Abstract
Induced pluripotent stem cell technology has attracted enormous interests for potential application in regenerative medicine. Here, we reported that a specific glycogen synthase kinase 3 (GSK-3) inhibitor, CHIR99021, can induce the reprogramming of mouse embryonic fibroblasts (MEFs) transduced by only Oct4 and Klf4 two factors. When combined with Parnate (also named tranylcypromine), an inhibitor of lysine-specific demethylase 1, CHIR99021 can result in the reprogramming of human primary keratinocyte transducted with Oct4 and Klf4 two factors. To our knowledge, this is the first time to generate human iPS cells from somatic cells without exogenous Sox2 expression. Our studies suggest that the GSK-3 inhibitor might have a general application to replace transcription factors in both mouse and human reprogramming.
INTRODUCTION
Pioneering work showed that virus-mediated expression of four transcription factors, Oct4, Klf4, Sox2 and c-Myc 1, reprograms mouse somatic cells into induced pluripotent stem (iPS) cells which closely resemble embryonic stem (ES) cells 1, 2. Reprogramming human somatic cells had also been achieved through similar strategy 3, 4. The iPS cell technology has attracted enormous interests with respect to its potential practical applications. By reprogramming and differentiation processes, patient-specific pluripotent stem cells could be created and further differentiated into functional autologous cells for cell-based therapy with alleviated immunocompatibility issues and ethical concerns. However, iPS cell applications are hindered by safety concerns and its complexity as the generation of iPS cells typically involves integration of exogenous DNA sequences. The key advances aimed at overcoming these safety concerns have been achieved by using non-integrating gene delivery approaches (such as adenovirus or episomal plasmid transfection) 5–7, or using cell membrane permeable proteins to trigger the reprogramming8, 9. However, reprogramming is extremely slow and inefficient under such conditions, which presents significant hurdles and potential risks to generate human iPS cells. Identification of small molecules or novel conditions that can enhance reprogramming or compensate the requirement of certain reprogramming factors will be highly desirable. We and others have shown it is possible to generate iPS cells with fewer factors by exploiting the endogenous gene expression 10–12. Neural progenitor cells (NPCs) with endogenous Sox2 expression13, could be reprogrammed into authentic iPS cells with only Oct4 and Klf4 transduction, however with a lower efficiency 10, 11. Using a chemical screen, a G9a histone methyltransferase inhibitor, BIX-0129414 was identified to enhance the reprogramming efficiency over 8 fold or replace the requirement of Oct4 transduction in NPC reprogramming 11. Importantly, BIX-01294 was also shown to enable the reprogramming of MEFs (which do not express Sox2) under Oct4 and Klf4 two factor conditions 15. From a subsequent synergistic screen, other small molecules, e.g. DNA methyltransferase (DNMT) inhibitor RG108 and L-type calcium channel agonist BayK8644, were identified to enhance MEF reprogramming. Similarly, another DNMT inhibitor, 5-AZA, was shown to improve the reprogramming efficiency in MEF cells up to 4 folds by transiting partially reprogrammed cells to become fully pluripotent. In another study, histone deacetylase (HDAC) inhibitors such as valproic acid (VPA) were shown to be able to enhance the reprogramming efficiency 16. In particular, VPA enabled reprogramming of human fibroblasts with only Oct4 and Sox2 17.
Previous studies showed that Wnt3a conditioned media promotes reprogramming of MEF cells 18. Wnt signaling entails inhibition of glycogen synthase kinase 3 (GSK-3) and stabilization of cytoplasmic β-catenin. Small molecule inhibitors of GSK-3 can mimic the activation of Wnt signaling, and maintain the pluripotent state of mES cells19–21. Lluis F. et al. reported that BIO, a GSK-3 inhibitor, could promote the reprogramming of somatic cells after fusion with mES cells22. Silva et al. reported inhibition of MEK and GSK-3 (using PD0325901 and CHIR99021, respectively) could transit “pre-iPS cells” into fully reprogrammed pluripotent cells23. More recently, Lyssiotis et al. identified another GSK-3/CDK2 inhibitor, kenpaullone, which could substitute Klf4 in reprogramming of MEFs in the presence of Oct4, Sox2 and cMyc. However, as a more specific GSK-3 inhibitor, CHIR99021, failed in producing the same positive effects on inducing the reprogramming of MEF cells under the Oct/Sox2/c-Myc transduction, kenpaullone’s effect may not result from its GSK-3 inhibition and its precise mechanism remains elusive.
Here, we reported that a specific GSK-3 inhibitor, CHIR99021, could allow the reprogramming of both mouse and human somatic cells without Sox2 transgene. Our studies suggest that the GSK-3 inhibitor might have a general application to replace transcription factors in both mouse and human somatic cell reprogramming.
