Abstract
Collagen is one of the most widely used biomaterials for tissue engineering and regenerative medicine. Fish collagen peptides (FCP) have been used as a dietary supplement, but their effects on the cellular function are still poorly understood. The objective of this study was to investigate the effects of FCP on collagen synthesis, quality and mineralization using an osteoblastic MC3T3-E1 cell culture system. Cells treated with FCP significantly upregulated the gene expression of several collagen modifying enzymes and more collagen was deposited in the cultures. Collagen in the treated group showed a greater extent of lysine hydroxylation, higher levels of hydroxylysine-aldehyde derived cross-links and accelerated cross-link maturation compared with the untreated group. Furthermore, the treated group showed accelerated matrix mineralization. These results indicate that FCP exerts a positive effect on osteoblastic cells in terms of collagen synthesis, quality and mineralization, thereby suggesting the potential utility of FCP for bone tissue engineering.
Keywords: Fish collagen peptides, Collagen post-translational modification, Collagen cross-link, Maturation of collagen, Mineralization
INTRODUCTION
Collagens are a large family of structurally related extracellular matrix proteins accounting for about 30% of the total proteins in the human body. Collagen has been widely used as a biomaterial, e.g. scaffold of cell and growth factors, wound dressing, soft tissue augmentation, dietary supplement, etc1–4), due to its biocompatibility, stability and bioactivity. One of the major commercial sources of collagen has been bovine tissues but other species have also been sought as an alternative source of collagen, because of the occurrence of Bovine Spongiform Encephalopathy (BSE). Considerable attention has been drawn to the collagen-rich fish solid waste that constitutes 50–70% of the original raw material for the effective utilization of resources5). Fish collagen or collagen peptides derived from the skin, bones, swim bladder and scales has been used as a functional food or dietary supplement6,7). However, their biological effects at the cellular and biochemical levels are still not clearly understood. The fibrillar type I collagen functions as a structural organizer of mineralization in bone, and its proper post-translational modifications such as cross-linking pattern are crucial for this function8–13). This study used an osteoblastic cell culture system to examine the effects of fish collagen peptides (FCP) on the gene expression of collagen modifying enzymes that play critical roles in covalent intermolecular cross-linking, collagen content, the extent of lysine (Lys) hydroxylation, cross-links and matrix mineralization.
MATERIALS AND METHODS
Partial characterization of fish collagen peptides
The FCP powder used in this study was kindly provided by Yaizu Suisankagaku Industry (Shizuoka, Japan). This product has been used as a food additive for food companies and food processing manufacturers, and it is the product of enzymatic digestion of purified skin collagen mainly from Gadiformes and Pleuronectidae by Bacillus species-derived proteinase. Aliquots of FCP were subjected to amino acid, SDS-PAGE and mass spectrometric analyses to partially characterize FCP. Approximately 1 mg of FCP powder was hydrolyzed with 6 N HCl and an aliquot of the hydrolysate was subjected to amino acid analysis as described previously14). The analysis was performed in duplicate. An aliquot of FCP was also separated by 15–20% SDS-PAGE and then the gel was stained by Coomassie brilliant blue (CBB).
The molecular mass of FCP was evaluated by a Matrix Assisted Laser Desorption/Ionization-Time of Flight (MALDI-TOF) mass spectrometer (Voyager DE PRO, Applied Biosystems, CA, USA) in the linear positive ion mode with delayed extraction. The extraction and guide wire voltages were set at 25 kV and 0.15%, respectively. Three profiles containing 300 shots each from the same spot were acquired. Insulin was used as an external standard. Ionization was achieved with a nitrogen laser (377 nm) using sinapinic acid as a matrix.
Preparation of fish collagen peptides solution
A FCP solution (5% w/v) containing α-modified essential medium (α-MEM, Gibco, Carlsbad, CA, USA) was prepared, the pH was adjusted to 7.4 and filtered using a 0.2 µm filter. membrane (Nalge Company, Rochester, NY, USA).
Cell culture
The mouse calvaria-derived MC3T3-E1 cells (subclone 4) purchased from American Type Culture Collection (CRL-2593) were used in this study. The cells were maintained in α-MEM supplemented with 10% fetal bovine serum (FBS, Atlanta, Lawrenceville, GA, USA), 100 U/mL penicillin G sodium and 100 µg/mL streptomycin sulfate in a humidified atmosphere of 5% CO2 at 37°C. The medium was changed every 3 days.
Quantitative real-time polymerase chain reaction analysis
MC3T3-E1 cells were seeded in a 6-well plate at a density of 2×105 cells/well and cultured under the aforementioned conditions until confluent. The medium was then replaced with those containing 0.05, 0.1, 0.2 and 0.5% (w/v) of FCP prepared by the addition of appropriate volumes of 5% FCP solution based on the findings of a previous report15). The cells cultured with α-MEM without FCP served as a control.
