Significance
This study investigated the expression and function of peroxisome proliferator-activated receptor alpha (PPARα) in the retina and its role in diabetic retinopathy. In both type 1 and type 2 diabetes models, expression of PPARα was significantly down-regulated in the retina. PPARα knockout exacerbated diabetes-induced retinal vascular leakage and retinal inflammation, while over-expression of PPARα in the retina of diabetic rats significantly alleviated diabetic retinopathy. This study reveals that PPARα has an anti-inflammatory function in the retina. These findings also suggest that diabetes-induced down-regulation of PPARα plays an important role in diabetic retinopathy and represents a novel therapeutic target for diabetic retinopathy.
Abstract
Two independent clinical studies have reported that fenofibrate, a peroxisome proliferator-activated receptor α (PPARα) agonist, has robust therapeutic effects on microvascular complications of diabetes, including diabetic retinopathy (DR) in type 2 diabetic patients. However, the expression and function of PPARα in the retina are unclear. Here, we demonstrated that PPARα is expressed in multiple cell types in the retina. In both type 1 and type 2 diabetes models, expression of PPARα, but not PPARβ/δ or PPARγ, was significantly down-regulated in the retina. Furthermore, high-glucose medium was sufficient to down-regulate PPARα expression in cultured retinal cells. To further investigate the role of PPARα in DR, diabetes was induced in PPARα knockout (KO) mice and wild-type (WT) mice. Diabetic PPARα KO mice developed more severe DR, as shown by retinal vascular leakage, leukostasis, pericyte loss, capillary degeneration, and over-expression of inflammatory factors, compared with diabetic WT mice. In addition, overexpression of PPARα in the retina of diabetic rats significantly alleviated diabetes-induced retinal vascular leakage and retinal inflammation. Furthermore, PPARα overexpression inhibited endothelial cell migration and proliferation. These findings revealed that diabetes-induced down-regulation of PPARα plays an important role in DR. Up-regulation or activation of PPARα may represent a novel therapeutic strategy for DR.
Peroxisome proliferator-activated receptors (PPARs) are a group of nuclear hormone receptors that function as transcription factors regulating the expression of a number of genes involving lipid metabolism and insulin resistance (1). There are three PPAR isotypes—PPARα, PPARβ/δ, and PPARγ—that play important roles in the regulation of cell differentiation, proliferation, development, and metabolism (carbohydrate, lipid, protein) (2, 3). PPARα is also crucial in the regulation of inflammation and angiogenesis (4–6) and is expressed in the liver, kidney, heart, muscle, adipose tissue, and other organs with significant fatty-acid catabolism (7, 8). PPARα is primarily activated through binding of ligands (9), which include lipids and synthetic fibrate drugs, such as fenofibrate (10, 11).
Diabetic microvascular complications include diabetic nephropathy, neuropathy, and retinopathy, which can occur in both type 1 and type 2 diabetes mellitus (12–15). Recently, fenofibrate, a specific PPARα agonist, has displayed surprising and robust efficacy in arresting the progression of microvascular complications in type 2 diabetes in the FIELD and ACCORD studies (16, 17). Further, PPARα knock-out (KO) mice with diabetes developed more severe nephropathy, compared with diabetic wild-type (WT) mice (18). Moreover, our recent study showed that fenofibrate had therapeutic effects on diabetic retinopathy (DR) via a PPARα-dependent mechanism (19). Also, PPARα ligands inhibited endothelial cell proliferation and migration (20) and reduced angiogenesis in a porcine model (6). However, the function of PPARα in the retina and its role in DR have not been clearly understood.
The present study determined the expression of PPARα in the retina and demonstrated down-regulation of PPARα in the retina of diabetes models. Further, we also induced diabetes in PPARα KO mice that displayed more severe retinopathy than diabetic WT mice. Moreover, we also demonstrated that overexpression of PPARα has therapeutic effects on DR. Our findings reveal the function of PPARα in the retina and may extend a novel therapeutic strategy of clinical relevance.
Results
PPARα Is Expressed in Multiple Cells in Human and Rat Retinas.
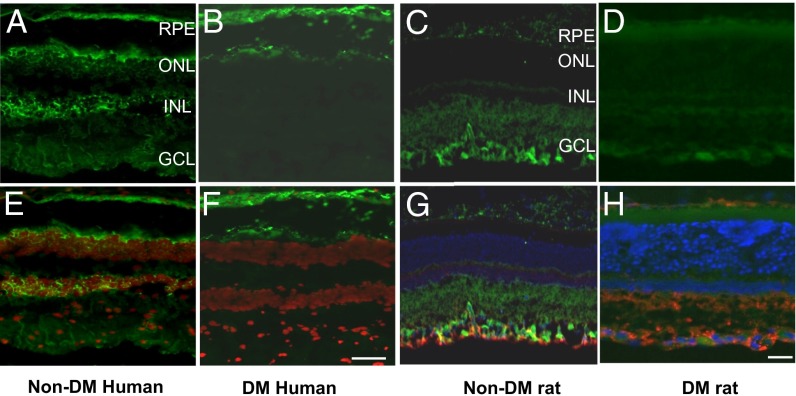
To determine whether PPARα is expressed in the retina, we first performed immunostaining of PPARα in human retinal sections. Immunostaining detected PPARα in the retinal pigment epithelium (RPE), outer nuclear layer, inner nuclear layer, and ganglion cell layer (Fig. 1 A and E). Further, we compared PPARα levels in the retinas of nondiabetic human donors (Fig. 1 A and E) with those of type 2 diabetic human donors with nonproliferative DR (Fig. 1 B and F). PPARα levels in the retina were substantially lower in the human donors with DR, compared with those in nondiabetic human donors.
Fig. 1.
Expression of PPARα in the human and rat retinas. (A, B, E, and F) Immunostaining of PPARα in human retinas. PPARα was immunostained (green) in the retina section from six nondiabetic human donors (A) and six type 2 diabetic donors with nonproliferative retinopathy (B). The nuclei were counterstained with DAPI (red) and merged with PPARα staining (green) (E and F). (C, D, G, and H) Immunostaining of PPARα in rat retinas. Retinal sections from nondiabetic rats (16 wk old) (C and G) and age-matched rats with 8 wk of STZ-induced diabetes (D and H) were double stained with an anti-PPARα antibody and anti-GFAP antibody. The nuclei were counterstained with DAPI. (G and H) Merged signals of PPARα (green), GFAP (red), and DAPI (blue). RPE, retinal pigment epithelium; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. (Scale bar: 50 µm.)
