Significance
The recycling of many signaling receptors requires specific sequences on their C-terminal tail. Why is this so, when other proteins can use a “bulk” recycling pathway without apparent sequence requirements? Our results suggest that sequence requirements provide control points for cells to reprogram receptor recycling and cellular sensitivity. We show that the beta-2 adrenergic receptor (B2AR), a prototypical receptor, can switch between sequence-dependent and bulk recycling pathways based on adrenergic signaling through protein kinase A (PKA). This switch is driven by PKA-mediated direct phosphorylation of B2AR, which partitions between physically and functionally distinct endosomal microdomains based on its phosphorylation status. This partitioning modulates cellular sensitivity to adrenergic signaling and might help cells adapt to changes in extracellular environment.
Keywords: endosome, sorting, catecholamine receptor, endosomal tubule
Abstract
The postendocytic recycling of signaling receptors is subject to multiple requirements. Why this is so, considering that many other proteins can recycle without apparent requirements, is a fundamental question. Here we show that cells can leverage these requirements to switch the recycling of the beta-2 adrenergic receptor (B2AR), a prototypic signaling receptor, between sequence-dependent and bulk recycling pathways, based on extracellular signals. This switch is determined by protein kinase A-mediated phosphorylation of B2AR on the cytoplasmic tail. The phosphorylation state of B2AR dictates its partitioning into spatially and functionally distinct endosomal microdomains mediating bulk and sequence-dependent recycling, and also regulates the rate of B2AR recycling and resensitization. Our results demonstrate that G protein-coupled receptor recycling is not always restricted to the sequence-dependent pathway, but may be reprogrammed as needed by physiological signals. Such flexible reprogramming might provide a versatile method for rapidly modulating cellular responses to extracellular signaling.
How proteins are sorted in the endocytic pathway is a fundamental question in cell biology. This is especially relevant for signaling receptors, given that relatively small changes in rates of receptor sorting into the recycling pathway can cause significant changes in surface receptors, and hence in cellular sensitivity (1–3). Our knowledge of receptor signaling and trafficking comes mainly from studying examples such as the beta-2 adrenergic receptor (B2AR), a prototypical member of G protein-coupled receptor (GPCR) family, the largest family of signaling receptors (2–5). B2AR activation initiates surface receptor removal and transport to endosomes, causing cellular desensitization (6, 7). The rate and extent of resensitization is then determined by B2AR surface recycling (1–3, 8, 9).
Interestingly, the recycling of signaling receptors is functionally distinct from the recycling of constitutively cycling proteins like the transferrin receptor (TfR) (1, 6, 10, 11). TfR recycles by “bulk” geometric sorting, largely independent of specific cytoplasmic sequences (12, 13). B2AR recycling, in contrast, requires a specific PSD95-Dlg1-zo-1 domain (PDZ)-ligand sequence on its C-terminal tail, which links the receptor to the actin cytoskeleton (14, 15). Recent work has identified physically and biochemically distinct microdomains on early endosomes that mediate B2AR recycling independent of TfR (14–16). Although the exact mechanisms of B2AR sorting into these domains remain under investigation, this sorting clearly requires specific sequence elements on B2AR (1, 10, 11, 17). Importantly, why signaling receptor sorting is subject to such specialized requirements, considering that cargo like TfR apparently can recycle without specific sequence requirements, is not clear (1, 12–16). One possibility is that these requirements allow signaling pathways to regulate and redirect receptor trafficking between different pathways as needed (17–19). Although this is an attractive idea, whether and how physiological signals regulate receptor sorting remain poorly understood (7, 19).
Here we show that adrenergic signaling can switch B2AR recycling between the sequence-dependent and bulk recycling pathways. Adrenergic activation, via protein kinase A (PKA)-mediated B2AR phosphorylation on the cytoplasmic tail, restricts B2AR to spatially defined PDZ- and actin-dependent endosomal microdomains. Dephosphorylation of B2AR switches B2AR to the bulk (PDZ-independent) recycling pathway, causing faster recycling of B2AR and increased cellular sensitivity. Our results suggest that cells may leverage sequence requirements for rapid adaptive reprogramming of signaling receptor trafficking and cellular sensitivity.
Results
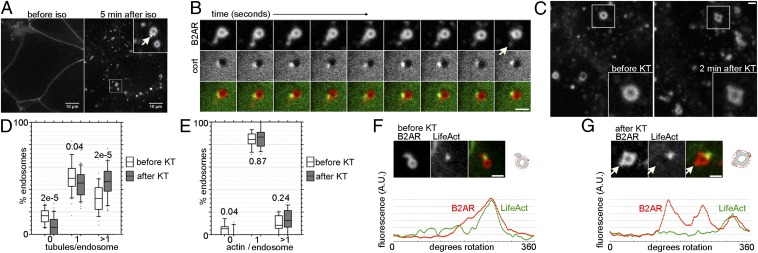
To test whether endosomal sorting of B2AR is regulated, we started with PKA signaling. Downstream activation of PKA has a homeostatic effect on B2AR delivery to the surface, making it a good candidate (20). We first optimized a live-cell imaging assay to directly visualize and resolve B2AR endosomal sorting at the level of discrete events mediating B2AR recycling. HEK293 cells expressing tagged B2AR were generated, and cells expressing a moderate level (∼1–2 pmol/mg) were chosen, because this level of expression has been shown to preserve the signaling and trafficking characteristics of B2AR (14, 20). When imaged under high-resolution confocal microscopy, fluorescently labeled B2AR was readily detected on the cell surface before activation (Fig. 1A). On addition of the B2AR agonist isoproterenol (iso), receptors were rapidly internalized and redistributed to internal spherical membranes that have been characterized as early endosomes (10, 15, 16, 21). Within these membranes, B2AR was concentrated in tubular domains (Fig. 1A). Several observations indicate that these tubules represent specialized microdomains mediating B2AR recycling. First, these tubules pinched off and generated vesicles (Fig. 1B and Movie S1) that traveled to the cell surface (15). Second, the tubules were devoid of the delta opioid receptor, a nonrecycling GPCR (Fig. S1). Third, the domains were spatially and biochemically distinct from bulk recycling domains (12, 15). Imaging TfR as a marker for bulk recycling along with B2AR revealed TfR recycling via multiple tubules extruding from an endosome, consistent with geometric sorting (e.g., Fig. 2F). The B2AR tubules represent a specific subset of these tubules marked by a highly localized actin cytoskeleton, a hallmark of B2AR recycling domains (15) (Fig. 1B).
