Significance
Mitosis is a highly regulated cell division process in eukaryotes. Assembly of the spindle and segregation of chromosomes in mitosis enable the mother cells to distribute the genetic materials equally to their daughter cells. Before chromosome segregation, the kinetochores at the chromosomes must be correctly captured by the microtubules. The mechanisms underlying the initiation of this process and the proper formation of the kinetochore fibers remain largely unknown. This study shows that transforming acidic coiled-coil–containing protein 3 (TACC3) is essential for proper kinetochore capture and kinetochore fiber formation. Our findings reveal a critical TACC3-dependent acentrosomal microtubule nucleation and sorting process to regulate kinetochore–microtubule connections.
Keywords: centrosome, noncentrosomal, cell cycle
Abstract
Kinetochore capture by dynamic kinetochore microtubule fibers (K fibers) is essential for proper chromosome alignment and accurate distribution of the replicated genome during cell division. Although this capture process has been extensively studied, the mechanisms underlying the initiation of this process and the proper formation of the K fibers remain largely unknown. Here we show that transforming acidic coiled-coil–containing protein 3 (TACC3) is essential for kinetochore capture and proper K-fiber formation in HeLa cells. To observe the assembly of acentrosomal microtubules more clearly, the cells were released from higher concentrations of nocodazole into zero or lower concentrations. We find that small acentrosomal TACC3–microtubule aster formation near the kinetochores and binding of the asters with the kinetochores are the initial steps of the kinetochore capture by the acentrosomal microtubules, and that the sorting of kinetochore-captured acentrosomal microtubules with centrosomal microtubules leads to the capture of kinetochore by centrosomal microtubules from both spindle poles. We demonstrate that the sorting of the TACC3-associated microtubules with the centrosomal microtubules is a crucial process for spindle assembly and chromosome movement. These findings, which are also supported in the unperturbed mitosis without nocodazole, reveal a critical TACC3-dependent acentrosomal microtubule nucleation and sorting process to regulate kinetochore–microtubule connections and provide deep insight into the mechanisms of mitotic spindle assembly and chromosome alignment.
To ensure proper segregation of the chromosomes into its two daughter cells during proliferation, the chromosomes of a mother cell must be captured by its assembling mitotic spindle through attachment of the chromosome kinetochores and the dynamic spindle microtubules (1). A “search-and-capture” model was proposed long ago, in which the dynamic spindle microtubules nucleated from the centrosomes search for and capture the chromosome kinetochores (2). Previous studies showed that the kinetochores are initially captured by the spindle-pole–nucleated microtubules with their lateral side (3, 4). Once captured, the kinetochores with their chromosomes are transported along the microtubules toward a spindle pole, and the microtubules shrink at their plus ends until the establishment of the end-on attachment (4, 5). However, this model is insufficient to explain the initial connection of the kinetochore and the spindle microtubules in the centrosome-independent spindle assembly process. Recent studies in Xenopus extracts indicated that microtubules are nucleated near the chromosomes and self-organize into a spindle (6). A new model for acentrosomal spindle assembly has been raised in mouse oocytes, in which self-organized microtubule organizing centers (MTOCs) replace the centrosome function (7). The somatic cells may also use the centrosome-independent pathway for their spindle assembly (8–10). In Drosophila cells, the centrosome-independent assembled kinetochore fibers can be captured by centrosomal microtubules (11–13).
Previous studies have shown that transforming acidic coiled-coil–containing protein 3 (TACC3) is essential for the mitotic spindle assembly and chromosome alignment, but the mechanism remains largely unknown (14–18). Here we reveal that TACC3-dependent small microtubule aster formation and sorting near the kinetochores contribute to correct microtubule–kinetochore connections.
Results and Discussion
TACC3 Regulates de Novo Assembly of Acentrosomal Microtubules.
Several groups reported recently that TACC3 is essential for chromosome alignment and spindle assembly in mitosis, but the clear mechanism remains unknown (14–18). To test how TACC3 regulates the spindle formation, we firstly knocked down TACC3 in HeLa cells using small interfering RNA against TACC3 (siTACC3) (>95% efficiency) (Fig. 1A). Then we treated TACC3-knockdown and the irrelevant-knockdown control cells with 1 μg/mL nocodazole for 5–8 h to abolish microtubule nucleation and mitotic spindle assembly, followed by releasing these cells into medium without nocodazole to allow them to reassemble their microtubules and spindles. As shown (Fig. 1B), in control cells, microtubules were quickly nucleated from both the separating centrosomes to form two big centrosomal asters; meanwhile, other microtubules were also quickly (within 1.5 min) nucleated in the cytoplasm to form many small acentrosomal asters. Then, these small asters quickly “fused” with each other and sorted into the big centrosomal asters, and finally these big and small microtubule asters assembled into a bipolar mitotic spindle within 15 min (3.5–15 min). In contrast, in TACC3-knockdown cells, although microtubules were nucleated around the separating centrosomes to form the two big centrosomal microtubule asters and finally assemble an immature bipolar mitotic spindle, there was nearly no other microtubule nucleation to form the small acentrosomal microtubule asters in the cytoplasm (Fig. 1 B–D and Fig. S1 A and B). These results suggested that TACC3 might contribute to the acentrosomal microtubule aster assembly and the microtubule–kinetochore connection. To further confirm these, we released these cells into the medium containing 15 ng/mL nocodazole and also verified that TACC3 is required for acentrosomal microtubule assembly (Fig. 1 C and D and Fig. S1 C and D).
Fig. 1.

