory neurotransmitter in the mammalian central nervous system and the GABAA receptors are expressed in cerebral cortex, hippocampus, basal ganglia, thalamus, cerebellum, and brainstem (Young and Chu, 1990). Midazolam, like benzodiazepines in general, promotes transient anterograde amnesia (Bulach et al., 2005; Fisher et al., 2006; Merritt et al., 2005; Reder et al., 2006a) and as such, provides a promising tool for studying human memory that finesses problems inherent in patient populations. Some of our previous work has suggested that midazolam-induced memory effects occur by inhibiting the formation of new associations (Park et al., 2004; Reder et al., 2007; 2009). Less clear, however, are the neural underpinnings of this effect. For example, previous research has highlighted the role of the hippocampus in relational binding and its critical role in explaining anterograde amnesia (Davachi, 2006; Henke, 2010; Squire et al., 2007). It is also known that the hippocampus is one of the regions affected by benzodiazepines due to the high density of GABAA receptors in this region. Consequently, there is a reason to believe that midazolam impairs formation of associations because it impairs hippocampal functioning. The goal of this paper is to combine the use of Arterial Spin Labeling (ASL) perfusion MRI with midazolam in order to examine the neural mechanisms of memory and possible causes of anterograde amnesia.
Why use ASL with midazolam?
Neuroimaging studies that use fMRI and measure the blood oxygenation level dependent (BOLD) signal may not be optimal when sedatives such as benzodiazepines are involved. Oxygen is extracted from blood in the capillaries, and the resulting deoxyhemoglobin travels into the venous circulation. Because of this, the BOLD signal may be localized to veins that may be as far as a few centimeters from the site of neuronal activity.
In contrast to BOLD fMRI, ASL directly measures cerebral blood flow (CBF) by using arterial blood water as an endogenous contrast agent (Liu et al., 2007). The ASL signal is mainly localized to arteries, capillaries, and brain tissue, and its localization is believed to be closer in space to the true sites of neuronal activity than the BOLD signal (Kim et al., 2002).
Other advantages of ASL over BOLD fMRI include the lower inter-subject and inter-session variation and minimal sensitivity to magnetic-field inhomogeneity effects (Wang et al., 2003; 2004). The administration of a drug often increases the inter-subject variability due to the differences in the rate a given drug is metabolized by subjects. The sum of drug-related inter-subject variability and scanning-related variability (that characterizes the BOLD fMRI) might hinder a true signal. This makes ASL especially useful for neuroimaging of psychopharmacological effects.
Review of Possible Regions influenced by Midazolam
Studies involving benzodiazepines that have focused on the regional specificity of drug-induced memory impairment effects found a significantly diminished repetition-related attenuation effect in extrastriate, prefrontal (Thiel et al., 2001) and occipito-temporal (Stephenson et al., 2003) regions. Such studies have also found a decrease in the extent and magnitude of activation within the hippocampal, fusiform, and inferior prefrontal cortices during encoding of face-name associations (Sperling et al., 2002). Furthermore Mintzer et al. (2001) found a dose-related deactivation in encoding-associated areas, such as right prefrontal cortex, left parahippocampal gyrus and left anterior cingulate cortex. However, no previous study used ASL to examine the effect of midazolam nor related changes in memory performance with changes in activation induced by the injection of the drug.
Relating Memory Performance to CBF changes under the influence of Midazolam
In this experiment, a long list of word-pairs was divided into four short lists, such that one-fourth of the word pairs were studied prior to injection, another quarter immediately after injection, another quarter of the pairs mid-way through an unrelated task and the final quarter of the word-pairs at the completion of the unrelated task. Performance should be very poor for word pairs studied immediately after injection in the midazolam condition but performance should slowly improve over time for the next two lists (see Reder, et al., 2007 for more details). An obvious prediction is that performance will be much better for the words shown before injection, regardless of drug condition. In order to understand the relationship between the drug-induced changes in CBF and memory performance, we plan to correlate subjects’ neural activity with accuracy on the cued-recall test separately for the two different drug/saline sessions.
Method
Subjects
Nine (4 female) healthy paid volunteers (18–35 years old) participated in the study. All were screened by a medical doctor and gave their written informed consent for a protocol approved by the Institutional Review Boards (IRBs) of Carnegie Mellon University and the University of Pittsburgh. They received $150 compensation for their participation over two sessions.