Fig. 1.
PKA inhibition sorts B2AR into actin-independent tubules on the endosome. (A) A representative HEK293 cell expressing FLAG-tagged B2AR imaged live before and after activation with iso. B2AR redistributes to tubular domains (arrow in Inset) on endosomes after iso. (Scale bar: 10 µm.) (B) Images from a two-color confocal time-lapse series, taken 0.3 s apart. The arrow indicates a vesicle generated from a cortactin-labeled microdomain. (C) Representative field from the same cell showing endosomes before and after PKA inhibition by KT. (D) Boxplots showing distributions of the percentage of endosomes per cell with 0, 1, or >1 B2AR tubules before (white) and after (gray) the addition of KT in the same cells, with P values (n = 440). (E) Similar boxplots of endosomes with 0, 1, or >1 actin spots (n = 250). (F) (Upper) Example image of a typical endosome with a B2AR tubule associated with LifeAct. The cartoon shows the mode of measurement of fluorescence along the endosome perimeter. (Lower) B2AR and LifeAct fluorescence traces. (G) Example endosome after the addition of KT (compare with before KT in F). The arrow denotes a tubule devoid of actin. (Scale bar: 1 μm.)
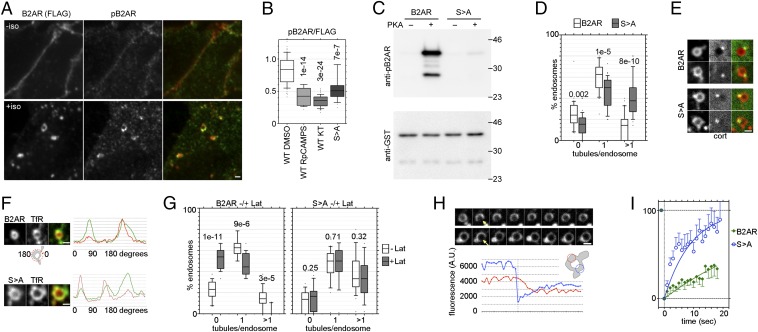
Fig. 2.
PKA-mediated B2AR phosphorylation restricts the entry of B2AR into bulk recycling tubules. (A) Immunofluorescence images of B2AR-expressing HEK293 cells double-labeled with anti-FLAG and anti-phosphoSer346 B2AR, before and after 10 min of iso treatment. (B) The ratio of pB2AR/FLAG in B2AR cells treated with iso and vehicle (DMSO), RpcAMPS (10 μM), or KT-5720 (10 μM) for 10 min, and in B2ARS>A cells treated with iso and vehicle. P values are with respect to B2AR. (C) In vitro phosphorylation of GST-B2AR tail with or without the S > A mutation. Representative blots from three experiments with anti-pB2AR (Upper) and anti-GST (Lower) are shown. (D) Percentages of B2AR (white) and B2ARS>A (gray) endosomes with 0, 1, or >1 receptor tubules, calculated from paired experiments (n = 266 for B2AR, n = 336 for S > A), with P values. (E) Examples of dual-color confocal images of B2AR (Upper) and B2ARS>A (Lower) endosomes with cortactin, showing B2ARS>A tubules lacking cortactin. (F) Typical B2AR (Upper) and B2ARS>A (Lower) endosomes imaged with TfR, showing a subset of TfR tubules devoid of B2AR but accessed by B2ARS>A. Oval Profile plots, as in Fig 1F, are shown to the right (Movie S3). (G) Percentage of B2AR (Left) and B2ARS>A (Right) endosomes with 0, 1, or >1 receptor tubules from the same cells before (white) and 5 min after (gray) lat-A. (H) (Upper) Frames taken 1 s apart from a time-lapse movie showing FRAP of B2AR (Top) and B2ARS>A (Bottom) endosomes, demonstrating slower recovery for B2AR. (Lower) Fluorescence values from the bleached region (blue) and a control region on the same endosome (blue), from another B2ARS>A example (Movie S4). (I) Quantitation of recovery rates of B2AR and B2ARS>A from multiple endosomes fitted to a single-phase exponential curves showing slow recovery of B2AR (t1/2 = 25.9 s, n = 10, R2 = 0.813) and rapid recovery of B2ARS>A (t1/2 = 5.9 s, n = 14, R2 = 0.779). Error bars are SEM. (Scale bars: 1 µm.)
