Abstract
Studies of evolution in wild populations often find that the heritable phenotypic traits of individuals producing the most offspring do not increase proportionally in the population. This paradox may arise when phenotypic traits influence both fecundity and viability and when there is a tradeoff between these fitness components, leading to opposing selection. Such tradeoffs are the foundation of life history theory, but they are rarely investigated in selection studies. Timing of breeding is a classic example of a heritable trait under directional selection that does not result in an evolutionary response. Using a 22-y study of a tropical parrot, we show that opposing viability and fecundity selection on the timing of breeding is common and affects optimal breeding date, defined by maximization of fitness. After accounting for sampling error, the directions of viability (positive) and fecundity (negative) selection were consistent, but the magnitude of selection fluctuated among years. Environmental conditions (rainfall and breeding density) primarily and breeding experience secondarily modified selection, shifting optimal timing among individuals and years. In contrast to other studies, viability selection was as strong as fecundity selection, late-born juveniles had greater survival than early-born juveniles, and breeding later in the year increased fitness under opposing selection. Our findings provide support for life history tradeoffs influencing selection on phenotypic traits, highlight the need to unify selection and life history theory, and illustrate the importance of monitoring survival as well as reproduction for understanding phenological responses to climate change.
Keywords: reproductive success, juvenile survival, adult survival, Forpus passerinus
Meta-analyses of selection often report strong and consistent directional selection on heritable traits without accompanying changes in the trait means over generations (1–4). A variety of alternative hypotheses have been suggested to explain this paradox, such as selection on correlated traits, fluctuating selection caused by environment, low genetic variance, and interactions between environment and genetics (2–6). Another possibility, advanced from theory but rarely shown, is that opposing selection may inhibit directional changes (2, 4, 5). Opposing fecundity and viability (adult survival) selection is predicted to arise from tradeoffs between fitness components, which life history theory suggests should be common (5). The tension on a trait imposed by opposing selection should weaken and constrain directional selection by pushing and pulling the trait in different directions, but few studies examine multiple fitness components (2, 4, 7, 8).
Opposing selection has primarily been shown on sexually selected traits, over single selection episodes, or across different life history stages (8–9). These studies often assume that opposing selection does not fluctuate in time and space. However, variation in environmental conditions or individual phenotypes may result in shifts in the magnitude and occurrence of opposing selection (10). If the drivers of selection act equally on viability and fecundity selection, then opposing selection could constrain changes in trait distributions. However, if multiple drivers differentially affect viability and fecundity selection, they could influence the magnitude of total selection and the occurrence of opposing selection. Multiple ecological drivers of selection are rarely examined (11), but identification of drivers and their influence on selection are necessary to determine whether they catalyze or inhibit directional changes in a trait, which could lead to small-scale fluctuations in the optimal and average phenotypes.
We examined the patterns of selection, drivers of selection, and optimal timing of breeding in two populations of green-rumped parrotlets (Forpus passerinus) in the llanos of Venezuela over 22 y (12) (SI Materials and Methods). Breeding date is highly responsive to environmental change, is heritable, and affects population growth (13–16), making it useful for testing the existence, causes, and consequences of selection. Timing of breeding is heritable in parrotlets (h2 = 0.22), and selection acts independently and more strongly on this trait than on other parrotlet reproductive traits (SI Materials and Methods and Table S1). Parrotlet females nest multiple times per year, and the time when a female initiates her first nest of the year influences the number of breeding attempts and number of fledglings that she produces over the entire year (r2 = 0.26, P < 0.001, n = 615). Thus, timing of breeding may be particularly important for fitness.
We examined three drivers that could influence variation in selection on the timing of breeding—breeding experience, rainfall, and breeding density. Life history theory predicts that experienced (i.e., older) breeders should have stronger fecundity selection and weaker viability selection than first-time breeders, because experienced breeders typically produce more offspring and their survival probability may be less vulnerable to environmental conditions (17, 18). The environmental condition most commonly examined in studies of timing of breeding is temperature, because it influences food availability in northern temperate regions, where climate warming has resulted in shifts in the timing of breeding in some species (13–16, 19, 20). In tropical regions, however, food availability is more strongly affected by rainfall than temperature fluctuations, which has been shown in parrotlets; therefore, rainfall may influence selection on timing of breeding in tropical species (21–23). Rainfall is also expected to respond to climate change and may have a greater effect on tropical species than climate warming (21, 22, 24). If rainfall before breeding is positively related to food availability (21), then fecundity selection for breeding earlier in the year should occur. Breeding density influences demography in many species, including parrotlets, and may alter selection on timing of breeding by affecting the level of competition for resources required during breeding (25, 26). Higher densities often favor earlier breeding (27). If rainfall primarily affects fecundity selection and breeding density primarily affects viability selection, then fluctuations in these environmental conditions could result in unexpected changes in the timing of breeding.