TACC3 is required for de novo assembly of acentrosomal microtubules in mitosis. (A) Detection of TACC3 RNAi depletion efficiency by Western blotting. HeLa cells were transfected with control and TACC3 siRNAs, respectively. The blots were probed with anti-TACC3 (Upper) and anti–α-tubulin (Lower). (B and C) Representative images of 1 μg/mL nocodazole-arrested normal control or TACC3-knockdown HeLa cells by siRNA followed by release into medium without nocodazole (B) or with 15 ng/mL nocodazole (C) at different time points (0.5, 1.5, 3.5, 7.5, and 15 min). The data are shown as maximum intensity projections of different z sections. TACC3 is in red, tubulin in green, and DNA in blue. (D) Statistics of numbers of acentrosomal microtubule seeds in control and TACC3 knockdown cells after nocodazole treatment and release for 1.5 min. More than 50 cells for each treatment were counted. (E) Control, nocodazole-treated (50 ng/mL for 8 min), and cold-treated (10 min on ice) HeLa cells were stained with anti-TACC3 (green) and anti-Hec1 (red) antibodies. (F) Staining of HeLa cells with TACC3 (green) and indicated proteins (Hec1, Aurora B, and clathrin) (red) after nocodazole treatment for 16 h. (G) HeLa cells were transfected with different siRNAs (control, siTACC3, siAurora A) as indicated. Cells were treated with 50 ng/mL nocodazole for 16 h before fixation. (H and I) Live imaging of HeLa cells expressing GFP–tubulin under indicated different treatments (siRNA control, siTACC3, and Aurora A inhibitor MLN8237). The mitotic cells were arrested with 500 ng/mL nocodazole and released into medium without nocodazole (H) or with 15 ng/mL nocodazole (I). (J) Illustration of TACC3-dependent acentrosomal microtubule assembly and clustering. During mitosis, TACC3 containing small microtubule seeds/asters were assembled around the chromosomes and kinetochores, and these small microtubule asters were further assembled and clustered into a complete bipolar spindle structure. (Scale bar, 10 μm.)
To understand why TACC3 is required for the acentrosomal microtubule aster assembly, we treated the mitotic HeLa cells with nocodazole or cold temperatures and observed that TACC3 stayed with the stable kinetochore-connected fibers (K fibers) (Fig. 1E and Fig. S1E). We treated HeLa cells with nocodazole (50 ng/mL) for 16 h to allow the cells to enter into mitosis without centrosomal microtubule nucleation and verified that TACC3 connected to kinetochore markers, i.e., Hec1 and Aurora B (Fig. 1F). Meanwhile, a fraction of clathrin heavy chain also colocalized with TACC3 on their fibers, supporting the notion that clathrin and TACC3 cofunction as a complex in mitosis (Fig. 1F) (15–18). Moreover, knockdown of Aurora A, which functions upstream of TACC3 (19), clearly abolished the acentrosomal microtubule aster formation (Fig. 1G).
To further examine the roles of TACC3 in regulating acentrosomal microtubule assembly, we performed live-imaging assays in HeLa cells expressing GFP–tubulin to analyze the dynamic assembly of spindle microtubules. The cells were released from 500 ng/mL nocodazole into medium with 0 (Fig. 1H and Movies S1–S6) or 15 ng/mL nocodazole (Fig. 1I and Movies S7–S12). As shown (Fig. 1H and Movies S1 and S2), in control cells, microtubules were assembled both around the centrosomes and in the acentrosomal regions. In contrast, TACC3 knockdown had little effect on centrosomal microtubule nucleation while strongly inhibiting the formation of acentrosomal microtubules (Fig. 1H and Movies S3 and S4). Moreover, we treated the cells with MLN8237, a small-molecule inhibitor of Aurora A, and found it suppressed both the centrosomal and acentrosomal microtubule assembly (Fig. 1H and Movies S5 and S6). To specifically analyze the formation of acentrosomal microtubules, the cells were released into 15 ng/mL nocodazole. Similar as what we observed in fixed samples (Fig. 1 C and G), the live tracking of microtubules also revealed that TACC3 and Aurora A is crucial for acentrosomal microtubule formation (Fig. 1I and Movies S7–S12). Together, we propose that TACC3-dependent acentrosomal microtubule nucleation is regulated by Aurora A, and TACC3-containing acentrosomal microtubule small aster assembly contributes to microtubule–kinetochore connections (Fig. 1J).
TACC3-Dependent Acentrosomal Microtubule Nucleation Is Required for Proper Kinetochore–Microtubule Capture and Correct Mitosis.
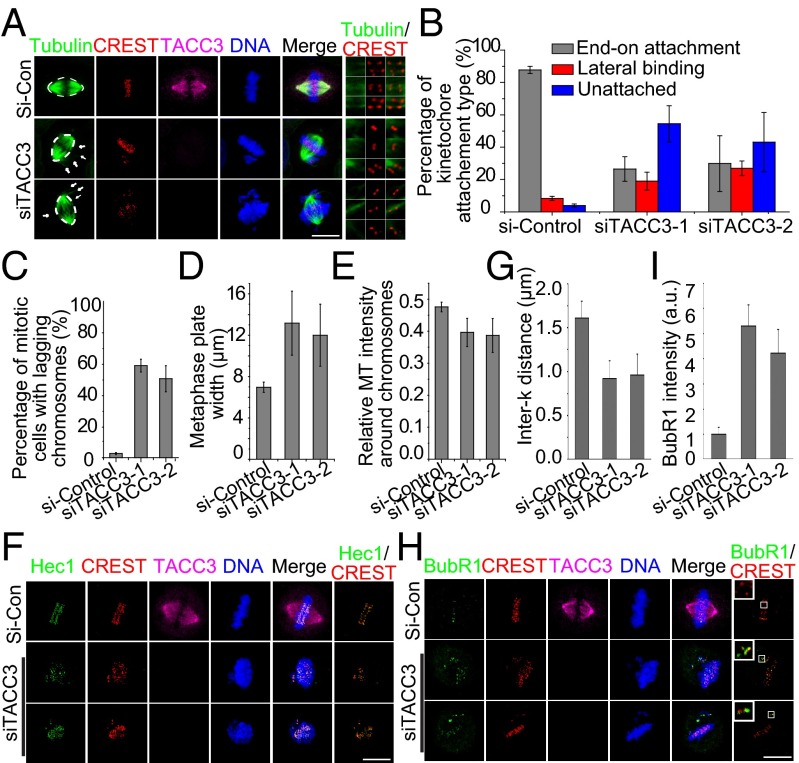
Because the previous conclusions were mainly based on nocodazole-treated cells, we next investigated the function of TACC3-dependent acentrosomal microtubule nucleation in kinetochore–microtubule capture during unperturbed mitosis in HeLa cells without nocodazole (Fig. 2). In normal metaphase cells, proper end-on attachments between kinetochores and microtubules were formed, whereas in TACC3-knockdown cells, many kinetochores were not connected by microtubules or only bound laterally with few microtubules (Fig. 2 A and B and Fig. S2 A and D). As shown (Fig. 2 C and D and Fig. S2B), TACC3 knockdown resulted in an increase of lagging chromosomes and metaphase plate width. Meanwhile, ablation of TACC3 led to abundant centrosome-nucleated astral microtubules outside the “spindle frame” as indicated by arrows, suggesting deficiency of the search-and-capture process (Fig. 2A). The microtubule intensity around the chromosomes in TACC3-depleted cells was much less than control (Fig. 2E and Fig. S2C), suggesting insufficient assembly of acentrosomal microtubules. Furthermore, we found that TACC3 depletion resulted in marked decrease of distances between paired kinetochores (Fig. 2 F and G), indicating that TACC3 is required for maintenance of proper interkinetochore tension. Moreover, TACC3 knockdown led to increased BubR1 levels at the kinetochores, suggesting the activation of spindle assembly checkpoint (Fig. 2 H and I). Because TACC3 localizes to acentrosomal microtubules and is not essential for centrosomal microtubule nucleation as discussed above (Fig. 1), we proposed that TACC3-dependent acentrosomal microtubule nucleation and small aster formation mainly contribute to the regulation of kinetochore function, including kinetochore–microtubule attachment and maintenance of interkinetochore tension.
