Abstract
Our previous studies have shown that aberrant arachidonic acid metabolism, especially the 5-lipoxygenase (5-Lox) pathway, is involved in oral carcinogenesis, and can be targeted for cancer prevention. In order to develop potent topical agents for oral cancer chemoprevention, five known 5-Lox inhibitors from dietary and synthetic sources, Zileuton, ABT-761, Licofelone, Curcumin and Garcinol, were evaluated in silico for their potential efficacy. Garcinol, a polyisoprenylated benzophenone from the fruit rind of Garcinia spp., was found to be a promising agent based on the calculation of a theoretical activity index. Computer modeling showed that garcinol well fit the active site of 5-Lox, and potentially inhibited enzyme activity through interactions between the phenolic hydroxyl groups and the non-heme catalytic iron. In a short-term study on 7,12-dimethylbenz[a]anthracene (DMBA)-treated hamster cheek pouch, topical garcinol suppressed leukotriene B4 (LTB4) biosynthesis and inhibited inflammation and cell proliferation in the oral epithelium. In a long-term carcinogenesis study, topical garcinol significantly reduced the size of visible tumors, the number of cancer lesions, cell proliferation, and LTB4 biosynthesis. These results demonstrated that topical application of a 5-Lox inhibitor, garcinol, had chemopreventive effect on DMBA-induced hamster cheek pouch carcinogenesis.
Keywords: Oral cancer, Chemoprevention, Garcinol, 5-Lipoxygenase, Topical
Introduction
Oral cancer is a common neoplasm worldwide, over 400,000 new cases being found each year, especially in developing countries (1). In the United States, approximately 39,400 new cases and 7,900 deaths are expected in 2011. Of those newly diagnosed individuals, only slightly more than half will be alive in 5 years. This situation has not significantly improved in decades (2, 3). Hence it is important to develop novel and effective chemopreventive strategies.
Arachidonic acid (AA) metabolism plays an important role in inflammation and inflammation-associated diseases including oral cancer. Among the AA-metabolizing pathways, the 5-lipoxygenase (5-Lox) pathway produces potent pro-inflammatory leukotrienes such as leukotriene B4 (LTB4), and promotes oral carcinogenesis (4-6). Alcohol, one of the major risk factors of oral cancer, promotes cancer, in part, through activation of the 5-Lox pathway (7). Targeting the 5-Lox pathway with single compounds or mixtures was effective in preventing oral carcinogenesis in animal models (6, 8-10). As we look for novel agents for oral cancer chemoprevention, we focused on 5-Lox inhibitors from dietary sources because of their relatively low toxicity and the potential for long-term use. Along this line we have established a strategy to identify 5-Lox inhibitors for topical use in oral cancer chemoprevention using 7,12-dimethylbenz[a]anthracene (DMBA)-induced hamster cheek pouch model (6, 10, 11).
In the present study, we first evaluated in silico five compounds with 5-Lox inhibitory activities (Zileuton, ABT-761, Licofelone, Curcumin and Garcinol) for their potential efficacy. Computer modeling of the 5-Lox-garcinol complex helped us to identify key functional groups of garcinol for its 5-Lox inhibitory activity. With a short-term and a long-term experiment of DMBA-induced hamster cheek pouch carcinogenesis, we investigated the anti-inflammatory and chemopreventive effect of topical garcinol.
Materials and Methods
In silico Prediction of physico-chemical properties of 5-Lox inhibitors
Aqueous solubility and partition coefficient (logP) were predicted by the ACD Suite (Version 8.0, Advanced Chemical Development Inc., Toronto, Canada). Permeability coefficient Kp and flux (Jmax) were calculated using the Potts and Guy equation (12): Log kp (cm/h)= −2.7+0.71× log P-0.0061× MW, and Jmax = kp × solubility. IC50 values for 5-Lox were estimated on the basis of in vitro assays of individual compounds in the literature. Theoretical activity index, Jmax/IC50, was calculated to evaluate the 5-Lox inhibitory activities of compounds.
Docking of garcinol to human 5-Lox
The coordinates of 5-Lox obtained from the X-ray structure of human 5-Lox (PDB code: 3O8Y) was used to explore garcinol docking poses by employing the genetic algorithm-based flexible docking program, GOLD (13, 14). For the substrate, an initial structure of garcinol was built by the Build Fragment tool in Discovery Studio (Accelrys, San Diego, CA). The lowest energy conformation of garcinol obtained by the Generate Conformations tool, employing the BEST conformation algorithm as implemented in Discovery Studio, was used for docking. For the GOLD docking experiment, we used the pre-defined default GOLD generic algorithm settings. GOLDScore was used for evaluating garcinol docking modes. The GOLD cavity detection algorithm was used to locate the substrate within the substrate binding site located near the C-terminus of the protein. The core fragment of garcinol was firstly used for docking, and the bulky groups were gradually added back to obtain plausible docking poses. We identified two distinct binding modes of garcinol in 5-Lox whose solvent exposure appeared to be minimum. Applying the selection rule that one of oxygen atoms of garcinol should be close to the coordinated Fe2+ in the substrate binding site near the C-terminus, one pose was selected as the garcinol binding mode.