Results
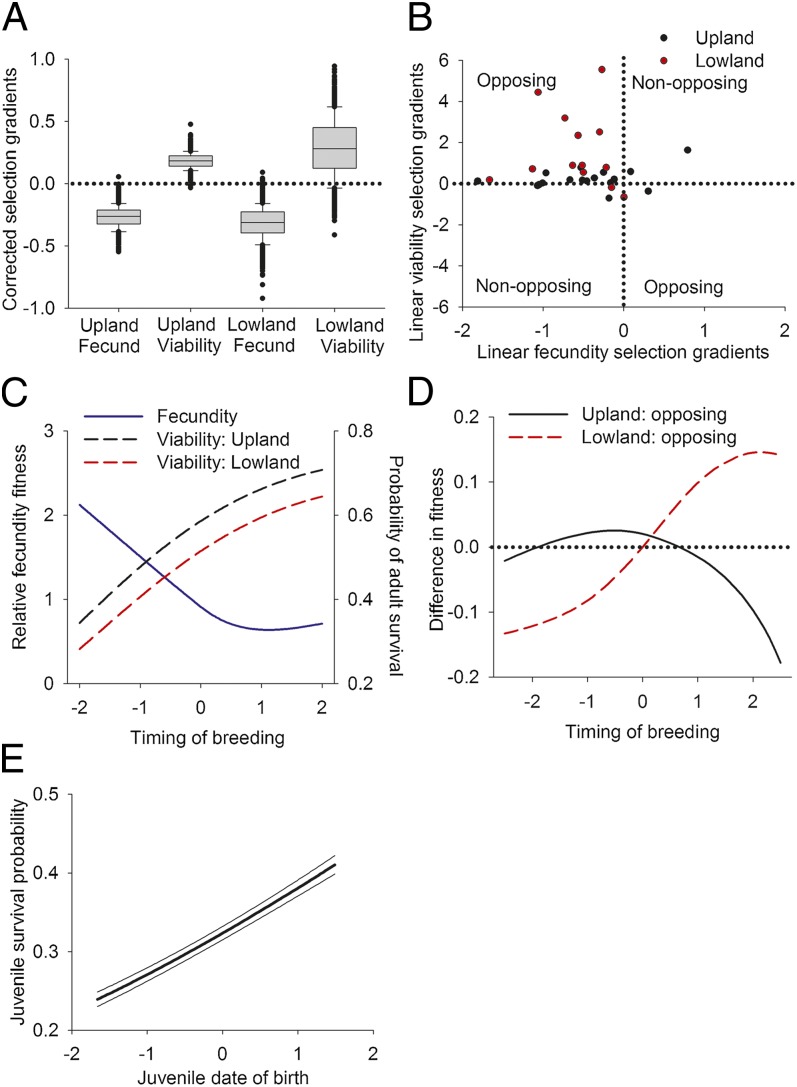
Opposing selection was the most common pattern in both parrotlet populations. Females that bred earlier in the year fledged more offspring (negative fecundity selection) but had a lower probability of survival to the next year (positive viability selection). The probability that selection gradients were opposing was high (Fig. 1A) based on 95% credible intervals (95% CIs) from the posterior probability distribution of selection gradients corrected for sampling error and estimated over the 22-y study period (95% CIs: Upland fecundity: −0.50 to −0.09, Upland viability: 0.08 to 0.31, Lowland fecundity: −0.59 to −0.09, Lowland viability: −0.19 to 0.80). The probability of opposing selection was 99.8% for the Upland population and 98.2% for the Lowland population. The prevalence of opposing selection was also evident when considering uncorrected selection gradients estimated annually (Fig. 1B); opposing selection occurred more often than expected by chance (binomial test: P < 0.001; Upland: 12 of 18 y; Lowland: 12 of 13 y) (Tables S2 and S3). A slight nonlinearity in the form of selection was observed across the entire 22-y study (Fig. 1C), and nonlinear selection was supported in 23–28% of years based on annual uncorrected estimates (Tables S2 and S3). Based on corrected estimates for nonlinear selection, we observed that fecundity selection was typically stabilizing (Upland: −1.14, 95% CI: −1.86 to −0.53, Lowland: −0.91, 95% CI: −1.94 to 0.23) and viability selection was disruptive (Upland: 0.36, 95% CI: 0.02 to 0.72, Lowland: 1.41, 95% CI: 0.15 to 2.75), demonstrating again their opposing nature. Moreover, the magnitude of linear selection varied over years from very weak (0.00) selection on timing of breeding to >0.47 (maximum based on 95% CI was 0.55, 0.47, 0.92, and 1.25 for fecundity and viability selection gradients in the Upland and Lowland populations, respectively). Opposing selection was not caused by a tradeoff between adult survival and the number of offspring produced per year, which does not occur in parrotlets (28), but instead, it resulted from a tradeoff mediated by timing of breeding.