Fig. 2.
TACC3 facilitates kinetochore–microtubule capture, interkinetochore tension, and spindle assembly checkpoint. Micrographs are presented as maximum intensity projections of different z sections. (A) Ablation of TACC3 led to defects in chromosome alignment and kinetochore–microtubule attachment. (Right) Representative paired kinetochores in single z sections. The oval lines indicate the spindle frame and the arrows indicate the microtubules outside the oval frame. Kinetochores stained with serum from patients with CREST syndrome are in red (CREST), tubulin in green, TACC3 in magenta, and DNA in blue. (B) Quantification of kinetochore attachment types in different groups. Percentages of end-on attachment, lateral binding status, and unattached kinetochores are shown. (C) Quantification of the percentages of metaphase cells with lagging chromosomes. (D) Measurement of the metaphase plate width in control and TACC3-depleted cells. (E) Quantification of the relative microtubule intensity around chromosomes. (F) HeLa cells were stained with anti-Hec1 (green), CREST (red), and TACC3 (magenta) antibodies. Representative images are shown. (Right) Micrographs in single z sections. (G) Statistics of interkinetochore distances in control and TACC3-ablated cells. The interkinetochore distances were measured according to paired Hec1 and CREST fluorescent signals. The distances between the centers of two paired Hec1 dots were measured with ImageJ. More than 150 kinetochore pairs in the same z sections in each group were analyzed. Data are presented as means plus SEs. (H) Cells were stained with anti-BubR1 (green), CREST (red), and TACC3 (magenta) antibodies. DNA is in blue. (I) Statistics of BubR1 fluorescence intensities in control and TACC3-ablated cells. The BubR1 intensities were quantified by ImageJ software, and the average cytoplasmic immunofluorescence intensity was subtracted as background. Data are presented as means plus SEs. (Scale bar, 10 μm.)
TACC3-dependent acentrosomal microtubule nucleation must be regulated by other mitotic regulators in performing its function, such as clathrin and Aurora A (20). So, here we checked the roles of other mitotic regulators in HeLa cells. Knockdown of TPX2, which activates Aurora A (21), reduced TACC3 targeting to the spindle, whereas inhibition of Aurora B by small molecule ZM447439 (22) or ablation of centromere protein E had no or little effect on the localization of TACC3 to the spindle (Fig. S3A). Moreover, unlike eg5 inhibition by monastral (23), inhibition of Plk1 by small molecule BI2536 (24) and knockdown of Nedd1 (25) both inhibited the targeting of TACC3 to the spindles (Fig. S3B). Overexpression of HSET, which mainly functions on centrosomal microtubules (26), had no effect on the spindle localization of TACC3 (Fig. S3C). We also knocked down Hec1 and found the location of TACC3 to the spindles was partially reduced (Fig. S3D). Taken together, these results indicate that inhibiting or knocking down upstream regulators for TACC3 (i.e., TPX2) or interfering with the kinetochore microtubule assembly activity (i.e., Plk1, Nedd1, and Hec1) can down-regulate the targeting of TACC3 to the spindles and lead to the defects in bipolar spindle assembly and chromosome alignment due to kinetochore–microtubule attachment failure.
The Sorting of the Small Acentrosomal Microtubule Seeds/Asters into Big Centrosomal Asters Is a General Mechanism for Spindle Assembly and Kinetochore Movement.