Design and Materials
In a within-subject, double-blind, cross-over design, subjects received midazolam in one session and saline in the other, with the two sessions occurring approximately one week apart. Stimuli consisted of 192 different English concrete nouns that were randomly paired to make 96 unique word pairs (e.g., table-cat) for the two study sessions, half used at each session. The 48 word pairs were divided into 4 sub-lists of 12 word pairs each.
Procedure
Prior to entering the scanner, subjects were instructed as to the nature of the paired associate learning task and subsequent cued-recall test. They were told that they would passively view word pairs and should try to remember them for a later memory test outside the scanner. Word-pairs were presented individually for 15 seconds each while subjects lay still in the scanner. While viewing the word pairs, subject’s brain activity was imaged using ASL. Each short list was presented over a period of 3 minutes.
The first study list was shown immediately after structural images were taken and immediately before the injection of the drug or saline. The second list of 12 pairs was shown immediately after injection. Each word pair was shown on the screen for 15 seconds such that each study block lasted 3 min.
Following the presentation of the second of the four lists, subjects began a different task (a visual search task, using BOLD) that will not be reported here. After completing half of the visual search task, subjects studied the third list of 12 word pairs (again using ASL). The final 12 word pairs were studied after all trials of the other task were completed. The time in the scanner was approximately one hour, including 10 minutes for structural data.
Each session was followed by several tests outside the scanner including the cued recall test. Subjects were given a sheet of paper with the 48 stimulus (left-hand) words on a different line of the page. Subjects were asked to write down the corresponding response (right-hand) word of the pair if it could be recalled. The presentation order of the word pairs was a different random order than the study order of the word pairs in the scanner.
Drug Administration
After the first ASL block that involved viewing the first list of 12 word pairs and while still lying in the bore, the subject was given a single bolus injection, within a 2-min period, of either midazolam (0.03 mg/kg of the subject’s body mass) or a matching volume of saline.
Imaging-data Acquisition
MRI data were collected on a 3T Siemens Tim Trio MRI scanner equipped with a standard transmit/receive head coil. A pulsed arterial spin labeling (PASL) sequence was used for perfusion fMRI scans. Interleaved images with and without labeling were acquired using a gradient echo planar imaging sequence (TR/TE/TI=3000/20/1800 ms; flip angle=90°). The tagging/control duration was 0.7 seconds. 19 oblique slices (thickness/gap=5/1 mm, field of view=224×224 mm2, matrix=70×70, voxel=3.2×3.2×5 mm3) covered the whole brain. For registration purposes, high-resolution anatomical images were acquired using a 3D magnetization prepared rapid gradient echo (MPRAGE) T1-weighted sequence (TR=2100 ms, TE=3.63 ms, inversion time (TI)=1100 ms, flip angle=8°, 192 contiguous slices of 1.0 mm thickness; The images were reconstructed as a 192×416×512 matrix with a 1.0×0.5×0.5 mm3 spatial resolution) for each subject. The total length of scan time lasted ~1 hour including the perfusion scan (Each block with 60 acquisitions lasted 3 min), anatomic scan, and other scans for BOLD imaging.
Data Analysis
Perfusion fMRI data were analyzed offline using the ASL Data Processing Toolbox (Wang et al., 2008) and the SPM5 software package. Data analysis focused on trials immediately before and immediately after intravenous midazolam injection so that the effect due to the drug was maximal (i.e., had not yet started to wear off, Schwagmeier et al., (1998)).
The steps of ASL data analysis were similar to those in Wang et al. (2005). MR image series were first realigned to correct for head movements, co-registered with each subject’s structural MRI, and spatial smoothed with a 12-mm full-width at half-maximum (FWHM) Gaussian kernel. Subjects’ head motion was less than 1.5 mm in any of the x, y, or z directions and less than 1.5° of any angular motion throughout the course of scan. Perfusion-weighted images series were generated by pair-wise subtraction of the label and control images, followed by conversion to absolute CBF image series based on a single compartment continuous arterial spin labeling perfusion model (Wang et al., 2005). Individual mean CBF images for each block were normalized into a canonical space (Montreal Neurological Institute standard brain) with re-sampling to 3×3×3 mm3. A paired t-test was performed using SPM5 to examine the effect of the MZ injection (before the MZ injection vs. after MZ injection (pre_MZ vs. post_MZ)) and the effect of the saline injection ((before the saline injection vs. after saline injection (pre_SA vs. post_SA)) under a combined threshold of P < 0.005 and cluster size >= 675 mm3. This yields a corrected threshold of p < 0.05, determined by Monte Carlo simulation using the AlphaSim program (FWHM=12 mm, with a mask of the whole brain gray matter tissues). Then, the two contrasts were compared using a random-effect two-sample t-test in the voxels activated in the pre_MZ vs. post_MZ or pre_SA vs. post_SA contrasts. The resulting images were thresholded at a combined threshold of p < 0.01 and cluster size >=135 mm3, which yields a corrected threshold of p < 0.001, determined by the AlphaSim program (FWHM=12 mm and the contrasts of pre_MZ vs. post_MZ or pre_SA vs. post_SA as masks). Based on the activation clusters from the above contrasts, we defined functional regions of interest (ROIs) using the WFU PickAtlas toolbox. The CBF changes extracted from each subject’s data from these ROIs were used for the Pearson’s correlation analysis of the drug-induced changes in neural and behavioral performance.