Fig. 1.
Frequency and impact of opposing selection on timing of breeding. (A) Box plots of corrected viability and fecundity selection gradients on timing of breeding. (B) The strength and direction of annual fecundity and viability selection based on uncorrected estimates. When fecundity and viability selection are in the opposite direction (+/−), selection is opposing. Nonopposing selection occurs when selection is in the same direction. Selection gradients are in Tables S2 and S3. (C) Viability and fecundity selection gradients over the course of the entire study. Selection gradients are in Table S4. (D) Optimal timing of breeding under opposing selection (for all breeders) in the Upland and Lowland populations. (E) Juvenile survival as a function of birth date (no population differences).
Opposing selection strongly influenced the optimal timing of breeding. Under opposing selection, the optimal breeding date (where fitness [e.g., λ or population growth] was maximized) was midyear for the Upland population and late in the year for the Lowland population (Fig. 1D). This result is in contrast with Northern Hemisphere bird species, in which reproductive success is usually greatest when breeding early (14). In parrotlets, the benefits to females of breeding early were reduced, because viability and fecundity selections were similar in magnitude and early-born juveniles had a lower probability of survival to the next year (Fig. 1E). The difference in optimal breeding date between the two populations was caused by stronger viability selection in the Lowland than the Upland population (Fig. 1A).
Both the timing of breeding and total selection (direct and indirect) fluctuated strongly in each population but fit predicted differences (Fig. 2). The average breeding date varied across years by 62 and 72 d in the Lowland and Upland populations (Fig. 2A), respectively, which is longer than the breeding season for many Northern Hemisphere birds. Breeding should occur earlier in the Upland than the Lowland population, because opposing selection resulted in lower fitness for early-breeding Lowland females but higher fitness for early-breeding Upland females (Fig. 1D). This prediction was supported, because breeding typically occurred 3.5 wk earlier in the Upland than the Lowland population (Fig. 2A) (Upland: median = 187 d; Lowland: median = 211 d; Mann–Whitney U test, P = 0.01, n = 1,352 nests). Total selection on breeding date (i.e., selection differentials calculated from the change in the mean timing of breeding between adjacent years) oscillated among years between positive and negative values (Fig. 2B). Fluctuations in breeding date may have arisen because of variation among years in the drivers of selection.
Fig. 2.
Fluctuations in selection and observed changes in breeding date. (A) Changes in mean breeding date over the study (mean ± SE). Dotted line is average date over the entire study. (B) Selection differentials on timing of breeding. *Significant change (P < 0.05) in the distributions of timing of breeding in years t and t − 1 (Tables S5). The years 2006–2009 are not included in the Lowland because of small sample sizes.
Environmental variation strongly influenced the magnitude of selection and both the optimal and observed timing of breeding. Rainfall before nesting was the only factor in the top model for fecundity selection [cumulative Akaike information criterion corrected for small sample sizes (AICc) WT = 0.58], with more than two times the weight of evidence as the next-best model (cumulative AICc WT = 0.27); breeding density and population had little effect (Table 1). Higher rainfall years resulted in stronger selection to breed early (MCMCglmm: β = −0.13) (Fig. 3A). In contrast, the best model for viability selection included breeding density and population (AICc = 0.62). Both terms had a cumulative AICc weight of 1.0 (Table 1), which was 2.5 times the weight of evidence for rainfall (cumulative AICc WT = 0.38). In years of lower breeding density, there was stronger selection to initiate nesting later (MCMCglmm: β = −0.18) (Fig. 3B). Thus, initiating breeding early in the year increased fitness in years of high rainfall, whereas optimal timing of breeding was later in years of low rainfall and low breeding density (Fig. 3 C and D). Rainfall and breeding density also influenced the average timing of breeding (Fig. S1).
Table 1.
AICc model selection results for the potential drivers of selection in parrotlets
| Models | AICc | ΔAICc | AICc WT |
| Fecundity | |||
| Rainfall | 20.71 | 0.00 | 0.58 |
| Intercept only | 22.56 | 1.55 | 0.27 |
| Rainfall + population | 25.25 | 4.54 | 0.06 |
| Rainfall + density | 26.23 | 5.53 | 0.04 |
| Population | 26.87 | 6.16 | 0.03 |
| Survival | |||
| Density + population | 6.45 | 0.00 | 0.62 |
| Density + rainfall + population | 8.05 | 1.60 | 0.27 |
| Density + rainfall × population | 9.96 | 3.52 | 0.11 |
Models > 7 ΔAICc not shown.