To define the cell types expressing PPARα in the retina, we double stained the retinal sections using antibodies for PPARα and for glial fibrillary acidic protein (GFAP), a glial cell marker, in normal (Fig. 1 C and G) and diabetic rats (Fig. 1 D and H). The result demonstrated the colocalization of PPARα and GFAP in the ganglion cell layer, suggesting the expression of PPARα in glial cells, including Müller cells. Further, PPARα is also expressed in the inner retina in mice, similar to that in rats (Fig. S1). Under the same staining conditions, diabetic rat retina showed less intense PPARα staining, compared with that in nondiabetic rat retina. Taken together, these results demonstrate that PPARα is expressed in the retinas, and its levels are reduced in the retinas with DR.
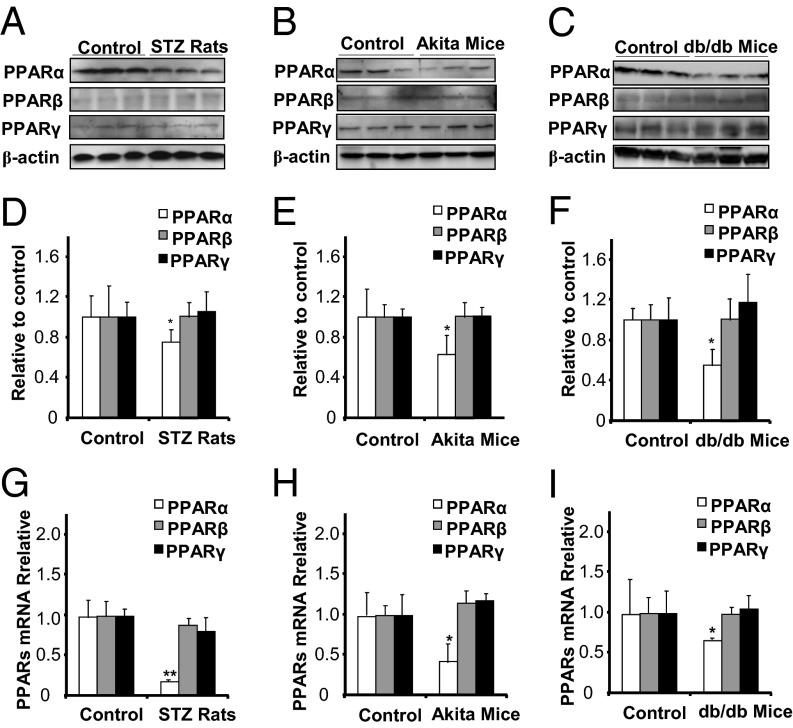
STZ-Induced Diabetic Rats, Akita Mice, and db/db Mice Have Down-Regulated PPARα Expression but Unchanged PPARβ and PPARγ Expression in the Retina.
We next investigated whether PPARα expression is changed in the retina under diabetic conditions in animal models. Western blot analysis showed that protein levels of PPARα were significantly lower in the retina of STZ-induced diabetic rats, compared with those in nondiabetic rats, whereas PPARβ and PPARγ levels showed no significant differences (Fig. 2 A and D). Similar results were observed in the retina of Akita mice, a genetic type 1 diabetic model, compared with their WT control mice (Fig. 2 B and E). Further, PPARα levels were also significantly decreased in the retina of db/db mice, a type 2 diabetic model, compared with their WT controls (Fig. 2 C and F). Similar to that in STZ-diabetic rats, PPARβ and PPARγ expression was not significantly changed in the retinas of Akita or db/db mice. Taken together, these results indicated that, among the PPAR family members, PPARα is selectively down-regulated in the retinas of both type 1 and type 2 diabetes models.
Fig. 2.
Down-regulation of PPARα expression in the retinas of STZ-diabetic rats, Akita mice, and db/db mice. (A and D) The same amount (50 µg) of retinal proteins from rats with 8 wk of STZ-induced diabetes and age-matched nondiabetic rats was used for Western blot analysis of PPARα, PPARβ, and PPARγ (A), semiquantified by densitometry and normalized by β-actin levels (D). (B and E) The same amount (50 µg) of retinal proteins from Akita mice (age of 16 wk) and WT controls was used for Western blot analysis of PPARα, PPARβ, and PPARγ (B), semiquantified by densitometry and normalized by β-actin levels (E). (C and F) The same amount (50 µg) of retinal proteins from db/db mice (age of 12 wk) and WT controls was used for Western blot analysis of PPARα, PPARβ, and PPARγ (C) and normalized by β-actin levels (F). (G–I) Real-time RT-PCR was performed to measure mRNA levels of PPARα, PPARβ, and PPARγ in the retinas of STZ-induced diabetic rats (G), Akita mice (H),and db/db mice (I) and their respective nondiabetic controls at the ages indicated above. All mRNA levels were expressed as fold of respective control (mean ± SD, n = 4, *P < 0.05, **P < 0.01, compared with control group).
PPARα mRNA Levels Are Decreased in the Retinas of STZ-Diabetic Rats, Akita Mice, and db/db Mice.
To determine whether the decreases in retinal PPARα levels in diabetes occur at the gene expression level, PPARα, PPARβ, and PPARγ mRNA levels were measured using real-time RT-PCR in the retinas of STZ-diabetic rats, Akita mice, db/db mice, and their respective nondiabetic controls. PPARα mRNA levels were significantly decreased in the retina of STZ-diabetic rats compared with normal rats whereas PPARβ and PPARγ mRNA levels were unchanged (Fig. 2G). Similar results were observed in the retinas of Akita mice (Fig. 2H) and db/db mice (Fig. 2I). These results demonstrated that PPARα mRNA expression is down-regulated in the retinas in both type 1 and type 2 diabetic animal models, consistent with the observation of the protein levels.
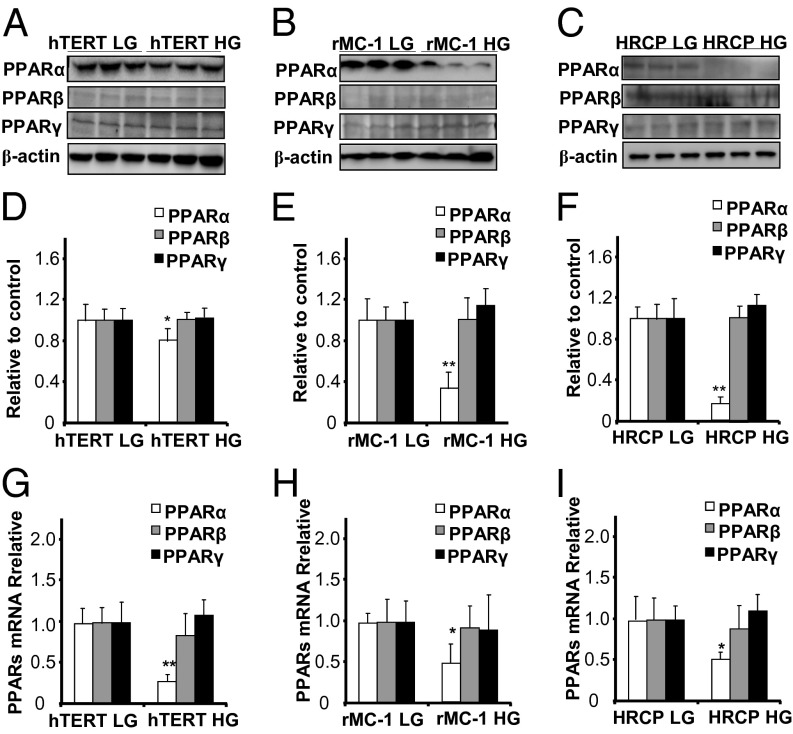
PPARα Expression Is Down-Regulated by High Glucose in Retinal Cells.
