Significance
The olfactory system of male moths exhibits the ability to detect minute amounts of sex pheromones. How this extreme sensitivity is achieved remains unclear. Using optogenetic techniques to activate a pheromone-responsive olfactory receptor neuron, our results reveal that weak olfactory inputs, but not strong inputs, are temporally integrated in second-order projection neurons to promote behavioral responsiveness. Furthermore, temporal integration of strong olfactory inputs is inhibited by GABAergic mechanisms, indicating that GABA signaling suppresses the amplification of strong stimuli. The timescale of this temporal integration corresponds well to the temporal dynamics of odor signals in the natural environment, suggesting that the olfactory systems of male moths use this mechanism to detect weak pheromone signals in the air.
Keywords: optogenetics, pheromone orientation behavior, transgenic silkmoth, olfaction
Abstract
The olfactory system of male moths has an extreme sensitivity with the capability to detect and recognize conspecific pheromones dispersed and greatly diluted in the air. Just 170 molecules of the silkmoth (Bombyx mori) sex pheromone bombykol are sufficient to induce sexual behavior in the male. However, it is still unclear how the sensitivity of olfactory receptor neurons (ORNs) is relayed through the brain to generate high behavioral responsiveness. Here, we show that ORN activity that is subthreshold in terms of behavior can be amplified to suprathreshold levels by temporal integration in antennal lobe projection neurons (PNs) if occurring within a specific time window. To control ORN inputs with high temporal resolution, channelrhodopsin-2 was genetically introduced into bombykol-responsive ORNs. Temporal integration in PNs was only observed for weak inputs, but not for strong inputs. Pharmacological dissection revealed that GABAergic mechanisms inhibit temporal integration of strong inputs, showing that GABA signaling regulates PN responses in a stimulus-dependent fashion. Our results show that boosting of the PNs’ responses by temporal integration of olfactory information occurs specifically near the behavioral threshold, effectively defining the lower bound for behavioral responsiveness.
Olfaction is a key element in many aspects of animal behavior, such as foraging, oviposition, and mate recognition. In many moth species, a special class of odorants called sex pheromones plays a critical role for identification of and orientation to potential mates. Because sex pheromones emitted by females are greatly diluted and dispersed in the air, sophisticated olfactory systems to detect minute amounts of sex pheromones and processing systems to translate subtle peripheral sensory responses into appropriate behavioral responses have evolved in male moths. Theoretical calculations have shown that the detection of 170 molecules of the sex pheromone bombykol [(E,Z)-10,12-hexadecadienol] can trigger sexual behavioral responses in males of the silkmoth Bombyx mori (1). The physical and chemical mechanisms in the antennae, the sensilla, and the olfactory receptor neurons (ORNs) responsible for this remarkably high sensitivity, which can detect even a single molecule, are well understood (2, 3). However, it is unclear how a small number of spikes from a small number of ORNs is processed centrally to allow for high behavioral responsiveness.
In moths, pheromone molecules are detected by specialized antennal ORNs that express particular pheromone receptor genes (4–10). The axons of ORNs convey pheromone information to the first olfactory center, the antennal lobe (AL; an analog of the vertebrate olfactory bulb). The AL is composed of a number of glomeruli where ORNs establish connections with two types of neurons: projection neurons (PNs), which relay olfactory information to higher brain regions, and local interneurons (LNs), which are involved in processing olfactory information within the deutocerebrum (11). In particular, male ALs have a specialized pheromone-processing unit called the macroglomerular complex (MGC), which comprises several glomeruli (12). In the silkmoth, the toroid glomerulus in the MGC is known to exclusively process bombykol information (13).
Previous reports have shown that the sensitivity of pheromone-responsive ORNs is ∼1,000-fold lower than that of PNs tuned to the same pheromone compound (14, 15), suggesting that the AL network amplifies ORN inputs. One possible source of amplification is the high convergence ratio between ORNs and PNs (16). Considering the small size of most antennae, spatial integration would be likely to occur; however, the fact that weak stimuli activate a few ORNs at best calls for additional mechanisms to explain the high behavioral sensitivity.
One candidate explanation is temporal integration, which is a fundamental mechanism for contributing to the amplification of synaptic inputs (17–19). In the olfactory system, the temporal integration properties of AL neurons and their relevance for the initiation of behavior are unknown. Temporal integration in the olfactory system has been challenging to investigate in detail because of the difficulty of accurately controlling olfactory stimuli within a given time period. Under natural conditions, odor molecules are distributed in odor plumes with intermittent local dynamics of odor exposure lasting >100 ms, followed by odor-free air lasting several hundreds of milliseconds (20–24). However, another study showed that “bursts” of odor within a plume structure can be as little 10–20 ms (25, 26). Therefore, the stimulation of pheromone-responsive ORNs must be controlled in the millisecond range.