Total RNA was isolated after 48 h of culture using Trizol reagent (Invitrogen, Carlsbad, CA, USA)16) and first-strand cDNA was synthesized using an Omniscript Reverse Transcriptase Kit (Qiagen, Valencia, CA, USA). The mRNA expression levels of type-I collagen α2 chain (COL1A2) and collagen modifying enzyme genes, i.e. lysyl hydroxylase (LH)1–3, lysyl oxidase (LOX), LOX-like protein (LOXL)1–4 and glycosyltransferase 25 domain containing 1 (GLT25D1), were quantitatively analyzed by real-time polymerase chain reaction (PCR). The expression of GLT25D1 in MC3T3-E1 cell line was recently reported17). PCR was performed by the ABI Prism 7000 Sequence detection system (Applied Biosystems, Foster City, CA, USA) using the specific primer-probes: COL1A2 (Applied Biosystems, ABI assay number: Mm00483888_m1), GLT25D1 (Mm00600638_m1), LH1 (Mm00599925_m1), LH2 (Mm00478767_m1), LH3 (Mm00478798_m1), LOX (Mm00495386_m1), LOXL1 (Mm01145738_m1), LOXL2 (Mm00804740_m1), LOXL3 (Mm00442953_m1) and LOXL4 (Mm00446385_m1) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH, ABI assay number: 4308313). The fold changes in gene expression relative to GAPDH were calculated using the values obtained from the control group as a calibrator by using the 2−ΔΔCT method18). All measurements were performed in triplicate and confirmed by three independent experiments. The concentration of FCP for the following experiments was determined based on the gene expression pattern.
Cell proliferation study
The cells were seeded in a 24-well plate at a density of 2×104 cells/well and cultured overnight. The medium was then replaced with that containing 0.2% FCP, the concentration determined by the real-time PCR analysis (see above). The cells were cultured with α-MEM with or without FCP, and then the cell number was counted at 2, 5, 7, 10 and 14 days, using a hemocytometer under a phase contrast microscope. All measurements were confirmed by three independent experiments.
Amino acid and collagen cross-link analyses
The cells were seeded in a 6-well plate at a density of 2×105 cells/well and cultured. The cells grew to confluence, and the medium was replaced with that containing 50 µg/mL of ascorbic acid with or without 0.2% FCP. The cells were cultured for 14 days. The cell/ matrix layers were scraped, washed with phosphate buffered saline (PBS) and deionized distilled water, and lyophilized. Aliquots of dried samples (about 2 mg) were reduced with standardized NaB3H4 as reported previously19). After flushing with N2, the reduced samples were hydrolyzed with 6N HCl in vacuo at 110°C for 22 h. The hydrolysates were then dried by a speed vacuum concentrator (Savant, Phoenix, AR, USA), dissolved in distilled water and filtered using a 0.22 µm membrane. An aliquot of each hydrolysate was then subjected to amino acid and cross-link analyses on a Varian high-performance liquid chromatography (HPLC) system as described previously14). Collagen derived from the MC3T3-E1 cells was calculated as µg of collagen/mg of dried cell matrix based on a value of 300 residues of Hyp per collagen and the molecular weight of collagen. The extent of Lys hydroxylation (Hyl) was calculated as moles of Hyl/mole of collagen based on a value of 300 residues of Hyp per collagen molecule. The analyses were performed in duplicate in two independent experiments.
The hydrolysates with known amounts of Hyp were then subjected to cross-link analysis with the system described previously14). The cross-link precursor aldehydes (i.e. Hylald and Lysald) and the major reducible cross-links (i.e. dehydrodihydroxylysinonorleucine/its ketoamine [deH-DHLNL], dehydrohydroxylysinonorleucine/ its ketoamine [deH-HLNL]) were analyzed as reduced forms, i.e. dihydroxynorleucine (DHNL), hydroxynorleucine (HNL), DHLNL and HLNL, respectively. The non-reducible cross-links, i.e. Pyr and deoxypyridinoline (d-Pyr) were also analyzed simultaneously14). The contents of these compounds were quantified as moles/mole of collagen. The total number of aldehydes (free and those involved in cross-links) was calculated as a sum of DHNL, HNL, DHLNL, HLNL and 2×(Pyr+d-Pyr). The cross-link maturation was calculated as (Pyr/DHLNL)×100. The analyses were performed in duplicate in two independent experiments.
In vitro mineralization assay
The cells were seeded in a 6-well plate at a density of 2×105 cells/well and cultured until confluence. The medium was then replaced with that containing 50 µg/ mL of ascorbic acid and 2 mM β-glycerophosphate (Sigma Chemical, St. Louis, MO, USA) with or without FCP. The medium was changed every 3 days. The cells were cultured for 21 days. The cells were washed with PBS, fixed with 100% methanol and stained with 1% alizarin red S (Sigma Chemical, St. Louis, MO, USA). Alizarin red S in a stained cell monolayer was semiquantified by the method described previously20,21). Briefly, 10% (v/v) acetic acid was added to each well and incubated at room temperature for 30 min. The monolayer was then scraped, transferred into a microcentrifuge tube, heated at 85°C for 10 min and cooled in ice for 5 min. The slurry was then centrifuged at 20,000 g for 15 min and 10%(v/v) ammonium hydroxide was added to the supernatant. Aliquots of the supernatant were read in triplicate at 405 nm using Powerwave X340 microplate spectrophotometer (BioTek Instruments, Tigan Street Winooski, VT, USA). The analyses were performed in triplicate in three independent experiments.
Statistical analysis
The data regarding the mRNA expression were statistically evaluated using the one-way analysis of variance (ANOVA) and Fisher’s PLSD test as a post-hoc test.
Statistical analyses for the other studies were performed using Student’s t-test. All data were expressed as the means±standard error and a p value less than 0.05 was considered to be statistically significant.