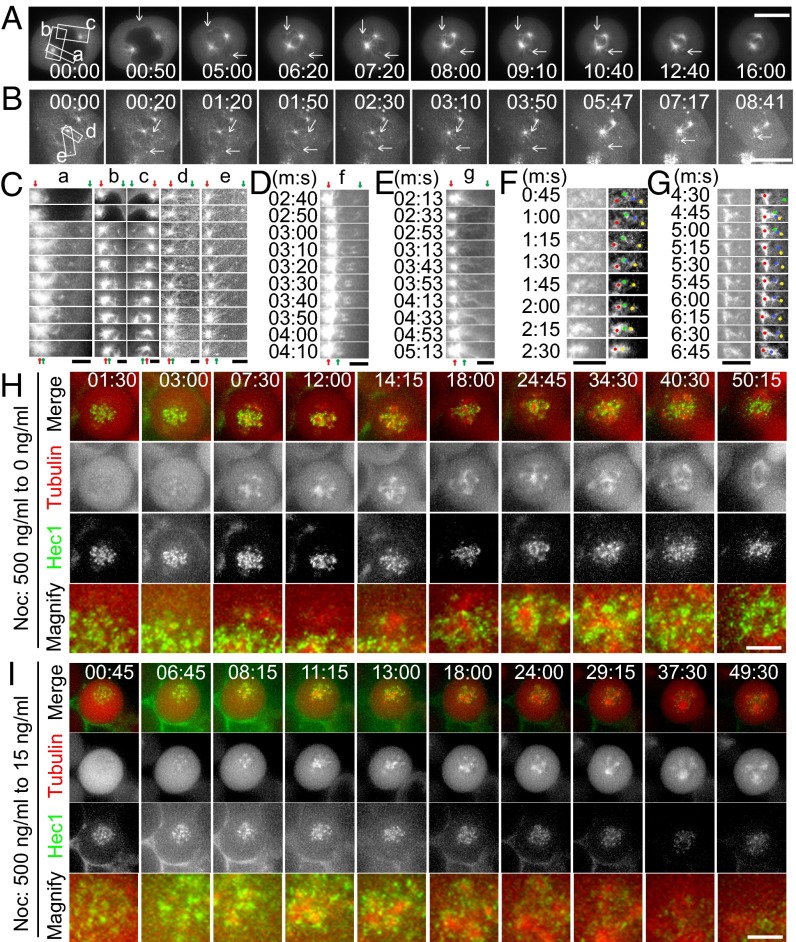
To better illustrate the roles for TACC3 in chromosome alignment and mitotic spindle assembly, we analyzed the microtubule behaviors by live imaging of HeLa cells expressing GFP–α-tubulin. We found that the sorting of the small acentrosomal asters into the big centrosomal asters is one key process for the mitotic spindle assembly (Fig. 3 A and B, Figs. S4 and S5, and Movies S13 and S14). During mitosis, both the centrosomal microtubules and the small acentrosomal aster microtubules kept growing in the cytoplasm, and when they encountered, these acentrosomal microtubules were immediately sorted into the microtubule arrays of the centrosomal asters. Five examples of microtubule sorting from the two movies mentioned above (Movies S13 and S14) are shown in magnified images (Fig. 3C, boxes a–e). As indicated, during mitosis, acentrosomal microtubules can be captured by and sorted into microtubules from one centrosome (Fig. 3C, boxes a, d, and e) or both of the two centrosomes (Fig. 3C, boxes b and c). The sorting of acentrosomal microtubules into centrosomal asters was also confirmed in other cases (Fig. 3 D and E, Fig. S6 A–C, and Movies S15 and S16). Together, these data indicate that the microtubule sorting of the small acentrosomal asters with the big centrosomal asters is a general mechanism for spindle assembly.
Fig. 3.
Sorting of TACC3-associated acentrosomal microtubules is a general mechanism for spindle assembly and kinetochore movement. (A–E) Live imaging of HeLa cells expressing GFP–tubulin. Red arrows indicate centrosomal asters and green arrows are acentrosomal microtubules. (A and B) Sorting of acentrosomal microtubules into centrosomal asters. (C) Magnified microtubule sorting regions (boxes a–e) as shown in A and B. (D and E) Another two examples of microtubule sorting existed in normal mitosis. (F and G) Assembly and sorting of multiple acentrosomal microtubule seeds when HeLa cells expressing GFP–tubulin were released from 500 ng/mL nocodazole into medium without nocodazole. (H and I) Live imaging of HeLa cells coexpressing mcherry–tubulin and GFP–Hec1. The cells were released from 500 ng/mL nocodazole into 0 (H) or 15 ng/mL (I) nocodazole as indicated. (Scale bars in A, B, H, and I, 10 μm and in C–G, 2 μm.)
To further characterize the acentrosomal microtubule sorting process, we analyzed the microtubule behaviors when the HeLa cells were released from 500 ng/mL nocodazole to medium without nocodazole (Fig. 1H and Movies S1 and S2) or with 15 ng/mL nocodazole (Fig. 1I and Movies S7 and S8). It can be easily seen in the movies that multiple large microtubule asters and spindle poles were assembled and fused with each other (also see Fig. S6D showing sorting of two spindle poles, derived from Movie S7). Fig. 3 F and G are two cases showing the assembly and sorting of individual acentrosomal foci when nocodazole was removed to observe the de novo assembly of microtubules. With 25 ng/mL nocodazole, the sorting process was also observed and reorganizations of spindle poles are shown (Fig. S6E and Movie S17). These data further demonstrate that acentrosomal microtubule sorting is a basic process for constructing the mitotic spindle and spindle poles during mitosis.
Furthermore, by using live cell imaging, we analyzed the behaviors of acentrosomal microtubules and kinetochores when the cells were released from 500 ng/mL nocodazole into 0 ng/mL. As shown (Fig. 3H and Movie S18), acentrosomal microtubules were preferentially assembled around and attached to the kinetochores, and sorting of these microtubule structures further resulted in kinetochore capture and movement. Similarly, when the cells were released into 15 ng/mL nocodazole, the acentrosomal microtubule assembly and sorting process was accompanied by chromosome kinetochore movement (Fig. 3I and Movie S19). Considering that TACC3 is initially associated with acentrosomal microtubules and kinetochores (Fig. S7 A–D), we propose that the TACC3-dependent acentrosomal microtubule assembly and sorting process facilitate kinetochore movement and capture.
A cell normally assembles its mitotic spindle into a bipolar structure, which is regulated by multiple and complex mechanical strengths (27). A mathematical model has been proposed for anastral spindle assembly in Drosophila oocytes (28). To illustrate the role of the microtubule sorting in establishing the spindle bipolarity, here we hypothesized a simplified mathematic model based on our abovementioned results (Fig. S8). For sorting of microtubules or asters, parallel microtubule motors mainly function to bundle these microtubules into paralleled bundles and clusters, whereas antiparallel microtubule motors usually make these microtubules slide and separate (Fig. S8 A and B). To simply address this, we hypothesized that there exists a minus-end–directed pulling force (F) for generating microtubule sorting power (Fig. S8C). For sorting of two microtubule structures (m, n): If 0° ≤θ <90° (where θ indicates the angle between Fm and Fn), fsorting
(m,n) function results in the formation of one clustered force: F(m,n)
(new) = (Fm + Fn) (|Fm|+|Fn|)/|Fm + Fn|. If 90° ≤θ ≤180°, fsorting
(m,n) results in formation of two antiparallel forces: Fm
(new) = (Fm − Fn)|Fm|/|Fm − Fn|; Fn
(new) = (Fn − Fm)|Fn|/|Fm − Fn|. For sorting of all of the microtubule structures: 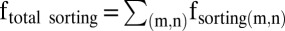 .