Materials and Methods
Cell Culture and Viral Transduction
MEFs were derived from 129S2/SvPasCrlf and ROSA26+/−/OG2+/− mice according to the protocol reported on WiCell Research Institute website: “Introduction to human embryonic stem cell culture methods”. ROSA26+/−/OG2+/− heterozygous transgenic mice carry GFP reporter gene under the control of the Oct4 promoter (Oct4-GFP) and the ubiquitously expressed neo/lacZ transgene 24. Animal experiments were performed according to the Animal Protection Guidelines of the Max Planck Institute for Biomolecular Research, Germany. MEFs were transduced by Oct4, Klf4 and Sox2 three factors, or two-factor combinations of the pMXs-based retroviruses encoding mouse Oct4, Klf4 and Sox2 (Addgene) as previously described 1. Twenty four hours later, transduced MEFs were seeded in 6-well plate and incubated with mESC growth medium: KnockoutTM DMEM, 7 % ES Cell-Qualified fetal bovine serum, 10 % Knockout Serum Replacement, 1% Glutamax, 1% Non-essential amino acids, 1% penicillin/streptomycin, 0.1 mM β-mercaptoethanol and 103 U/ml mLIF (Millipore). MEFs transduced with Oct4/Klf4/Sox2 (1×104 cells/well together with 105 cells/well CF1 feeders in 6-well plates) were then treated with GSK-3 inhibitor CHIR99021 (Stemgent) for two weeks, and EGFP positive colonies were picked up at the third week after treatment. MEFs transduced with Oct4/Klf4 (1×105 cells/well in 6-well plates) were treated with 10 μM CHIR99021 for four weeks, GFP positive colonies were picked up and expanded at the fourth to fifth week after treatment.
Neonatal Human Epidermal Keratinocytes (NHEKs, Lonza) were cultured and transduced with two-factor combinations of lentiviruses encoding human Oct4, Sox2 (pSin-EF2-Puro-based) and mouse Klf4 (pLOVE-based) as previously described 4, 25. Lentiviral vectors were obtained from Addgene. Twenty four hours later, 1×105 transduced NHEKs were seeded on the irradiated X-ray inactivated CF1 MEF feeder cells in a 100 mm dish by keratinocyte medium (Lonza). One week after, the media was changed to human ES cell medium: DMEM/F12, 20 % Knockout serum replacement, 1% Glutamax, 1% Non-essential amino acids, 1% penicillin/streptomycin, 0.1 mM β-mercaptoethanol and 100 ng/ml bFGF and treated with GSK-3 inhibitor CHIR99021 (Stemgent) (10 μM) alone or combined with valproic acid (0.5~2 mM), BIX-01294 (Stemgent) (1~2 μM), RG108 (Stemgent) (1~5 μM), Parnate (Sigma) (2~4 μM), PD0325901 (Stemgent) (0.5μM) and SB431542 (Tocris) (2μM). The media containing above small molecule combinations were changed every day. Two week after treatment, the cells were sub-cultured (1:1) on new feeder cells (PD0325901 and SB431542 were only used in the first two-week treatment). After another two weeks, the small molecules were removed and the cells were stained with Alexa Fluor 555-conjugated Mouse anti-Human TRA-1-81 antibody (BD Pharmingen). The positive colonies were marked and picked up for expansion on feeder cells in human ES cell medium about 7 weeks after transduction. The human iPSCs were sub-cultured regularly by Accutase (Chemicon). All cell culture products were from Invitrogen/Gibco BRL except where mentioned.
Cytochemistry and Immunofluorescence Assay
Alkaline Phosphatase staining was performed according to the manufacturer’s protocol using the Alkaline Phosphatase Detection Kit (Millipore). For immunofluorescence assay, cells were fixed in 4% paraformaldehyde for 10 minutes and washed three times with PBS containing 0.1% Triton X-100 (Sigma-Aldrich). The fixed cells were then incubated in blocking buffer, 0.1% Triton X-100 and 10% normal donkey serum (Jackson ImmunoResearch Laboratories Inc) in PBS (Invitrogen/Gibco BRL), for 30 min at room temperature. The cells were then incubated with primary antibody overnight at 4°C in blocking buffer. The day after, cells were washed with PBS and incubated with secondary antibody in PBS containing 0.1% Triton X-100 for one hour at room temperature. Mouse anti-Oct4 antibody (1:250) (Santa Cruz Biotechnology), rabbit anti-Sox2 antibody (1:2000) (Chemicon), mouse anti-SSEA1 antibody (1:250) (Santa Cruz Biotechnology), rabbit anti-Nanog antibody (1:250) (Abcam), rat anti-SSEA3 antibody (1:1000) (Chemicon), mouse anti-SSEA4 antibody (1:1000) (Chemicon), mouse anti-TRA-1-81 antibody (1:1000) (Chemicon), goat anti-Sox17 (1:200) (R&D), mouse anti-βIII-Tubulin (Tuj1) antibody (1:1000) (Covance Research Products), rabbit anti-Brachyury antibody (1:200) (Santa Cruz) were used as primary antibodies. Secondary antibodies were Alexa Fluor 486/555 donkey anti-mouse, anti-rat, anti-goat or anti-rabbit IgG (1:500) (Invitrogen). Nuclei were visualized by DAPI staining (Sigma-Aldrich). Images were captured using a Nikon Eclipse TE2000-U microscope.
Differentiation of iPSCs in Vitro
The in vitro differentiation of miPSCs-OK and hiPSCs-OK was carried out by the standard embryoid body (EB) differentiation method. The iPSCs were dissociated by either 0.05 % Trypsin-EDTA (miPSCs-OK) or Accutase (hiPSCs-OK), and then cultured in ultra-low attachment 100-mm dish in DMEM medium supplemented with 10% FBS to form EBs. The medium was changed every other day. One week later, the EBs were harvested and transferred into Matrigel-coated 6-well plate in DMEM medium with 10% FBS. Three to seven day later, the cells were fixed for immunocytochemistry analysis.