Fig. 3.
Influence of environmental conditions on timing of breeding. (A) The influence of prebreeding rainfall on corrected fecundity selection gradients. (B) The influence of breeding density on corrected viability selection gradients. (C) Optimal breeding date under high- and low-rainfall years. (D) Optimal breeding date under high- and low-density years.
Annual fluctuations in selection gradients were secondarily modified by an individual’s breeding experience. Opposing selection was again the most common pattern observed for both types of females, but both positive and negative viability selection occurred in first-time breeders in the Lowland and experienced breeders in the Upland populations (Fig. 4 A and B). Variation in the direction of selection resulted in small differences in optimal breeding dates compared with the influence of breeding experience. For experienced breeders, breeding earlier in the year was better than later under opposing and negative selection, whereas breeding later in the year was optimal for first-time breeders in the Upland population (Fig. 4C). In the Lowland population (Fig. 4D), breeding later in the year had strong fitness advantages for experienced breeders, whereas fitness of first-time breeders differed less with breeding date, with a slight increase over the breeding season under opposing selection and no difference under negative selection. Although the number of first-time breeders relative to experienced breeders varied (Lowland: 0.22–0.80; Upland: 0.35–0.78), it had little influence on average breeding date (Fig. S1), because breeding mid- to late year was typically optimal.
Fig. 4.
Influence of breeding experience on timing of breeding. (A and B) Box plots of the corrected viability and fecundity selection gradients on timing of breeding for EBs (experienced breeders) and FBs (first-time breeders) in the (A) Upland (95% CIs: EB fecundity: −0.81 to 0.01, EB viability: −0.21 to 0.39, FB fecundity: −0.35 to −0.01, FB viability: 0.06 to 0.43) and (B) Lowland populations (95% CIs: EB fecundity: −0.80 to −0.01, EB viability: −0.61 to 2.76, FB fecundity: −1.18 to −0.17, FB viability: −0.55 to 0.99). (C) Optimal breeding date in the Upland population when EBs have opposing selection, EBs have negative selection, and FBs have opposing selection. (D) Optimal breeding date in the Lowland population when EBs have opposing selection, FBs have opposing selection, and FBs have negative selection.
Discussion
Our results indicate the importance of incorporating life history theory in studies of selection and phenotypic evolution by showing that opposing selection on reproductive traits is possible and that tradeoffs can be environmentally dependent. Opposing selection may explain why phenotypic traits do not change as expected when only a single fitness metric is examined. Unequal magnitudes of fecundity and viability selection resulted in total selection remaining strong, despite the occurrence of opposing selection (2). Therefore, opposing selection does not necessarily inhibit changes in phenotypic trait distributions; instead, it may lead to trait fluctuations depending on the relative magnitude of selection on multiple fitness components, environmental conditions, and individual heterogeneity (e.g., breeding experience). As a result, mean breeding date in parrotlets oscillated between earlier and later depending on the year.
Like other studies conducted primarily on Northern Hemisphere species (13, 14, 29), tropical parrotlets exhibited negative fecundity selection, but unlike other studies, parrotlets experienced viability selection that was in opposition to and similar in strength to fecundity selection (1, 2). Moreover, viability and fecundity selection gradients corrected for sampling error were larger in parrotlets compared with most other taxa (based on the review in ref. 4; survival: 0.1; fecundity: 0.2). Selection on timing of breeding may be stronger in tropical parrotlets than temperate birds for three reasons. (i) Timing of first breeding by multibrooded parrotlet females influences the number of nesting attempts and offspring that they produce in a year, whereas most temperate birds nest one time and raise a single brood annually (30). (ii) Longer tropical breeding seasons may alter the relationships between adult and juvenile survival, food availability, and breeding date (22, 31). (iii) Nonbreeding males and females are common in parrotlets (and other tropical species), causing strong competition for nest sites and infanticide (12, 31, 32). Although females that began breeding early in the year fledged more offspring, juvenile survival was higher for individuals born later in the year. Higher survival of late-born juveniles reduced the importance of nesting early on the optimal timing of breeding and underscores the need to examine how timing of breeding influences offspring fitness after they leave the nest.