RESULTS
Amino acid, SDS-PAGE and MALDI-TOFMS analyses
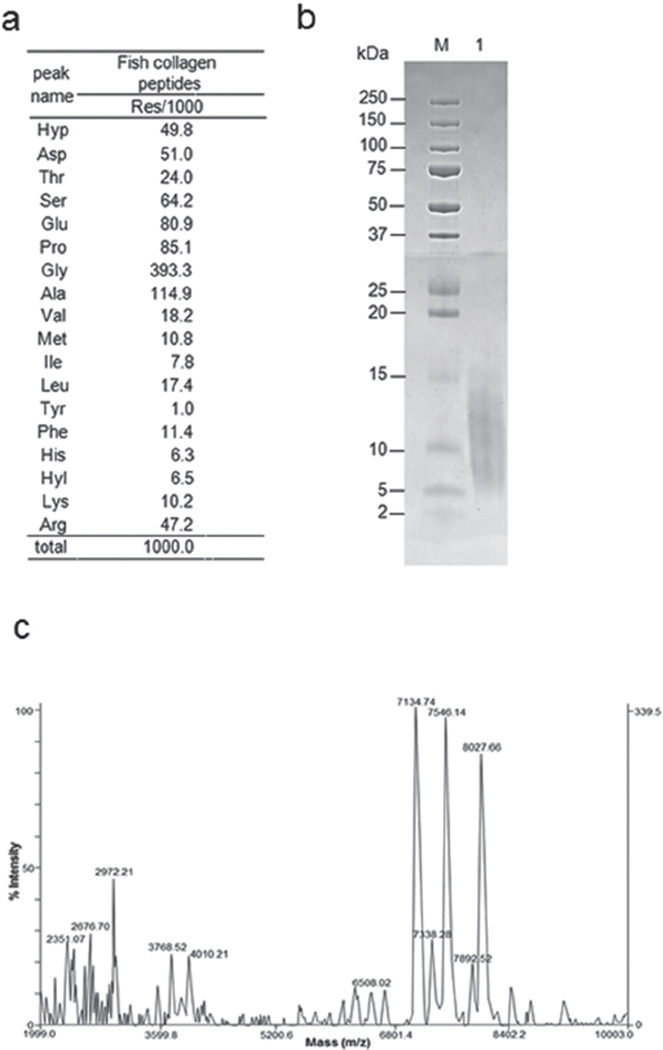
The hydroxyproline content was ~5% of the total amino acids and glycine ~39% (Fig. 1a). The relatively low hydroxyproline (Hyp) content in this fish collagen was consistent with the findings of a previous report22). The SDS-PAGE analysis showed a smear band migrating from ~5 k to 13 kDa (Fig. 1b). The TOF-MS data identified many peptides with varied molecular sizes ranging from ~2 k to ~8 kDa (Fig. 1c).
Fig. 1. Biochemical characterization of FCP.
(a) Amino acid composition of FCP expressed as residues per 1,000 total amino acid residues. Hyp, hydroxyproline; Asp, aspartic acid; Thr, threonine; Ser, serine; Glu, glutamic acid; Pro, proline; Gly, glysine; Ala, alanine; Val, valine; Met, methionine; Ile, isoleucine; Leu, leucine; Tyr, tyrosine; Phe, phenylalanine; His, histidine; Hyl, hydroxylysine; Lys, lysine; Arg, arginine. (b) SDS-PAGE profile of FCP separated on a 15–20% polyacrylamide gel and stained with CBB. Lane M, broad range molecular weight standard. Lane 1, FCP. (c) MALDI-TOF mass spectrometry obtained from FCP analyzed in the positive mode. The analysis was performed in the linear mode using insulin as an external standard. The effective peak patterns are shown in the values.
Gene expression of type I collagen, LH isoforms, LOX, LOXL isoforms and GLT25D1
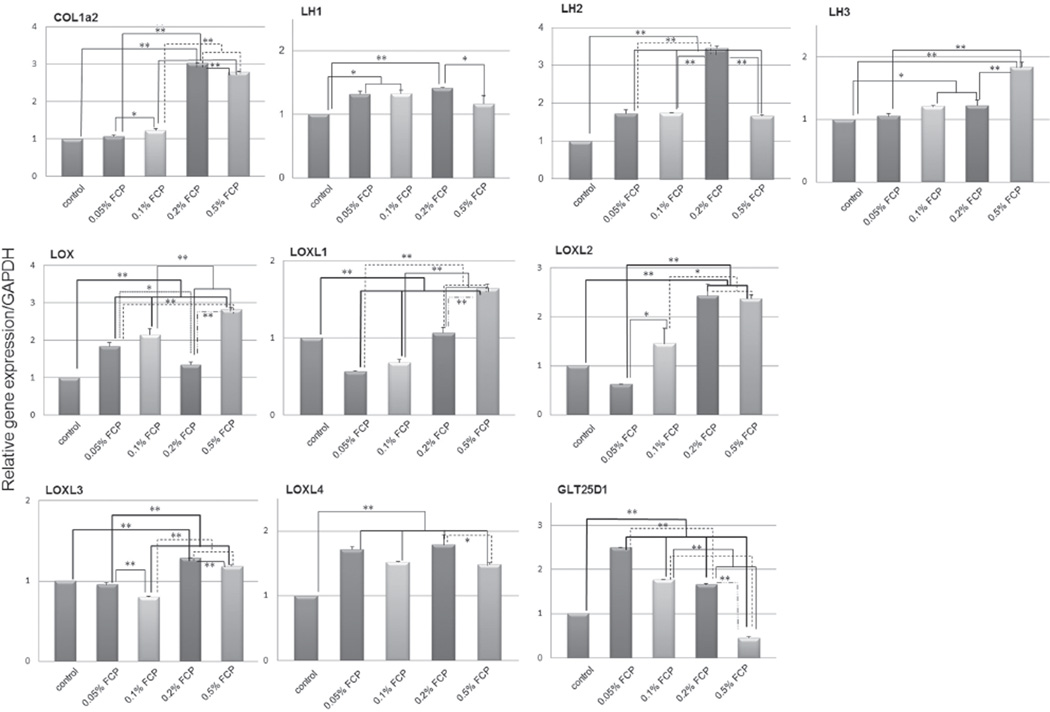
The mRNA expression patterns are shown in Fig. 2. The expression of COL1A2, all LHs, LOXL2–4 and GLT25D1 mRNAs were significantly upregulated by the FCP treatment. LH2 mRNA expression in the 0.2% (w/v) FCP treated group showed the highest increase reaching to 3.4-fold that of the controls (p<0.01), followed by COL1A2, LOXL2, LOXL4, GLT25D1, LH1, LOXL3 and LH3 (3.0, 2.4, 1.8, 1.7, 1.4, 1.3 and 1.2-folds, respectively: p<0.01 except p<0.05 in LH3). The LOX and LOXL1 mRNA expression tended to increase (1.3 and 1.2-fold increases, respectively) but did not reach significant levels. 0.2% (w/v) FCP was used for the subsequent studies, based on these results.