PCR analysis
To detect the expression of pluripotency genes by MEFs and NHEKs that were treated with small molecules, untransduced MEFs and NHEKs were treated for three days in mESC growth medium with 10 μM CHIR99021 or in hES cell medium with either combination of 10 μM CHIR99021 and 2 μM Parnate or combination of 10 μM CHIR99021, 2 μM Parnate, 0.5 μM PD0325901 and 2 μM SB431542. For the semi-quantitative and quantitative RT-PCR analyses, RNA was extracted from miPSCs-OK, hiPSCs-OK, MEFs, treated MEFs and treated NHEKs using the RNeasy Plus Mini Kit in combination with QIAshredder (Qiagene). Reverse transcription was performed with 1μg RNA using iScriptTM cDNA Synthesis Kit (BioRad). The expression of pluripotent markers by miPSCs-OK was analyzed by RT-PCR using Platinum PCR SuperMix (Invitrogen). The primers for the endogenous Oct4, Sox2, Klf4 and Nanog were as reported 1. Amplification of viral transduced genes was done using the gene specific forward primers (Klf4: 5′-GCG AAC TCA CAC AGG CGA GAA ACC-3′; Sox2: 5′-GGT TAC CTC TTC CTC CCA CTC CAG-3′ and Oct4: 5′-TTG GGC TAG AGA AGG ATG TGG TTC-3′) and common reverse primer pMXs-L3205 (5′-CCC TTT TTC TGG AGA CTA AAT AAA-3′)3. The RT-PCR was performed in 30 (amplification of pluripotent markers) or 35 (amplification of viral transduced genes) cycles (94°C for 30s, annealing temperature for 30s, and 72°C for 30s). Real-time PCR was carried out using iQ SYBR Green Supermix (BioRad). The primers for the human endogenous Oct4, total Oct4, endogenous Sox2, total Sox2, Nanog, Klf4, GDF-3 and Cripto were as reported 4, 26, 27. The primer for viral Klf4 was 5′-CAC CTT GCC TTA CAC ATG AAG AGG-3′ and 5′-CGT AGA ATC GAG ACC GAG GAG A-3′. The primer for FGF-4 was 5′-GAC ACC CGC GAC AGC CT -3′ and 5′-TCA CCA CGC CCC GCT-3′. The expression of genes of interest was normalized to that of GAPDH in all samples.
Genomic DNA was extracted from miPSCs-OK using DNeasy Blood & Tissue Kit (QIAGEN). In order to analyze the viral integration in miPSCs-OK, the genomic DNA of miPSCs-OK was subjected to PCR analysis using the same primers employed to amplify the viral transduced genes in the RT-PCR experiments. For the methylation analysis of Oct4 promoter by bisulfite-sequencing, DNA samples from hiPSC-OK were isolated using the Non Organic DNA Isolation Kit (Millipore) and were then treated with the EZ DNA Methylation-Gold Kit (Zymo Research Corp., Orange, CA). The treated DNA samples were then used as templates to amplify targets of interest. Primers used for the amplification of the Oct4 promoter fragment (406bp, from −2192~−1786) were 5′-GGA TGT TAT TAA GAT GAA GAT AGT TGG-3′ and 5′-CCT AAA CTC CCC TTC AAA ATC TAT T-3′28. The resulting fragments were cloned using the TOPO TA Cloning Kit for sequencing (Invitrogen) and sequenced.
Aggregation of iPSCs with zona-free embryos
miPSCs-OK were aggregated with denuded post-compacted eight-cell stage embryos to obtain aggregate chimeras. Eight-cell embryos (B6C3F1) were flushed from females at 2.5 dpc and cultured in microdrops of KSOM medium (10% FCS) under mineral oil. Clumps of iPSCs (10–20 cells) after short treatment of trypsin were chosen and transferred into microdrops containing zona-free eight-cell embryos. Eight-cell embryos aggregated with iPSCs were cultured overnight at 37 °C, 5% CO2. Aggregated blastocysts that developed from eight-cell stage were transferred into one uterine horn of a 2.5 dpc pseudopregnant recipient. The recipient mice were sacrificed at ED 13.5 day. The embryos were analyzed by x-gal staining to reveal the contribution of iPS cells.
Teratoma Formation
Three to five million hiPSC-OK (passage 8, clone 1) were injected under the kidney capsule of SCID mice (n=3). After 6–8 weeks, the neoplasm was removed and then histologically analyzed.