.
During spindle assembly without centrosomes, the bipolarity is formed through sorting of multiple acentrosomal microtubules (Fig. S8D) (7, 29). In spindle assembly with centrosomes, centrosomes function as the main MTOCs with high sorting forces (Fa and Fb) to generate the bipolarity. When centrosomes are artificially removed in some situations, spindle bipolarity can still be established through acentrosomal MTOCs (8, 27, 30). In nocodazole-treated cells, due to the reduced sorting force, some acentrosomal forces cannot be sorted, resulting in extra acentrosomal MTOCs, i.e., formation of multipolar microtubule structures (Fig. S8 C and D).
Loading of Preformed Acentrosomal TACC3–Microtubule Seeds/Asters to Kinetochores Followed by Sorting Are Crucial Steps for Kinetochore Capture.
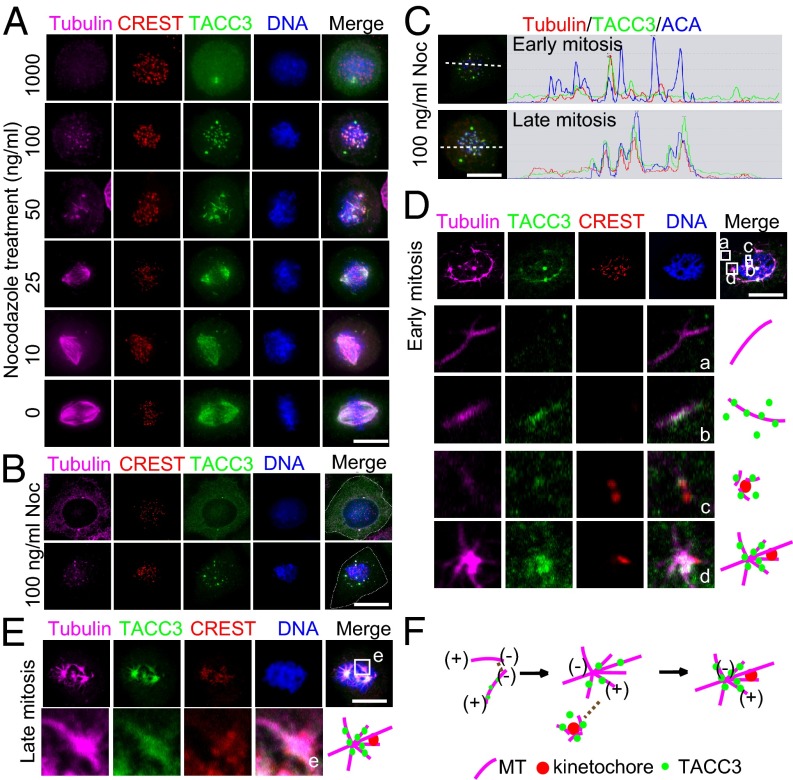
As indicated above, TACC3-dependent acentrosomal microtubule assembly and sorting facilitate proper kinetochore capture. However, how TACC3 regulates microtubule and kinetochore behaviors remains unknown. To address this, we analyzed the behaviors of TACC3, microtubules, and kinetochores in mitotic HeLa cells in the presence of different concentrations (0–1 μg/mL) of nocodazole. With the decrease of nocodazole concentration, the microtubule nucleation activity and kinetochore capture by the nucleated microtubules were remarkably increased (Fig. 4A and Fig. S9 A and B). Data showed that microtubule plus-end tracking protein EB1 spread through the kinetochore-targeted TACC3-containing microtubule asters to spindle poles in the presence of nocodazole (Fig. S9C). Next, we confirmed TACC3 dynamically associated with microtubules and kinetochores in various treatments using nocodazole (Fig. S10 A–G). To understand how these TACC3–microtubule seeds/asters and kinetochores get connected, we detected the behaviors of TACC3–microtubule complexes during early mitosis in the presence of 100 ng/mL nocodazole (Fig. 4B). Before nuclear envelope breakdown, TACC3 and tubulin stayed outside the nucleus and almost no TACC3 or tubulin located on the kinetochores. With nuclear envelope breakdown in early mitosis, partial TACC3 was loaded onto the short microtubules to form TACC3–microtubule seeds/asters, and the seeds/asters quickly connected with the kinetochores (Fig. 4 A–C). We also analyzed the TACC3–microtubule behaviors in HeLa cells with 25 ng/mL nocodazole. There were at least four kinds of microtubule structures in early mitosis, the microtubules without TACC3, the dissociative TACC3–microtubules, the laterally kinetochore-bound TACC–microtubules, and the kinetochore-attached TACC3–microtubule asters (Fig. 4D). In contrast, most TACC3–microtubule structures attached to kinetochores in late mitosis (Fig. 4E), suggesting that the TACC3–microtubule seeds/asters had been sorted into the kinetochore-attached K fibers. Thus, we propose that the capture of the kinetochores by spindle microtubules is facilitated through stepwise assembly of TACC3–microtubule seeds/asters near the kinetochores (Fig. 4F) and sorting of the TACC3–microtubule seeds/asters into the big centrosomal asters during the mitotic spindle assembly.
Fig. 4.