We further refined the 5-Lox-garcinol complex by molecular dynamics simulation. The enzyme structure in complex with garcinol was immersed in a rectangular cell of water molecules to produce a periodic box of dimension 96 × 83 × 118 Å3. The system was subjected to 5,000 steps of minimization of two sequential stages: (1) the solvent in the system was minimized with the protein fixed; (2) the entire system was minimized with the Cα atoms of the protein constrained, using the CHARMm force field as implemented in Discovery Studio (Accelrys, San Diego, CA). The minimized structure, then, was simulated at 300 K for 100 ps in the constant volume (NVT) ensemble with the Cα atoms of the protein constrained. MD simulations were performed by the Dynamics module as implemented in Discovery Studio, using the CHARMm force field. The van der Waals interactions were switched at 14 Å and zero smoothly at 16 Å. Electrostatic interactions were treated using the spherical cutoff method. The timestep size for integration of each step of the simulation was 1 fs. The resulting structure was subjected to a full energy minimization of 2,500 steps after removing all constraints applied to the Cα atoms of the protein.
Short-term animal experiment
All procedures involving the use of hamsters were approved by the Institutional Animal Use and Care Committee, Beijing Hospital for Stomatology, Beijing, China. Male Syrian golden hamsters aged 6-8 weeks weighing 60–80 g were purchased from Vital River Laboratory Animal Company (Beijing, China) and housed 4 per cage in a room with controlled temperature and humidity with 12 h light/dark cycles. All animals were given lab chow (Keaoxieli Diet Co., Beijing, China) and water ad libitum. Animals were treated with 0.5% DMBA (0.1ml in mineral oil) topically on the left cheek pouch three times per week for three consecutive weeks. After the last treatment of DMBA, the hamsters were randomly divided into four groups, with one group as positive control (Group 1B) and the other three groups topically applied treated with 0.5mM, 5mM, 50mM garcinol (0.1ml in mineral oil), respectively, three times within the following week. Garcinol was prepared according to a method reported previously (15). The purity of garcinol was determined to be >95% by high performance chromatography.
Hamsters were sacrificed at the forth week to obtain tissue samples of the left cheek pouch. They were injected with BrdU (50 mg/kg, i.p.) two hours before sacrifice. A piece of tissue from each hamster was snap-frozen in liquid nitrogen for analysis of LTB4. The remaining samples were fixed with 10% PBS-buffered formalin. Formalin-fixed tissues were sectioned and stained with hematoxylin and eosin for histopathological diagnosis of basal cell hyperplasia, dysplasia, and squamous cell carcinoma (SCC) (16, 17).
The number of inflammatory cells per mm2 was then calculated after three noncontiguous randomly selected fields were counted at 400× magnification. BrdU immunostaining was performed using the ABC kit (Vector Labs, Burlingame, CA) and a mouse monoclonal anti-BrdU (1:1000; Sigma, St. Louis, MO). BrdU labeling index was calculated as a parameter of epithelial cell proliferation by counting at least 1,000 epithelial cells in each section at 200× magnification. Microscopic evaluation of tissue sections were performed in a blinded fashion.
Long-term animal experiment
Ten hamsters were used as negative control group (Group 2A). 128 hamsters were treated topically on the left cheek pouch with 0.5% DMBA solution (0.1ml in mineral oil), 3 times per week for 6 weeks. Starting from the 7th week, DMBA-treated hamsters were randomly divided into 4 groups, Group 2B (positive control), Group 2C (0.5mM garcinol), Group 2D (5mM garcionol), and Group 2E (50mM garcinol). Hamsters were sacrificed at the end of 24th week and the left cheek pouches were examined for histopathology and BrdU immunohistochemisty. A piece of tissue was also snap-frozen in liquid nitrogen for analysis of prostaglandin E2 (PGE2) and LTB4. The pouches were flattened on a hard plastic membrane for counting the number of visible tumors. The length, width and height of each tumor were measured with a caliper and the tumor volume calculated using the formula: volume= 4/3πr3 (where r was the average radius of the three diameter measurements in cm).
Enzyme immunoassay for PGE2 and LTB4
Frozen hamster cheek pouch epithelium was analyzed immediately after being taken out of −80°C freezer. After pulverization and homogenization in a buffer containing 10 mM of Zileuton, a part of the sample was used for analyzing the protein concentration while the other was extracted with an organic solvent. The organic extract was dried under nitrogen and reconstituted in the enzyme immunoassay buffer for analysis with EIA kits according to the manufacturer's instructions (Cayman Chemical, Ann Arbor, MI). The tissue levels of PGE2 and LTB4 were expressed as pg/mg protein.
Statistical analysis
The tumor incidence was compared by the Chi square test. One-way ANOVA test was used to compare body weight, the number of visible tumors, and the numbers of oral lesions. Tumor volume was analyzed using the Wilcoxon signed rank test. Tissue levels of LTB4 and PGE2 were analyzed using the Student's t-test. A P value <0.05 was regarded as statistically significant.