RESULTS
CHIR99021 can significantly promote the reprogramming of MEFs transduced by Oct4, Sox2 and Klf4
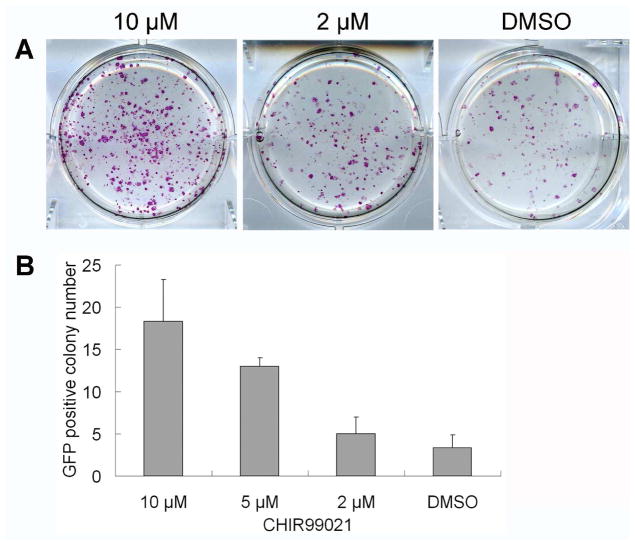
It had been shown that Oct4/Sox2/Klf4-infected MEFs could be reprogrammed into pluripotent state with higher efficiency when cultured under Wnt3a-conditioned medium18. However, small molecule activators of Wnt signaling pathway were not found to have similar effects. Combination of CHIR99021, a GSK-3 inhibitor which can activate Wnt signaling pathway, with PD0325901, a MEK inhibitor, was shown to promote partially reprogrammed iPSCs to full pluripotency23. Concurrent with those studies, we found that CHIR99021 could significantly promote reprogramming of murine fibroblasts. Treating Oct4/Sox2/Klf4 transduced MEFs with CHIR99021 for two weeks significantly increased the number of alkaline phosphase (AP)-positive mESC-like colonies in a dose-dependent manner (AP staining was done at the third week after treatment) (Figure 1A). CHIR99021 treatment of Oct4/Sox2/Klf4-transduced MEFs (ROSA26+/−/OG2+/−), which express GFP under the control of Oct4 promoter and also ubiquitously LacZ, also increased the number of GFP-positive colonies, which could be observed as early as two weeks after treatment. CHIR99021 showed the greatest effects at about 10 μM, which increase efficiency from 0.03–0.08 % to 0.2–0.4 % of transduced MEFs. (Figure 1B). Our results therefore suggest that CHIR99021 can significantly improve reprogramming efficiency of MEFs transduced with Oct4, Sox2, and Klf4. These mouse iPS cell colonies could be stably expanded under conventional mESC growth condition and express typical pluripotency markers, such as AP, Oct4, Sox2, Nanog, SSEA1 by cytochemistry and immunostaining.
Figure 1.
CHIR99021 promoted the reprogramming of MEFs transduced by Oct4, Sox2 and Klf4. MEFs from 129 strain were transduced with Oct4, Sox2 and Klf4 by retroviruses, and treated the next day with increasing concentrations of CHIR99021 for two week. Three weeks later, AP was detected by staining cells in monolayer (A). ROSA26+/−/OG2+/− MEFs transduced with Oct4, Sox2 and Klf4 were seeded into 6-well plates at the density of 1x104 cells/well (together with 105 cells/well CF1 feeders) and treated with CHIR99021 for two weeks. After 3 weeks of treatment, the GFP positive colonies in each well were counted (B). Error bars represent standard deviation for N=3.
CHIR99021 enabled the reprogramming of MEFs transduced by Oct4/Klf4
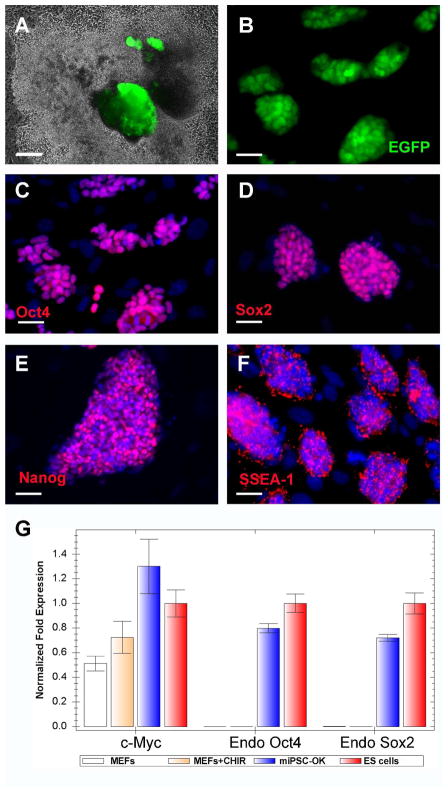
We had previously identified BIX01294, a small molecule inhibitor of a histone methyltransferase G9a, which enabled reprogramming of both mouse NPCs and MEFs infected by only Oct4 and Klf411, 15. We then investigated whether iPS cells could be generated from MEFs with fewer reprogramming factors in the presence of CHIR99021. OG2 MEFs transduced with different two-factor combinations (Oct4/Klf4, Oct4/Sox2 and Sox2/Klf4) were treated with 10 μM CHIR99021. GFP positive iPS cell colonies were identified only when MEFs were transduced with the combination of Oct4 and Klf4, but not with any other combination. On average, about six GFP positive colonies were identified out of 105 OG2 MEFs 4~5 weeks after Oct4/Klf4 transduction and CHIR99021 treatment. Stable iPS cell lines (miPSC-OK) were established by picking up the GFP positive colonies (Figure 2A, 2B). Immunocytochemistry revealed that miPSC-OK express typical pluripotency markers, such as Oct4, Sox2, Nanog and SSEA-1 (Figure 2C–F). MEFs do not express Sox2 endogenously, and real-time PCR analysis revealed that CHIR99021 treatment did not induce the expression of Sox2 and Oct4 in MEFs (Figure 2G). Therefore, the mechanisms by which CHIR99021 promotes the reprogramming of MEFs transduced by Oct4/Klf4 are independent of direct Sox2 induction.