Targeting of preassembled acentrosomal TACC3–microtubule seeds/asters to kinetochore facilitates kinetochore capture by centrosomal microtubules. (A) TACC3–microtubule complex dynamically associated with kinetochores upon nocodazole treatment. Staining of mitotic HeLa cells arrested by different concentrations (1 μg/mL, 100 ng/mL, 50 ng/mL, 25 ng/mL, 10 ng/mL, and 0 ng/mL) of nocodazole. Tubulin is in magenta, CREST in red, TACC3 in green, and DNA in blue. (B) The total of 100 ng/mL nocodazole-arrested HeLa cells in early mitosis were stained with tubulin (magenta), CREST (red), TACC3 (green), and DNA (blue). The white dashed lines indicate the cell boundary. (C) Illustration of acentrosomal TACC3–microtubule (MT) seeds/asters in 100 ng/mL nocodazole-arrested early and late mitotic HeLa cells. (D and E) Different types of acentrosomal microtubules assemble the mitotic spindle. HeLa cells were treated with 25 ng/mL nocodazole for 5 h. The representative early (D) and late (E) mitotic cells are shown. The maximum intensity projections of 3D images are shown. In early mitosis (D), four different types of acentrosomal microtubule structures (a–d) are indicated by projection of selected z stacks followed by magnification. In contrast, only one type of acentrosomal microtubules (e in E) was in late mitosis. The square e in E is magnified and illustrated (Below). (F) Illustration of the acentrosomal microtubule nucleation, TACC3–microtubule seeds/asters assembly and the end-on capture of the kinetochores by the small aster tubules during transition from early mitosis to late mitosis. (Scale bar, 10 μm.)
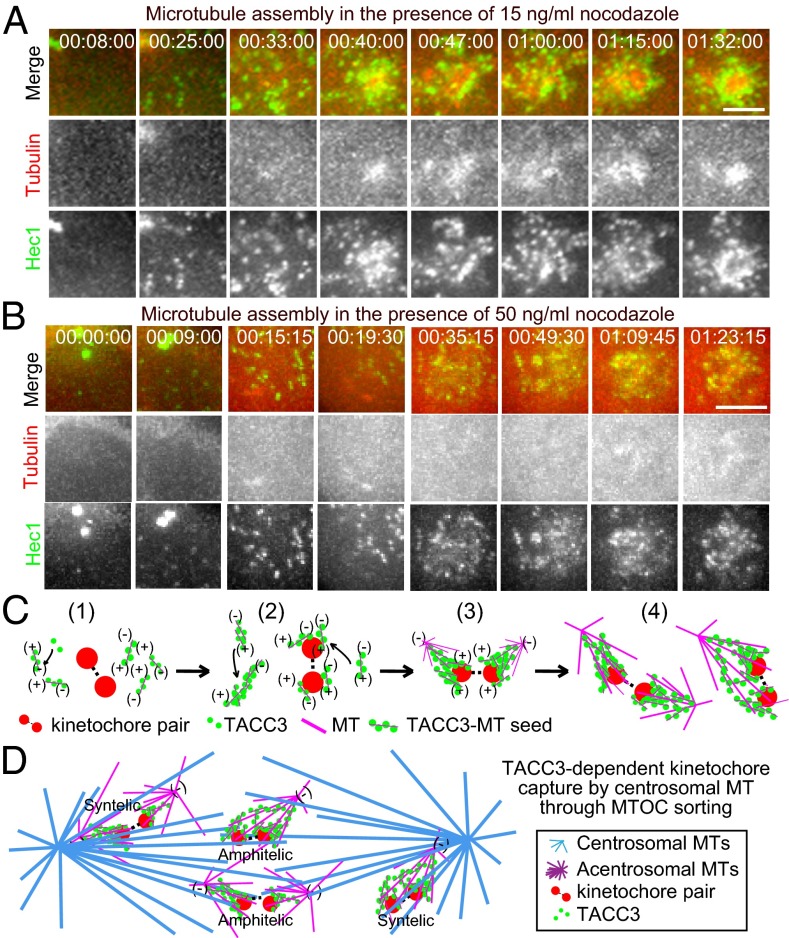
As indicated previously (Fig. 3 H and I and Movies S18 and S19), the de novo assembly and sorting of microtubules in the cytoplasm facilitate kinetochore association. To further investigate the initial kinetochore capture process, we analyzed the dynamic behaviors of kinetochores and microtubules during the progression from late G2 to mitosis in the presence of 15 ng/mL (Fig. 5A, Fig. S11A, and Movie S20) and 50 ng/mL nocodazole (Fig. 5B, Fig. S11B, and Movie S21) in HeLa cells. In late G2 or early mitosis (e.g., before 00:30:00 in Fig. 5A), there were a few polymerized microtubules that were not attached with kinetochores. With the time increase, the microtubule seeds/asters began to assemble and sort around the kinetochores. In agreement with what we saw in fixed samples (Fig. 4C), these data indicated that loading and self-assembly of microtubules at the kinetochores facilitate the progression of mitosis and spindle formation.
Fig. 5.
A unique model for kinetochore capture by microtubules during mitotic spindle assembly in somatic cells with centrosomes. (A and B) Live imaging of HeLa cells coexpressing mcherry–tubulin and GFP–Hec1 in the presence of 15 ng/mL (A) or 50 ng/mL (B) nocodazole. Magnified images are shown. Tubulin is in red and Hec1 is in green. (Scale bar, 5 μm.) (C) Illustration of initial kinetochore capture process by microtubules in a TACC3-dependent way. The small green dots represent TACC3 proteins, the magenta lines represent the microtubules (MT), and the large red dots stand for the kinetochore pair. The initial kinetochore capture by adjacent acentrosomal microtubules can be divided into four steps: (i) microtubule assembly followed by formation of TACC3–microtubule seeds; (ii) binding of TACC3–microtubule seeds with kinetochores and further nucleation of the microtubules; (iii) further assembly and sorting of the TACC3–microtubule seeds lead to the formation of small TACC3–microtubule seeds/asters; and (iv) clustering of TACC3–microtubule seeds/asters and further nucleation of the acentrosomal microtubules produces the initial capture of the kinetochores by the acentrosomal aster microtubules. (D) Illustration of TACC3-dependent kinetochore capture in establishing bipolar spindle assembly. The acentrosomal microtubule-captured kinetochores are further captured by centrosomal microtubules through sorting the small acentrosomal asters into the big centrosomal microtubule asters to generate amphitelic and synthelic attachments before final chromosome biorientation.