Importantly, the foregoing approach provided an assay for comparing rapid changes in B2AR sorting into these recycling domains in the same cells, thereby eliminating variability across cells. In cells exposed to iso for 5 min, inhibition of kinase by the addition of KT-5720 (KT), a PKA inhibitor, resulted in a significant increase in the mean number of B2AR tubules per endosome in the same set of cells (from 1.19 ± 0.03 to 1.51 ± 0.05; P = 6 × 10−7) within 1 min. To better characterize this effect, we binned endosomes based on the number of B2AR tubules in the same cells before and after the addition of KT. KT increased the proportion of endosomes in which B2AR was sorted into more than one tubule (Fig. 1D). Furthermore, in an assay detecting surface fusion of recycling vesicles (20), KT increased the number of B2AR recycling vesicles fusing with the surface (Fig. 3E). Taken together, the data from these experiments indicate that signaling through PKA restricts the sorting of B2AR to a smaller number of endosomal recycling tubules.
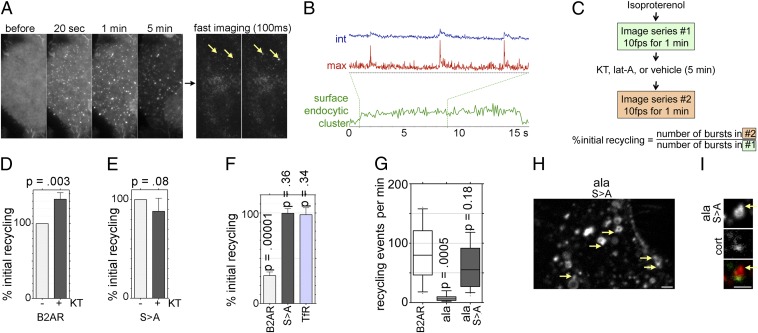
Fig. 3.
Phosphorylation restricts B2AR surface delivery to the actin-dependent recycling pathway. (A) Time frames from a TIR-FM movie of SpH-B2AR–expressing cells exposed to iso, showing slower endocytic clustering and loss of surface fluorescence over 5 min. Imaging at 10 fps shows abrupt bursts of B2AR fluorescence (arrows) appearing in a single 100-ms frame. (B) Fluorescence traces of a region of the plasma membrane showing integrated (blue) and maximum (red) intensity of recycling events. The integrated intensity trace of a surface endocytic cluster (green) shows the differences in time scale and profile. Dotted lines indicate the time period in which recycling events were traced. (C) Schematic showing the experimental workflow for estimating the effect of PKA and actin inhibition on B2AR recycling in the same cells. (D) Percent change (± SEM) in number of B2AR recycling events in the same cells over unit time in response to KT. (E) Percent change in recycling with B2ARS>A in response to KT. (F) Percent change in recycling of B2AR, B2ARS>A, and TfR after lat-A compared with before lat-A, demonstrating that B2AR recycling is actin-dependent, but B2ARS>A recycling and TfR recycling are not. (G) Number of recycling events per minute for B2AR, B2ARala, and B2ARalaS > A, showing that the loss of recycling of B2ARala is rescued by S > A. (H) Representative image of B2ARalaS > A endosomes. Arrows indicate tubules. (I) Representative endosome showing B2ARalaS > A in a tubule devoid of cortactin (arrow). (Scale bars: 1 µm.)
We hypothesized that there are two potential modes by which PKA inhibition could increase the number of B2AR recycling tubules: by generating more actin-based recycling microdomains (15), and by allowing access of B2AR to bulk recycling tubules. To test the first possibility, we directly visualized actin domains using coronin1C-GFP or LifeAct-RFP (22). We found no increase in the number of actin microdomains per endosome after the addition of KT (P = 0.24 for % endosomes with more than one microdomain), indicating that PKA inhibition does not generate new actin microdomains (Fig. 1E). This finding suggested the second possibility. To test this, we directly visualized B2AR and the actin cytoskeleton using multiple established markers for F-actin, including actin, coronin1C, cortactin, and LifeAct (22) (Fig. 1 B and F). Before the addition of KT, B2AR was detected exclusively in tubules that colocalized with actin markers (15, 16) (Fig. 1B). After the addition of KT, B2AR entered multiple tubules, many of which were devoid of actin (Fig. 1F), suggesting that PKA signaling ensured the sorting of B2AR into actin-dependent microdomains. Furthermore, the fact that KT pretreatment was not required suggests that this sorting is a dynamic process that responds to changes at physiologically relevant time scales.
We next attempted to identify the kinase target that regulates B2AR sorting. Interestingly, B2AR itself contains two cytoplasmic consensus PKA sites that are phosphorylated in response to an agonist (2, 23, 24). One of these two sites, Ser-261/262, located in the third cytoplasmic loop, is not involved in endocytic trafficking (24–27); thus, we focused on Ser-345/346 in the cytoplasmic tail, which has been implicated in surface delivery of B2AR (20). We first detected iso-induced B2AR phosphorylation at this site, using a phospho-specific Ser346 antibody. Before iso, when B2AR (detected by anti-FLAG) was on the membrane, only minimal phospho-S346 B2AR (pB2AR) was present (Fig. 2A, Upper). At 10 min after iso, a strong pB2AR signal was detected on the endosomes marked by anti-FLAG (Fig. 2A, Lower). The ratios of pB2AR to FLAG (uncorrected values without background subtraction) from multiple endosomes showed significantly decreased pB2AR fluorescence on cotreatment with KT or the competitive PKA inhibitor Rp-cAMPS compared with the DMSO control (Fig. 2B).