Figure 2.
CHIR99021 enable the reprogramming of MEFs transduced with Oct4 and Klf4 only. ROSA26+/−/OG2+/− MEFs transduced with Oct4 and Klf4 were splited into 6-well plates at the density of 105 cells/well and treated with 10 μM CHIR99021 for 4 weeks. Panel A shows GFP-positive colonies before picking. Total of four miPSCs-OK lines were established (B). The expression of Oct4 (C), Sox2 (D), Nanog (E) and SSEA-1(F) by miPSCs-OK was detected by immunocytochemistry. The expression of pluripotency genes by MEFs after CHIR99021 treatment was analyzed by real-time PCR (G). Scale bars, 20μm. Error bars represent standard deviation for N=3.
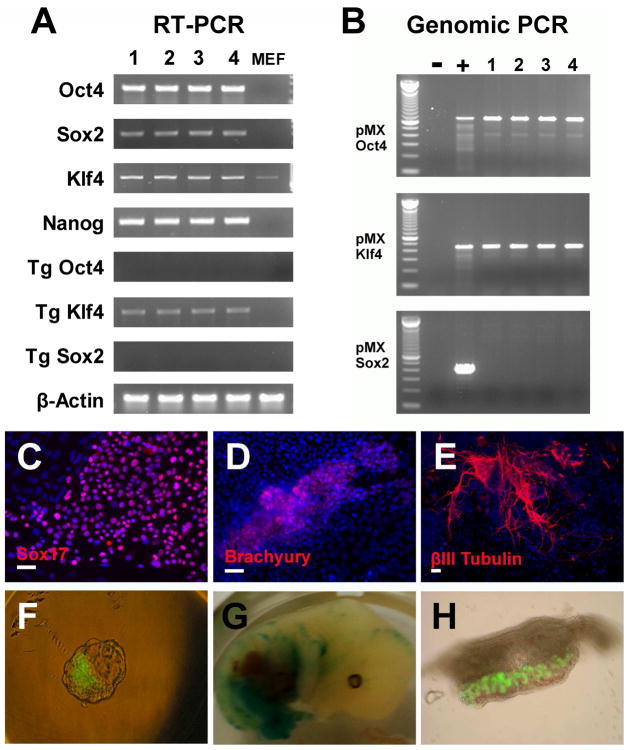
RT-PCR analysis confirmed the reactivation and expression of the endogenous mouse Oct4, Sox2, Nanog, and Klf4 (Figure 3A). By using the specific primers for transgenes, RT-PCR analysis revealed that the viral genes were largely silenced (Figure 3A). PCR of genomic DNA of miPSC-OK confirmed the integration of retroviral Oct4 and Klf4, but no other reprogramming genes (Figure 3B). To examine the developmental potential of miPSC-OK, in vitro differentiation assay was preformed. Immunostaining showed miPSC-OK could differentiate into endoderm (Sox17), mesoderm (brachyury) and neuroectoderm (βIII-tubulin) derivatives under the standard embryoid body differentiation methods (Figure 3C–E). Most importantly, miPSC-OK (clone 1 and clone 2) could efficiently incorporate into the inner cell mass (ICM) of blastocysts after aggregation with 8-cell embryos, lead to mid-gestational chimerism (ED13.5) after the aggregated embryos were transplanted into mice, and contribute to germline cells in vivo (Figure 3F–H). However, no adult chimeric mice were found after twenty embryos aggregated with miPSC-OK (clone 1) were transplanted. These in vitro and in vivo characterizations confirm that the miPSC-OK are molecularly, morphologically, and functionally similar to the original four-factor iPS cells and the mouse ESCs.
Figure 3.
PCR analysis and in vitro differentiation of miPSCs-OK. The expression of typical endogenous pluripotency genes and transduced genes (Tg) were analyzed by RT-PCR (A). Genomic PCR revealed integration of Oct4 and Klf4 retrovirues (B). 1~4 referred to the four established miPSCs-OK lines. 100bp DNA ladder (invitrogen) was used as a marker. Rat iPSCs generated by Oct4/Klf4/Sox2 transductions were used as positive control (+) and MEFs were used as negative control (−). Under standard EB differentiation methods, the in vitro pluripotency of miPSCs-OK was analyzed by Immunostaining (C–E). miPSCs-OK efficiently incorporated into the ICM of a blastocyst after aggregation with an 8-cell embryo (F). Chimeric embryo (13.5 dpc) was obtained after the transfer of the aggregated embryos into a pseudo-pregnant mouse. LacZ staining showed the contribution of miPSCs-OK (G). miPSCs-OK contributed to the germline cells (GFP–positive) in male gonad tissue isolated from chimeric embryos (H). Scale bars, 20 μm.