To determine which type of retinal cells has impaired expression of PPARα in diabetes, we mimicked the diabetic condition using high-glucose medium in cultured retinal cells. Expression of PPARα was significantly down-regulated in hTERT RPE cells treated with high glucose (30 mM glucose) for 72 h, compared with low glucose control (5 mM glucose and 25 mM mannitol) whereas PPARβ and PPARγ expression levels showed no significant differences (Fig. 3 A and D). Similar results were observed in high glucose-treated rMC-1, a cell line derived from rat Müller cells (Fig. 3 B and E) and primary human retinal capillary pericytes (HRCP) (Fig. 3 C and F). Taken together, these results indicated that high glucose is a diabetic stressor that down-regulates PPARα expression in multiple retinal cells.
Fig. 3.
Down-regulation of PPARα expression by high glucose in retinal cells. hTERT RPE cells, rMC-1 cells, and HRCP were exposed to 30 mM d-glucose for 72 h, with 5 mM d-glucose plus 25 mM l-glucose as control. (A–F) Total cell lysates were used for Western blot analysis of PPARα, PPARβ, and PPARγ in hTERT RPE cells (A), rMC-1 cells (B), and HRCP (C). The results were semiquantified by densitometry and normalized by β-actin levels (D–F). (G–I) Real-time RT-PCR was performed to measure mRNA levels of PPARα, PPARβ, and PPARγ in hTERT RPE cells (G), rMC-1 cells (H), and HRCP (I). All values are fold of respective control (mean ± SD, n = 4, *P < 0.05, **P < 0.01).
PPARα mRNA Levels Are Decreased by High Glucose in Retinal Cells.
To confirm the down-regulation of PPARα mRNA expression by high glucose, PPARα, PPARβ, and PPARγ mRNA levels were measured by real-time RT-PCR. PPARα mRNA levels were significantly decreased in high glucose-treated hTERT RPE (Fig. 3G), rMC-1 cells (Fig. 3H), and HRCP (Fig. 3I), compared with their respective controls. However, PPARβ and PPARγ mRNA levels showed no significant changes between these groups (Fig. 3 G–I).
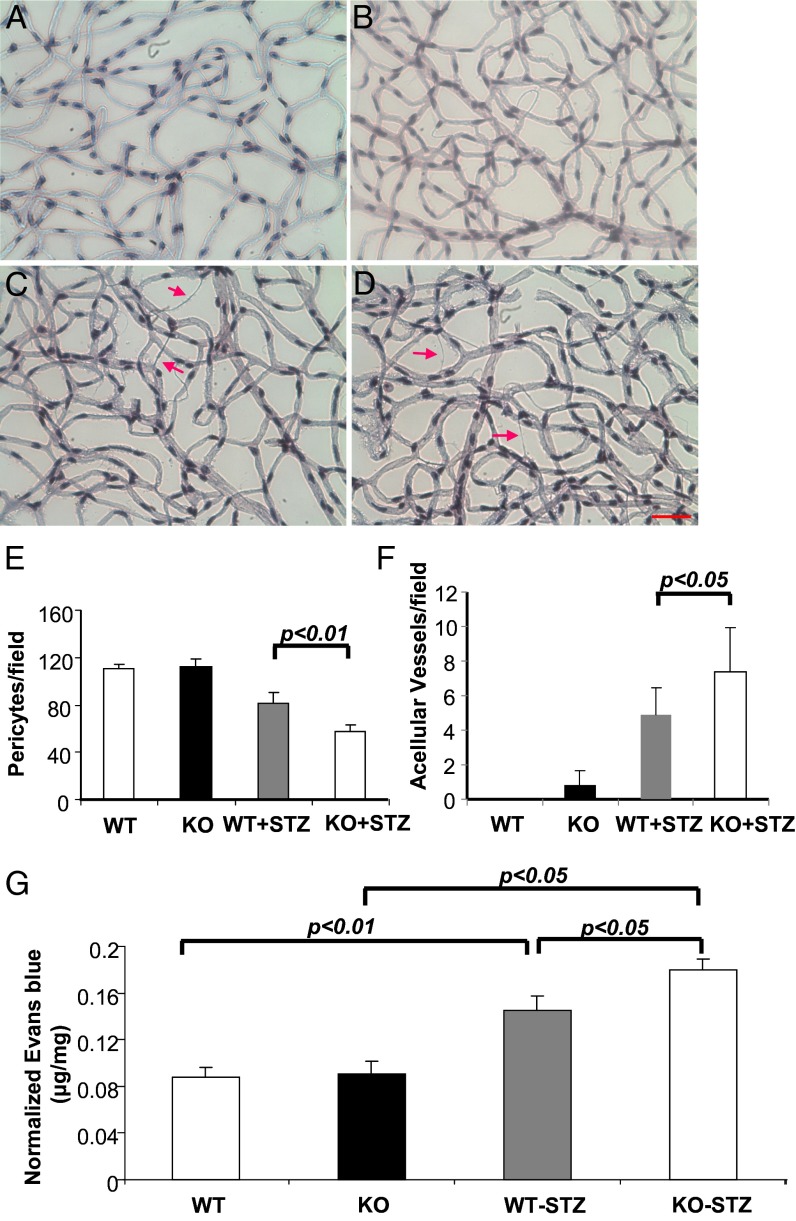
Diabetic PPARα KO Mice Show More Severe Retinal Vessel Impairment and Higher Vascular Leakage Compared with Diabetic WT Mice.