Results
Due to technical failures, data from two subjects were incomplete and could not be analyzed, leaving seven complete data sets (two sessions per subject) to be analyzed.
Behavioral Data
A 2×2 within subjects repeated measures ANOVA was performed on the cued-recall data (Figure S1). There was a main effect of drug session, F(1,6)=7.1, p <0.01, whether studied pre- or post-injection, F(1,6)=5.2, p <0.1, and an interaction between these two factors F(1,6)=15.1, p <0.01. A planned comparison of pre- vs. post- injection conditions revealed a significant difference under midazolam F(1,6)=16.4, p <0.01, but not under saline F(1,6)=0.3, p >0.1.
Imaging Data
We first compared CBF pre- vs. post- injection in the midazolam condition. This analysis revealed decreases in the left middle frontal gyrus (BA 46), right superior and middle frontal gyrus (BA 9, 10), left inferior temporal gyrus (BA 20), left cingulate gyrus (BA 24), left PCu (BA 31, 7), right precentral gyrus (BA 4), right thalamus and right caudate (Table S1; Figure S2). There was also an effect on CBF of pre- vs. post- injection in the saline condition: The contrasts were reliable in the left inferior temporal gyrus/fusiform gyrus (BA 37), left caudate/insula (BA 13), right putamen/caudate/inferior frontal gyrus (BA 47) and right superior temporal gyrus (BA 13/41/42) (Table S2; Figure S3).
Given our interest in the effects of midazolam on brain activity as opposed to the effects of injection per se (e.g., emotion experience due to the injection), we contrasted the effect of midazolam injection with the effect of the saline injection. The results of this contrast are shown in Table 1 (Figure S4). Even after controlling for the effect of injection, midazolam-induced decreases still remain in the left middle frontal gyrus (BA 46) (Figure 1), left cingulate gyrus (BA 24) and left posterior cingulate/precuneus (PCC/PCu, BA 31, 7).
Table 1.
Regions significantly activated between pre- and post-injection of midazolam after controlling for corresponding changes between pre- and post-injection of saline. Loci of maxima are in MNI coordinates in mm. Lt, left.
| Regions | BA | Cluster | MNI Coordinates | T-score | ||
|---|---|---|---|---|---|---|
| (pre_MZ vs post_MZ) vs (pre_SA vs post_SA) | ||||||
| Lt. middle frontal gyrus | 46 | 5 | −57 | 33 | 27 | 3.43 |
| Lt. cingulate gyrus | 24 | 9 | −3 | −12 | 39 | 3.24 |
| Lt. posterior cingulate | 31 | 54 | −9 | −60 | 21 | 6.17 |
| Lt. precuneus | 7 | −9 | −63 | 39 | 4.24 | |
| (post_MZ vs pre_MZ) vs (post_SA vs pre_SA) | ||||||
| None | ||||||
Figure 1.
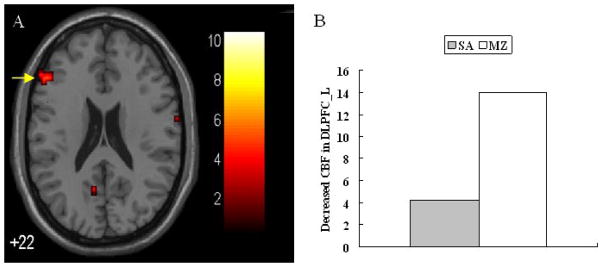
(A) Midazolam-induced decrease in activation in left DLPFC ROI defined from clusters shown in Table S1; (B) A plot of the decreased CBF changes after injection in the left DLPFC in the midazolam and saline conditions.
i Given that the drug wears off over time, for most of the analyses, we opted to focus on the contrast that would give us the biggest effect, namely immediately before vs. immediately after injection.