Fig. 2. mRNA expression levels of type I collagen and collagen modifying enzymes with or without FCP treatment in MC3T3-E1 cells analyzed by real-time PCR.
The expression levels were normalized to GAPDH. The values are shown as the mean±SE from triplicate assays. *p<0.05, **p<0.01. LH: lysyl hydroxylase, LOX: lysyl oxidase, FCP: fish collagen peptide.
Cell growth
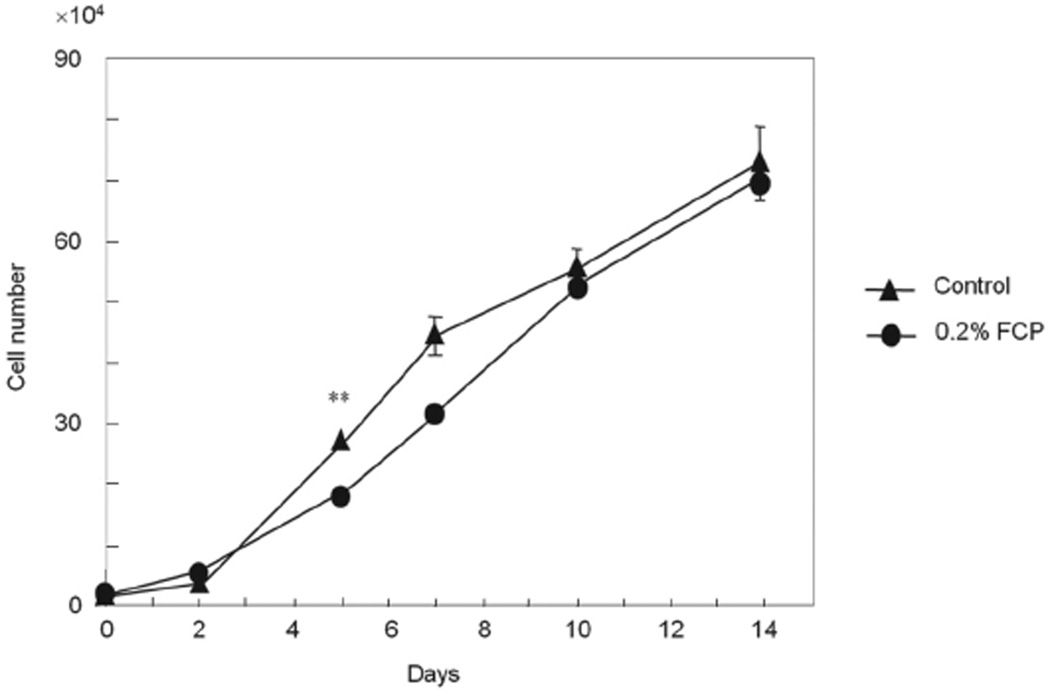
The number of cells in both groups was counted up to 14 days and is shown in Fig. 3. Cell morphology was not changed by the addition of FCP (data not shown). The cell numbers were essentially the same between the FCP treated and control groups during the course of the study except at day 5 when the former showed a lower number of cells in comparison to the control (p<0.01).
Fig. 3. Effect of FCP on cell growth.
The values are shown as the mean±SE from triplicate assays. **p<0.01.
Collagen content, lysine hydroxylation of collagen and collagen cross-links
The collagen contents of MC3T3-E1 cells and Hyl are shown in Table 1. The collagen contents significantly increased (14.8%) by FCP treatment in comparison to the controls (p<0.05).
Table 1.
Contents of collagen, hydroxylysine, cross-link precursor aldehydes and cross-links of collagen
| control | 0.2% FCP | |
|---|---|---|
| Collagen content | 265.783 (±8.488) | 305.220 (±8.550)* |
| Hyl | 42.3 (±0.4) | 52.8 (±3.3)* |
| DHNL | ND | ND |
| HNL | ND | ND |
| DHLNL | 0.766 (±0.026) | 0.839 (±0.037) |
| HLNL | 0.284 (±0.009) | 0.281 (±0.006) |
| Pyr | 0.013 (±0.001) | 0.023 (±0.002)* |
| d-Pyr | ND | ND |
Collagen content is expressed in µg of collagen/mg of cell matrix. The other values are expressed in moles/mole of collagen. Hyl, hydroxylysine; DHNL, dihydroxynorleusine; HNL, hydroxynorleucine; DHLNL, dihydroxylysinonorleucine; HLNL, hydroxylysinonorleucine; Pyr, pyridinoline; d-Pyr, deoxypyridinoline; ND, not detected. The values are shown as the mean±SE.
p<0.05 (n=4).