To examine the role of temporal integration in the generation of low thresholds for pheromone-induced behavioral responses, we generated GAL4/UAS transgenic silkmoths expressing channelrhodopsin-2 (ChR2), a light-gated ion channel from green algae, in bombykol-responsive ORNs. The transgenic silkmoths showed light-activated pheromone orientation behavior, and we succeeded in controlling the activity of bombykol-responsive ORNs with single-spike resolution. Using paired-pulse photostimulation at various interstimulus intervals (ISIs), we tested whether temporal integration in the AL network could be the basis of the high behavioral sensitivity.
Results
Replacing Pheromone Stimuli with Light.
We generated a GAL4/UAS transgenic silkmoth line expressing ChR2 under the control of a putative promoter sequence of Bombyx mori olfactory receptor-1 (BmOR1) (27), the gene encoding the olfactory receptor for the major sex pheromone (bombykol) in the silkmoth. RT-PCR with ChR2 gene-specific primers showed that ChR2 was expressed only in male antennae bearing both the BmOR1-GAL4 and UAS-ChR2 transgenes (Fig. 1A). Two-color fluorescence whole-mount in situ hybridization of male antennae revealed that ChR2 was expressed in almost all (96.8%) BmOR1-expressing cells in transgenic silkmoths containing BmOR1-GAL4 and UAS-ChR2 (Fig. S1 and Table S1). In the antennae of BmOR1-GAL4/UAS-ChR2 male moths, fluorescence signals of mCherry fused with ChR2 were detected in ORNs whose dendrites innervated pheromone-responsive sensilla (Fig. 1B). Axons of ChR2-expressing neurons terminated exclusively in the toroid (Fig. 1C), which is the bombykol-processing glomerulus in the silkmoth MGC (13). These results show that ChR2 expression was restricted to bombykol-responsive ORNs.
Fig. 1.
Light stimulation triggers pheromone orientation behavior in ChR2-expressing transgenic silkmoths. (A) RT-PCR analysis of the ChR2 gene. RT-PCR was conducted with total RNA from the antennae of male moths carrying BmOR1-GAL4 and UAS-ChR2, UAS-ChR2 alone, or BmOR1-GAL4 alone. The minus sign (−) indicates negative controls without reverse transcriptase. Actin was used as a positive control. (B) The expression of ChR2 in the antennae of male moths bearing both BmOR1-GAL4 and UAS-ChR2 was visualized by fluorescence of mCherry fused with ChR2. White arrows indicate the dendrites of ORNs that innervate olfactory sensilla. No mCherry signals were detected in the proximal axon. (Scale bar: 50 μm.) (C) Terminal axonal arborization of ChR2-expressing neurons visualized by fluorescence of mCherry fused with ChR2 (magenta) in a representative confocal section. The label was confined to the toroid (dotted line) of the MGC. The overall neuropil structure was imaged by autofluorescence (green). T, toroid; D, dorsal, M; medial. (Scale bar: 100 μm.) (D) Blue-light stimulation induced pheromone orientation behavior only in the ATR-injected BmOR1-GAL4/UAS-ChR2 male moths (n = 20). A single 100-ms pulse of 1.19 mW/mm2 blue light was used as a stimulus. (E) Light intensity-dependent increase in the percentage of ChR2-expressing male moths responding to blue-light stimulation (n = 20). A series of blue-light intensities (100-ms duration; 0.04–1.19 mW/mm2) was applied. (F) Illumination-duration-dependent increase in the percentage of ChR2-expressing male moths (n = 35) responding to blue-light stimulation (1.69 mW/mm2). (G) Dynamics of the cumulative body-orientation angle during tethered walking in a representative ChR2-expressing male moth in response to photostimulation (Upper) or bombykol stimulation (Lower). Unilateral stimuli of 200-ms duration with 1.69-mW/mm2 blue light (Upper) or 100 ng of bombykol (Lower) triggered similar zigzag behavioral patterns. Stimulus onset (at t = 0) is indicated by arrows; an angle of 0° indicates the initial forward bearing of the moth.
Upon blue-light stimulation, ChR2-expressing male moths exhibited wing-flapping behavior, which always accompanies pheromone orientation behavior in silkmoths (Fig. 1D and Movie S1). The initiation of the behavioral response was dependent on the intensity and duration of illumination (Fig. 1 E and F). No behavioral response was observed in ChR2-expressing moths without all-trans retinal (ATR) injection or devoid of antennae, in response to yellow-light stimulation, in parental control moths bearing either the GAL4 or UAS transgene alone or in normal moths (Fig. 1D, Figs. S2 and S3, and Movie S2).
The analysis of the body orientation direction after single-pulsed photostimulation demonstrated that the moths displayed zigzag turning typical of pheromone orientation behavior (Fig. 1G) (28). Based on different behavioral parameters, there were no significant differences between stimulation with bombykol and with blue light (Fig. 1G and Table S2). Therefore, blue-light stimulation in the ChR2-expressing male silkmoths can be used to mimic olfactory stimulation with pheromones.
To confirm that ChR2-assisted photoactivation can control the activity of bombykol-responsive ORNs, we recorded the electrophysiological responses to blue-light stimulation in male long sensilla trichodea that contained a bombykol-responsive ORN. All of the tested long sensilla trichodea in transgenic moths responded to both bombykol and blue-light stimulation with the same spike amplitude, indicating that light-evoked spikes were derived from the bombykol-responsive ORNs; long sensilla trichodea in parental control moths showed no responses to blue light (Fig. 2A and Fig. S4). The responses of bombykol-responsive ORNs to light stimulation were dependent on the intensity and duration of the light stimulus (Fig. 2 B and C).