As a control for phosphorylation, and to test the function of these residues, we mutated Ser-345 and 346 to alanines (B2ARS>A). The decrease on PKA inhibition was comparable to that seen in B2ARS>A (Fig. 2B). To directly test whether PKA could phosphorylate serine 346 of B2AR, we generated GST-fusion proteins containing aa 320–413 of B2AR and a corresponding S > A version. When these were incubated with purified PKA, the B2AR tail, but not the B2ARS>A tail, was phosphorylated (Fig. 2C). Taken together, these results indicate that PKA was required and sufficient for iso-mediated B2AR phosphorylation.
We next imaged the endosomal sorting of the nonphosphorylatable receptor B2ARS>A. Strikingly, this mutant was sorted into a significantly higher number of tubules per endosome compared with WT B2AR (1.4 ± 0.05 vs. 0.91 ± 0.04; P = 9 × 10−10) in paired sets of experiments performed on the same days under the same conditions. A significant fraction (41.4 ± 3%) of endosomes showed more than one tubule containing B2ARS>A. In comparison, WT B2AR was sorted mostly into one tubule per endosome, with only 14.5 ± 2% of endosomes showing more than one B2AR tubule (Fig. 2D). This increase was comparable to that seen with KT described above, suggesting sorting of the B2ARS>A into bulk recycling tubules. Three additional observations support this interpretation. First, B2AR S > A entered tubules devoid of cortactin, which marks B2AR recycling tubules, but not bulk recycling tubules (Fig. 2E and Movie S2). Second, B2ARS>A showed higher colocalization with TfR tubules on the endosome compared with WT B2AR (Fig. 2F and Movie S3). Third, the entry of B2ARS>A into endosomal tubules was independent of actin, with no significant increase in endosomes lacking B2ARS>A tubules after actin depolymerization by latrunculin-A (lat-A; Fig. 2G). In addition, there was no decrease in endosomes with multiple B2ARS>A tubules after lat-A. In contrast, the fraction of endosomes with B2AR tubules decreased significantly after lat-A (Fig. 2G), as expected for actin-dependent B2AR recycling.
One proposed role of endosomal actin is to stabilize sequence-dependent recycling tubules, to allow for kinetic sorting of B2AR (15). This function depends on a key property of B2AR—its mobility on the endosome is slower than that of bulk recycling proteins like TfR, which prevents its access to the transient bulk recycling tubules not stabilized by actin. We directly tested whether B2AR phosphorylation changed its mobility by comparing B2AR and B2ARS>A using the fluorescence recovery after photobleaching (FRAP) technique. When a small part of the endosomal membrane was bleached, we found recovery of receptor fluorescence in the bleached region and decreased receptor fluorescence in nonbleached regions, consistent with receptor movement into the bleached area (Fig. 2H and Movie S4). When recovery rates were quantitated across multiple experiments, B2ARS>A recovered much faster than B2AR. Single-phase exponential fits showed a t1/2 of ∼6 s for B2ARS>A recovery, compared with t1/2 = 26.9 s for B2AR recovery (Fig. 2I). These experiments suggest that PKA phosphorylation of B2AR ensures accurate kinetic sorting of B2AR into sequence-dependent recycling tubules and prevents missorting of B2AR into bulk recycling tubules.
To test whether the foregoing effect changes B2AR surface recycling, we directly visualized the rate of B2AR delivery to the cell surface at the level of individual events that mediate recycling. To do this, we tagged the N-terminal domain of B2AR with a pH-sensitive GFP (SpH), which is fluorescent when exposed to neutral extracellular medium but quenched in the acidic environment of intracellular vesicles and endosomes (28). To obtain better signal-to-noise ratios and detect individual fusion events, we imaged receptor dynamics at the surface using total internal reflection fluorescence microscopy (TIR-FM). SpH-B2AR clustered and internalized rapidly in response to agonist (Fig. 3A), as described previously (20, 29). After 5 min, serial acquisition of images at 10–30 frames per second (fps) revealed transient bursts of SpH-B2AR fluorescence in the plasma membrane (Fig. 3A), denoting fusion of vesicles from the rapid B2AR recycling pathway (Movie S5) (20). Maximum fluorescence traces showed a transient spike followed by an exponential drop within a few hundred milliseconds, whereas integrated intensity in the region increased over a sustained period, consistent with an increased local pool of receptors on the surface (Fig. 3B and Fig. S2). Fluorescence traces from the same region confirmed that the profiles of these events were rapid and distinct from those of endocytic clusters (Fig. 3B). Quantitation of multiple events showed a rapid spike in fluorescence and a sustained increase in fluorescence (Fig. S2), as would be expected for recycling events delivering receptors to the cell surface (20).
To test whether PKA regulated B2AR insertion, we imaged iso-treated cells for 1 min as above, applied KT for 5 min, and reimaged for another 1 min (Fig. 3C). This allowed us to monitor the changes induced by PKA signaling in the same cells without cell-to-cell variability. PKA inhibition increased the number of recycling events observed over a unit time period (Fig. 3D). This increase was not seen for B2ARS>A, confirming B2AR as the target of PKA (Fig. 3E). Consistent with restriction of B2AR recycling to the actin-mediated recycling pathway, lat-A significantly reduced the rate of B2AR recycling. Importantly, however, actin depolymerization had no effect on the rate of B2ARS>A recycling (Movie S6), much like TfR (Fig. 3F). Together with Fig. 2G, these results indicate that surface recycling of B2ARS>A is independent of actin.