Selection processes in parrotlets were modulated by rainfall and breeding density, but these ecological drivers differently affected viability and fecundity selection, highlighting the importance of examining more than one driver (11). In years of high rainfall, there was strong selection to breed early, perhaps because peak food availability shifted earlier or food availability remained high throughout the year, resulting in a longer breeding season. Corresponding demographic outcomes include greater success for first nests and more nesting attempts for early-breeding individuals. In contrast, there was strong selection to breed later in the years of low breeding density, because it increased the chance of adult survival. Lower breeding density reduces competition for nest sites, which can be strong in parrotlets (33), and it increases the chance of breeding when opportunities are limited (34). Delaying the start of breeding until later in the year might increase adult survival of parrotlets under low breeding densities if low densities are a result of poor conditions early in the breeding season (35). Correspondingly, parrotlet density was positively correlated with adult survival in the same year (β = 0.005, SE = 0.0007, P < 0.001), suggesting that breeding conditions may be better in years of higher density.
Fluctuations in llanos rainfall, population density, and breeding experience maintained large differences in the onset of breeding among parrotlet individuals (May to November), populations (0–62 d), and years (June to September). Moreover, different optimal breeding dates were observed for experienced and first-time breeders in all years in the Upland population and 40% of years in the Lowland population, which might be expected for individual optimization based on previous breeding experience and age (36). As a result, no single strategy within an individual’s lifetime would optimize fitness, and parrotlets should adjust timing of breeding to match environmental conditions and breeding experience. These results combined with a low estimate of heritability imply a strong role of phenotypic plasticity in timing of breeding in parrotlets; another potential reason why heritable traits do not always change as expected (3).
Parrotlets nesting in the Upland and Lowland populations exhibited similar patterns of opposing selection and timing of breeding in response to rainfall and breeding density. These populations are spatially segregated with strong site fidelity of breeding adults, are connected by dispersing juveniles, and their individuals exhibit different demographic rates (12, 32, 37). The fact that these populations had similar patterns of selection on the timing of breeding lends support to the importance of opposing selection and its environmental drivers (rainfall and breeding density). The primary difference between the two populations was how selection on timing of breeding responded to breeding experience. The Upland population fit predictions based on life history theory; experienced breeders had stronger fecundity selection and weaker viability selection (17, 18). However, these predictions were not upheld in the Lowland population; experienced breeders had stronger viability selection than first-time breeders. Nest site selection has a much larger influence on predation rate in the Lowland than the Upland population (32), which results in lower survival of breeding females in the Lowland (37) and may yield greater viability selection.
The timing of breeding is a key phenotypic trait that has been strongly influenced by climate change, because many species have responded to shifts in the phenology of their food supply or other environmental conditions by breeding earlier (15, 16). To truly understand how the timing of life history events will respond to climate change, we need to assess the evolutionary basis of phenological shifts. This approach requires individual-level monitoring of survival in addition to the typical approach of monitoring fecundity. Viability selection should be especially important in medium- and long-lived organisms, because it has large effects on population growth and individual fitness (4, 38). Phenological shifts are expected under directional selection but could also occur under opposing selection if the strength of viability and fecundity selection differs in response to climatic variation. Although studies have primarily focused on temperature warming, changes in rainfall may have greater effects on tropical populations (21). Rainfall is projected to decline over the next century in the llanos of Venezuela (24), which for parrotlets, would reduce the strength of fecundity selection and result in fewer offspring recruited. This change could create a positive feedback loop: lower offspring production results in lower breeding densities, which increases the strength of viability selection and pushes breeding date later, resulting in fewer offspring produced. Such a scenario requires additional evaluation using integrated approaches to assess the effects of climate change and the genetic architecture of opposing selection (39).
Materials and Methods
Field Methods.
Green-rumped parrotlets are small (24–36 g), are plumage-dimorphic, and can have multiple nesting attempts per year. We have studied two color-banded populations in 106 nest boxes in the Venezuelan llanos since 1988 (12). Breeding females exhibit high site fidelity (28). Separated at their closest point by 600 m of inhospitable habitat, the Lowland population is 1–2 m lower than the Upland, and this difference results in substantial differences in vegetation, flooding, and demographic traits (12, 37). Additional details are in SI Materials and Methods.
Selection Analyses.
Selection analyses were conducted in R 2.14 (R Development Core Team 2011). Selection gradients were defined as partial effects from a multiple regression (the slope), and selection differentials were defined as changes in mean timing of breeding between years t and t − 1 (40). Only females were included, because paternity is unknown (SI Materials and Methods). Timing of breeding was defined as the date of the first egg of the first nesting attempt of the year for a female. Timing of breeding was standardized per year and per population to control for environmental covariance. Viability selection was the probability that an adult female survived to the next breeding season, and it was analyzed using a binomial error structure and logit link function. Parrotlets can breed multiple times and have multiple successful broods in 1 y. Therefore, fecundity selection was quantified from the total number of female offspring that a breeding female fledged during the year standardized by the yearly average number of fledged female offspring per adult female (i.e., relative fecundity fitness). It was analyzed with a Gaussian distribution and identity link function. We used the number of fledged young rather than the number that recruited, because it is a standard metric of avian fitness and does not confound local survival with natal dispersal, which strongly influences lifetime reproductive success in parrotlets (26); also, only a small proportion of females recruit locally because of natal dispersal. Instead, fecundity selection was translated into local recruitment, which is described in SI Materials and Methods, through the estimation of local juvenile survival and the incorporation of both terms into a matrix population model to estimate optimal timing of breeding.