Fig. 2.
Light-evoked ORN responses in BmOR1-GAL4/UAS-ChR2 transgenic moths. (A) Representative recordings of bombykol-responsive ORNs to blue-light stimulation (1.69 mW/mm2) for 100-ms duration, as indicated by the bars under the recording traces. The genotypes are given on each trace. (B) Intensity-dependent increase in spike counts induced by blue-light stimulation for 100-ms duration in bombykol-responsive ORNs expressing ChR2. Data are shown as means ± SEM (n = 18). (C) Duration-dependent increase in spike counts induced by blue-light stimulation at 1.69 mW/mm2 in bombykol-responsive ORNs expressing ChR2. Data are shown as means ± SEM (n = 48).
In the brain, ChR2-assisted photoactivation of bombykol-responsive ORNs triggered the same typical neural activity patterns as bombykol stimulation in toroid PNs and neck motor neurons, whose activity is indicative of steering during pheromone orientation (29). We thus confirmed that the response of bombykol-responsive ORNs to ChR2-assisted photostimulation mimics the bombykol-induced response in the brain (Fig. S5).
Temporal Integration Lowers the Behavioral Threshold.
To determine whether intermittent ORN inputs could be effectively integrated to induce behavior, we observed behavioral responses to 3-ms paired-pulse photostimulation separated by various ISIs. Only 17–28% of moths showed a behavioral response at ISIs from 100 to 180 ms, but behavioral responsiveness was elevated more than twofold at ISIs of <80 ms (Fig. 3). Thus, ISIs of <80 ms in paired-pulse photostimulation led to temporal integration and enhanced behavioral responsiveness. Where in the brain does this behaviorally relevant temporal integration occur? One potential candidate is the AL, and we therefore proceeded to analyze temporal integration properties of AL PNs.
Fig. 3.
Temporal integration in the initiation of pheromone orientation behavior induced by paired-pulse photostimulation. Shown are percentages of moths responding behaviorally to paired-pulse photostimulation (3-ms duration and 1.69 mW/mm2) at various ISIs. Data groups labeled with different letters differ significantly (P < 0.05; Fisher’s exact test). The percentage of moths displaying a behavioral response was significantly increased with ISIs of <80 ms (n = 36).
Temporal Integration in PN Responses at Behaviorally Relevant Timescales.
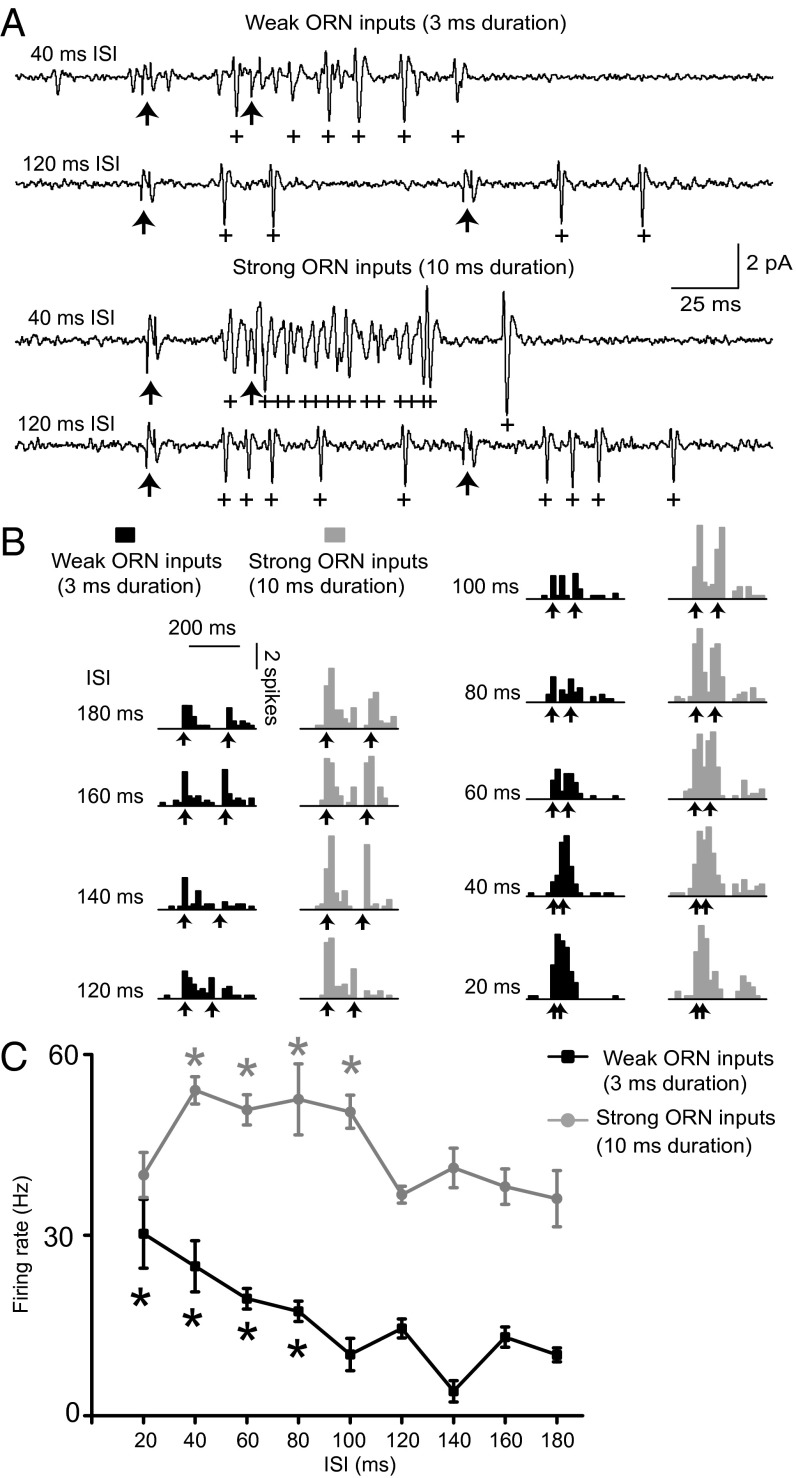
We recorded PN responses to paired-pulse photostimulation separated by various ISIs using the same paradigm as in the behavioral experiments (Fig. 4). At longer ISIs (100–180 ms), PN responses to the stimuli were clearly temporally segregated and similar in amplitude. This segregation represents the condition in which responses to successive stimuli do not interact (Fig. 4C). In contrast, PN spike responses tended to increase at an ISI of 80 ms and were significantly elevated at ISIs of ≤60 ms, compared with single responses at long ISIs (Fig. 4C). These results show that ORN inputs are temporally integrated in PNs on a behaviorally relevant timescale.
Fig. 4.
Temporal integration in PN responses to paired-pulse photostimulation. (A) Representative PN responses to paired-pulse photostimulation of 3-ms (Upper) or 10-ms (Lower) duration at an ISI of 40 or 120 ms. With 3-ms duration, paired-pulsed stimulation induced a greater spike count at 40-ms ISI than at 120-ms ISI. Arrows indicate the timing of stimulus presentation. The plus symbol (+) indicates spike timing. (B) Peristimulus time histograms (PSTHs; 20-ms bin width) of PN spike responses averaged over individuals (n = 12). Arrows indicate the timing of stimulus presentation. (C) Relationship between ISIs of paired-pulsed photostimulation and PN spike output. The firing rate was calculated by counting spikes during 500 ms from the onset of the first light pulse. A paired-pulse stimulus with 3-ms duration evoked significantly more spikes at ISIs from 20 to 60 ms than at an ISI of 180 ms (n = 12). *P < 0.05 (Wilcoxon signed rank test). No increase in spike number was observed with stimulation of 10-ms duration (n = 13). Data are shown as means ± SEM.