The Hyl content per collagen derived from MC3T3-E1 cells in the FCP treated group was also significantly higher in comparison to that in the control group (p<0.05). These findings indicate that FCP treatment not only increased collagen synthesis and deposition in the cultures but also elevated the extent of Lys hydroxylation of collagen.
Collagen cross-link analysis
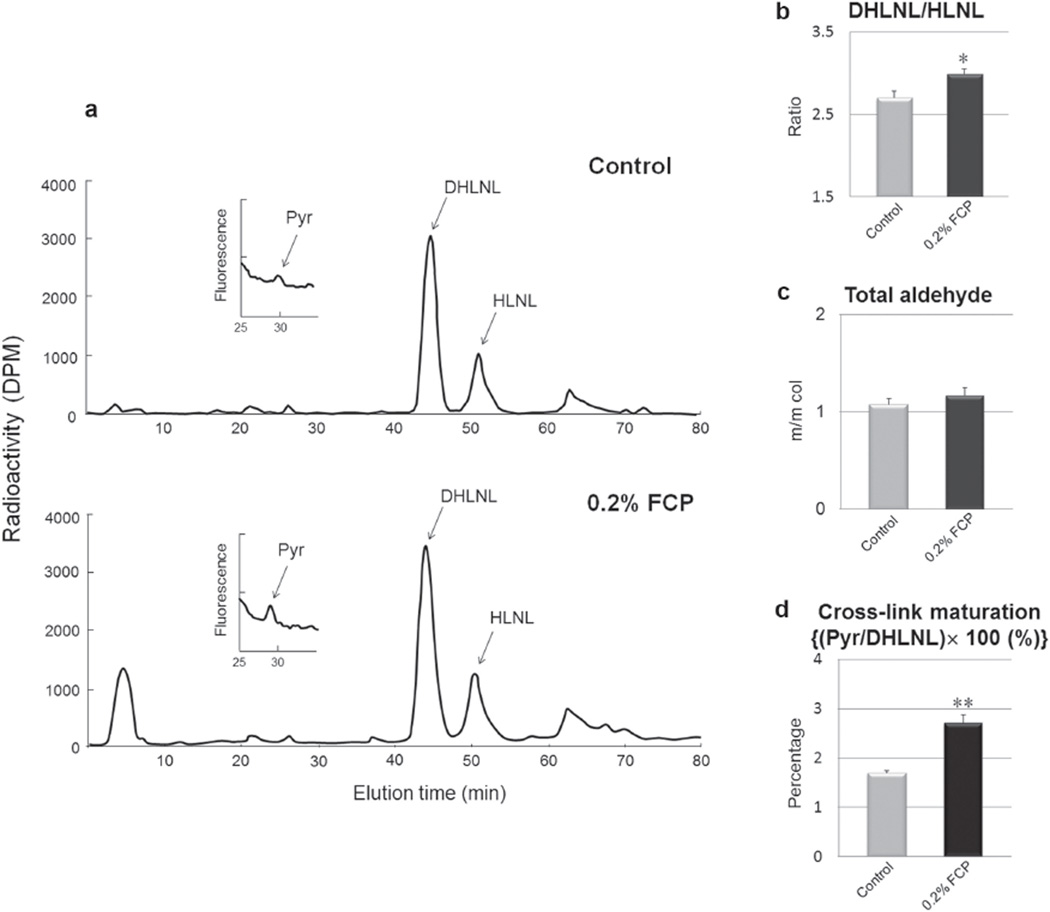
The contents of cross-link precursor aldehydes and collagen cross-links are shown in Table 1. Neither crosslink precursor aldehydes (DHNL and HNL) nor a nonreducible/mature cross-link, d-Pyr, were detected in any of the groups examined. Two major reducible cross-links (DHLNL and HLNL) were identified in both groups, and the levels were comparable between the two groups, although there was a trend toward a higher DHLNL in the FCP treated group. In contrast, the Pyr content in the FCP treated group was significantly higher than that in the control group (p<0.05). The ratio of DHLNL to HLNL, total number of aldehydes and the maturation of collagen cross-links are shown in Fig. 4. The ratio of DHLNL to HLNL in the FCP treated group was significantly higher than that in the control group (2.988±0.067 vs. 2.701±0.078) (p<0.05). The total number of aldehydes was not significantly different between the two groups (1.165±0.084 vs. 1.076±0.058 in control). The cross-link maturation (Pyr/DHLNL ×100) in the FCP treated group was significantly higher than that in the control group (2.702±0.175 vs. 1.690±0.046) (p<0.01).
Fig. 4. Effect of FCP on collagen cross-linking in MC3T3-E1 cell/matrices at 2 weeks of cultures.
(a) Representative chromatographs of collagen cross-links and their precursor aldehydes. (b) DHLNL/HLNL. (c) A total number of aldehydes calculated as a sum of DHLNL+HLNL+2×Pyr. m/m col: moles/mole of collagen. (d) Collagen cross-link maturation evaluated as the ratio of the mature (Pyr) to the immature (DHLNL) cross-links. FCP: Fish collagen peptides, *p<0.05, **p<0.01 (n=4).