The actin-independence of B2ARS>A suggests that PKA-mediated phosphorylation is required to restrict B2AR to the sequence-dependent recycling pathway. A key prediction of this model is that the B2ARS>A would recycle independent of the PDZ ligand sequence. To test this prediction, we generated a double mutant (B2ARalaS > A) in which both the PKA phosphorylation sites and the PDZ ligand are mutated and compared its recycling to a mutant in which just the PDZ ligand is disrupted (B2ARala). Strikingly, B2ARalaS > A recycled robustly, comparable to the WT B2AR, in contrast to the poorly recycling B2ARala (Fig. 3G and Fig. S3A). Further, B2ARalaS > A readily entered endosomal tubues that were devoid of actin (Fig. 3 H and I), suggesting that the S > A mutation rescues recycling of the B2ARala by allowing entry into bulk recycling tubules. Interestingly, the net recycling of this mutant was comparable to that of B2AR, because it lacks a PDZ ligand and is not concentrated in endosomal tubules (Fig. S3 B–D). Taken together, our results indicate that PKA-mediated phosphorylation restricts B2AR recycling to the PDZ-dependent recycling pathway and support a two-step hierarchical model for B2AR endosomal sorting, as discussed below (Fig. S4).
What is the significance of restricting B2AR recycling to the PDZ-dependent pathway? To explore this question, we first measured the effect of B2AR phosphorylation on total surface receptor levels. Because SpH is fluorescent only when exposed to the extracellular surface and not in the interior of endosomes (20), we monitored ensemble SpH-B2AR fluorescence in live cells over time. This provided a highly time-resolved assay for measuring surface B2AR in the same set of cells in response to agonist and washout (Fig. 4A). Iso induced an exponential decrease in surface B2AR fluorescence, as predicted with agonist-mediated B2AR internalization (Fig. 4B). The levels reached a plateau after ∼10 min, indicating equilibration of internalization and recycling and steady-state partitioning of receptors between endosomes and the surface (Movie S7) (30, 31). Inhibition of PKA at this stage by the addition of KT led to an increase in B2AR fluorescence, indicating increased surface B2AR (Fig. 4B). Given that PKA inhibition has no effect on B2AR internalization (24–27, 32), this increase in surface B2AR on PKA inhibition likely reflects an increase in B2AR recycling rates from steady state (Fig. 3D).
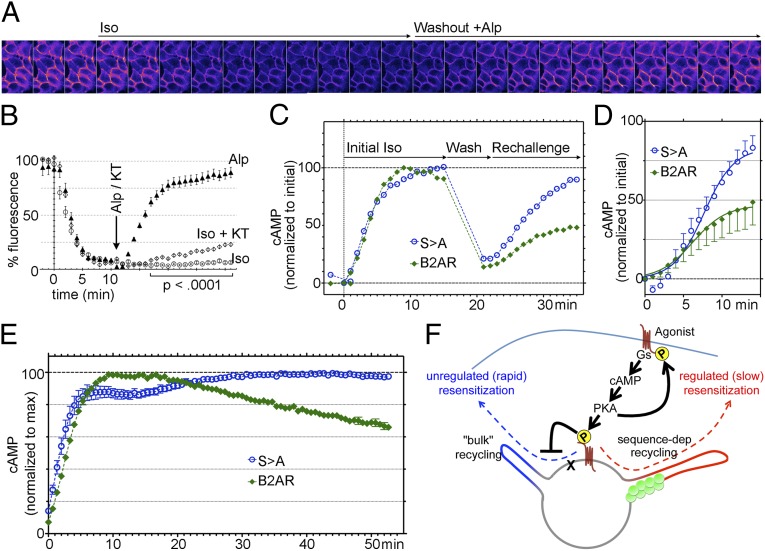
Fig. 4.
Phosphorylation regulates B2AR resensitization. (A) Frames from a movie of cells expressing SpH-B2AR, imaged 1 min apart, showing rapid loss of surface B2AR fluorescence in response to iso and recovery in response to washout with alprenolol. (B) Quantitation of fluorescence changes normalized to initial values from multiple cells (n > 500 in each case). (C) cAMP levels measured in B2AR- or B2ARS>A-expressing cells in response to an initial iso challenge for 15 min, washout for 5 min, and a rechallenge to estimate the extent of recycling and resensitization. Example traces from one experiment, both normalized to the maximum of the initial iso. (D) cAMP responses for the rechallenge, normalized to the maximum of the initial iso across multiple experiments (n = 9), with curve fits (R2 = 0.98 and 0.99). (E) cAMP response to persistent iso in B2AR- or B2ARS>A-expressing cells. (F) Model for homeostatic regulation of B2AR recycling. PKA-mediated phosphorylation of B2AR in response to agonist prevents entry of B2AR into bulk recycling tubules and ensures PDZ-dependent recycling of B2AR.
To determine the functional effect of this change in recycling to adrenergic signaling, we measured the rate of resensitization of cells to adrenergic signaling through detection of cAMP levels in living cells in response to adrenergic stimulation, using a luciferase-based reporter. Cells expressing B2AR or B2ARS>A were exposed to iso for 15 min to allow for steady-state partitioning (30, 31) and signaling. Agonist was then washed out for 5 min to allow for receptor recycling to the surface. The rate of resensitization, which depends on the receptors recycled during this period (18), was determined as the increase in cAMP levels in response to a second challenge with iso. In contrast to the cells expressing B2AR, which recovered to only ∼46% of the initial signal, B2ARS>A recovered to ∼83% over the same time frame (Fig. 4 C and D). Furthermore, in contrast to WT B2AR, cAMP levels in B2ARS>A-expressing cells did not decrease significantly in response to persistent adrenergic signaling, suggesting increased recycling at steady state (Fig. 4). Taken together, the results of these experiments indicate that PKA regulates the rate of resensitization of cells to adrenergic signaling by controlling the rate of B2AR recycling.