We estimated selection gradients using three methods. First, we calculated annual linear and nonlinear selection gradients using the approaches by Lande and Arnold (41) and generalized linear models. Populations and years were analyzed separately, and female age was included as a covariate, because age influenced timing of breeding and survival (SI Materials and Methods). Linear and nonlinear models were compared using AICc; the model with the lowest AICc value best explained the form of selection (42) for that year (Tables S2 and S3). Second, we conducted random effect meta-analyses to take into account sampling error and uncertainty in the selection gradient estimates (here called “corrected” estimates, refs. 4 and 8 contain methodological details). These analyses were done using a Bayesian modeling framework and the package MCMCglmm (43). The annual linear and nonlinear selection gradients estimated above and their SE values were used in the meta-analyses models. All models included flat priors, an intercept-only model, no random effects, and 10,000 iterations. These analyses provided an estimate of the overall selection gradient after accounting for sampling error and 95% CIs based on the posterior probability distributions. We assessed the probability of obtaining positive, negative, and opposing selection from the posterior probability distributions. Third, we analyzed selection across the entire study using generalized linear mixed models and generalized additive mixed models. Female age was included as a covariate, and female identity was included as a random factor, because females may breed in more than 1 y. Estimates corrected for sampling error were not examined, because (i) we wanted to test for nonlinearity using generalized additive mixed models, which is not possible with MCMCglmm, and (ii) only one estimate per fitness metric, based on large sample sizes (Table S4), was calculated, greatly reducing sampling error (4). AICc model selection was used to test the form of selection and whether population, year, or interaction was important (Table S6). The best model was used to explain selection across the entire study.
Drivers of Selection.
Three potential drivers of selection were examined: breeding experience, rainfall, and breeding density (SI Materials and Methods). We examined variation in selection between first-time (FBs) and experienced breeders (EBs) by first calculating annual linear selection for EBs and FBs using a generalized linear model. We then used these estimates and their SEs in the meta-analysis technique described above (with the same parameters) to examine selection for EBs and FBs corrected for sampling error (Fig. 4). We examined the influence of rainfall and breeding density using a two-step approach. Because of the lack of a unified theory for methods of Bayesian model selection, we used the procedure suggested by Royle and Dorazio (44) and employed a frequentist method of model selection to identify the best model and a Bayesian method to estimate parameters. First, we used uncorrected linear selection gradients to obtain a top model based on AICc model selection (Table 1). Second, the fixed effects in the top model were used in the meta-analysis method to test how environmental drivers influence selection using corrected gradients. Year was included as a random effect for fecundity but not viability selection based on the results of log-likelihood ratio tests.
Optimal Timing of Breeding.
To examine the optimal timing of breeding, we used a matrix population model (SI Materials and Methods and Figs. S2 and S3) to quantify when fitness is maximized over different breeding dates. The matrix model was adapted from a model developed previously for parrotlets (37), and it included three stages—nonbreeders, FBs, and EBs. Corrected selection gradients and the effect of timing of breeding on juvenile survival were incorporated into the model. The selection gradients and mean number of offspring used in the model varied depending on the comparisons being made (opposing vs. nonopposing and EB vs. FB).
Supplementary Material
Acknowledgments
We thank the many Forpus field workers over the years and acknowledge the long-term contributions by Karl Berg, Virginia Saenz, and Scott Stoleson. The laboratory of S.R.B., J. Patrick Kelley, Stephanie Carlson, Shripad Tuljapurkar, Sutirth Dey, and two anonymous reviewers provided helpful comments on the manuscript. The late Tomas Blohm gave us permission to work and live on his ranch. The research described here was compliant with the current laws of Venezuela. Decades of parrotlet research were funded primarily by multiple grants from each of our long-term sponsors: National Science Foundation Grants IBN-9407349, DEB-9503194 and IBN-0113173, the Smithsonian Institution, the National Geographic Society, and the A. Starker Leopold Chair in Wildlife Biology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303821110/-/DCSupplemental.