Temporal integration may impede the capability to resolve odor dynamics within plumes at high stimulus intensities. Thus, we tested whether temporal integration occurs specifically when the stimulus amplitude is near the behavioral threshold (3-ms duration; Fig. 1F), but not at stimulus levels that induce maximum behavioral responsiveness (10-ms duration; Fig. 1F). We defined ORN inputs induced by 3- and 10-ms stimulus durations as “weak” and “strong” ORN inputs, respectively, based on the behavioral responsiveness they elicited. In contrast to the results for weak ORN inputs, no significant difference in spike output was observed between long and short ISIs with strong ORN inputs (Fig. 4). These results suggest that supralinear integration of weak, but not strong, ORN inputs occurred in PNs.
We confirmed that ChR2-expressing ORNs showed sublinear integration of individual responses to paired-pulse photostimulation at all ISIs and stimulus durations used (Fig. S6) and that no adaptation or facilitation occurred in the ORNs. Latency scatter due to conduction times in the antennal nerve was also negligible (Fig. S7).
Origins of Temporal Integration in PNs.
AL PN firing patterns are generated by ORN and AL LN inputs, both of which may be involved in controlling temporal integration. The LNs are predominantly GABAergic and play an important role in modifying PN temporal dynamics (30–32). To examine the involvement of GABAergic mechanisms in PN temporal integration, we applied two different GABA receptor blockers, picrotoxin as a GABAA receptor blocker and 3-N-[1-(3,4-dichlorophenyl)ethylamino]-2-hydroxypropyl cyclohexylmethyl phosphinic acid as a GABAB receptor blocker (32), and conducted the same paired-pulse photostimulation during PN recordings (Fig. 5). Application of these blockers caused increases in the spontaneous firing rate, light-evoked spike count, and peak instantaneous spike frequency of PNs (Fig. S8), suggesting a disinhibitory effect by GABA receptor blockade. In response to weak ORN inputs, PN temporal integration properties in the presence of these blockers were similar to those in saline controls; a paired-pulse stimulus evoked a greater spike output than that attributable to linear summation for ISIs of 20–80 ms (Fig. 5C). This result implies that temporal integration may be an intrinsic property of the PNs. In response to strong ORN inputs, PN temporal integration, which was not evident at ISIs of <80 ms in saline controls, became significant in the presence of the GABA receptor blockers (Fig. 5C). Previous work has suggested that GABAergic signaling can regulate temporal precision of PN response to ORN stimulation (31). We thus examined this issue, using our optogenetic ORN stimulation. As shown in Fig. S8, the interquartile range of PN spike latency is increased in the presence of GABA receptor blockers, suggesting that temporal precision of PN responses is dependent on GABA signaling. Thus, our data suggest that temporal integration is abolished by GABAergic mechanisms in the presence of strong ORN inputs to favor increased temporal resolution for plume tracking and that GABA signaling is important for temporal precision of PN responses.