Discussion
How proteins are sorted in the endosome into functionally distinct pathways is a fundamental question in cell biology. This study demonstrates that a single cargo molecule can be sorted into biochemically, functionally, and physically distinct pathways in the endosome based on specific modifications, providing key support for a hierarchical model for endosomal sorting (Fig. S4). As an initial sorting step, restricted cargo mobility on the endosome, combined with the rapid formation and fission of bulk recycling tubules, acts as a “kinetic” retention mechanism for keeping cargo out of the bulk pathway. For B2AR, PKA-mediated phosphorylation of B2AR at Ser-345/346 is responsible for this retention (Fig. 2I), and absence of this phosphorylation makes B2AR recycling independent of its recycling sequence (Fig. 3 G–I). A downstream sorting step, based on affinity interactions of sequence elements (e.g., a PDZ domain), then sorts cargo into physically distinct domains that mediate sequence-dependent recycling or degradation (17).
Our results also suggest a physiological basis for why B2AR recycling is subject to multiple requirements, even though other proteins apparently can recycle without specific requirements (12, 13). PKA-mediated phosphorylation of B2AR on Ser-345/346 in response to adrenergic signaling (2, 23, 24) (Fig. 2 A–C) regulates the first sorting step (Fig. S4 and Fig. 4F). The second step might be regulated by GPCR kinase (GRK)-mediated phosphorylation of Ser-411, a residue in the PDZ ligand that is required for B2AR recycling (11, 33). Because the rate and requirements of recycling through these pathways are distinct (Figs. 3G and 4B) (1, 15, 20), these multiple requirements potentially provide control points for cells to modulate the rate of resensitization to adrenergic signaling (Fig. 4 C–E).
Both of the foregoing downstream phosphorylation steps provide homeostatic negative feedback to adrenergic signaling, by keeping receptors out of the bulk recycling pathway (PKA) or by slowing sequence-dependent recycling by impairing PDZ interactions (GRK) (11). This action is relevant during persistent sympathetic activation, such as in the heart, where rapid B2AR recycling might be disadvantageous for normal signal attenuation (Fig. 4E). In acute activation, such as in neurotransmitter signaling, a rapid decrease in agonist triggers bulk recycling and quick recovery of sensitization. Thus, such combinatorial phosphorylation can ensure a normal signaling profile by rapidly reprogramming the trafficking itinerary of B2AR in different physiological settings.
Interestingly, recent evidence suggests that B2AR, in addition to early signaling at the cell surface, can initiate a second wave of G protein signaling from the endosome (34). Considered together with our findings in the present study, this suggests the exciting possibility that signaling-mediated sorting of GPCRs into fate-specific endosomal microdomains, in addition to changing trafficking rates, also might direct receptors to specific signaling complexes that are spatially segregated and activated on the endosome. In this case, PKA phosphorylation of B2AR not only might change the rate and extent of signaling, but also might generate different signatures of downstream signals depending on the complexes localized to these domains.
Such kinase-mediated switching of receptor sorting might represent a general principle for the rapid plasticity of signaling pathways. Members of the GPCR family are heavily phosphorylated by multiple kinases, including PKA and GRKs, and PDZ-dependent recycling has been recognized by several groups working with various GPCRs (3, 35). Interestingly, recruitment of PKA to B2AR and the beta-1 adrenergic receptor (B1AR) by the A-kinase anchoring protein (AKAP) family of scaffolds has been reported to enhance the resensitization of these receptors (36–39), although the mechanisms underlying this are unclear. B1AR and B2AR have been reported to have different trafficking itineraries and biochemical interactions, including with PDZ proteins (40–42). In our experiments, B1AR was sorted into endosomal tubules marked by coronin, and PKA inhibition did not reduce the entry of B1AR into tubules and resulted in slightly increased B1AR recycling in a quantitative assay (Fig. S5). This finding is consistent with our previous observations that a PDZ and actin association is sufficient for targeting GPCRs to endosomal recycling microdomains (15). It is possible that scaffolding of larger complexes by AKAP plays a role in resensitizing receptors through convergent mechanisms, such as deubiquitination or conformational changes, or by facilitating PDZ interactions on the endosome. In addition, PKA can phosphorylate many components of the vesicle trafficking machinery (43, 44). Nevertheless, direct modification of the cargo provides a way to selectively control the trafficking of physiologically relevant proteins like signaling receptors without modifying trafficking at a global level.
By restricting cargo to the actin-dependent pathway, this kinase switch also provides a potential mode for signaling cross-talk to change the sensitivity to signals. Given that actin assembly on endosomes is highly regulated by a specialized set of proteins, including Arp2/3 and WASH (15, 45, 46), this provides an additional point of control for regulation of adrenergic recycling by other signaling pathways. In turn, dephosphorylation of B2AR by switching receptors out of this actin-dependent pathway may generate receptors that are insensitive to heterologous control by external signaling. Further analysis of other GPCRs that use the sequence-dependent recycling pathway and the kinases that modify them, with advanced imaging techniques as used in the present study, will refine our understanding of the mechanisms and significance of reprogramming of receptor trafficking in the context of homologous and heterologous regulation.
Materials and Methods
Reagents.
Cell culture, constructs, and pharmacologic reagents have been described previously (15) and are discussed in detail in SI Materials and Methods.
Imaging.