In summary, mainly based on the results observed in nocodazole-treated cells, our present work leads us to hypothesize a unique model to illustrate the mechanism of the kinetochore capture by microtubules (Fig. 5 C and D). First, once the cell enters mitosis and the nuclear envelope breaks down, TACC3 and the short microtubules bind together to form small TACC3–microtubule seeds near the kinetochores along with the assembly of two big centrosomal asters, and the seeds grow gradually into small TACC3–microtubule asters through the nucleation of the short microtubules bound on the seeds. Second, the small TACC3–microtubule seeds/asters get connected with different parts of the kinetochores; and meanwhile, the TACC3–microtubule seeds/asters keep growing. Third, continuous nucleation and sorting of the TACC3–microtubule seeds/asters enable the kinetochores to be captured by the small TACC3–microtubule asters through lateral binding. Fourth, by skating along the microtubules of the TACC3–microtubule asters, the kinetochores are firmly captured through end-on connection of the microtubules and the kinetochores. Simultaneously, the small acentrosomal asters are sorted and join the two big centrosomal asters. And finally, depending on the transport of acentrosomal microtubule asters toward the centrosomes by the microtubule sorting mechanism, the acentrosomal asters are totally fused in the centrosomal asters, and a bipolar spindle is eventually established. Noticeably, although the abovementioned results are mainly based on observations in nocodazole-treated cells, TACC3 is also required for kinetochore capture in unperturbed mitosis without nocodazole treatment (Fig. 2). Because acentrosomal microtubule seeds in normal mitosis act similarly as in nocodazole-treated samples (Fig. 3 A–E), and TACC3 targets to acentrosomal microtubules (Fig. 1E and Fig. S7) and may regulate acentrosomal microtubule assembly in normal mitosis (Fig. 2E), the acentrosomal TACC3–microtubule seeds/asters mechanisms may be true in normal mitosis.
Efficient chromosome capture requires a bias to complete the capture process during mitotic spindle assembly (31). Meanwhile, chromosome movements and rotations are required to accelerate mitotic spindle assembly according to computer simulations (32). How does a cell speed up the process of kinetochore capture? The lateral surface area of a microtubule is larger than that of its tip and enables the fast kinetochore capture (3, 4). The formation of TACC3–microtubule seeds/asters near the kinetochore may speed up the kinetochore capture process through forming acentrosomal microtubule-attached kinetochores, thus increasing the microtubule capture surface of the kinetochores and accelerating the movement of kinetochores/chromosomes along the microtubules. Meanwhile, the growth and sorting of the kinetochore-bound TACC3–microtubule asters also promote the cluster formation and separation of the acentrosomal MTOCs as well as the movement and rotation of chromosome kinetochores. In agreement with the previous study that TACC3 depletion delays the mitotic progression (18), our study further highlights the role of the acentrosomal TACC3 in establishing kinetochore capture by microtubules in a fast and accurate way.
In mammalian somatic cells, Ran GTPase activity promotes microtubule nucleation at kinetochores (33), and Ran effectors such as microspherule protein 1 and γ-TuRC also function in K fibers (34, 35). As TACC3 is also a target of the Ran GTPase system (20), it may coordinate with Ran to regulate acentrosomal microtubules for kinetochore capture. Together these provide deep insights into the key events during the kinetochore capture process by the microtubules. However, further analyses at a higher resolution level to elucidate the kinetochore behaviors and functions are still needed in the future.
Materials and Methods
All experiments were done in HeLa cells. Living cell imaging data was acquired by a DeltaVision system (Applied Precision). For microscopy of the fixed samples, the images were acquired by the DeltaVision or Zeiss LSM710 microscope. Details of cell culture and drug treatment, RNA interference, small-molecule inhibitors, antibodies, live cell imaging and microscopy, Western blotting, immunofluorescence, and statistical analyses are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Xuebiao Yao for HeLa cells stably expressing mcherry-tubulin and plasmids and Jennifer Deluca, Jun Zhou, Xueliang Zhu, Michael Lampson, and Wen-Hwa Lee for plasmids and reagents. This work was supported by funds from the State Key Basic Research and Development Plan (2010CB833705) and the National Natural Science Foundation of China (31071188, 31030044, and 90913021).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312382110/-/DCSupplemental.