Results
Garcinol is a potent 5-Lox inhibitor
By using the Potts and Guy equation, we calculated the physico-chemical properties of five chemical compounds from dietary and synthetic sources, Zileuton, ABT-761, Licofelone, curcumin and garcinol. Water solubility and Kp were calculated with the ACD software. Table 1 showed that garcinol had the highest values of permeability (Kp), total flux through the oral mucosa (Jmax) and the theoretical activity index (Jmax/IC50). To target the 5-Lox enzyme in oral epithelial cells, it is desirable to maximize the theoretical activity index, the ratio of Jmax and IC50 of a 5-Lox inhibitor (Jmax/IC50). As a result, garcinol has the highest value of theoretical activity index (3,084), whereas curcumin, Zileuton, Licofelone, and ABT-761 have the values of 0.0084, 0.65, 4.57, and 106.09, respectively. Therefore, garcinol was predicted as a potent 5-Lox inhibitor, which may potentially be used for oral cancer chemoprevention through topical application.
Table 1.
Predicted physico-chemical properties of chemical compounds with inhibitory effects on 5-Lox
| Compound | Molecular weighta | Solubility in pure water (μg/ml)a | Partition coefficient Log Pa | Permeability coefficient Kp (cm/h)b | Flux (Jmax) (μg/cm2/h)b | IC50 (5-Lox) (μM)c | Theoretical activity index (Jmax/IC50) |
|---|---|---|---|---|---|---|---|
| Zileuton | 236.29 | 10 | 3.74 | 3.27×10−2 | 0.33 | 0.5 | 0.65 |
| ABT-761 | 318.37 | 62 | 4.56 | 3.94×10−2 | 2.44 | 0.023 | 106.09 |
| Licofelone | 379.88 | 1.82 | 6.73 | 5.77×10−1 | 1.05 | 0.23 | 4.57 |
| Curcumin | 368.38 | 50 | 2.92 | 1.34×10−3 | 0.07 | 8 | 0.0084 |
| Garcinol | 602.80 | 85 | 11.57 | 6.88×101 | 5,860 | 1.9 | 3,084.21 |
Interaction between garcinol and human 5-Lox
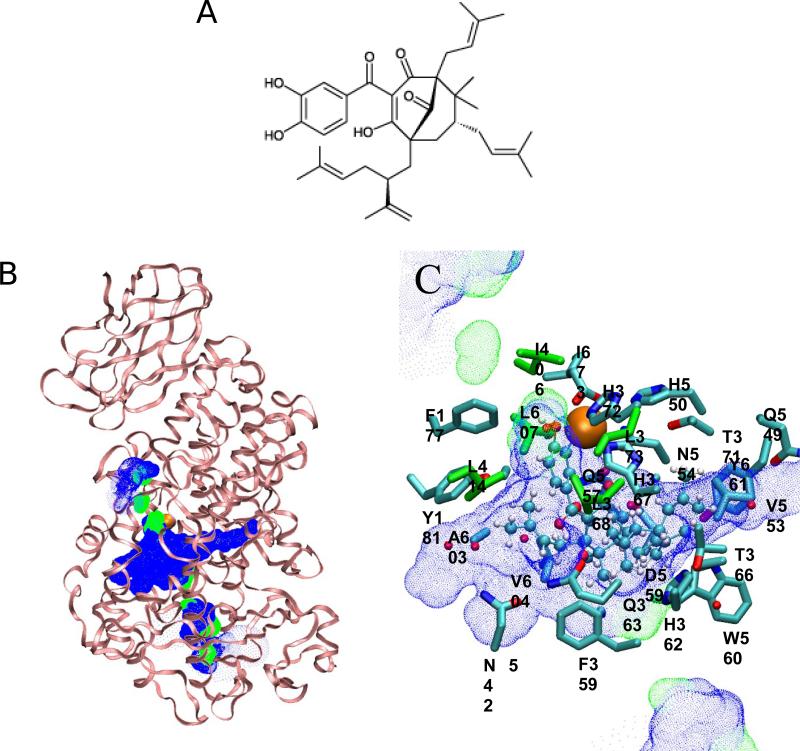
We targeted the active site in the catalytic domain of 5-Lox, where the catalytic iron was coordinated by three conserved His residues (H367, H372, and H550) as well as the C-terminal I673, as the binding site for garcinol. According to the present binding mode of garcinol, two hydroxyl groups of garcinol were coordinated to the catalytic iron that is also coordinated to H367, H372, and I673. Interestingly, H550 was loosely coordinated to the catalytic iron, possibly due to the newly introduced hydroxyl groups of garcinol in place of H550. The aromatic ring of garcinol was close to F177 and Y181 of helix a2, the helix that forms part of an arch and defines the catalytic site.19 A possible aromatic stacking of the aromatic ring of garcinol to these aromatic residues could stabilize the bound substrate, securing a geometry ideal for coordination of the catalytic iron. Additionally, the bound garcinol appeared to be stabilized by forming hydrogen bonds to several polar residues at the catalytic site (Figure 1): 1) The oxygen atom of one of the hydroxyl groups of garcinol forms a hydrogen bond to the side chain N atom of N554; 2) The oxygen atom at the C3 position forms one hydrogen bond to the oxygen atom of Q363 and another to a coordinated water; and 3) the oxygen atom of the carbonyl group connecting the C1 and C5 carbons forms a hydrogen bond to the side chain N of Q557. With much occluded from solvent, the hydrophobic alkenyl substituents of garcinol appeared to favorably interact with the residues at the catalytic binding site. The 3-methyl-2-butenyl moiety of garcinol, in particular, positioned near the region formed by several hydrophobic residues, including L368, L373, I406, L414, and L607, conserved in many AA-metabolizing lipoxygenases.