Fig. 5.
GABAergic mechanisms in the AL network inhibit PN temporal integration of strong ORN inputs. (A) A representative PN response under blockade of GABAA and GABAB receptors. With 3- and 10-ms duration, paired-pulsed stimulation induced a greater spike count at 40-ms ISI than at 120-ms ISI. Arrows indicate the timing of stimulus presentation. The plus symbol (+) indicates spike timing. (B) PSTHs of PN spike responses under blockade of GABAA and GABAB receptors. Spikes were counted in 20-ms bins and averaged (n = 6). (C) Effect of GABA receptor blockade. The firing rate was calculated by counting spikes during 500 ms from the onset of the first light pulse (n = 6). *P < 0.05 (Wilcoxon signed rank test). Data are shown as means ± SEM.
Discussion
The extreme sensitivity of male moths to female pheromones arises from pushing olfactory systems nearly to their physical and chemical limits. We show that information from ORNs is further processed and that temporal integration in AL PNs contributes to enhanced behavioral sensitivity at low stimulus intensities, whereas GABAergic mechanisms inhibit temporal integration at high stimulus intensities to increase temporal resolution. Taking advantage of the high temporal resolution of ChR2-assisted photoactivation, we demonstrated that ChR2 makes it possible to control ORN activity on a millisecond timescale.
In response to weak ORN inputs, PN responses showed supralinear integration properties at ISIs of <80 ms in paired-pulse photostimulation (Fig. 4). This supralinear integration likely occurs when the first afferent synaptic potential is temporally integrated with a subsequent one (17), indicating that the time window within which ORN inputs can effectively interact in PNs is in the range of 40 to 80 ms. The timescale of integration in PNs appears to be relatively long compared with the temporal characteristics of cortical neurons in the somatosensory and visual systems of mammals (33, 34). This long integration time allows nonsimultaneous synaptic inputs to effectively generate spike outputs, and thus PNs act as temporal integrators to amplify synaptic inputs from ORNs.
In the present study, temporal integration was not evident in response to strong ORN inputs (Fig. 4), but supralinear integration could be obtained by applying GABA receptor blockers (Fig. 5). Previous studies have reported that GABAergic LNs have synaptic connectivity with both ORNs and PNs (35–37) and that GABA release from LNs inhibits synaptic transmission from ORNs to PNs (31, 38). Indeed, pharmacological blockade of GABA receptors is likely to have a disinhibitory effect on PN responses (31, 32, 39), correlated with the strength of ORN inputs (39–41). Consistent with these observations, our results showed that inhibition by GABAergic LNs contributed to shaping PN responses only in the presence of strong, but not weak, ORN inputs. The details of this GABAergic mechanism remain to be elucidated.
Although the application of GABA receptor blockers induced temporal integration of strong ORN inputs at ISIs of 40–100 ms, supralinear output was not observed at the shortest ISI of 20 ms even in the presence of blockers (Fig. 5C). This result raises the possibility that paired-pulse stimulation induces short-term synaptic depression, as shown in the AL of Drosophila (42).
The integration time window for PN responses in the present study was identical to that during which behavioral responses could be enhanced by paired-pulse photostimulation. This finding suggests that temporal integration of successive spikes by PNs is a basic mechanism contributing to the high behavioral sensitivity of moths to pheromone stimuli. It has been shown that within a plume, the concentration of odor is highly dynamic, with bursts of odor lasting as little as 10–20 ms (25, 26). Temporal integration is limited to a time window of up to 80 ms, which approximately corresponds to the duration of multiple bursts. In contrast, the durations of the odor plumes themselves are significantly longer, and previous work has examined the behavioral and electrophysiological responses of insect olfactory systems to these stimuli (43–46). Thus, the insect olfactory system is able to integrate ORN inputs between multiple bursts within single odor plumes for enhanced sensitivity as well as to retain temporal information of plume dynamics occurring at longer timescales (23–26). Our use of optogenetic techniques has allowed us to describe the cellular and behavioral responses at timescales relevant to “intraplume” odor dynamics, which provides insights into the signal processing properties of the olfactory system.