Live cell imaging was performed as described previously (15) and as detailed in SI Materials and Methods. Images acquired directly from the camera without adjustments were analyzed with ImageJ. Quantitation was performed double-blinded with scrambled file names. Simple statistical analyses were done in Microsoft Excel, and curve fits were done using GraphPad Prism. In vitro phosphorylation and signaling assays are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank D. Shiwarski, A. Soohoo, C. Almaguer, and S. Bowersox for essential technical help and comments, and Drs. S. Mukhopadhyay, A. Linstedt, T. Lee, V. Faundez, M. von Zastrow, A. Hanyaloglu, P. Friedman, G. Romero, A. Bisello, J.-P. Vilardaga, A. Sorkin, and D. Drubin for reagents, comments, and helpful discussions. R.V. was supported by National Institutes of Health/National Institute on Drug Abuse Grant T90-DA023420, and M.A.P. was supported by National Institutes of Health Grant R00-DA024698.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1306340110/-/DCSupplemental.
References
- 1.Hanyaloglu AC, McCullagh E, von Zastrow M. Essential role of Hrs in a recycling mechanism mediating functional resensitization of cell signaling. EMBO J. 2005;24(13):2265–2283. doi: 10.1038/sj.emboj.7600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lefkowitz RJ, Pitcher J, Krueger K, Daaka Y. Mechanisms of beta-adrenergic receptor desensitization and resensitization. Adv Pharmacol. 1998;42:416–420. doi: 10.1016/s1054-3589(08)60777-2. [DOI] [PubMed] [Google Scholar]
- 3.Magalhaes AC, Dunn H, Ferguson SS. Regulation of GPCR activity, trafficking and localization by GPCR-interacting proteins. Br J Pharmacol. 2012;165(6):1717–1736. doi: 10.1111/j.1476-5381.2011.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drake MT, Shenoy SK, Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circ Res. 2006;99(6):570–582. doi: 10.1161/01.RES.0000242563.47507.ce. [DOI] [PubMed] [Google Scholar]
- 5.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3(9):639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 6.Marchese A, Paing MM, Temple BR, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol. 2008;48:601–629. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonnord P, Blouin CM, Lamaze C. Membrane trafficking and signaling: Two sides of the same coin. Semin Cell Dev Biol. 2012;23(2):154–164. doi: 10.1016/j.semcdb.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Kurz JB, Perkins JP. Isoproterenol-initiated beta-adrenergic receptor diacytosis in cultured cells. Mol Pharmacol. 1992;41(2):375–381. [PubMed] [Google Scholar]
- 9.Pippig S, Andexinger S, Lohse MJ. Sequestration and recycling of beta 2-adrenergic receptors permit receptor resensitization. Mol Pharmacol. 1995;47(4):666–676. [PubMed] [Google Scholar]
- 10.Seachrist JL, Anborgh PH, Ferguson SS. Beta 2-adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases. J Biol Chem. 2000;275(35):27221–27228. doi: 10.1074/jbc.M003657200. [DOI] [PubMed] [Google Scholar]
- 11.Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the β2-adrenergic receptor. Nature. 1999;401(6750):286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- 12.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5(2):121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 13.Marsh EW, Leopold PL, Jones NL, Maxfield FR. Oligomerized transferrin receptors are selectively retained by a lumenal sorting signal in a long-lived endocytic recycling compartment. J Cell Biol. 1995;129(6):1509–1522. doi: 10.1083/jcb.129.6.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lauffer BE, et al. SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J Cell Biol. 2010;190(4):565–574. doi: 10.1083/jcb.201004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puthenveedu MA, et al. Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell. 2010;143(5):761–773. doi: 10.1016/j.cell.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Temkin P, et al. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol. 2011;13(6):715–721. doi: 10.1038/ncb2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanyaloglu AC, von Zastrow M. A novel sorting sequence in the beta2-adrenergic receptor switches recycling from default to the Hrs-dependent mechanism. J Biol Chem. 2007;282(5):3095–3104. doi: 10.1074/jbc.M605398200. [DOI] [PubMed] [Google Scholar]
- 18.Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- 19.Sorkin A, von Zastrow M. Endocytosis and signalling: Intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10(9):609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yudowski GA, Puthenveedu MA, Henry AG, von Zastrow M. Cargo-mediated regulation of a rapid Rab4-dependent recycling pathway. Mol Biol Cell. 2009;20(11):2774–2784. doi: 10.1091/mbc.E08-08-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore RH, et al. Ligand-stimulated beta 2-adrenergic receptor internalization via the constitutive endocytic pathway into rab5-containing endosomes. J Cell Sci. 1995;108(Pt 9):2983–2991. doi: 10.1242/jcs.108.9.2983. [DOI] [PubMed] [Google Scholar]
- 22.Riedl J, et al. Lifeact: A versatile marker to visualize F-actin. Nat Methods. 2008;5(7):605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trester-Zedlitz M, Burlingame A, Kobilka B, von Zastrow M. Mass spectrometric analysis of agonist effects on posttranslational modifications of the beta-2 adrenoceptor in mammalian cells. Biochemistry. 2005;44(16):6133–6143. doi: 10.1021/bi0475469. [DOI] [PubMed] [Google Scholar]