Figure 1.
Interaction between garcinol and 5-Lox. (A) Structure of garcinol. (B) The structure of 5-Lox represented by ribbon (in pink). The solvent accessible pore created by using HOLE at the catalytic binding region at the end of the MD simulations are also represented by blue dots for low radius surface and green dots for mid radius surface. (C) The key interactions between garcinol and the residues at the catalytic binding site. Garcinol is represented by ball-and-stick (in atom type) while the amino acid residues by stick (in atom type). The active site hydrophobic residues, including L368, L373, I406, L414, and L607, conserved in many AA-metabolizing lipoxygenases are colored green.
Short-term effect of topical garcinol on cell proliferation and LTB4 biosynthesis in DMBA-treated hamster cheek pouch (Table 2)
Table 2.
Inhibitory effects of short-term treatment of topical garcinol on cell proliferation and aberrant AA metabolism in DMBA-treated hamster cheek pouch
| Group | Treatment | No | Body weight (g) | No. of hyperplasia | No. of dysplasia | Inflammatory cells (/mm2) | BrdU-labeling index | LTB4 (pg/mg) |
|---|---|---|---|---|---|---|---|---|
| 1A | Negative control | 3 | 162.27±23.44 | - | - | 47.78±19.73** | 2.49±1.73** | 2.75±0.54* |
| 1B | DMBA | 6 | 147.17±9.66 | 5.17±1.32 | 5.00±2.00 | 320.51±155.88 | 4.26±2.21 | 6.16±4.16 |
| 1C | DMBA+ 0.5mM Garcinol | 6 | 154.00±10.99 | 3.67±0.82* | 2.83±0.98** | 197.72±83.77** | 3.79±1.03 | 1.50±0.70** |
| 1D | DMBA + 5mM Garcinol | 6 | 148.50±14.56 | 3.50±1.05** | 3.17±1.16* | 184.39±64.16** | 3.30±1.37* | 0.86±0.76** |
| 1E | DMBA + 50mM Garcinol | 6 | 141.67±9.18 | 3.00±1.89** | 2.00±0.89** | 100.41±52.53** | 3.01±1.78** | 1.59±2.38** |
P <0.05 as compared with Group 1B
P<0.01 as compared with Group 1B.
All animals appeared healthy throughout the short-term study. Body weights of animals increased steadily and there was no statistical difference among the 5 groups. DMBA treatment (Group 1B) for 3 weeks produced hyperproliferation in hamster cheek pouch as shown by increased BrdU-labeling index. Topical garcinol (Groups 1C, 1D and 1E) for 1 week suppressed hyperproliferation in epithelial cells. In comparison to the positive control group (Group 1B) both the number of epithelial hyperplasia and the number of dysplasia were significantly reduced by topical application of 0.5mM garcinol (Group 1C), 5mM garcinol (Group 1D) and 50mM garcinol (Group 1E). There were very few of neutrophils in the submucosa of the negative control group (Group 1A). DMBA treatment caused severe inflammation in the epithelium as shown by infiltration of a large number of lymphocytes and neutrophils into the submucosa in the positive control group (Group 1B). Topical garcinol significantly suppressed infiltration of inflammatory cells in the submucosa (Group 1C, 1D and 1E).
The 5-Lox pathway was evidently activated by DMBA treatment as shown by increased LTB4 biosynthesis in the oral epithelium (Group 1B). Corresponding to its anti-inflammatory effect, topical garcinol treatment (Group 1C, 1D and 1E) significantly suppressed LTB4 biosynthesis. These data suggested that topical garcinol was effective in suppressing DMBA-induced inflammation and hyperproliferation of oral epithelium through inhibition of 5-Lox.
Long-term chemopreventive effect of topical garcinol on DMBA-induced oral carcinogenesis, cell proliferation and LTB4 biosynthesis in hamster cheek pouch (Table 3; Table 4)
Table 3.