In addition to temporal structure of stimuli, the properties of the PNs appear to be sensitive to odor concentration, because at high stimulus intensities, temporal integration is inhibited by GABAergic signaling, thus making this system responsive to a wide range of odor concentrations. These features may be behaviorally advantageous for male silkmoth pheromone-plume tracking, particularly at close range of a female. Together, our results demonstrate that temporal integration properties of PNs are matched to the temporal properties of odor signals and play an important role in enhancing behavioral sensitivity.
Materials and Methods
For details, see SI Materials and Methods.
Construction of the pBacUAS-ChR2 Vector.
The DNA fragment encoding mammalian codon-optimized ChR2 fused with mCherry was amplified by PCR using pLenti-CaMKIIa-hChR2-mCherry-WPRE (47) as a template and the primers 5′-CTCCTAGGATGGACTATGGCGGCGCTTTG-3′ and 5′-CTCCTAGGTTACTTGTACAGCTCGTCCATG-3′. The recognition sequence for BlnI was added to the 5′ ends of both primers. PCR was performed with PrimeSTAR HS DNA polymerase (R010A; Takara Bio) under the following thermal program: 98 °C for 2 min and 30 cycles of 98 °C for 10 s, 64 °C for 15 s, and 72 °C for 2 min, followed by a final extension at 72 °C for 5 min. The amplified DNA was digested with BlnI and inserted into the BlnI site of the pBacMCS[UAS-A3-kynurenine 3-monooxygenase (KMO)] vector (48). The resultant vector was named pBacUAS-ChR2.
Generation of Transgenic Moths.
The pBacUAS-ChR2 vector was purified by using a HiSpeed Midi Plasmid Purification kit (Qiagen), dissolved at a concentration of 0.2 mg/mL in 0.5 mM phosphate buffer (pH 7.0) containing 5 mM KCl, and mixed with an equal volume of 0.2 mg/mL helper DNA encoding piggyBac transposase. This mixture was microinjected into eggs of the w1-pnd strain that were collected 3–6 h after oviposition, as described (49). The injected eggs were reared to adults and crossed to obtain G1 progeny. The screening of positive clones was performed immediately after the hatch by detecting the body pigmentation induced by Bombyx KMO expression under the control of the cytoplasmic actin gene (A3) promoter, as described (48). G1 adults were crossed with individuals of the BmOR1-GAL4 line (27), and G2 adults were used as a GAL4/UAS strain.
Injection of Exogenous ATR.
ATR injection was performed to provide a chromophore as a cofactor for ChR2. ATR is essential for ChR2 activation, and insects do not intrinsically possess ATR. Therefore, we injected exogenous ATR into the abdomen of silkmoths. For the experiments, we used moths 2–5 h after ATR injection because this time window coincided with high ATR concentration in the moth antennae as confirmed by HPLC (Fig. S2).
Light Stimulation.
A high-power blue LED with peak wavelength of 465 nm (LBW5AP-JYKY-35-Z; Osram Opto Semiconductors) generated the photostimulation in all experiments.
ORN Recordings.
The moth was immobilized on a plastic plate, and the antennae were stabilized by dental wax. Spikes were recorded by inserting an electrolytically sharpened tungsten wire electrode into the base of a sensillum trichodeum. As a reference electrode, a platinum plate was inserted in the neck of the moth. Photostimulation was generated by the blue LED system described above.
PN Recordings.
Two methods were used to record PN responses, intracellular recordings (Fig. S4) and cell-attached recordings (Figs. 4 and 5).
Supplementary Material
Acknowledgments
We thank Dr. Karl Deisseroth (Stanford University) for providing the pLenti-CaMKIIa-hChR2-mCherry-WPRE plasmid; Dr. Shigeru Matsuyama (University of Tsukuba) for purification of bombykol; Kenji Higo (Tokyo Institute of Technology) for technical advice about optical components; and Tomoki Kazawa (University of Tokyo) and Dr. Mark Wu (Johns Hopkins University) for comments on the manuscript. This work was supported by the Japan Society for the Promotion of Science (JSPS) (M.T., S.S.H., and R.M.) and JSPS Scientific Research (B) Grant 21370029 (to R.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Kaissling KE, Priesner E. Die riechschwelle des seidenspinners. Naturwissenschaften. 1970;57(1):23–28. doi: 10.1007/BF00593550. [DOI] [PubMed] [Google Scholar]
- 2.Kaissling KE. R. H. Wright Lectures on Insect Olfaction. Burnaby, VC, Canada: Simon Fraser Univ; 1987. [Google Scholar]
- 3.Adam G, Delbrück M. Reduction of dimensionality of biological diffusion processes. In: Rich A, Davidson N, editors. Structural Chemistry and Molecular Biology. San Francisco: Freeman; 1968. pp. 198–215. [Google Scholar]