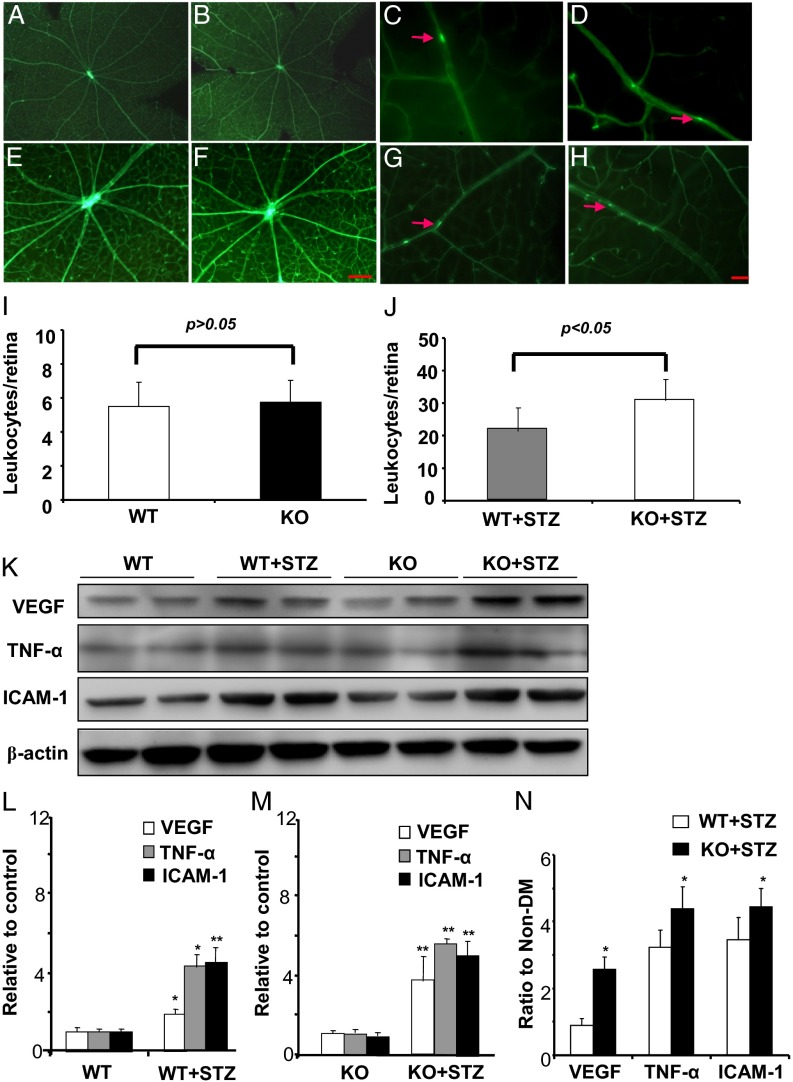
To test the hypothesis that down-regulated PPARα expression may exacerbate DR, we induced diabetes in PPARα KO mice and age- and genetic background-matched WT mice. Retina trypsin digestion was performed in nondiabetic WT mice (Fig. 4A), nondiabetic PPARα KO mice (Fig. 4B), STZ-induced diabetic WT mice (Fig. 4C), and STZ-induced diabetic PPARα KO mice (Fig. 4D) at 8 wk after the onset of diabetes. Pericytes and acellular capillaries were counted in a double-blind manner, which showed that, whereas there was no significant difference in numbers of pericytes between nondiabetic WT mice and nondiabetic PPARα KO mice, diabetic PPARα KO mice had significantly lower numbers of pericytes, compared with diabetic WT mice, with similar hyperglycemia levels and the same duration of diabetes (Fig. 4E), suggesting more severe diabetes-induced pericytes loss. Further, numbers of acellular capillaries were significantly higher in diabetic PPARα KO mice, compared with diabetic WT mice, suggesting aggravated capillary degeneration (Fig. 4F).
Fig. 4.
PPARα knockout exacerbates diabetic microvascular damage by diabetes. Diabetes was induced in PPARα KO mice and WT control by STZ injections. At 12 wk after diabetes onset, retina trypsin digestion was performed in age-matched nondiabetic WT mice (A), nondiabetic PPARα KO mice (KO) (B), diabetic WT mice (WT-STZ) (C), and diabetic PPARα KO mice (KO+STZ) (D). (Scale bar: 25 µm.) Red arrows indicate acellular capillaries. (E) Pericytes were quantified in five random fields per retina and averaged. (F) Acellular capillaries were quantified in five random fields and averaged. Retina vascular leakage was measured in the same groups by permeability assay (G). All values are mean ± SD (n = 4).
To determine severity of the diabetes-induced retinal vascular leakage, a retinal vascular permeability assay was performed in the same four groups (Fig. 4G). Retinal vascular permeability showed no significant differences between nondiabetic WT mice and PPARα KO mice; both diabetic WT mice and diabetic PPARα KO mice had significantly increased retina vascular leakage compared with respective nondiabetic controls. Diabetic PPARα KO mice had significantly higher retina vascular leakage than diabetic WT mice. Taken together, these results indicated that diabetic PPARα KO mice developed more severe vascular impairment compared with diabetic WT mice.
Diabetic PPARα KO Mice Develop More Severe Retina Inflammation Compared with Diabetic WT Mice.
To determine the difference of retinal inflammation in PPARα KO mice and WT mice under normal/diabetic conditions, a retinal leukostasis assay was performed in nondiabetic WT mice (Fig. 5 A and C), nondiabetic PPARα KO mice (Fig. 5 B and D), STZ-diabetic WT mice (Fig. 5 E and G), and STZ-diabetic PPARα KO mice (Fig. 5 F and H). Quantification of adherent leukocytes in flat-mounted retinas showed no significant difference between nondiabetic WT mice and nondiabetic PPARα KO mice (Fig. 5I); in contrast, diabetic PPARα KO mice had significantly more adherent leukocytes per retina than diabetic WT mice (Fig. 5J). Further, retinal levels of inflammatory factors including VEGF, TNF-α, and ICAM-1 were compared by Western blot analysis (Fig. 5K) and ELISA (Fig. S2). Both diabetic WT mice and diabetic PPARα KO mice displayed elevated retinal levels of VEGF, TNF-α, and ICAM-1, compared with their nondiabetic controls (Fig. 5 L and M and Fig. S2). Diabetic PPARα KO mice showed higher inductions of VEGF, TNF-α, and ICAM-1 expression by diabetes, compared with diabetic WT mice (Fig. 5N and Fig. S2). Taken together, these results demonstrated that diabetic PPARα KO mice had more severe diabetes-induced retinal inflammation, compared with diabetic WT mice.
Fig. 5.