Long-term chemopreventive effect of topical garcinol on DMBA-induced oral carcinogenesis in hamster cheek pouch
| Group | Treatment | No. | Visible tumors |
Microscopic changes |
||||
|---|---|---|---|---|---|---|---|---|
| Incidence (%) | Number | Volume (cm3) | No. of dysplasia | Incidence of SCC | No. of SCC | |||
| 2A | Negative control | 5 | - | - | - | - | - | - |
| 2B | DMBA | 25 | 96.0 (24/25) | 3.70±2.79 | 7.64±18.67 | 1.52±1.40 | 96.0 (24/25) | 3.00±2.28 |
| 2C | DMBA + 0.5mM garcinol | 21 | 81.0 (17/21) | 2.18±1.70** | 0.90±1.80* | 1.37±1.30 | 81.0 (17/21) | 1.63±1.34** |
| 2D | DMBA + 5mM garcinol | 23 | 82.6 (19/23) | 2.45±2.01* | 0.52±1.44* | 1.06±0.25 | 87.0 (20/23) | 1.56±1.04** |
| 2E | DMBA + 50mM garcinol | 25 | 80.0 (20/25) | 2.13±1.75** | 0.58±1.41* | 0.85±0.18* | 84.0 (21/25) | 1.23±0.81** |
P <0.05 as compared with Group 2B
P<0.01 as compared with Group 2B.
Table 4.
Inhibitory effects of long-term treatment of topical garcinol on cell proliferation and aberrant AA metabolism in DMBA-treated hamster cheek pouch
| Group | Treatment | BrdU-labeling index (%) |
AA metabolites |
||||
|---|---|---|---|---|---|---|---|
| Normal | Hyperplasia | Dysplasia | SCC | PGE2 (pg/mg) | LTB4 (pg/mg) | ||
| 2A | Negative control | 2.26±0.85** | - | - | - | 23.04±6.23** | 3.02±1.67** |
| 2B | DMBA | 5.61±2.61 | 5.16±1.88 | 6.91±3.71 | 10.85±7.94 | 205.31±145.49 | 35.26±25.03 |
| 2C | DMBA + 0.5mM garcinol | 3.30±1.91** | 4.08±1.68* | 4.57±1.09** | 6.81±3.00* | 128.08±101.58* | 18.57±19.04* |
| 2D | DMBA + 5mM garcinol | 3.58±2.12** | 4.21±2.40* | 4.14±1.95** | 5.66±2.30* | 109.31±86.79* | 19.89±18.78* |
| 2E | DMBA + 50mM garcinol | 3.57±2.65** | 3.56±1.21** | 4.40±1.82** | 5.89±2.49* | 102.54±76.44** | 14.25±8.67** |
P <0.05 as compared with Group 2B
P<0.01 as compared with Group 2B.
The majority of DMBA-treated animals developed visible tumors at the end of experiment. Topical garcinol treatment did not significantly reduce the incidence of visible tumors. Instead, it significantly reduced the number and the volume of visible tumors. Under microscope, although the incidence of SCC was not significantly decreased by topical garcinol, the number of dysplasia and the number of SCC were significantly reduced. These data clearly indicated a chemopreventive effect of topical garcinol on DMBA-induced oral carcinogenesis in hamster cheek pouch.
Consistent with the histological changes, topical garcinol suppressed proliferation of oral epithelial cells as the BrdU-labeling index significantly decreased in histologically normal epithelium, hyperplasia, dysplasia and SCC. Not only so, LTB4 biosynthesis in the oral epithelium was dramatically inhibited by topical garcinol, as well as PGE2 biosynthesis.
Discussion
This study was aimed to evaluate the efficacy of garcionol, a potent 5-Lox inhibitor for topical use in oral cancer chemoprevention. Five compounds with 5-Lox inhibitory activities were first evaluated in silico for their potential potency: 1) Zileuton, a clinically proven medicine, has been shown to be chemopreventive against oral carcinogenesis in our previous study (6). 2) ABT-761 is a more potent 5-Lox inhibitor than Zileuton (18). 3) Licofelone is a dual inhibitor of 5-Lox and cyclooxygenase 2(19). Our previous study has shown that combination of a 5-Lox inhibitor and a cycloxygenase 2 inhibitor is more effective in preventing oral carcinogenesis than single inhibitors used alone (5). 4) Curcumin, as a relatively weak inhibitor of 5-Lox, yet has been showed to be slightly effective in preventing oral carcinogenesis in our previous study (8). 5) Garcinol is found in Guttiferae plants, which are shrubs native to India and South East Asia (20, 21). As one of the major constituents of G. indica extract, garcinol is a polyisoprenylated benzophenone derivative structurally similar to curcumin. It has been used as a food ingredient, garnish and cosmetic constituent, as well as a traditional medicine for treating inflammation and other disorders. A recent study clearly demonstrated garcinol as a potent 5-Lox inhibitor (22). Among these five compounds, garcinol was a potent compound based on our calculation of the theoretical activity index (Table 1).
Docking analysis was performed to demonstrate how garcinol interacts with 5-Lox. According to our computer modeling, the catalytic active site of 5-Lox appears to be well protected from solvent (Fig. 1B). Our data suggested that two hydroxyl groups of garcinol play a key role in blocking the enzyme function by competitively forming iron coordination at the enzyme active site. It was a concern whether the 5-Lox active site would not be large enough to accommodate a relatively large substrate like garcinol (molecular weight 602). However, our data showed that garcinol fits into the active site quite well (Fig. 1C), partly due to the flexible alkenyl substituents adaptable to the geometric constraint(s) of the enzyme binding pocket. In the present 5-Lox-garcinol model, the 3-methyl-2-butenyl moiety of garcinol closely interacted with L414 and L607 (Fig. 1C). Considering the hydrophobic residues in the catalytic binding pocket (L368, L373, I406, L414, and L607) which are conserved in many AA-metabolizing lipoxygenases for substrate binding, the 3-methyl-2-butenyl moiety is important in retaining the enzymatic inhibitory activity of garcinol. On the other hand, the 5-methyl-2-(1-methylethenyl)-4-hexenyl group at C5 position of garcinol appears to cause an unfavorable interaction with 5-Lox, as the pocket region where this moiety is positioned appeared too narrow to fully accommodate this moiety (Fig. 1C).