Correlation between Behavioral and Imaging Data
Three functional ROIs that survived the correction for the effect of injection (Table 1) were defined based on the corresponding clusters in Table S1. We then correlated the changes in CBF from pre- to post- injection in these three ROIs with the difference in cued recall accuracy for word-pairs studied pre- vs. post- injection. In the midazolam condition there was a remarkably strong correlation in the left middle frontal gyrus (r=0.80, p<0.05), but not in the saline condition (r=0.002) (Figure S5). The correlations for the other two ROIs were not reliable for either drug condition (MZ: left cingulate gyrus, r=0.07; left PCC/PCu, r=−0.34; SA: left cingulate gyrus, r=−0.21; left PCC/PCu, r=0.55).
The effect of midazolam on memory performance immediately after injection was huge (no subject recalled any words studied in that block). That means that the correlations in the midazolam condition were driven by changes from the baseline in the pre-injection condition. To examine whether the strong correlation was somehow caused by the floor effect for items studied immediately after the midazolam injection, we also correlated CBF changes between the first (pre-injection) and fourth (final) encoding blocks, using the difference in memory performance between first and fourth list. The final block memory performance was not near the floori because the drug had begun to wear off (approximately 40 minutes after injection) and the delay from study to test was shortest (The post-test recall accuracy for word-pairs is plotted as a function of all four study blocks and drug condition in Figure S6). The correlation in the left DLPFC was strong, r=0.63, but only marginally significant (one-tailed test). The correlation of CBF and memory performance in the left cingulate gyrus was moderate but not significant, r=−0.39, while in the left PCC/PCu it was strong, r=−0.73, and reached significance at p <0.05, one-tailed.
Discussion
In this study, healthy subjects were scanned, using ASL, while encoding word pairs under midazolam in one session and under saline in another. Of interest was the effect of the drug manipulation on CBF during encoding of pair associates. As expected, the injection of midazolam severely impaired subjects’ memory for word pairs. Consistent with previous BOLD fMRI and PET studies, the analysis of the ASL data revealed midazolam-induced CBF decreases in frontal, temporal, parietal and some subcortical regions (Veselis et al., 1997; Reinsel et al., 2000; Sperling et al., 2002). Unlike previous studies, this experiment was a within-subject, double-blind design, we thus could compare changes pre- vs. post-injection under saline for any effects due to anticipation of a drug or fear from an injection. The regions that showed an effect, post-injection under midazolam after controlling for the injection of saline, are arguably due only to the drug itself. We still found that the left DLPFC, the left cingulate gyrus and the left PCC/PCu were activated.
We also investigated whether the neural decreases in the ROIs listed above (by subject) were correlated with a given subject’s difference in memory performance pre- vs. post- injection of midazolam. We did not find a reliable correlation between memory performance and neural effects in either the left cingulate gyrus or the left PCC/PCu, suggesting that these regions may contribute more to the drug’s sedative effect than to memory impairment. However, a significant positive correlation between the drop in memory performance and the decrease in the CBF was found in the left DLPFC. Previous non-drug studies also have implicated left DLPFC in encoding of associations between items (Murray and Ranganath, 2007; Blumenfeld et al., 2006), providing converging evidence for this interpretation. This finding also provides further support for our view that midazolam blocks the formation of long-term memory associations (Park et al., 2004; Reder et al., 2007).
One alternative explanation for the effect of midazolam on memory is that the memory failures reflect an impairment of consolidation of newly formed associations in long-term memory rather than their formation per se, (c.f., Reder et al., 2006b; Curtis et al., 2003). This interpretation seems unlikely because retrieval of associations formed just prior to the midazolam injection were unaffected by the drug.
Some previous studies have found that left DLPFC is a part of the attention network (Fan et al., 2005). This suggests that decreases in DLPFC under midazolam may adversely affect attention to a stimulus during encoding rather than blocking the formation of new bindings/associations. Although we did not measure sedation using related tests such as Visual Analogue Scales (VAS) (Wezenberg et al., 2007), we have a reason to believe that memory impairment should not be attributed to a lack of attention. First, subjects’ performance in other tasks involving midazolam (Park et al., 2004) showed no evidence of impairment in attention (e.g., speed and accuracy in a visual search task). Second, subjects received a very low-dose of midazolam (i.e., 0.03 mg/kg of the subject’s body mass). Third, the anesthesiologists and experimenters typically could not identify whether the subjects’ drug condition was midazolam or saline.