In vitro mineralization assay
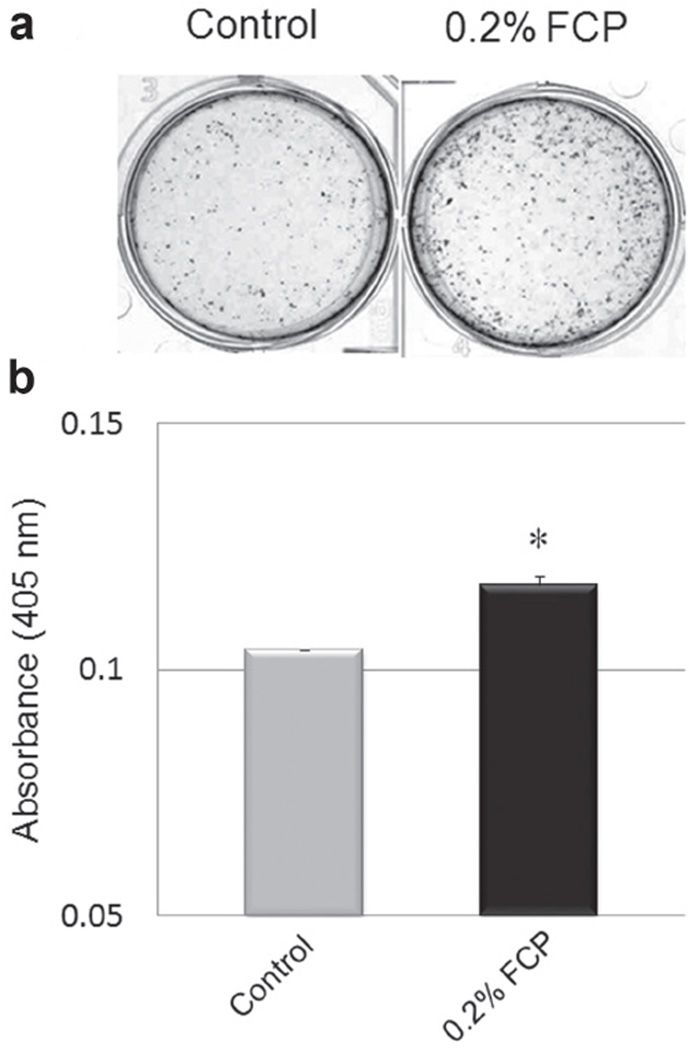
Alizarin red S staining was carried out to assess the extent of matrix mineralization. The representative Alizarin red S staining pattern at day 21 is shown in Fig. 5a. The formation of mineralized nodules was evident in both groups. However, the number of mineralized nodules that formed in the FCP treated group appeared to be significantly higher than that of the controls. A semiquantitative analysis of Alizarin red S staining indeed confirmed that MC3T3-E1 cells treated with FCP produced significantly more stained mineral than the untreated cells (p<0.05; Fig. 5b).
Fig. 5. Effect of FCP on matrix mineralization in MC3T3-E1 cell culture system at 21 day.
(a) The cultures were stained with alizarin red S to visualize mineralized nodules. (b) Semi-quantification of the mineral content in the cultures shown in (a). The values are shown as the mean±SE. *p<0.05 (n=3).
DISCUSSION
Previous studies have indicated that collagen peptides derived from fish skin might have a positive effect on bone by increasing bone mineral density and by exerting anti-inflammatory activity in Osteoarthritis23,23).
The present study explored the effects of FCP on the collagen synthesis, post-translational modifications and mineralization in an osteoblastic cell culture system. The mRNA expression levels of COL1A2, LH1–3 (especially LH2), LOXL2–4 and GLT25D1 were all significantly upregulated in the FCP treated group. Recent studies have indicated that LH1 is the helical LH25), LH2 telopetidyl LH26–28), and LH3 and GLT25D1 collagen glycosyltransferases29,29). Therefore, the biochemical findings of collagen synthesized by the FCP-treated cells, i.e. higher collagen content, higher extent of Lys hydroxylation in collagen, higher levels of Hylald-derived collagen cross-links (i.e. higher DHLNL/HLNL ratio, slightly higher DHLNL and significantly higher Pyr) in comparison to the control cells, are consistent with the gene expression pattern. The upregulation of the gene expression for LH3 and a recently identified glycosyltransferase, GLT25D1, in the FCP treated group indicate that the collagen synthesized in this group could be more glycosylated31). We have recently reported that LH3 primarily functions as a glucosyltransferase for type I collagen and possibly GLT25D1 as a galactosyltransferase in an osteoblastic cell culture system17). We have also suggested a regulatory role of LH3-mediated glucosylation in cross-link maturation when occurred at the specific molecular site, i.e. residue 8732). The current finding that the maturation of collagen cross-links was accelerated in the FCP treated group (Fig. 4) seems inconsistent with this notion. However, to address this, further studies are warranted to determine whether or not the extent of collagen glycosylation in the FCP-treated group is indeed increased at the protein level, and if so, what type of glycosylation (i.e. mono-and/or di-glycosylation) and where in the molecule the increases occur. These data will certainly provide more insight into the nature of collagen produced by FCP-treated cells and the role of glycosylation in cross-linking.
The expression levels of LOX and LOXL1 were unchanged by the FCP treatment, and this is likely the reason why the total number of aldehydes was also comparable between the FCP treated and control groups. The biological functions of LOXL2–4 in osteoblasts are unknown, the results obtained in this study suggest their contribution to the formation of collagen crosslinks in this culture system may not be significant. Therefore, the major effect of FCP on collagen cross-linking in osteoblasts could be the change in the cross-linking pathway toward the Hylald-derived pathway14), but not the quantity of cross-links.
The increased collagen content and the modifications described above may favor the occurrence of matrix mineralization by providing a stable structural template, thus resulting in an increased formation of mineralized nodules.