PPARα knockout exacerbates retinal inflammation induced by diabetes. Diabetes was induced in PPARα KO mice and WT mice by STZ injections. (A–J) A retina leukostasis assay was performed 12 wk after the onset of diabetes in age-matched nondiabetic WT mice (A and C), nondiabetic PPARα KO mice (B and D), diabetic WT mice (E and G), and diabetic PPARα KO mice (F and H). [Scale bars: 100 µm (A, B, E, and F) and 25 µm (C, D, G, and H).] (I and J) Adherent leukocytes per retina were quantified and compared between nondiabetic WT and PPARα KO mice (I) and between diabetic WT and diabetic PPARα KO mice (J) (mean ± SD, n = 4). (K–N) The same amount (50 µg) of retina protein from these four groups was used for Western blot analysis of VEGF, TNF-α, and ICAM-1 (K). These protein levels were semiquantified with densitometry, normalized by β-actin levels, and compared between nondiabetic and diabetic WT mice (L) (mean ± SD, n = 4, *P < 0.05, **P < 0.01) and between nondiabetic and diabetic PPARα KO mice (M) (mean ± SD, n = 4, **P < 0.01). The increased folds of VEGF, TNF-α, and ICAM-1 levels induced by diabetes in WT mice were compared with those in PPARα KO (N) (mean ± SD, n = 4, *P < 0.05, compared with diabetic WT group).
PPARα Overexpression Reduces Vascular Leakage and Retina Inflammation in STZ-Diabetic Rats.
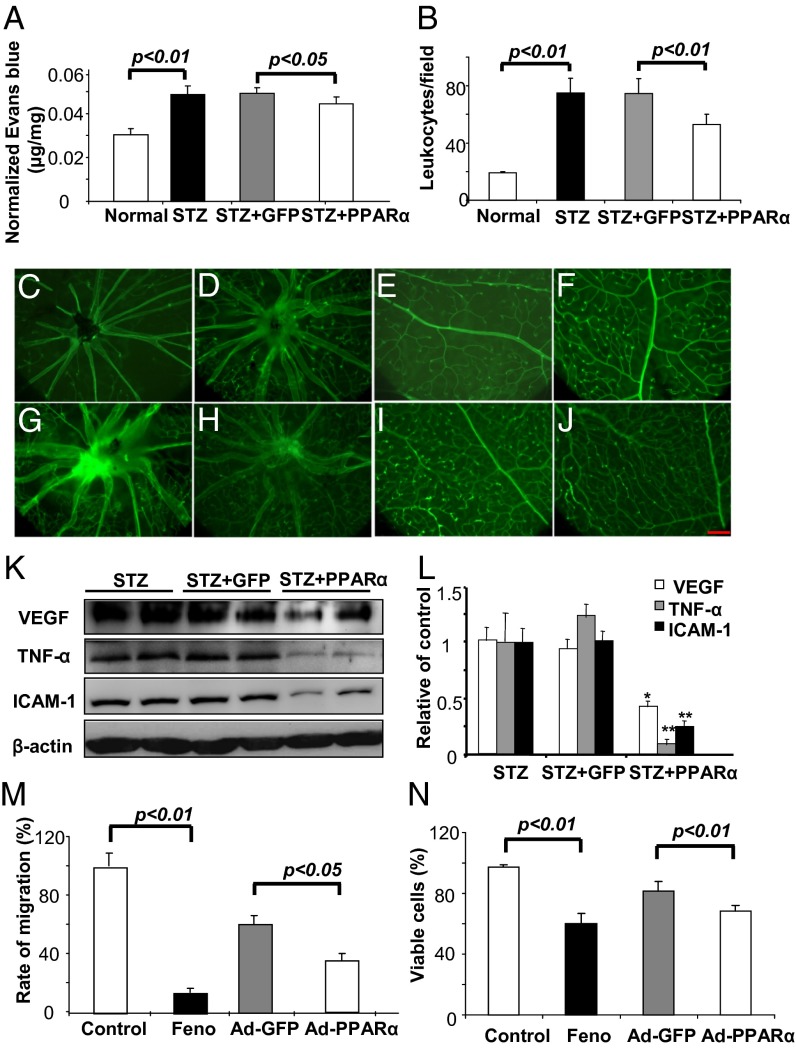
To investigate whether overexpression of PPARα in the retina may rescue the retinal vascular defects in DR, we overexpressed PPARα in the retina of diabetic rats. Adenovirus-expressing PPARα (Ad-PPARα) was injected intravitreally into nondiabetic and STZ-induced diabetic rats at 8 wk after the onset of diabetes; Ad-GFP was injected as control. A retinal vascular permeability assay was performed in normal rats, untreated STZ-diabetic rats, STZ-diabetic rats with an intravitreal injection of Ad-GFP (control adenovirus), and STZ-diabetic rats injected with Ad-PPARα at 4 wk after the injection. The results showed that the intravitreal injection of Ad-PPARα significantly reduced vascular leakage, compared with Ad-GFP injection and untreated diabetic groups (Fig. 6A). Further, to determine the retina inflammation change, a retinal leukostasis assay was performed in normal rats (Fig. 6 C and G), untreated diabetic rats (Fig. 6 D and H), diabetic rats with Ad-GFP injection (Fig. 6 E and I), and diabetic rats with Ad-PPARα injection (Fig. 6 F and J). Numbers of adherent leukocytes per retina were significantly decreased in the Ad-PPARα–treated diabetic rats, compared with the Ad-GFP–treated diabetic rats (Fig. 6B). Moreover, expression levels of VEGF, TNF-α, and ICAM-1 in the retina were measured by Western blot analysis and ELISA. The quantified data showed that Ad-PPARα treatment significantly down-regulated the expression of VEGF, TNF-α, and ICAM-1, compared with the Ad-GFP group (Fig. 6 K and L and Fig. S3). Taken together, these results showed that PPARα overexpression in the retina alleviated vascular leakage and retinal inflammation in diabetic rats.
Fig. 6.
PPARα overexpression ameliorates vascular leakage, leukostasis, and overexpression of angiogenic and inflammatory factors in the retinas of diabetic rats and inhibits endothelial cell migration and proliferation. Diabetic rats with 8 wk of STZ-induced diabetes received an intravitreal injection of Ad-PPARα or Ad-GFP (control). (A) Four weeks after the injection, retinal vascular leakage was measured by permeability assay and normalized by total retinal protein concentration (mean ± SD, n = 4). (B–J) Retina leukostasis assay was performed in age-matched nondiabetic rats (C and E), untreated diabetic rats (D and F), Ad-GFP-treated diabetic rats (G and I), and Ad-PPARα–treated diabetic rats (H and J). Adherent leukocytes per retina were quantified and compared (B) (mean ± SD, n = 4). [Scale bars: 100 µm (A, B, E, and F) and 25 µm (C, D, G, and H).] (K and L) The same amount (50 µg) of retina proteins was used for Western blot analysis of VEGF, TNF-α, and ICAM-1 (K). These protein levels were semiquantified with densitometry and normalized by β-actin levels (L) (mean ± SD, n = 4, *P < 0.05, **P < 0.01, compared with the Ad-GFP treatment group). (M and N) HRCECs were exposed to 25 μM fenofibrate, with DMSO as control or infected with Ad-PPARα at multiplicity of infection (MOI) of 20, with Ad-GFP as control, for 24 h and then subjected to in vitro scratch-wound healing assay with images captured at 0 and 8 h after the scratch using phase-contrast microscope. The rate of migration was measured and expressed as % of the vehicle control (M) (mean ± SD, n = 6). HRCECs were treated similarly as described above for 24 h and then changed to normal culture medium for 48 h. Viable cells were quantified by trypan blue exclusion assay and expressed as % of vehicle control (N) (mean ± SD, n = 4).