The hippocampus is considered to be critical to associative memory (Davachi, 2006; Henke, 2010; Squire et al., 2007). However, contrary to these expectations, we failed to observe decreases in hippocampal activation under midazolam. The failure to detect changes in hippocampal activity is sometimes reported in the neuroimaging literature. For example, Veselis et al. (1997) failed to detect hippocampal deactivation under midazolam; however, in a later study they found a dose-dependent hippocampal effect (Reinsel et al., 2000). One reason for a lack of a hippocampal effect is a low signal to noise ratio due to the shape and location of hippocampus (Zeineh et al., 2003). Another reason for the failure to observe a decreased CBF in hippocampus in our study may be related to the relatively large voxel size (5 × 5 × 3) we used. To isolate the small hippocampal region, it may be necessary to use smaller voxels.
To our knowledge, a combined psychopharmacological and ASL methodology has not been previously implemented to investigate the neural mechanisms of memory. In this double-blind, within-subject design experiment, we used ASL to understand the midazolam-induced episodic memory impairments. Our results suggest that midazolam disrupts activation in left DLPFC, thus impairing the formation of new associations. In addition, we also identified novel patterns of neural activity due to enhanced spatial localization and lower variability between and within imaging sessions.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China 61105118 (to Peipeng Liang), the National Institutes of Health to L. Reder: 5R01MH052808 and T32MH019983 (supported A. Manelis).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blumenfeld RS, Ranganath C. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. J Neurosci. 2006;26:916–925. doi: 10.1523/JNEUROSCI.2353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bulach R, Myles PS, Russnak M. Double-blind randomized controlled trial to determine extent of amnesia with midazolam given immediately before general anaesthesia. BJA. 2005;94:300–305. doi: 10.1093/bja/aei040. [DOI] [PubMed] [Google Scholar]
- 3.Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Science. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- 4.Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. NeuroImage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Fisher J, Hirshman E, Henthorn T, Arndt J, Passannante A. Midazolam amnesia and short-term/working memory processes. Conscious Cognition. 2006;15:54–63. doi: 10.1016/j.concog.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Henke K. A model for memory systems based on processing modes rather than consciousness. Nat Rev Neurosci. 2010;11:523–532. doi: 10.1038/nrn2850. [DOI] [PubMed] [Google Scholar]
- 8.Hirshman E, Passannante A, Arndt J. Midazolam amnesia and conceptual processing in implicit memory. J Exp Psychol Gen. 2001;130:453–465. doi: 10.1037//0096-3445.130.3.453. [DOI] [PubMed] [Google Scholar]
- 9.Kim SG, Duong TQ. Mapping cortical columnar structures using fMRI. Physiol Behav. 2002;77:641–644. doi: 10.1016/s0031-9384(02)00901-0. [DOI] [PubMed] [Google Scholar]
- 10.Liu TT, Brown GG. Measurement of cerebral perfusion with arterial spin labeling: Part 1. Methods. J Int Neuropsychol Soc. 2007;13:517–525. doi: 10.1017/S1355617707070646. [DOI] [PubMed] [Google Scholar]
- 11.Merritt P, Hirshman E, Hsu J, Berrigan M. Metamemory without the memory: are people aware of midazolam-induced amnesia? Psychopharmacology. 2005;177:336–343. doi: 10.1007/s00213-004-1958-8. [DOI] [PubMed] [Google Scholar]
- 12.Mintzer MZ, Griffiths RR, Contoreggi C, Kimes AS, London ED, Ernst M. Dose effects of triazolam on brain activity during episodic memory encoding: a PET study. Neuropsychopharmacology. 2001;25:744–756. doi: 10.1016/S0893-133X(01)00280-9. [DOI] [PubMed] [Google Scholar]