As expected, topical garcinol significantly was effective in inhibiting DMBA-induced inflammation, cell proliferation, LTB4 biosynthesis and the number of precancerous lesions. Consistent with our data from the short-term experiment, our long-term experiment also demonstrated cancer chemopreventive effect of garcinol on DMBA-inudced cheek pouch carcinogenesis. As a 5-Lox inhibitor, topical garcinol inhibited LTB4 biosynthesis, as well as PGE2 biosynthesis. These data are consistent with its inhibitory effect on 5-Lox and microsomal prostaglandin E synthase 1, a critical enzyme downstream to cyclooxygenases for PGE2 biosynthesis (22).
Other than its 5-Lox inhibitory activity, garcinol may exert its chemopreventive effects through other mechanisms as well. It is known that garcinol inhibits not only 5-Lox (IC50=0.1μM) (22), but also other enzymes such as, microsomal prostaglandin E synthase 1 (IC50=0.3μM) (22), histone acetyltransferase p300 (IC50=7μM) (23), P300/CBP-associated factor (IC50=5μM) (23), acetylcholine esterase (IC50=0.7μM) (24). Garcinol can potentiate TRAIL-induced apoptosis through upregulation of death receptors and downregulation of antiapoptotic proteins (25). Recent studies also showed that garcinol inhibited constitutive NFκB activity, which was consistent with down-regulation of NFκB-regulated genes. Garcinol exhibited dose-dependent cancer cell-specific growth inhibition with a concomitant induction of apoptosis, and had no effect on non-tumorigenic MCF-10A cells (26). This anti-proliferative and apoptosis-promoting mechanism may contribute to its anti-cancer activity in various cancer cells (27-30). In vivo, garcinol has been shown to prevent azoxymethane-induced colon carcinogenesis in rats (31), and 4NQO-induced oral carcinogenesis in rats (32). One potential drawback of this study is that the keratinized oral mucosa in hamster cheek pouch may be less permeable to topical compound. Pharmacokinetics after topical application may be less predictable. Some areas of oral cavity are better than other areas for topical delivery (33, 34). Further studies are needed to improve permeability of an effective compound across oral epithelium for oral cancer chemoprevention.
Two recent studies on 4NQO-induced oral carcinogenesis failed to show the chemopreventive effect of a 5-Lox inhibitor (Zileuton) in rats (35), and inhibition of carcinogenesis in 5-Lox knockout mice (7). Differences in experimental designs, animal species (mouse, rat, or hamster), methods of disruption (genetic or pharmacological), and routes of administration (topical or oral), may contribute to the discrepancy. A more probable explanation, however, may lie with different mechanisms of the 4NQO model and the DMBA model. Although both models produce lesions similar to those found in humans, the 4NQO model is known to produce less severe inflammation than the DMBA model. Populations of infiltrating inflammatory cells in the oral epithelium are also different in these two models (36). These data suggest that 5-Lox inhibitors (i.e., garcinol) may be more relevant to chemoprevention of oral cancer associated with inflammation.
Acknowledgements
This study was supported by research grants from the Beijing Municipal Natural Science Foundation (7102066), the National Natural Science Foundation of China (30973325), Ph.D. Programs Foundation of Ministry of Education of China (No. 20101107110009), the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions, and NIH (U56 CA092070 and U54 CA156735).