PPARα Overexpression Inhibits Migration and Proliferation of Human Retinal Capillary Endothelial Cell.
Next, we investigated the direct effects of PPARα on endothelial cell migration and proliferation, important steps in angiogenesis. An in vitro scratch-wound healing assay was performed in primary retinal capillary endothelial cells (HRCECs) to measure cell migration. HRCECs were treated with fenofibrate (positive control group) and with DMSO as the vehicle control and were infected with Ad-PPARα, with Ad-GFP as the control virus (Fig. S4). The quantified data showed that fenofibrate and Ad-PPARα both significantly inhibited HRCEC migration, compared with DMSO and Ad-GFP, respectively (Fig. 6M). Further, HRCEC growth was measured using trypan blue staining and quantified in the same four groups. Fenofibrate and PPARα overexpression both significantly decreased viable HRCEC numbers compared with DMSO and Ad-GFP (Fig. 6N). Taken together, these results showed that PPARα overexpression also had an antiangiogenic effect by directly inhibiting HRCEC migration and proliferation.
Discussion
PPARα is an important transcription factor known to regulate lipid metabolism (1). Its function in the retina has not been investigated. The results presented here demonstrated that PPARα, but not PPARβ or PPARγ, is down-regulated in the retinas of both type 1 and type 2 diabetic models and that high glucose is a direct cause of the PPARα down-regulation. The present study also showed that PPARα KO exacerbates diabetes-induced vascular impairment, vascular leakage, and inflammation in the retina. Furthermore, PPARα overexpression can rescue vascular leakage and inflammation in the retina of diabetic rats and inhibit endothelial cell migration and proliferation. These observations suggest that the diabetes-induced down-regulation of PPARα plays an important role in DR and may represent a new therapeutic target for the treatment of diabetic microvascular complications.
PPARα, PPARβ, and PPARγ have been shown to have key roles in regulation of lipid metabolism through regulating related gene expression (21–23); however, these PPAR family members show tissue-specific distribution (24, 25) and have different roles in signaling modulation in microvascular (26–28) and macrovascular cells (29). Our results demonstrated that, unlike PPARα, expression of PPARβ and PPARγ has no significant changes in the retina in both type 1 and type 2 diabetic models, suggesting that PPARα down-regulation by diabetes is selective and that PPARα rather than PPARβ and PPARγ is implicated in DR. This assumption is supported by the clinical findings that agonist of PPARα, but not PPARγ agonist, has beneficial effects on DR (16).
To define the direct cause of PPARα down-regulation under diabetic conditions, we used high-glucose media in cultured retinal cells. The results showed that high glucose alone is sufficient to cause PPARα down-regulation in multiple cell types. Previously, PPARα expression was found to be down-regulated by hypoxia inducible factor-1 (HIF-1) under hypoxia condition in intestinal epithelial cells (30). To investigate whether HIF-1 mediates down-regulation of PPARα induced by high glucose, we applied hypoxia in vitro and in vivo. However, our results showed that PPARα expression levels were not significantly altered by CoCl2-induced hypoxia in hTERT RPE cells and rat Müller cells at the time points analyzed although HIF-1 was activated (Fig. S5 A and B). Moreover, PPARα expression was not down-regulated in the retina of oxygen-induced retinopathy (OIR) mice (Fig. S5C) and OIR rats (Fig. S5D), an ischemia-induce retinal neovascularization (NV) model with activation of HIF-1. These results demonstrate that PPARα is down-regulated by chronic diabetes stressors but not by acute ischemia. Furthermore, PPARα KO mice with OIR did not develop more severe retinal NV, compared with OIR WT mice (Fig. S6). Taken together, these observations suggest that acute hypoxia alone is not sufficient to lead to down-regulation of PPARα under diabetic conditions. The mechanism and signaling pathways responsible for the PPARα down-regulation in DR remain to be elucidated in the future.
Chronic inflammation is believed to play a key role in DR (31). Inflammation has been shown to contribute to endothelium impairment, vascular leakage, pericyte loss, and increased capillary degeneration, leading to increased acellular vessels in DR (32). Although under normal conditions PPARα KO mice did not show detectable vascular phenotypes in the retina at the ages analyzed, PPARα KO significantly exacerbated leukostasis and overexpression of inflammatory factors induced by diabetes, suggesting more severe diabetes-induced retinal inflammation in the absence of PPARα. Consistently, PPARα KO mice with diabetes showed increased retinal vascular leakage, more severe pericyte loss, and increased acellular vessels, a result of capillary degeneration induced by retinal inflammation in DR. Taken together, these observations suggest that PPARα has an anti-inflammatory activity under diabetic conditions, which is responsible for its beneficial effects on DR.
Our immunohistochemical analysis in the retina and Western blot analysis in cultured retinal cells both demonstrated that PPARα is expressed in multiple retinal cell types. PPARα overexpression inhibited endothelial cell proliferation and migration. PPARα up-regulation also suppressed expression of ICAM-1, an adhesion molecule responsible for leukocyte adherence. These results support a direct effect of PPARα on vascular cells. Our results also showed that PPARα suppressed expression of inflammatory factors such as TNF-α and VEGF in other retinal cell types, such as Müller cells and RPE cells, which are major producers of inflammatory cytokines under diabetic conditions. It is likely that diabetes stressors down-regulate PPARα expression in these retinal cells, leading to increased expression and secretion of inflammatory cytokines (33–35) whereas PPARα overexpression suppresses overexpression of inflammatory factors in these cells. Thus, the pathological role of PPARα down-regulation in diabetic microvascular complications may be through both direct effects on vascular cells and indirect effects on vascular homeostasis, involving secretion of inflammatory cytokines from nonvascular cells. To investigate the molecular mechanism or signaling pathway by which the PPARα regulates inflammation factors, we investigated the interactions of PPARα with the NF-κB signaling pathway. The results showed that PPARα overexpression significantly increased IκBα levels, while decreasing phosphorylated NF-κB levels in high glucose-treated retinal cells (Fig. S7 A and B). Similarly, intravitreal injection of Ad-PPARα also elevated IκBα levels and decreased phosphorylated NF-κB levels in the retina of STZ-induced diabetic rats (Fig. S7 C and D). These observations are consistent with previous studies using PPARα agonist (36). Taken together, these findings suggest that the anti-inflammatory activity of PPARα is, at least in part, through inhibition of NF-κB signaling under diabetic stress.