CHIR99021 enabled the reprogramming of human neonatal keratinocytes transduced with Oct4/Klf4 when combined with Parnate
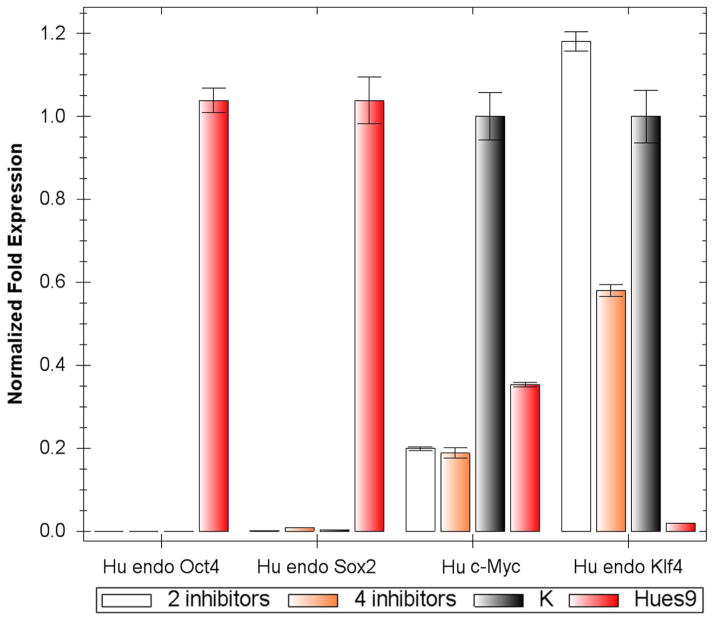
We next investigated whether human iPS cells could be generated with fewer transcription factors in the presence of CHIR99021 and/or direct epigenetic modifiers including inhibitors of DNA methyltransferase (RG108), histone methyltransferase (BIX01294), histone deacetylase (valproic acid/VPA) and lysine-specific demethylase 1 (Parnate). To this end, we selected primary human neonatal epidermal keratinocytes (HNEKs), concurrent with recent studies suggesting that keratinocytes transduced with four factors could be reprogrammed into iPS cells more efficiently and rapidly in comparison to other somatic cell types26. Primary keratinocytes were transduced with different two-factor combinations (Oct4/Klf4, Oct4/Sox2 and Sox2/Klf4), treated with CHIR99021 alone, or combined with epigenetic modifiers, and then stained with the human pluripotency cell-surface marker TRA-1-81 5 weeks post-infection. Tra-1-81 positive human ESC-like colonies could only be identified from culture infected by Oct4 and Klf4 in the presence of CHIR99021 and Parnate. On average, about two Tra-1-81 positive colonies could be identified out of 105 transduced HNEKs, which was at lease 100 times less efficient than 4-factor transduced keratinocytes. Stable human iPS cells could be established and long-term expanded by picking up these colonies (named hiPSC-OK). In addition, we have also found that combined treatment using inhibitors of MEK (PD0325901) and TGFβ receptor (SB431542) could improve the reprogramming efficiency of human fibroblasts transduced by Oct4/Sox2/Klf4/c-Myc (unpublished data). By using CHIR99021 (10 μM) and Parnate (2 μM) as the basal condition, addition of PD0325901 (0.5 μM) and SB431542 (2 μM) could further increase the TRA-1-81 positive colonies from human keratinocytes transduced with Oct4/Klf4 (about 5–10 Tra-1-81 positive colonies could be identified out of 105 transduced HNEKs), but the detailed mechanisms underlying this observation still need to be revealed. Nine TRA-1-81 positive colonies were expanded, and three stable human iPS cells (hiPSC-OK), one from CHIR99021 and Parnate condition (hiPSC-OK 1) and another two from CHIR99021/Parnate plus PD0325901/SB431542 condition (hiPSC-OK 2, 3), were further studied and long-term cultured for over 20 passages. hiPSC-OK express typical pluripotency markers, such as AP, Oct4, Sox2, Nanog, TRA-1-81, SSEA3 and SSEA-4 (Figure 4A–D). Real-time PCR analysis confirmed expression of the endogenous human Oct4, Sox2, Nanog, Cripto, GDF-3 and FGF4 (Figure 4E). Although the viral Oct4 and Klf4 expression was not completely silenced, bisulfite sequencing analysis revealed that the Oct4 promoter of hiPSC-OK is largely demethylated (Figure 4F). Similar to the CHIR99021 treatment of MEFs, real-time PCR analysis indicated neither CHIR99021/Parnate (2 inhibitors) nor CHIR99021/Parnate/PD0325901/SB431542 (4 inhibitors) treatment induced the expression of Sox2 and Oct4 in keratinocytes immediately (Figure 5). The terminal differentiation of keratinocytes induced by the human ES cell culture media may result in the significant down-regulation of c-Myc expression after treatment (Figure 5).
Figure 4.