References
- 1.Cheeseman IM, Desai A. Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol. 2008;9(1):33–46. doi: 10.1038/nrm2310. [DOI] [PubMed] [Google Scholar]
- 2.Kirschner M, Mitchison T. Beyond self-assembly: From microtubules to morphogenesis. Cell. 1986;45(3):329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- 3.Hayden JH, Bowser SS, Rieder CL. Kinetochores capture astral microtubules during chromosome attachment to the mitotic spindle: Direct visualization in live newt lung cells. J Cell Biol. 1990;111(3):1039–1045. doi: 10.1083/jcb.111.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka K, et al. Molecular mechanisms of kinetochore capture by spindle microtubules. Nature. 2005;434(7036):987–994. doi: 10.1038/nature03483. [DOI] [PubMed] [Google Scholar]
- 5.Gandhi SR, et al. Kinetochore-dependent microtubule rescue ensures their efficient and sustained interactions in early mitosis. Dev Cell. 2011;21(5):920–933. doi: 10.1016/j.devcel.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heald R, et al. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 1996;382(6590):420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- 7.Schuh M, Ellenberg J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell. 2007;130(3):484–498. doi: 10.1016/j.cell.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Khodjakov A, Cole RW, Oakley BR, Rieder CL. Centrosome-independent mitotic spindle formation in vertebrates. Curr Biol. 2000;10(2):59–67. doi: 10.1016/s0960-9822(99)00276-6. [DOI] [PubMed] [Google Scholar]
- 9.Tulu US, Rusan NM, Wadsworth P. Peripheral, non-centrosome-associated microtubules contribute to spindle formation in centrosome-containing cells. Curr Biol. 2003;13(21):1894–1899. doi: 10.1016/j.cub.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Tulu US, Fagerstrom C, Ferenz NP, Wadsworth P. Molecular requirements for kinetochore-associated microtubule formation in mammalian cells. Curr Biol. 2006;16(5):536–541. doi: 10.1016/j.cub.2006.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maiato H, Rieder CL, Khodjakov A. Kinetochore-driven formation of kinetochore fibers contributes to spindle assembly during animal mitosis. J Cell Biol. 2004;167(5):831–840. doi: 10.1083/jcb.200407090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goshima G, Nédélec F, Vale RD. Mechanisms for focusing mitotic spindle poles by minus end-directed motor proteins. J Cell Biol. 2005;171(2):229–240. doi: 10.1083/jcb.200505107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goshima G, Vale RD. The roles of microtubule-based motor proteins in mitosis: Comprehensive RNAi analysis in the Drosophila S2 cell line. J Cell Biol. 2003;162(6):1003–1016. doi: 10.1083/jcb.200303022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider L, et al. The transforming acidic coiled coil 3 protein is essential for spindle-dependent chromosome alignment and mitotic survival. J Biol Chem. 2007;282(40):29273–29283. doi: 10.1074/jbc.M704151200. [DOI] [PubMed] [Google Scholar]
- 15.Booth DG, Hood FE, Prior IA, Royle SJ. A TACC3/ch-TOG/clathrin complex stabilises kinetochore fibres by inter-microtubule bridging. EMBO J. 2011;30(5):906–919. doi: 10.1038/emboj.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu W, et al. Clathrin recruits phosphorylated TACC3 to spindle poles for bipolar spindle assembly and chromosome alignment. J Cell Sci. 2010;123(Pt 21):3645–3651. doi: 10.1242/jcs.075911. [DOI] [PubMed] [Google Scholar]
- 17.Hubner NC, et al. Quantitative proteomics combined with BAC TransgeneOmics reveals in vivo protein interactions. J Cell Biol. 2010;189(4):739–754. doi: 10.1083/jcb.200911091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin CH, Hu CK, Shih HM. Clathrin heavy chain mediates TACC3 targeting to mitotic spindles to ensure spindle stability. J Cell Biol. 2010;189(7):1097–1105. doi: 10.1083/jcb.200911120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katayama H, Sasai K, Kloc M, Brinkley BR, Sen S. Aurora kinase-A regulates kinetochore/chromatin associated microtubule assembly in human cells. Cell Cycle. 2008;7(17):2691–2704. doi: 10.4161/cc.7.17.6460. [DOI] [PubMed] [Google Scholar]
- 20.Fu W, Jiang Q, Zhang C. Novel functions of endocytic player clathrin in mitosis. Cell Res. 2011;21(12):1655–1661. doi: 10.1038/cr.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu J, Bian M, Liu J, Jiang Q, Zhang C. A single amino acid change converts Aurora-A into Aurora-B-like kinase in terms of partner specificity and cellular function. Proc Natl Acad Sci USA. 2009;106(17):6939–6944. doi: 10.1073/pnas.0900833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ditchfield C, et al. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161(2):267–280. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapoor TM, Mayer TU, Coughlin ML, Mitchison TJ. Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J Cell Biol. 2000;150(5):975–988. doi: 10.1083/jcb.150.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steegmaier M, et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr Biol. 2007;17(4):316–322. doi: 10.1016/j.cub.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, et al. Sequential phosphorylation of Nedd1 by Cdk1 and Plk1 is required for targeting of the gammaTuRC to the centrosome. J Cell Sci. 2009;122(Pt 13):2240–2251. doi: 10.1242/jcs.042747. [DOI] [PubMed] [Google Scholar]
- 26.Cai S, Weaver LN, Ems-McClung SC, Walczak CE. Kinesin-14 family proteins HSET/XCTK2 control spindle length by cross-linking and sliding microtubules. Mol Biol Cell. 2009;20(5):1348–1359. doi: 10.1091/mbc.E08-09-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumont S, Mitchison TJ. Force and length in the mitotic spindle. Curr Biol. 2009;19(17):R749–R761. doi: 10.1016/j.cub.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hallen MA, Endow SA. Anastral spindle assembly: A mathematical model. Biophys J. 2009;97(8):2191–2201. doi: 10.1016/j.bpj.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breuer M, et al. HURP permits MTOC sorting for robust meiotic spindle bipolarity, similar to extra centrosome clustering in cancer cells. J Cell Biol. 2010;191(7):1251–1260. doi: 10.1083/jcb.201005065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hornick JE, et al. Amphiastral mitotic spindle assembly in vertebrate cells lacking centrosomes. Curr Biol. 2011;21(7):598–605. doi: 10.1016/j.cub.2011.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wollman R, et al. Efficient chromosome capture requires a bias in the ‘search-and-capture’ process during mitotic-spindle assembly. Curr Biol. 2005;15(9):828–832. doi: 10.1016/j.cub.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Paul R, et al. Computer simulations predict that chromosome movements and rotations accelerate mitotic spindle assembly without compromising accuracy. Proc Natl Acad Sci USA. 2009;106(37):15708–15713. doi: 10.1073/pnas.0908261106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torosantucci L, De Luca M, Guarguaglini G, Lavia P, Degrassi F. Localized RanGTP accumulation promotes microtubule nucleation at kinetochores in somatic mammalian cells. Mol Biol Cell. 2008;19(5):1873–1882. doi: 10.1091/mbc.E07-10-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meunier S, Vernos I. K-fibre minus ends are stabilized by a RanGTP-dependent mechanism essential for functional spindle assembly. Nat Cell Biol. 2011;13(12):1406–1414. doi: 10.1038/ncb2372. [DOI] [PubMed] [Google Scholar]
- 35.Mishra RK, Chakraborty P, Arnaoutov A, Fontoura BM, Dasso M. The Nup107-160 complex and gamma-TuRC regulate microtubule polymerization at kinetochores. Nat Cell Biol. 2010;12(2):164–169. doi: 10.1038/ncb2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.