PPARα activity can be enhanced through two different mechanisms: increase of PPARα activity (primarily via ligand binding) (37) and up-regulation of its expression. There are endogenous ligands, such as intracellular fatty acids, and synthetic ligands, such as fibrates, that are clinically used for hyperlipidemia treatment (16, 38). Recently, two independent, perspective clinical studies reported that fenofibrate, a PPARα agonist, has therapeutic effects on diabetic microvascular complications (16, 17). Our recent study using diabetic animal models showed that the fenofibrate effect on DR is PPARα-dependent (19). We and other groups have reported that fenofibrate has anti-inflammatory effects (4, 5, 19), consistent with the anti-inflammatory activity of PPARα observed in this study. It is likely that fenofibrate induces PPARα activity, which compensates for the down-regulation of PPARα under diabetes conditions, leading to amelioration of retinal inflammation, a major pathogenic feature of DR. These observations suggest that PPARα is a promising drug target for the treatment of retinal inflammation and vascular dysfunctions in diabetic microvascular complications.
In summary, this study suggests that diabetes-induced PPARα down-regulation represents a unique pathogenic mechanism for diabetic microvascular complications. Up-regulation or activation of PPARα may become a new therapeutic strategy for DR.
Materials and Methods
Animals.
Care, use, and treatment of experimental animals were in strict agreement with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Male C57BL/6J mice, PPARα KO mice, Akita mice, db/db mice, and C57BLKS/J mice (The Jackson Laboratory) and female Brown Norway rats (Charles River) were used in this study.
Induction of Diabetes in Rats.
Diabetes was induced in female Brown Norway rats (8 wk old) by an i.p. injection of STZ (55 mg/kg in 10 mM citrate buffer, pH 4.5) as described previously (19).
Immunohistochemistry.
Human donor eyes were obtained from the National Diseases Research Interchange with full ethical approval for use in research. Diabetic eyes were categorized and stained according to a standardized protocol (39). Rat and mouse eyes were dissected and sectioned and stained as described previously (19).
Western Blot Analysis.
The retinas of each mouse/rat were dissected, combined, and homogenized. The equal amount (50 µg) of total protein from each sample was used for Western blot analysis as described previously (40).
Quantitative Real-Time Reverse Transcription-PCR.
Total RNA was isolated from the retina using TRIzol according to the manufacturer’s protocol (Invitrogen). The RNA was used for reverse-transcription (RT) and amplified by quantitative real-time PCR as described previously (40).
Retinal Vascular Permeability Assay.
Retinal vascular permeability was quantified using the Evans blue-albumin leakage method as described previously (19).
Retina Trypsin Digestion Assay.
Trypsin digestion of the retina was performed following the method of Cogan and Kuwabara (41) with modifications (42).
Retina Leukostasis Assay.
The leukostasis assay was performed by staining adherent leukocytes in the vasculature in flat-mounted retina as described previously (19).
Statistical Analysis.
All of the values in the results were expressed as mean ± SD. Statistical analyses were performed using the Student t test. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health Grants EY018659, EY012231, EY019309, EY018358, and P20GM104934, and by American Diabetes Association Grant 7-11-JF-10.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307211110/-/DCSupplemental.
References
- 1.Michalik L, et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev. 2006;58(4):726–741. doi: 10.1124/pr.58.4.5. [DOI] [PubMed] [Google Scholar]
- 2.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 3.Filip-Ciubotaru F, Foia L, Manciuc C, Grigore C. [PPARs: Structure, mechanisms of action and control. Note I] Rev Med Chir Soc Med Nat Iasi. 2011;115(2):477–484. [PubMed] [Google Scholar]
- 4.Cuzzocrea S, et al. The role of the peroxisome proliferator-activated receptor-alpha (PPAR-alpha) in the regulation of acute inflammation. J Leukoc Biol. 2006;79(5):999–1010. doi: 10.1189/jlb.0605341. [DOI] [PubMed] [Google Scholar]
- 5.Duval C, Fruchart JC, Staels B. PPAR alpha, fibrates, lipid metabolism and inflammation. Arch Mal Coeur Vaiss. 2004;97(6):665–672. [PubMed] [Google Scholar]
- 6.Kasai T, Miyauchi K, Yokoyama T, Aihara K, Daida H. Efficacy of peroxisome proliferative activated receptor (PPAR)-alpha ligands, fenofibrate, on intimal hyperplasia and constrictive remodeling after coronary angioplasty in porcine models. Atherosclerosis. 2006;188(2):274–280. doi: 10.1016/j.atherosclerosis.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 7.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347(6294):645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 8.Dreyer C, et al. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68(5):879–887. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- 9.Miyachi H, Uchiki H. Analysis of the critical structural determinant(s) of species-selective peroxisome proliferator-activated receptor alpha (PPAR alpha)-activation by phenylpropanoic acid-type PPAR alpha agonists. Bioorg Med Chem Lett. 2003;13(19):3145–3149. doi: 10.1016/s0960-894x(03)00715-7. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, et al. PPAR-α agonist fenofibrate upregulates tetrahydrobiopterin level through increasing the expression of guanosine 5′-triphosphate cyclohydrolase-I in human umbilical vein endothelial cells. PPAR Res. 2011;2011:523520. doi: 10.1155/2011/523520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castillero E, et al. Fenofibrate, a PPARalpha agonist, decreases atrogenes and myostatin expression and improves arthritis-induced skeletal muscle atrophy. Am J Physiol Endocrinol Metab. 2011;300(5):E790–E799. doi: 10.1152/ajpendo.00590.2010. [DOI] [PubMed] [Google Scholar]
- 12.Hanssen KF. Blood glucose control and microvascular and macrovascular complications in diabetes. Diabetes. 1997;46(Suppl 2):S101–S103. doi: 10.2337/diab.46.2.s101. [DOI] [PubMed] [Google Scholar]
- 13.Maji D. Prevention of microvascular and macrovascular complications in diabetes mellitus. J Indian Med Assoc. 2004;102(8):426–, 428, 430 passim. [PubMed] [Google Scholar]
- 14.Hiukka A, Maranghi M, Matikainen N, Taskinen MR. PPARalpha: An emerging therapeutic target in diabetic microvascular damage. Nat Rev Endocrinol. 2010;6(8):454–463. doi: 10.1038/nrendo.2010.89. [DOI] [PubMed] [Google Scholar]