Abbreviations
- AA
Arachidonic acid
- BrdU
5-bromodeoxyuridine
- DMBA
7,12-dimethylbenz[a]anthracene
- LTB4
leukotriene B4
- 5-Lox
5-lipoxygenase
- PGE2
prostaglandin E2
- SCC
squamous cell carcinoma
References
- 1.Magrath I, Litvak J. Cancer in developing countries: opportunity and challenge. J Natl Cancer Inst. 1993;85:862–874. doi: 10.1093/jnci/85.11.862. [DOI] [PubMed] [Google Scholar]
- 2.Funk GF, Karnell LH, Robinson RA, Zhen WK, Trask DK, et al. Presentation, treatment, and outcome of oral cavity cancer: a National Cancer Data Base report. Head Neck. 2002;24:165–180. doi: 10.1002/hed.10004. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Sood S, Yang CS, Li N, Sun Z. Five-lipoxygenase pathway of arachidonic acid metabolism in carcinogenesis and cancer chemoprevention. Curr Cancer Drug Targets. 2006;6:613–622. doi: 10.2174/156800906778742451. [DOI] [PubMed] [Google Scholar]
- 5.Li N, Sood S, Wang S, Fang M, Wang P, et al. Overexpression of 5-lipoxygenase and cyclooxygenase 2 in hamster and human oral cancer and chemopreventive effects of zileuton and celecoxib. Clin Cancer Res. 2005;11:2089–2096. doi: 10.1158/1078-0432.CCR-04-1684. [DOI] [PubMed] [Google Scholar]
- 6.Sun Z, Sood S, Li N, Ramji D, Yang P, et al. Involvement of the 5-lipoxygenase/leukotriene A4 hydrolase pathway in 7, 12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamster cheek pouch, and inhibition of carcinogenesis by its inhibitors. Carcinogenesis. 2006;27:1902–1908. doi: 10.1093/carcin/bgl039. [DOI] [PubMed] [Google Scholar]
- 7.Guo Y, Wang X, Zhang X, Sun Z, Chen X. Ethanol promotes chemically induced oral cancer in mice through activation of the 5-lipoxygenase pathway of arachidonic acid metabolism. Cancer Prev Res (Phila) 2011;4:1863–1872. doi: 10.1158/1940-6207.CAPR-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li N, Chen X, Liao J, Yang G, Wang S, et al. Inhibition of 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamsters by tea and curcumin. Carcinogenesis. 2002;23:1307–1313. doi: 10.1093/carcin/23.8.1307. [DOI] [PubMed] [Google Scholar]
- 9.Sun Z, Guan X, Li N, Liu X, Chen X. Chemoprevention of oral cancer in animal models and human patients with ZengShengPing, a mixture of medicinal herbs. Oral Oncol. 2010;45:105–110. doi: 10.1016/j.oraloncology.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Yang P, Sun Z, Chan D, Cartwright CA, Vijjeswarapu M, et al. Zyflamend reduces LTB4 formation and prevents oral carcinogenesis in a 7,12-dimethylbenz[alpha]anthracene (DMBA)-induced hamster cheek pouch model. Carcinogenesis. 2008;29:2182–2189. doi: 10.1093/carcin/bgn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sood S, Shiff SJ, Yang CS, Chen X. Selection of topically applied non-steroidal anti-inflammatory drugs for oral cancer chemoprevention. Oral Oncol. 2005;41:562–567. doi: 10.1016/j.oraloncology.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Potts RO, Guy RH. Predicting skin permeability. Pharm Res. 1992;9:663–669. doi: 10.1023/a:1015810312465. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert NC, Bartlett SG, Waight MT, Neau DB, Boeglin WE, et al. The structure of human 5-lipoxygenase. Science. 2011;331:217–219. doi: 10.1126/science.1197203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verdonk ML, Cole JC, Hartshorn MJ, Murray CW, Taylor RD. Improved protein-ligand docking using GOLD. Proteins. 2003;52:609–623. doi: 10.1002/prot.10465. [DOI] [PubMed] [Google Scholar]
- 15.Sang S, Pan MH, Cheng X, Bai N, Stark RE, et al. Chemical studies on antioxidant mechanism of garcinol: analysis of radical reaction products of garcinol and their antitumor activities. Tetrahedron. 2001;57:9931–9938. [Google Scholar]
- 16.Kramer I, Lucas R, Pindborg J, Sobin L. WHO, Collaborating center for oral precancerous lesions: definitions of leukoplakia and related lesions: an aid to studies on oral precancer. Oral Surg Oral Med Oral Pathol. 1978;46:518–589. [PubMed] [Google Scholar]
- 17.Leininger I, KJokinen M. Tumors of the oral cavity, pharynx, oesophagus and stomach. In: Turosov V, editor. Pathology of tumors in laboratory animals. Vol. 3. IARC; Albany, NY: 1982. pp. 167–169. [Google Scholar]
- 18.Bell RL, Harris RR, Malo PE, Bouska JB, Shaughnessy TK, et al. ABT-761 attenuates bronchoconstriction and pulmonary inflammation in rodents. J Pharmacol Exp Ther. 1997;280:1366–1373. [PubMed] [Google Scholar]
- 19.Alvaro-Gracia JM. Licofelone--clinical update on a novel LOX/COX inhibitor for the treatment of osteoarthritis. Rheumatology (Oxford) 2004;43(Suppl 1):i21–25. doi: 10.1093/rheumatology/keh105. [DOI] [PubMed] [Google Scholar]
- 20.Bakana P, Claeys M, Totte J, Pieters LA, Van Hoof L, et al. Structure and chemotherapeutical activity of a polyisoprenylated benzophenone from the stem bark of Garcinia huillensis. J Ethnopharmacol. 1987;21:75–84. doi: 10.1016/0378-8741(87)90096-1. [DOI] [PubMed] [Google Scholar]