The MS analysis identified several molecular weight species ranging from 2,351.07 to 8,027.66 (m/z). The apparent molecular weights based on the SDS-PAGE were ~5 k to 13 kDa. The larger values obtained from SDS-PAGE are likely due to the fact that the peptides with a collagenous sequence tend to migrate slower in the gel than other/non-collagenous peptides33,33).
At this point, it is not clear if the effect of FCP observed was exerted by the single, fish collagen-derived peptide or a combination of several peptides. Identifying such peptide(s) will require fractionation of the peptide mixture of FCP and testing for the effects, which will be the natural next step of this study.
CONCLUSIONS
The present study demonstrated that FCP exerts a positive effect on collagen synthesis, collagen quality and matrix mineralization in an osteoblastic cell culture system, likely by upregulating the gene expression of the respective collagen modifying enzymes. This suggests the potential utility of FCP as a biomaterial for bone healing and regeneration.
ACKNOWLEDGMENTS
This study was partially supported by a Grant-in-Aid for Young Scientists (B) 22791840 from the Ministry of Education, Culture, Sports, Science and Technology, of Japan, and by NIH grant AR060978. The authors thank Yaizu Suisankagaku Industry, Japan, for providing FCP and its product information.
REFERENCES
- 1.Ber S, Köse GT, Hasirci V. Bone tissue engineering on patterned collagen films: an in vitro study. Biomaterials. 2005;26:1977–1986. doi: 10.1016/j.biomaterials.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Hirata I, Nomura Y, Ito M, Shimazu A, Okazaki M. Acceleration of bone formation with BMP2 in frame-reinforced carbonate apatite-collagen sponge scaffolds. J Artif Organs. 2007;10:212–217. doi: 10.1007/s10047-007-0391-2. [DOI] [PubMed] [Google Scholar]
- 3.Koide M, Osaki K, Konishi J, Oyamada K, Katakura T, Takahashi A. A new type of biomaterial for artificial skin: Dehydrothermally cross-linked composites of fibrillar and denatured collagens. J Biomed Mater Res. 1993;27:79–87. doi: 10.1002/jbm.820270111. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka M, Koyama Y, Nomura Y. Effects of collagen peptide ingestion on UV-B-induced skin damage. Biosci Biotechnol Biochem. 2009;73:930–932. doi: 10.1271/bbb.80649. [DOI] [PubMed] [Google Scholar]
- 5.Jongjareonrak A, Benjakul S, Visessanguan W, Nagai T, Tanaka M. Isolation and characterisation of acid and pepsin-solubilised collagens from the skin of Brownstripe red snapper (Lutjanus vitta) Food Chem. 2005;93:475–484. [Google Scholar]
- 6.Ohara H, Matsumoto H, Ito K, Iwai K, Sato K. Comparison of quantity and structures of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. J Agric Food Chem. 2007;55:1532–1535. doi: 10.1021/jf062834s. [DOI] [PubMed] [Google Scholar]
- 7.Ohara H, Iida H, Ito K, Takeuchi Y, Nomura Y. Effects of Pro-Hyp, a collagen hydrolysate-derived peptide, on hyaluronic acid synthesis using in vitro cultured synovium cells and oral ingestion of collagen hydrolysates in a guinea pig model of osteoarthritis. Biosci Biotechnol Biochem. 2010;74:2096–2099. doi: 10.1271/bbb.100193. [DOI] [PubMed] [Google Scholar]
- 8.Yamauchi M. Collagen Biochemistry: an Overview. In: Phillips GO, editor. Advances in Tissue Banking. vol. 6. Singapore: World Scientific Publishing; 2002. pp. 445–500. [Google Scholar]
- 9.Fernandes RJ, Harkey MA, Weis M, Askew JW, Eyre DR. The post-translational phenotype of collagen synthesized by SAOS-2 osteosarcoma cells. Bone. 2007;40:1343–1351. doi: 10.1016/j.bone.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turecek C, Fratzl-Zelman N, Rumpler M, Buchinger B, Spitzer S, Zoehrer R, Durchschlag E, Klaushofer K, Paschalis EP, Varga F. Collagen cross-linking influences osteoblastic differentiation. Calcif Tissue Int. 2008;82:392–400. doi: 10.1007/s00223-008-9136-3. [DOI] [PubMed] [Google Scholar]
- 11.Knott L, Bailey AJ. Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance. Bone. 1998;22:181–187. doi: 10.1016/s8756-3282(97)00279-2. [DOI] [PubMed] [Google Scholar]
- 12.Hong HH, Pischon N, Santana RB, Palamakumbura AH, Chase HB, Gantz D, Guo Y, Uzel MI, Ma D, Trackman PC. A role for lysyl oxidase regulation in the control of normal collagen deposition in differentiating osteoblast cultures. J Cell Physiol. 2004;200:53–62. doi: 10.1002/jcp.10476. [DOI] [PubMed] [Google Scholar]
- 13.Rucker RB, Murray J. Cross-linking amino acids in collagen and elastin. Am J Clin Nutr. 1978;31:1221–1236. doi: 10.1093/ajcn/31.7.1221. [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi M, Shiiba M. Lysine hydroxylation and cross-linking of collagen. Methods Mol Biol. 2008;446:95–108. doi: 10.1007/978-1-60327-084-7_7. [DOI] [PubMed] [Google Scholar]