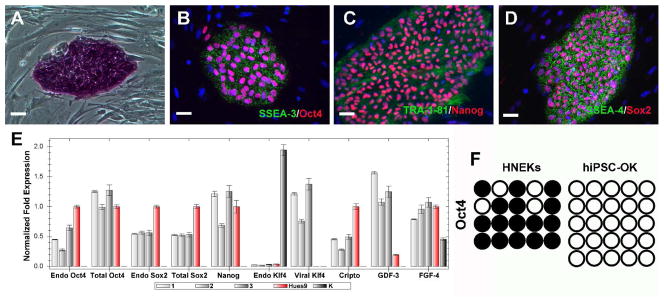
hiPSCs-OK are generated from primary human keratinocytes transduced by Oct4 and Klf4. Established hiPSC-OK clones express pluripotency markers AP (A), SSEA3 (green)/Oct4 (red; B), TRA-1-81 (green)/Nanog (red; C), and SSEA4(green)/Sox2 (red; D). Expression of endogenous (endo) markers and viral transgenes in hiPS-OK 1, 2 and 3 was determined by real-time PCR (E). Primary human keratinocytes (K) and Hues9 human ES cells were used as controls. Error bars represent standard deviation for N=3. The methylation status of the Oct4 promoter in primary human keratinocytes and hiPSC-OK was analyzed using bisulfite sequencing. Open circles indicate unmethylated, and filled circles indicate methylated CpG dinucleotides (F).
Figure 5.
Real-time PCR analysis of the expression of pluripotency genes by keratinocytes after small molecule treatment. Primary human keratinocytes (K), keratinocytes treated with either combination of 10 μM CHIR99021 and 2 μM Parnate (2 inhibitors), or combination of 10 μM CHIR99021, 2 μM Parnate, 0.5 μM PD0325901, and 2 μM SB431542 (4 inhibitors), and Hues9 human ES cells were analyzed. Error bars represent standard deviation for N=3.
To examine developmental potentials of hiPSC-OK, in vitro differentiation assays were preformed. Immunostaining confirmed that hiPSC-OK could differentiate into endoderm (Sox17), mesoderm (brachyury) and neuroectoderm (βIII-tubulin) (Figure 6A–C) derivatives in vitro. Furthermore, after transplanted into the SCID mice, hiPSC-OK formed teratoma consisting of representative derivatives of all three germ layers including epithelial tube structure (endoderm) (Figure 6D), cartilage-like structure (mesoderm) (Figure 6E) and neuroepithelium-like structure (ectoderm) (Figure 6F). These in vitro and in vivo characterizations confirm that the human iPS cells generated by Oct4 and Klf4 viral transduction closely resemble human ES cells in terms of typical pluripotency marker expression and differentiation potential.
Figure 6.
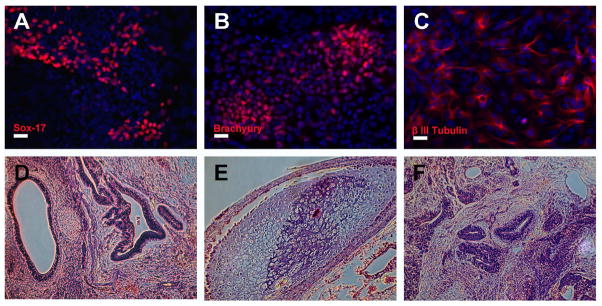
hiPSCs-OK showed pluripotent potential in vitro and in vivo. Using the standard EB differentiation method, the in vitro pluripotency of hiPSCs-OK was analyzed by immunostaining (A–C). hiPSCs-OK generated full teratoma in SCID mice. Hematoxylin and eosin staining of hiPSCs-OK teratoma sections showed epithelial tube structure (endoderm), cartilage-like structure (mesoderm) and neuroepithelium-like structure (ectoderm) appear in (D–F). Scale bars, 20 μm.
DISCUSSION
Reprogramming is a very slow and inefficient process. Such low efficiency and slow kinetics also present “hidden” risks in iPSCs, such as accumulated and selected subtle genetic and epigenetic abnormalities. Consequently, it is highly desirable to identify new conditions/small molecules that can promote reprogramming and/or replace certain factors.
In the present study we reported that the GSK-3 inhibitor CHIR99021 can significantly improve the reprogramming efficiency of MEFs transduced by Oct4/Sox2/Klf4, and also enable the reprogramming of MEFs transduced by only Oct4 and Klf4. When combined with Parnate, CHIR99021 can result in the reprogramming of human primary keratinocytes transducted with only Oct4 and Klf4 as well. Although previous studies showed that the activation of Wnt signaling promote somatic cell reprogramming, this study is the first report to show GSK-3 inhibitor could allow the reprogramming of both mouse and human somatic cell without Sox2. Recently it is reported that the target genes co-bounded by Oct4, Sox2 and Klf4 in ES cells showed a lower histone H3 lysine 4 (H3K4) trimethylation enrichment in partially reprogrammed cells than in ES/iPS cells, and this low histone H3K4 trimethylation may result in the lack of binding of many important regulators of pluripotency by Oct4, Sox2 and Klf4 29. Parnate, a monoamine oxidase inhibitor used as an antidepressant drug, showed potent inhibitory effect on lysine-specific demethylase 1 and inhibiting the H3K4 demethylation, but does not influence the acetylation of H3K9/K1430. Parnate may facilitate the full reprogramming of HNEKs transduced with only Oct4 and Klf4 by inhibiting H3K4 demethylation.
Particularly, this is also the first time to generate human iPS cells from somatic cells without exogenous Sox2 expression. Both Oct4 and Sox2 are critical regulators in human/mouse ES cell pluripotency and also the only common reprogramming factors used for generation of human iPS cells. Replacement of Sox2 in human cell reprogramming represents an important step toward identifying a chemically defined condition that could allow reprogramming human somatic cells by Oct4 only or without the forced expression of any exogenous factor. As HNEKs express Klf4 endogenously, it would be conceivable that HNEKs could perhaps be fully reprogrammed with only Oct4 transduction, but so far it was not achieved for unknown reasons. When Oct4 transduced HNEKs were treated under the same chemical condition, some human ES cell-like colonies were observed. After picking up these colonies, stable lines were established which could be long-term cultured under conventional human ES cell media. However, these cells are negative to AP staining, and expression of other pluripotency markers, such as Nanog and Sox2 could not be detected by immunostaining (data not shown).