- 21.Krishnamurthy N, Lewis YS. On the structures of garcinol, isogarcinol and camboginol. Tetrahedron Lett. 1981;2:793–796. [Google Scholar]
- 22.Koeberle A, Northoff H, Werz O. Identification of 5-lipoxygenase and microsomal prostaglandin E2 synthase-1 as functional targets of the anti-inflammatory and anti-carcinogenic garcinol. Biochem Pharmacol. 2009;77:1513–1521. doi: 10.1016/j.bcp.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Balasubramanyam K, Altaf M, Varier RA, Swaminathan V, Ravindran A, et al. Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. J Biol Chem. 2004;279:33716–33726. doi: 10.1074/jbc.M402839200. [DOI] [PubMed] [Google Scholar]
- 24.Lenta BN, Vonthron-Senecheau C, Weniger B, Devkota KP, Ngoupayo J, et al. Leishmanicidal and cholinesterase inhibiting activities of phenolic compounds from Allanblackia monticola and Symphonia globulifera. Molecules. 2007;12:1548–1557. doi: 10.3390/12081548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prasad S, Ravindran J, Sung B, Pandey MK, Aggarwal BB. Garcinol potentiates TRAIL-induced apoptosis through modulation of death receptors and antiapoptotic proteins. Mol Cancer Ther. 2010;9:856–868. doi: 10.1158/1535-7163.MCT-09-1113. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Shaari K, Suppaiah V, Wai LK, Stanslas J, Tejo BA, et al. Bioassay-guided identification of an anti-inflammatory prenylated acylphloroglucinol from Melicope ptelefolia and molecular insights into its interaction with 5-lipoxygenase. Bioorg Med Chem. 2011;19:6340–6347. doi: 10.1016/j.bmc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad A, Wang Z, Ali R, Maitah MY, Kong D, et al. Apoptosis-inducing effect of garcinol is mediated by NF-kappaB signaling in breast cancer cells. J Cell Biochem. 2010;109:1134–1141. doi: 10.1002/jcb.22492. [DOI] [PubMed] [Google Scholar]
- 28.Ahmad A, Wang Z, Wojewoda C, Ali R, Kong D, et al. Garcinol-induced apoptosis in prostate and pancreatic cancer cells is mediated by NF- kappaB signaling. Front Biosci (Elite Ed) 2011;3:1483–1492. doi: 10.2741/e349. [DOI] [PubMed] [Google Scholar]
- 29.Cheng AC, Tsai ML, Liu CM, Lee MF, Nagabhushanam K, et al. Garcinol inhibits cell growth in hepatocellular carcinoma Hep3B cells through induction of ROS-dependent apoptosis. Food Funct. 2010;1:301–307. doi: 10.1039/c0fo00134a. [DOI] [PubMed] [Google Scholar]
- 30.Parasramka MA, Gupta SV. Garcinol inhibits cell proliferation and promotes apoptosis in pancreatic adenocarcinoma cells. Nutr Cancer. 2011;63:456–465. doi: 10.1080/01635581.2011.535962. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka T, Kohno H, Shimada R, Kagami S, Yamaguchi F, et al. Prevention of colonic aberrant crypt foci by dietary feeding of garcinol in male F344 rats. Carcinogenesis. 2000;21:1183–1189. [PubMed] [Google Scholar]
- 32.Yoshida K, Tanaka T, Hirose Y, Yamaguchi F, Kohno H, et al. Dietary garcinol inhibits 4-nitroquinoline 1-oxide-induced tongue carcinogenesis in rats. Cancer Lett. 2005;221:29–39. doi: 10.1016/j.canlet.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Lesch CA, Squier CA, Cruchley A, Williams DM, Speight P. The permeability of human oral mucosa and skin to water. J Dent Res. 1989;68:1345–1349. doi: 10.1177/00220345890680091101. [DOI] [PubMed] [Google Scholar]
- 34.Shojaei AH. Buccal mucosa as a route for systemic drug delivery: a review. J Pharm Pharm Sci. 1998;1:15–30. [PubMed] [Google Scholar]
- 35.McCormick DL, Phillips JM, Horn TL, Johnson WD, Steele VE, et al. Overexpression of cyclooxygenase-2 in rat oral cancers and prevention of oral carcinogenesis in rats by selective and nonselective COX inhibitors. Cancer Prev Res (Phila) 2010;3:73–81. doi: 10.1158/1940-6207.CAPR-09-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matthews JB, Mason GI, Scully CM, Prime SS. In situ characterisation of the oral mucosal inflammatory cell response of rats induced by 4-nitroquinoline-N-oxide. Carcinogenesis. 1986;7:783–788. doi: 10.1093/carcin/7.5.783. [DOI] [PubMed] [Google Scholar]
- 37.Laufer SA, Augustin J, Dannhardt G, Kiefer W. (6,7-Diaryldihydropyrrolizin-5-yl)acetic acids, a novel class of potent dual inhibitors of both cyclooxygenase and 5-lipoxygenase. J Med Chem. 1994;37:1894–1897. doi: 10.1021/jm00038a021. [DOI] [PubMed] [Google Scholar]
- 38.Flynn DL, Rafferty MF, Boctor AM. Inhibition of 5-hydroxy-eicosatetraenoic acid (5-HETE) formation in intact human neutrophils by naturally-occurring diarylheptanoids: inhibitory activities of curcuminoids and yakuchinones. Prostaglandins Leukot Med. 1986;22:357–360. doi: 10.1016/0262-1746(86)90146-0. [DOI] [PubMed] [Google Scholar]