- 24.Hausdorff WP, et al. Phosphorylation sites on two domains of the beta 2-adrenergic receptor are involved in distinct pathways of receptor desensitization. J Biol Chem. 1989;264(21):12657–12665. [PubMed] [Google Scholar]
- 25.Iyer V, et al. Differential phosphorylation and dephosphorylation of beta2-adrenoceptor sites Ser262 and Ser355,356. Br J Pharmacol. 2006;147(3):249–259. doi: 10.1038/sj.bjp.0706551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seibold A, et al. Localization of the sites mediating desensitization of the beta(2)-adrenergic receptor by the GRK pathway. Mol Pharmacol. 2000;58(5):1162–1173. doi: 10.1124/mol.58.5.1162. [DOI] [PubMed] [Google Scholar]
- 27.Hausdorff WP, et al. A small region of the beta-adrenergic receptor is selectively involved in its rapid regulation. Proc Natl Acad Sci USA. 1991;88(8):2979–2983. doi: 10.1073/pnas.88.8.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394(6689):192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 29.Puthenveedu MA, von Zastrow M. Cargo regulates clathrin-coated pit dynamics. Cell. 2006;127(1):113–124. doi: 10.1016/j.cell.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 30.Morrison KJ, et al. Repetitive endocytosis and recycling of the beta 2-adrenergic receptor during agonist-induced steady state redistribution. Mol Pharmacol. 1996;50(3):692–699. [PubMed] [Google Scholar]
- 31.von Zastrow M, Kobilka BK. Ligand-regulated internalization and recycling of human beta 2-adrenergic receptors between the plasma membrane and endosomes containing transferrin receptors. J Biol Chem. 1992;267(5):3530–3538. [PubMed] [Google Scholar]
- 32.Bouvier M, Guilbault N, Bonin H. Phorbol ester-induced phosphorylation of the beta 2-adrenergic receptor decreases its coupling to Gs. FEBS Lett. 1991;279(2):243–248. doi: 10.1016/0014-5793(91)80159-z. [DOI] [PubMed] [Google Scholar]
- 33.Fredericks ZL, Pitcher JA, Lefkowitz RJ. Identification of the G protein-coupled receptor kinase phosphorylation sites in the human beta2-adrenergic receptor. J Biol Chem. 1996;271(23):13796–13803. doi: 10.1074/jbc.271.23.13796. [DOI] [PubMed] [Google Scholar]
- 34.Irannejad R, et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495(7442):534–538. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero G, von Zastrow M, Friedman PA. Role of PDZ proteins in regulating trafficking, signaling, and function of GPCRs: Means, motif, and opportunity. Adv Pharmacol. 2011;62:279–314. doi: 10.1016/B978-0-12-385952-5.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gardner LA, Delos Santos NM, Matta SG, Whitt MA, Bahouth SW. Role of the cyclic AMP-dependent protein kinase in homologous resensitization of the beta1-adrenergic receptor. J Biol Chem. 2004;279(20):21135–21143. doi: 10.1074/jbc.M313652200. [DOI] [PubMed] [Google Scholar]
- 37.Gardner LA, Tavalin SJ, Goehring AS, Scott JD, Bahouth SW. AKAP79-mediated targeting of the cyclic AMP-dependent protein kinase to the beta1-adrenergic receptor promotes recycling and functional resensitization of the receptor. J Biol Chem. 2006;281(44):33537–33553. doi: 10.1074/jbc.M601809200. [DOI] [PubMed] [Google Scholar]
- 38.Shih M, Lin F, Scott JD, Wang HY, Malbon CC. Dynamic complexes of beta2-adrenergic receptors with protein kinases and phosphatases and the role of gravin. J Biol Chem. 1999;274(3):1588–1595. doi: 10.1074/jbc.274.3.1588. [DOI] [PubMed] [Google Scholar]
- 39.Ritter SL, Hall RA. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat Rev Mol Cell Biol. 2009;10(12):819–830. doi: 10.1038/nrm2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gavi S, Yin D, Shumay E, Wang HY, Malbon CC. The 15-amino acid motif of the C terminus of the beta2-adrenergic receptor is sufficient to confer insulin-stimulated counterregulation to the beta1-adrenergic receptor. Endocrinology. 2005;146(1):450–457. doi: 10.1210/en.2004-0595. [DOI] [PubMed] [Google Scholar]
- 41.Liang W, Curran PK, Hoang Q, Moreland RT, Fishman PH. Differences in endosomal targeting of human (beta)1- and (beta)2-adrenergic receptors following clathrin-mediated endocytosis. J Cell Sci. 2004;117(Pt 5):723–734. doi: 10.1242/jcs.00878. [DOI] [PubMed] [Google Scholar]
- 42.Valentine CD, Haggie PM. Confinement of β(1)- and β(2)-adrenergic receptors in the plasma membrane of cardiomyocyte-like H9c2 cells is mediated by selective interactions with PDZ domain and A-kinase anchoring proteins but not caveolae. Mol Biol Cell. 2011;22(16):2970–2982. doi: 10.1091/mbc.E11-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baba T, Sakisaka T, Mochida S, Takai Y. PKA-catalyzed phosphorylation of tomosyn and its implication in Ca2+-dependent exocytosis of neurotransmitter. J Cell Biol. 2005;170(7):1113–1125. doi: 10.1083/jcb.200504055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirling H, Scheller RH. Phosphorylation of synaptic vesicle proteins: Modulation of the alpha SNAP interaction with the core complex. Proc Natl Acad Sci USA. 1996;93(21):11945–11949. doi: 10.1073/pnas.93.21.11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Derivery E, et al. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev Cell. 2009;17(5):712–723. doi: 10.1016/j.devcel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 46.Gomez TS, Billadeau DD. A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev Cell. 2009;17(5):699–711. doi: 10.1016/j.devcel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.