Our studies underscore the unique advantage of the chemical approach for improving reprogramming that may ultimately allow the generation of iPS cells or multipotent tissue-specific cells in completely chemically defined conditions without any permanent genetic modification. Ultimately, a completely chemically defined condition for efficient reprogramming of somatic cells would be highly favorable for various iPS cell applications.
Acknowledgments
This work was supported by funding from Fate Therapeutics and CIRM to SD, Larry L Hillblom Foundation to AH.
Footnotes
Author contribution: SD and WL conceived the study. WL, SD and RA wrote the manuscript. WL, HZ, RA, SZ conducted the experiments. EH generated teratomas and AH supervised EH. JYJ generated chimera data and HRS supervised JYJ. SD supervised the study.
References
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 5.Stadtfeld M, Nagaya M, Utikal J, et al. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okita K, Nakagawa M, Hyenjong H, et al. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 7.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim D, Kim CH, Moon JI, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou H, Wu S, Joo JY, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JB, Zaehres H, Wu G, et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454:646–650. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y, Do JT, Desponts C, et al. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;2:525–528. doi: 10.1016/j.stem.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Eminli S, Utikal J, Arnold K, et al. Reprogramming of neural progenitor cells into induced pluripotent stem cells in the absence of exogenous Sox2 expression. Stem Cells. 2008;26:2467–2474. doi: 10.1634/stemcells.2008-0317. [DOI] [PubMed] [Google Scholar]
- 13.Blelloch R, Wang Z, Meissner A, et al. Reprogramming efficiency following somatic cell nuclear transfer is influenced by the differentiation and methylation state of the donor nucleus. Stem Cells. 2006;24:2007–2013. doi: 10.1634/stemcells.2006-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubicek S, O’Sullivan RJ, August EM, et al. Reversal of H3K9me2 by a small-molecule inhibitor for the G9a histone methyltransferase. Mol Cell. 2007;25:473–481. doi: 10.1016/j.molcel.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y, Desponts C, Do JT, et al. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008;3:568–574. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Huangfu D, Maehr R, Guo W, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huangfu D, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 18.Marson A, Foreman R, Chevalier B, et al. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell. 2008;3:132–135. doi: 10.1016/j.stem.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato N, Meijer L, Skaltsounis L, et al. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 20.Bone HK, Damiano T, Bartlett S, et al. Involvement of GSK-3 in regulation of murine embryonic stem cell self-renewal revealed by a series of bisindolylmaleimides. Chem Biol. 2009;16:15–27. doi: 10.1016/j.chembiol.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Umehara H, Kimura T, Ohtsuka S, et al. Efficient derivation of embryonic stem cells by inhibition of glycogen synthase kinase-3. Stem Cells. 2007;25:2705–2711. doi: 10.1634/stemcells.2007-0086. [DOI] [PubMed] [Google Scholar]
- 22.Lluis F, Pedone E, Pepe S, et al. Periodic activation of Wnt/beta-catenin signaling enhances somatic cell reprogramming mediated by cell fusion. Cell Stem Cell. 2008;3:493–507. doi: 10.1016/j.stem.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Silva J, Barrandon O, Nichols J, et al. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Do JT, Scholer HR. Nuclei of embryonic stem cells reprogram somatic cells. Stem Cells. 2004;22:941–949. doi: 10.1634/stemcells.22-6-941. [DOI] [PubMed] [Google Scholar]
- 25.Blelloch R, Venere M, Yen J, et al. Generation of Induced Pluripotent Stem Cells in the Absence of Drug Selection. 2007;1:245–247. doi: 10.1016/j.stem.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aasen T, Raya A, Barrero MJ, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 27.Mateizel I, De Temmerman N, Ullmann U, et al. Derivation of human embryonic stem cell lines from embryos obtained after IVF and after PGD for monogenic disorders. Hum Reprod. 2006;21:503–511. doi: 10.1093/humrep/dei345. [DOI] [PubMed] [Google Scholar]
- 28.Deb-Rinker P, Ly D, Jezierski A, et al. Sequential DNA Methylation of the Nanog and Oct-4 Upstream Regions in Human NT2 Cells during Neuronal Differentiation. J Biol Chem. 2005;280:6257–6260. doi: 10.1074/jbc.C400479200. [DOI] [PubMed] [Google Scholar]
- 29.Sridharan R, Tchieu J, Mason MJ, et al. Role of the murine reprogramming factors in the induction of pluripotency. Cell. 2009;136:364–377. doi: 10.1016/j.cell.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mimasu S, Sengoku T, Fukuzawa S, et al. Crystal structure of histone demethylase LSD1 and tranylcypromine at 2.25 A. Biochem Biophys Res Commun. 2008;366:15–22. doi: 10.1016/j.bbrc.2007.11.066. [DOI] [PubMed] [Google Scholar]