References
- 1.Siepielski AM, DiBattista JD, Evans JA, Carlson SM. Differences in the temporal dynamics of phenotypic selection among fitness components in the wild. Proc Biol Sci. 2011;278(1711):1572–1580. doi: 10.1098/rspb.2010.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kingsolver JG, Diamond SE. Phenotypic selection in natural populations: What limits directional selection? Am Nat. 2011;177(3):346–357. doi: 10.1086/658341. [DOI] [PubMed] [Google Scholar]
- 3.Merilä J, Sheldon BC, Kruuk LEB. Explaining stasis: Microevolutionary studies in natural populations. Genetica. 2001;112–113(2001):199–222. [PubMed] [Google Scholar]
- 4.Kingsolver JG, Diamond SE, Siepielski AM, Carlson SM. Synthetic analyses of phenotypic selection in natural populations: Lessons, limitations and future directions. Evol Ecol. 2012;26(5):1101–1118. [Google Scholar]
- 5.Roff DA. Life History Evolution. Sunderland, MA: Sinauer; 2002. [Google Scholar]
- 6.Wilson AJ, et al. Environmental coupling of selection and heritability limits evolution. PLoS Biol. 2006;4(7):e216. doi: 10.1371/journal.pbio.0040216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrissey MB, Hadfield JD. Directional selection in temporally replicated studies is remarkably consistent. Evolution. 2012;66(2):435–442. doi: 10.1111/j.1558-5646.2011.01444.x. [DOI] [PubMed] [Google Scholar]
- 8.Schluter D, Price TD, Rowe L. Conflicting selection pressures and life-history trade-offs. Proc Biol Sci. 1991;246(1315):11–17. [Google Scholar]
- 9.Candolin U. Opposing selection on a sexually dimorphic trait through female choice and male competition in a water boatman. Evolution. 2004;58(8):1861–1864. doi: 10.1111/j.0014-3820.2004.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 10.Borash DJ, Gibbs AG, Joshi A, Mueller LD. A genetic polymorphism maintained by natural selection in a temporally varying environment. Am Nat. 1998;151(2):148–156. doi: 10.1086/286108. [DOI] [PubMed] [Google Scholar]
- 11.MacColl ADC. The ecological causes of evolution. Trends Ecol Evol. 2011;26(10):514–522. doi: 10.1016/j.tree.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Beissinger SR. Long-term studies of the green-rumped parrotlet (Forpus passerinus) in Venezuela: Hatching asynchrony, social system and population structure. Ornitol Neotrop. 2008;19:73–83. [Google Scholar]
- 13.Daan S, Dijkstra C, Tinbergen JM. Family-planning in the kestrel (Falco tinnunculus)—the ultimate control of covariation of laying date and clutch size. Behaviour. 1990;114(1–4):83–116. [Google Scholar]
- 14.Sheldon BC, Kruuk LEB, Merilä J. Natural selection and inheritance of breeding time and clutch size in the collared flycatcher. Evolution. 2003;57(2):406–420. doi: 10.1111/j.0014-3820.2003.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 15.Both C, et al. Large-scale geographical variation confirms that climate change causes birds to lay earlier. Proc Biol Sci. 2004;271(1549):1657–1662. doi: 10.1098/rspb.2004.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charmantier A, et al. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science. 2008;320(5877):800–803. doi: 10.1126/science.1157174. [DOI] [PubMed] [Google Scholar]
- 17.Forslund P, Pärt T. Age and reproduction in birds—hypotheses and tests. Trends Ecol Evol. 1995;10(9):374–378. doi: 10.1016/s0169-5347(00)89141-7. [DOI] [PubMed] [Google Scholar]
- 18.Nevoux M, Weimerskirch H, Barbraud C. Environmental variation and experience-related differences in the demography of the long-lived black-browed albatross. J Anim Ecol. 2007;76(1):159–167. doi: 10.1111/j.1365-2656.2006.01191.x. [DOI] [PubMed] [Google Scholar]
- 19.Husby A, Visser ME, Kruuk LEB. Speeding up microevolution: The effects of increasing temperature on selection and genetic variance in a wild bird population. PLoS Biol. 2011;9(2):e1000585. doi: 10.1371/journal.pbio.1000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst. 2006;37:637–669. [Google Scholar]
- 21.Williams SE, Middleton J. Climatic seasonality, resource bottlenecks, and abundance of rainforest birds: Implications for global climate change. Divers Distrib. 2008;14(1):69–77. [Google Scholar]
- 22.Senapathi D, Nicoll MA, Teplitsky C, Jones CG, Norris K. Climate change and the risks associated with delayed breeding in a tropical wild bird population. Proc Biol Sci. 2011;278(172):3184–3190. doi: 10.1098/rspb.2011.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoleson SH, Beissinger SR. Hatching asynchrony, brood reduction, and food limitation in a neotropical parrot. Ecol Monogr. 1997;67(2):131–154. [Google Scholar]