- 4.Miura N, Nakagawa T, Touhara K, Ishikawa Y. Broadly and narrowly tuned odorant receptors are involved in female sex pheromone reception in Ostrinia moths. Insect Biochem Mol Biol. 2010;40(1):64–73. doi: 10.1016/j.ibmb.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Forstner M, Breer H, Krieger J. A receptor and binding protein interplay in the detection of a distinct pheromone component in the silkmoth Antheraea polyphemus. Int J Biol Sci. 2009;5(7):745–757. doi: 10.7150/ijbs.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krieger J, Grosse-Wilde E, Gohl T, Breer H. Candidate pheromone receptors of the silkmoth Bombyx mori. Eur J Neurosci. 2005;21(8):2167–2176. doi: 10.1111/j.1460-9568.2005.04058.x. [DOI] [PubMed] [Google Scholar]
- 7.Sakurai T, et al. Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori. Proc Natl Acad Sci USA. 2004;101(47):16653–16658. doi: 10.1073/pnas.0407596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakagawa T, Sakurai T, Nishioka T, Touhara K. Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science. 2005;307(5715):1638–1642. doi: 10.1126/science.1106267. [DOI] [PubMed] [Google Scholar]
- 9.Mitsuno H, et al. Identification of receptors of main sex-pheromone components of three Lepidopteran species. Eur J Neurosci. 2008;28(5):893–902. doi: 10.1111/j.1460-9568.2008.06429.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang G, Vásquez GM, Schal C, Zwiebel LJ, Gould F. Functional characterization of pheromone receptors in the tobacco budworm Heliothis virescens. Insect Mol Biol. 2011;20(1):125–133. doi: 10.1111/j.1365-2583.2010.01045.x. [DOI] [PubMed] [Google Scholar]
- 11.Hildebrand JG, Shepherd GM. Mechanisms of olfactory discrimination: Converging evidence for common principles across phyla. Annu Rev Neurosci. 1997;20:595–631. doi: 10.1146/annurev.neuro.20.1.595. [DOI] [PubMed] [Google Scholar]
- 12.Hansson BS, Ljungberg H, Hallberg E, Löfstedt C. Functional specialization of olfactory glomeruli in a moth. Science. 1992;256(5061):1313–1315. doi: 10.1126/science.1598574. [DOI] [PubMed] [Google Scholar]
- 13.Kanzaki R, Soo K, Seki Y, Wada S. Projections to higher olfactory centers from subdivisions of the antennal lobe macroglomerular complex of the male silkmoth. Chem Senses. 2003;28(2):113–130. doi: 10.1093/chemse/28.2.113. [DOI] [PubMed] [Google Scholar]
- 14.Jarriault D, Gadenne C, Lucas P, Rospars JP, Anton S. Transformation of the sex pheromone signal in the noctuid moth Agrotis ipsilon: From peripheral input to antennal lobe output. Chem Senses. 2010;35(8):705–715. doi: 10.1093/chemse/bjq069. [DOI] [PubMed] [Google Scholar]
- 15.Hartlieb E, Anton S, Hansson BS. Dose-dependent response characteristics of antennal lobe neurons in the male moth Agrotis segetum (Lepidoptera: Noctuidae) J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1997;181(5):469–476. [Google Scholar]
- 16.Jarriault D, Gadenne C, Rospars JP, Anton S. Quantitative analysis of sex-pheromone coding in the antennal lobe of the moth Agrotis ipsilon: A tool to study network plasticity. J Exp Biol. 2009;212(8):1191–1201. doi: 10.1242/jeb.024166. [DOI] [PubMed] [Google Scholar]
- 17.Fortune ES, Rose GJ. Short-term synaptic plasticity as a temporal filter. Trends Neurosci. 2001;24(7):381–385. doi: 10.1016/s0166-2236(00)01835-x. [DOI] [PubMed] [Google Scholar]
- 18.Thomson AM. Molecular frequency filters at central synapses. Prog Neurobiol. 2000;62(2):159–196. doi: 10.1016/s0301-0082(00)00008-3. [DOI] [PubMed] [Google Scholar]
- 19.Thomson AM. Facilitation, augmentation and potentiation at central synapses. Trends Neurosci. 2000;23(7):305–312. doi: 10.1016/s0166-2236(00)01580-0. [DOI] [PubMed] [Google Scholar]
- 20.Murlis J, Elkinton JS, Cardé RT. Odor plumes and how insects use them. Annu Rev Entomol. 1992;37:505–532. [Google Scholar]
- 21.Koehl MA. The fluid mechanics of arthropod sniffing in turbulent odor plumes. Chem Senses. 2006;31(2):93–105. doi: 10.1093/chemse/bjj009. [DOI] [PubMed] [Google Scholar]
- 22.Vickers NJ. Winging it: Moth flight behavior and responses of olfactory neurons are shaped by pheromone plume dynamics. Chem Senses. 2006;31(2):155–166. doi: 10.1093/chemse/bjj011. [DOI] [PubMed] [Google Scholar]
- 23.Murlis J, Jones CD. Fine-scale structure of odour plumes in relation to insect orientation to distant pheromone and other attractant sources. Physiol Entomol. 1981;6(1):71–86. [Google Scholar]
- 24.Murlis J, Willis MA, Carde RT. Spatial and temporal structures of pheromone plumes in fields and forests. Physiol Entomol. 2000;25(3):211–222. [Google Scholar]
- 25.Jones CD. On the structure of instantaneous plumes in the atmosphere. J Hazard Mater. 1983;7(2):87–112. [Google Scholar]
- 26.Justus KA. Measurement of odor-plume structure in a wind tunnel using a photoionization detector and a tracer gas. Environ Fluid Mech. 2002;2(1):115–142. [Google Scholar]