- 15.Yamada S, Ganno T, Ohara N, Hayashi Y. Chitosan monomer accelerates alkaline phosphatase activity on human osteoblastic cells under hypofunctional conditions. J Biomed Mater Res A. 2007;83:290–295. doi: 10.1002/jbm.a.31234. [DOI] [PubMed] [Google Scholar]
- 16.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 17.Sricholpech M, Perdivara I, Nagaoka H, Yokoyama M, Tomer KB, Yamauchi M. Lysyl hydroxylase 3 glucosylates galactosylhydroxylysine residues in type I collagen in osteoblast culture. J Biol Chem. 2011;286:8846–8856. doi: 10.1074/jbc.M110.178509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC T method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi M, Katz EP, Mechanic GL. Intermolecular cross-linking and stereospecific molecular packing in type I collagen fibrils of the periodontal ligament. Biochemistry. 1986;25:4907–4913. doi: 10.1021/bi00365a027. [DOI] [PubMed] [Google Scholar]
- 20.Gregory CA, Gunn WG, Peister A, Prockop DJ. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329:77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Paristhiman D, Mochida Y, Duarte WR, Yamauchi M. Biglycan modulates osteoblast differentiation and matrix mineralization. J Bone Miner Res. 2005;20:1878–1886. doi: 10.1359/JBMR.050612. [DOI] [PubMed] [Google Scholar]
- 22.Bae I, Osatomi K, Yoshida A, Osako K, Yamaguchi A, Hara K. Biochemical properties of acid-soluble collagens extracted from the skins of underutilised fishes. Food Chem. 2008;108:49–54. [Google Scholar]
- 23.Kim MM, Mendis E, Rajapakse N, Lee SH, Kim SK. Effect of spongin derived from Hymeniacidon sinapium on bone mineralization. J Biomed Mater Res Part B Appl Biomater. 2009;90:540–546. doi: 10.1002/jbm.b.31315. [DOI] [PubMed] [Google Scholar]
- 24.Ryu B, Qian ZJ, Kim SK. Purification of a peptide from seahorse, that inhibits TPA-induced MMP, iNOS and COX-2 expression through MAPK and NF-kappaB activation, and induces human osteoblastic and chondrocytic differentiation. Chem Biol Interact. 2010;184:413–422. doi: 10.1016/j.cbi.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Eyre D, Shao P, Weis MA, Steinmann B. The kyphoscoliotic type of Ehlers-Danlos syndrome (type VI): differential effects on the hydroxylation of lysine in collagens I and II revealed by analysis of cross-linked telopeptides from urine. Mol Genet Metab. 2002;76:211–216. doi: 10.1016/s1096-7192(02)00036-7. [DOI] [PubMed] [Google Scholar]
- 26.Uzawa K, Grzesik WJ, Nishiura T, Kuznetsov SA, Robey PG, Brenner DA, Yamauchi M. Differential expression of human lysyl hydroxylase genes, lysine hydroxylation, and cross-linking of type I collagen during osteoblastic differentiation in vitro . J Bone Miner Res. 1999;14:1272–1280. doi: 10.1359/jbmr.1999.14.8.1272. [DOI] [PubMed] [Google Scholar]
- 27.Pornprasertsuk S, Duarte WR, Mochida Y, Yamauchi M. Lysyl hydroxylase-2b directs collagen cross-linking pathways in MC3T3-E1 cells. J Bone Miner Res. 2004;19:1349–1355. doi: 10.1359/JBMR.040323. [DOI] [PubMed] [Google Scholar]
- 28.Pornprasertsuk S, Duarte WR, Mochida Y, Yamauchi M. Overexpression of lysyl hydroxylase-2b leads to defective collagen fibrillogenesis and matrix mineralization. J Bone Miner Res. 2005;20:81–87. doi: 10.1359/JBMR.041026. [DOI] [PubMed] [Google Scholar]
- 29.Wang C, Luosujärvi H, Heikkinen J, Risteli M, Uitto L, Myllylä R. The third activity for lysyl hydroxylase 3: galactosylation of hydroxylysyl residues in collagens in vitro . Matrix Biol. 2002;21:559–566. doi: 10.1016/s0945-053x(02)00071-9. [DOI] [PubMed] [Google Scholar]
- 30.Schegg B, Hülsmeier AJ, Rutschmann C, Maag C, Hennet T. Core glycosylation of collagen is initiated by two beta(1-O) galactosyltransferases. Mol Cell Biol. 2009;29:943–952. doi: 10.1128/MCB.02085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Kovanen V, Raudasoja P, Eskelinen S, Pospiech H, Myllylä R. The glycosyltransferase activities of lysyl hydroxylase 3 (LH3) in the extracellular space are important for cell growth and viability. J Cell Mol Med. 2009;13:508–521. doi: 10.1111/j.1582-4934.2008.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sricholpech M, Perdivara I, Yokoyama M, Nagaoka H, Terajima M, Tomer KB, Yamauchi M. Lysyl Hydroxylase 3-mediated glucosylation in type I collagen. Molecular loci and biological significance. J Biol Chem. 2012;287:22998–23009. doi: 10.1074/jbc.M112.343954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furthmayr H, Timpl R. Characterization of collagen peptides by sodium dodecylsulfate-polyacrylamide electrophoresis. Anal Biochem. 1971;41:510–516. doi: 10.1016/0003-2697(71)90173-4. [DOI] [PubMed] [Google Scholar]
- 34.Eriksen HA, Sharp CA, Robins SP, Sassi ML, Risteli L, Risteli J. Differently cross-linked and uncross-linked carboxy-terminal telopeptides of type I collagen in human mineralised bone. Bone. 2004;34:720–727. doi: 10.1016/j.bone.2003.12.009. [DOI] [PubMed] [Google Scholar]