- 15.Tandon N, Ali MK, Narayan KM. Pharmacologic prevention of microvascular and macrovascular complications in diabetes mellitus: Implications of the results of recent clinical trials in type 2 diabetes. Am J Cardiovasc Drugs. 2012;12(1):7–22. doi: 10.2165/11594650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Keech AC, et al. FIELD study investigators Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): A randomised controlled trial. Lancet. 2007;370(9600):1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 17.Ginsberg HN, et al. ACCORD Study Group Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park CW, et al. Accelerated diabetic nephropathy in mice lacking the peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55(4):885–893. doi: 10.2337/diabetes.55.04.06.db05-1329. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, et al. Therapeutic effects of PPARα agonists on diabetic retinopathy in type 1 diabetes models. Diabetes. 2013;62(1):261–272. doi: 10.2337/db11-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goetze S, et al. PPAR activators inhibit endothelial cell migration by targeting Akt. Biochem Biophys Res Commun. 2002;293(5):1431–1437. doi: 10.1016/S0006-291X(02)00385-6. [DOI] [PubMed] [Google Scholar]
- 21.Barak Y, Sadovsky Y, Shalom-Barak T. PPAR signaling in placental development and function. PPAR Res. 2008;2008:142082. doi: 10.1155/2008/142082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeyama K, Kodera Y, Suzawa M, Kato S. [Peroxisome proliferator-activated receptor(PPAR)—structure, function, tissue distribution, gene expression] Nihon Rinsho. 2000;58(2):357–363. [PubMed] [Google Scholar]
- 23.Kawada T. [Lipid metabolism related nuclear receptor—the structure, function, expression and classification of peroxisome proliferation-activated receptor (PPAR)] Nihon Rinsho. 1998;56(7):1722–1728. [PubMed] [Google Scholar]
- 24.Wang F, et al. Tissue-specific expression of PPAR mRNAs in diabetic rats and divergent effects of cilostazol. Can J Physiol Pharmacol. 2008;86(7):465–471. doi: 10.1139/y08-043. [DOI] [PubMed] [Google Scholar]
- 25.Hegarty BD, Furler SM, Oakes ND, Kraegen EW, Cooney GJ. Peroxisome proliferator-activated receptor (PPAR) activation induces tissue-specific effects on fatty acid uptake and metabolism in vivo—a study using the novel PPARalpha/gamma agonist tesaglitazar. Endocrinology. 2004;145(7):3158–3164. doi: 10.1210/en.2004-0260. [DOI] [PubMed] [Google Scholar]
- 26.Araújo CV, et al. PPAR gamma activation protects the brain against microvascular dysfunction in sepsis. Microvasc Res. 2012;84(2):218–221. doi: 10.1016/j.mvr.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Madonna R, et al. Omega-3 fatty acids attenuate constitutive and insulin-induced CD36 expression through a suppression of PPAR α/γ activity in microvascular endothelial cells. Thromb Haemost. 2011;106(3):500–510. doi: 10.1160/TH10-09-0574. [DOI] [PubMed] [Google Scholar]
- 28.Biscetti F, et al. Selective activation of peroxisome proliferator-activated receptor (PPAR)alpha and PPAR gamma induces neoangiogenesis through a vascular endothelial growth factor-dependent mechanism. Diabetes. 2008;57(5):1394–1404. doi: 10.2337/db07-0765. [DOI] [PubMed] [Google Scholar]
- 29.Hsueh WA, Jackson S, Law RE. Control of vascular cell proliferation and migration by PPAR-gamma: A new approach to the macrovascular complications of diabetes. Diabetes Care. 2001;24(2):392–397. doi: 10.2337/diacare.24.2.392. [DOI] [PubMed] [Google Scholar]
- 30.Narravula S, Colgan SP. Hypoxia-inducible factor 1-mediated inhibition of peroxisome proliferator-activated receptor alpha expression during hypoxia. J Immunol. 2001;166(12):7543–7548. doi: 10.4049/jimmunol.166.12.7543. [DOI] [PubMed] [Google Scholar]
- 31.Fujita T, et al. Complement-mediated chronic inflammation is associated with diabetic microvascular complication. Diabetes Metab Res Rev. 2013;29(3):220–226. doi: 10.1002/dmrr.2380. [DOI] [PubMed] [Google Scholar]
- 32.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30(5):343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi G, et al. Control of chemokine gradients by the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2008;49(10):4620–4630. doi: 10.1167/iovs.08-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eichler W, Yafai Y, Wiedemann P, Reichenbach A. Angiogenesis-related factors derived from retinal glial (Müller) cells in hypoxia. Neuroreport. 2004;15(10):1633–1637. doi: 10.1097/01.wnr.0000133071.00786.a4. [DOI] [PubMed] [Google Scholar]
- 35.Holtkamp GM, Kijlstra A, Peek R, de Vos AF. Retinal pigment epithelium-immune system interactions: Cytokine production and cytokine-induced changes. Prog Retin Eye Res. 2001;20(1):29–48. doi: 10.1016/s1350-9462(00)00017-3. [DOI] [PubMed] [Google Scholar]
- 36.Okayasu T, Tomizawa A, Suzuki K, Manaka K, Hattori Y. PPARalpha activators upregulate eNOS activity and inhibit cytokine-induced NF-kappaB activation through AMP-activated protein kinase activation. Life Sci. 2008;82(15-16):884–891. doi: 10.1016/j.lfs.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Hertz R, Bar-Tana J. Peroxisome proliferator-activated receptor (PPAR) alpha activation and its consequences in humans. Toxicol Lett. 1998;102-103:85–90. doi: 10.1016/s0378-4274(98)00290-2. [DOI] [PubMed] [Google Scholar]
- 38.Varet J, et al. Fenofibrate inhibits angiogenesis in vitro and in vivo. Cell Mol Life Sci. 2003;60(4):810–819. doi: 10.1007/s00018-003-2322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, et al. Activation of the Wnt pathway plays a pathogenic role in diabetic retinopathy in humans and animal models. Am J Pathol. 2009;175(6):2676–2685. doi: 10.2353/ajpath.2009.080945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu Y, et al. Pathogenic role of the Wnt signaling pathway activation in laser-induced choroidal neovascularization. Invest Ophthalmol Vis Sci. 2013;54(1):141–154. doi: 10.1167/iovs.12-10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cogan DG, Kuwabara T. Comparison of retinal and cerebral vasculature in trypsin digest preparations. Br J Ophthalmol. 1984;68(1):10–12. doi: 10.1136/bjo.68.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michael JC, De Venecia G. Retinal trypsin digest study of cystoid macular edema associated with peripheral choroidal melanoma. Am J Ophthalmol. 1995;119(2):152–156. doi: 10.1016/s0002-9394(14)73867-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.