- 27.Sakurai T, et al. A single sex pheromone receptor determines chemical response specificity of sexual behavior in the silkmoth Bombyx mori. PLoS Genet. 2011;7(6):e1002115. doi: 10.1371/journal.pgen.1002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanzaki R. Self-generated zigzag turning of Bombyx mori males during pheromone-mediated upwind walking. Zoolog Sci. 1992;9(3):515–527. [Google Scholar]
- 29.Kanzaki R, Mishima T. Pheromone-triggered ‘flipflopping’ neural signals correlate with activities of neck motor neurons of a male moth, Bombyx mori. Zoolog Sci. 1996;13(1):79–87. [Google Scholar]
- 30.Christensen TA, Waldrop BR, Hildebrand JG. GABAergic mechanisms that shape the temporal response to odors in moth olfactory projection neurons. Ann N Y Acad Sci. 1998;855:475–481. doi: 10.1111/j.1749-6632.1998.tb10608.x. [DOI] [PubMed] [Google Scholar]
- 31.Christensen TA, Waldrop BR, Hildebrand JG. Multitasking in the olfactory system: Context-dependent responses to odors reveal dual GABA-regulated coding mechanisms in single olfactory projection neurons. J Neurosci. 1998;18(15):5999–6008. doi: 10.1523/JNEUROSCI.18-15-05999.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci. 2005;25(40):9069–9079. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293(5532):1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- 34.Cardin JA, Kumbhani RD, Contreras D, Palmer LA. Cellular mechanisms of temporal sensitivity in visual cortex neurons. J Neurosci. 2010;30(10):3652–3662. doi: 10.1523/JNEUROSCI.5279-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Distler PG, Boeckh J. Synaptic connections between identified neuron types in the antennal lobe glomeruli of the cockroach, Periplaneta americana: I. Uniglomerular projection neurons. J Comp Neurol. 1997;378(3):307–319. [PubMed] [Google Scholar]
- 36.Distler PG, Gruber C, Boeckh J. Synaptic connections between GABA-immunoreactive neurons and uniglomerular projection neurons within the antennal lobe of the cockroach, Periplaneta americana. Synapse. 1998;29(1):1–13. doi: 10.1002/(SICI)1098-2396(199805)29:1<1::AID-SYN1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 37.Sun XJ, Tolbert LP, Hildebrand JG. Synaptic organization of the uniglomerular projection neurons of the antennal lobe of the moth Manduca sexta: A laser scanning confocal and electron microscopic study. J Comp Neurol. 1997;379(1):2–20. doi: 10.1002/(sici)1096-9861(19970303)379:1<2::aid-cne2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 38.Waldrop B, Christensen TA, Hildebrand JG. GABA-mediated synaptic inhibition of projection neurons in the antennal lobes of the sphinx moth, Manduca sexta. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1987;161(1):23–32. doi: 10.1007/BF00609452. [DOI] [PubMed] [Google Scholar]
- 39.Lei H, Riffell JA, Gage SL, Hildebrand JG. Contrast enhancement of stimulus intermittency in a primary olfactory network and its behavioral significance. J Biol. 2009;8(2):21. doi: 10.1186/jbiol120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Root CM, et al. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59(2):311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452(7190):956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kazama H, Wilson RI. Homeostatic matching and nonlinear amplification at identified central synapses. Neuron. 2008;58(3):401–413. doi: 10.1016/j.neuron.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vickers NJ, Christensen TA, Baker TC, Hildebrand JG. Odour-plume dynamics influence the brain’s olfactory code. Nature. 2001;410(6827):466–470. doi: 10.1038/35068559. [DOI] [PubMed] [Google Scholar]
- 44.Christensen TA, Hildebrand JG. Coincident stimulation with pheromone components improves temporal pattern resolution in central olfactory neurons. J Neurophysiol. 1997;77(2):775–781. doi: 10.1152/jn.1997.77.2.775. [DOI] [PubMed] [Google Scholar]
- 45.Lei H, Hansson BS. Central processing of pulsed pheromone signals by antennal lobe neurons in the male moth Agrotis segetum. J Neurophysiol. 1999;81(3):1113–1122. doi: 10.1152/jn.1999.81.3.1113. [DOI] [PubMed] [Google Scholar]
- 46.Geffen MN, Broome BM, Laurent G, Meister M. Neural encoding of rapidly fluctuating odors. Neuron. 2009;61(4):570–586. doi: 10.1016/j.neuron.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 47.Zhang F, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446(7136):633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 48.Quan G, et al. Rescue of white egg 1 mutant by introduction of the wild-type Bombyx kynurenine 3–monooxygenase gene. Insect Sci. 2007;14(2):85–92. [Google Scholar]
- 49.Tamura T, et al. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat Biotechnol. 2000;18(1):81–84. doi: 10.1038/71978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.