- 13.Murray LJ, Ranganath C. The dorsolateral prefrontal cortex contributes to successful relational memory encoding. J Neurosci. 2007;27:5515–5522. doi: 10.1523/JNEUROSCI.0406-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park H, Quinlan JJ, Thornton ER, Reder LM. The effect of midazolam on visual search: Implications for understanding amnesia. Proc Natl Acad Sci U S A. 2004;101:17879–17883. doi: 10.1073/pnas.0408075101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reder LM, Oates JM, Thornton ER, Quinlan JJ, Kaufer A, Sauer J. Drug induced amnesia hurts recognition, but only for memories that can be unitized. Psychol Sci. 2006a;17:562–567. doi: 10.1111/j.1467-9280.2006.01744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reder LM, Proctor I, Anderson JR, Gyulai F, Quinlan JJ, Oates JM. Midazolam does not inhibit association formation, just its storage and strengthening. Psychopharmacology. 2006b;188:462–471. doi: 10.1007/s00213-006-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reder LM, Oates JM, Dickison D, Anderson JR, Gyula F, Quinlan JJ, Ferris JL, Dulik M, Jefferson BF. Retrograde facilitation under midazolam: The role of general and specific interference. Psychon Bull Rev. 2007;14:261–269. doi: 10.3758/bf03194062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reder LM, Park H, Kieffaber PD. Memory systems do not divide on consciousness: Reinterpreting memory in terms of activation and binding. Psychological Bulletin. 2009;135:23–49. doi: 10.1037/a0013974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reinsel RA, Veselis RA, Dnistrian AM, Feshchenko VA, Beattie BJ, Duff MR. Midazolam decreases cerebral blood flow in the left prefrontal cortex in a dose-dependent fashion. Int J Neuropsychopharmacol. 2000;3:117–127. doi: 10.1017/S1461145700001814. [DOI] [PubMed] [Google Scholar]
- 20.Schwagmeier R, Alincic S, Striebel HW. Midazolam pharmacokinetics following intravenous and buccal administration. Br J Clin Pharmacol. 1998;46:203–206. doi: 10.1046/j.1365-2125.1998.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sperling R, Greve D, Dale A, Killiany R, Holmes J, Rosas HD, Cocchiarella A, Firth P, Rosen B, Lake S, Lange N, Routledge C, Albert M. Functional MRI detection of pharmacologically induced memory impairment. Proc Natl Acad Sci U S A. 2002;99:455–460. doi: 10.1073/pnas.012467899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephenson CM, Suckling J, Dirckx SG, Ooi C, McKenna PJ, Bisbrown-Chippendale R, Kerwin RW, Pickard JD, Bullmore ET. GABAergic inhibitory mechanisms for repetition-adaptivity in large-scale brain systems. Neuroimage. 2003;19:1578–1588. doi: 10.1016/s1053-8119(03)00257-x. [DOI] [PubMed] [Google Scholar]
- 24.Thiel CM, Henson RNA, Morris JS, Friston KJ, Dolan RJ. Pharmacological modulation of behavioral and neuronal correlates of repetition priming. The Journal of Neuroscience. 2001;21:6846–6852. doi: 10.1523/JNEUROSCI.21-17-06846.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veselis RA, Reinsel RA, Beattie BJ, Mawlawi OR, Feshchenko VA, DiResta GR, Larson SM, Blasberg RG. Midazolam changes cerebral blood flow in discrete brain regions: An H215O positron emission tomography study. Anesthesiology. 1997;87:1106–1117. doi: 10.1097/00000542-199711000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Aguirre GK, Kimberg DY, Roc AC, Li L, Detre JA. Arterial spin labeling perfusion fMRI with very low task frequency. Magn Reson Med. 2003;49:796–802. doi: 10.1002/mrm.10437. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Aguirre GK, Kimberg DY, Roc AC, Li L, Detre JA. Reduced susceptibility effects in perfusion fMRI with single-shot spin-echo EPI acquisitions at 1.5 Tesla. Magn Reson Imaging. 2004;22:1–7. doi: 10.1016/S0730-725X(03)00210-8. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Aguirre GK, Rao H, Wang J, Fernández-Seara MA, Childress AR, Detre JA. Empirical ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging. 2008;26:261–269. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Zhang Y, Wolf RL, Roc AC, Alsop DC, Detre JA. Amplitude modulated continuous arterial spin labeling perfusion MR with single coil at 3T-feasibility. Radiology. 2005;235:218–228. doi: 10.1148/radiol.2351031663. [DOI] [PubMed] [Google Scholar]
- 30.Wezenberg E, Sabbe BGC, Hulstijn W, Ruigt GSF, Verkes RJ. The role of sedation tests in identifying sedative drug effects in healthy volunteers and their power to dissociate sedative-related impairments from memory dysfunctions. J Psychopharmacol. 2007;21:579–587. doi: 10.1177/0269881106071550. [DOI] [PubMed] [Google Scholar]
- 31.Young AB, Chu D. Distribution of GABAA and GABAB receptors in mammalian brain: Potential targets for drug development. Drug Dev Res. 1990;21:161–167. [Google Scholar]
- 32.Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299:577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