- 24.Christensen JH, et al. In: Climate Change 2007: The Physical Science Basis. Contribution of Working Group 1 to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Solomon S, et al., editors. Cambridge, United Kingdom: Cambridge Univ Press; 2007. pp. 847–940. [Google Scholar]
- 25.Garant D, Kruuk LEB, McCleery RH, Sheldon BC. The effects of environmental heterogeneity on multivariate selection on reproductive traits in female great tits. Evolution. 2007;61(7):1546–1559. doi: 10.1111/j.1558-5646.2007.00128.x. [DOI] [PubMed] [Google Scholar]
- 26.Tarwater CE, Beissinger SR. Dispersal polymorphisms from natal phenotype-environment interactions have carry-over effects on lifetime reproductive success of a tropical parrot. Ecol Lett. 2012;15(11):1218–1229. doi: 10.1111/j.1461-0248.2012.01843.x. [DOI] [PubMed] [Google Scholar]
- 27.Ahola MP, Laaksonen T, Eeva T, Lehikoinen E. Great tits lay increasingly smaller clutches than selected for: A study of climate- and density-related changes in reproductive traits. J Anim Ecol. 2009;78(6):1298–1306. doi: 10.1111/j.1365-2656.2009.01596.x. [DOI] [PubMed] [Google Scholar]
- 28.Sandercock BK, Beissinger SR, Stoleson SH, Melland RR, Hughes CR. Survival rates of a neotropical parrot: Implications for latitudinal comparisons of avian demography. Ecology. 2000;81(5):1351–1370. [Google Scholar]
- 29.Verhulst S, Vanbalen JH, Tinbergen JM. Seasonal decline in reproductive success of the great tit—variation in time or quality. Ecology. 1995;76(8):2392–2403. [Google Scholar]
- 30.Bohning-Gaese K, Halbe B, Lemoine N, Oberrath R. Factors influencing the clutch size, number of broods and annual fecundity of North American and European land birds. Evol Ecol Res. 2000;2(7):823–839. [Google Scholar]
- 31.Tarwater CE, Ricklefs RE, Maddox JD, Brawn JD. Pre-reproductive survival in a tropical bird and its implications for avian life histories. Ecology. 2011;92(6):1271–1281. doi: 10.1890/10-1386.1. [DOI] [PubMed] [Google Scholar]
- 32.Bonebrake TC, Beissinger SR. Predation and infanticide influence ideal free choice by a parrot occupying heterogeneous tropical habitats. Oecologia. 2010;163(2):385–393. doi: 10.1007/s00442-010-1566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beissinger SR, Tygielski S, Elderd B. Social constraints on the onset of incubation in a neotropical parrot: A nestbox addition experiment. Anim Behav. 1998;55(1):21–32. doi: 10.1006/anbe.1997.0576. [DOI] [PubMed] [Google Scholar]
- 34.Beissinger SR. On the limited breeding opportunities hypothesis for avian clutch size. Am Nat. 1996;147(4):655–658. [Google Scholar]
- 35.Greene CM, Stamps JA. Habitat selection at low population densities. Ecology. 2001;82(8):2091–2100. [Google Scholar]
- 36.Verhulst S, Nilsson JA. The timing of birds’ breeding seasons: A review of experiments that manipulated timing of breeding. Philos Trans R Soc Lond B Biol Sci. 2008;363(1490):399–410. doi: 10.1098/rstb.2007.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veran S, Beissinger SR. Demographic origins of skewed operational and adult sex ratios: Perturbation analyses of two-sex models. Ecol Lett. 2009;12(2):129–143. doi: 10.1111/j.1461-0248.2008.01268.x. [DOI] [PubMed] [Google Scholar]
- 38.Caswell H. Matrix Population Models: Construction, Analysis and Interpredation. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- 39.Visser ME, Caro SP, van Oers K, Schaper SV, Helm B. Phenology, seasonal timing and circannual rhythms: Towards a unified framework. Philos Trans R Soc Lond B Biol Sci. 2010;365(1555):3113–3127. doi: 10.1098/rstb.2010.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsumura S, Arlinghaus R, Dieckmann U. Standardizing selection strengths to study selection in the wild: A critical comparison and suggestions for the future. Bioscience. 2012;62(12):1039–1054. [Google Scholar]
- 41.Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983;37(6):1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 42.Burnham KP, Anderson DR. Model Selection and Inference: A Practical Information-Theoretic Approach. New York: Springer; 2002. [Google Scholar]
- 43.Hadfield JD. MCMC methods for multi-repsonse generalized linear mixed models: The MCMCglmm R package. J Stat Softw. 2010;33(2):1–22. [Google Scholar]
- 44.Royle JA, Dorazio RM. Hierarchical Modeling and Inference in Ecology: The Analysis of Data from Populations, Metapopulations and Communities. San Diego: Academic; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.