Abstract
Objectives
This study aims to identify a relationship between bone mineral density (BMD) of lumbar spine, and the weight and body mass index (BMI) in women.
Methods
The subjects were 1,143 females who visited the public health center. BMD (T-score), height and weight were measured and age, menopause, diabetes and hypertension, exercising status and smoking status were inquired by interview.
Results
Among the subjects, 362 (31.7%) were in the normal group and 781 (68.3%) were in the abnormal group. As the result of the logistic regression analysis with BMI (Model I), the odds ratio of getting into the abnormal BMD group as age increases by 1 year marked 1.044 (95% CI = 1.009-1.080). The odds ratio of getting into the abnormal BMD group due to menopause was 2.663 (1.516-4.679) and the odds ratio according to lack of walking exercise was 2.597 (1.878-3.591). The odds ratio with 1 kg/m2 of BMI increase was 0.909 (0.862-0.959). In the logistic regression analysis with weight (Model II), the odds ratio of getting into the abnormal BMD group as age increases by 1 year marked 1.044 (1.009-1.080). The odds ratio of getting into the abnormal bone density group due to menopause was 2.575 (1.472-4.507) and the odds ratio according to lack of walking exercise was 2.598 (1.881-3.587). The odds ratio with 1 kg of weight increase was 0.963 (0.942-0.984). The Akaike's information criterion (AIC) values of Model I and Model II were 1196.18 and 1197.14 respectively, indicating Model I has the better compatibility of regression analysis model.
Conclusion
Weight, BMI and BMD had a positive correlation. However, the coefficient of correlation between weight and BMD was higher than the coefficient between BMI and BMD, which means low weight is much more likely to be related to osteoporosis with no other factor considered. On the other hand, under the condition considering age, height, menopause and walking exercise smoking status, low BMI is much more compatible as a risk factor for osteoporosis.
Keywords: Body mass index, Bone mineral density, Osteoporosis, Weight
INTRODUCTION
Osteoporosis is the most common disease among metalic bone diseases; it weakens bone mass by destructing the microstructures of bones thereby increasing the risk of fractures.[1] As getting older, women lose 30-50% of trabecular bone mass and 25-30% of cortical bone mass and especially lose the largest volume of bone mass in pre- and postmenopause.[2]
Since osteoporosis gains more importance as elderly population increases, it becomes a social issue due to the financial burden as well as a main interest of modern medicine.[3] Approximately 2 million cases of fractures including femur fractures, spine fractures, and wrist fractures caused by osteoporosis are occurred annually in the US.[4] In 2005, the direct medical cost of osteoporosis was evaluated to be 137-203 billion dollars in the US. Until 2,025, more than 3 million cases of fractures are anticipated to be occurred so that 253 billion dollars of medical costs are expected to be consumed annually.[3]
The clinical importance of osteoporosis is a fracture occurrence and approximately more than a half of Caucasian women experience osteoporotic fractures in more than one body part if osteoporosis is not treated.[5] Femur fractures are the most severe thereby high in mortality rate, spine fractures are the most commonly happened, and wrist fractures become complications that the frequency increases rapidly in pre- and postmenopause.[6] First of all, in clinical trials, it is important to find the patients with osteoporosis and the risk groups which osteoporosis may occur. Possible risk factors of osteoporosis are height, weight, body mass index (BMI), a medical history, a family history at mother's side, smoking, an alcohol, and an exercise etc; these are widely analyzed.[7-12]
National Osteoporosis Foundation in the US determined the main risk factors of osteoporotic fractures; of the risk factors, there are correctable factors and uncorrectable factors and the risk of fractures increases as the risk factors increase. A fracture history in the past, a family history of factures, smoking, and low body weight (≤ 57.8 kg) are known as the main factors determining the risk of femur fractures.[4]
Also, European Osteoporosis Foundation reported that the risk of fractures increased approximately 30% in the same age group in case of possessing more than 2 risk factors among the clinical risk factors and performing the bone mineral density (BMD) examination considering the risk factors was an excellent screening examination in a clinical perspective.[11] The objectives of the study were to investigate which factors were more useful as the risk factors of osteoporosis among the risk factors of osteoporosis occurrences by reviewing the risk factors of osteoporosis occurrences that was found and discussed previously; of the risk factors, especially, to examine which was more useful as a risk factor for osteoporosis of body weight and BMI in women.
SUBJECTS AND METHODS
1. Subjects
The study was performed in subjects who visited a public health center in Seoul to have a BMD examination from May 2010 to March 2011 and the data was collected regarding as follows; BMI, body weight, height, and a BMD were measured and whether or not the subjects had hypertensions, diabetes, menopause, walking exercises, and smoking were examined by interviews. Since women with osteoporosis were the main interest of the study, men were excluded and the subjects who had female hormones in the past or currently, had steroids for a long time, had a thyroid disease or thyroid hormones, and had other osteoporosis treatments or currently took the medications for osteoporosis were excluded as well. Due to hysterectomy in the past, the cases where the exact age at the time of menopause was not able to be found were also excluded from the data.
2. Study variables
T-score, a BMD, was used; when T-score was more than -1 and less than -1, it was defined as a normal BMD and an abnormal BMD, respectively, and these were used as dependent variables in the study. Age, height, body weight, BMI, duration of diabetes, duration of hypertensions, a menopausal status, duration after menopause, the age of menopause, a smoking status, and a walking exercise status were determined as independent variables that could influence on the normal and abnormal BMDs.
Smoking was defined based upon a current smoking status by interviews and a walking exercise was defined in cases of performing a walking exercise at least 30 minutes per day for five days a week.
In present study, T-score was measured utilizing dual energy X-ray absorptionmetry (DXA) and the average scores of the BMDs in anterior and posterior lumbar of lumbar L1-L4. Lunar DPX Bravo® from GE Inc., was used in a BMD measurement.
3. Analysis methods
T-test and Chi-square test were carried out in order to examine the relationship between normal and abnormal T-scores and age, height, body weight, BMI, a hypertension status, a diabetes status, a walking exercise status, a smoking status, and a menopausal status. Logistic regression analysis was performed to analyze the relationship between normal and abnormal BMD groups and the independent variables including age, height, body weight, BMI, a hypertension status, a diabetes status, a walking exercise status, a smoking status, and a menopausal status. Statistical analysis was performed utilizing SAS 9.2 software (SAS Institute Inc., Cary, NC, USA).
RESULTS
1. General characteristics of the subjects
Total 1,143 subjects were participated in the study; the average age was 62.0 ± 9.7, the average BMI was 23.8 ± 2.9 kg/m2, 1,035 subjects were postmenopausal (90.6%), the average age at the time of menopause was 49.5 ± 4.7, 266 subjects were performed the walking exercises (23.2%), 128 subjects were smokers (11.2%), and 362 subjects were in the normal BMD group (31.7%) (Table 1).
Table 1.
General characteristics of study subjects
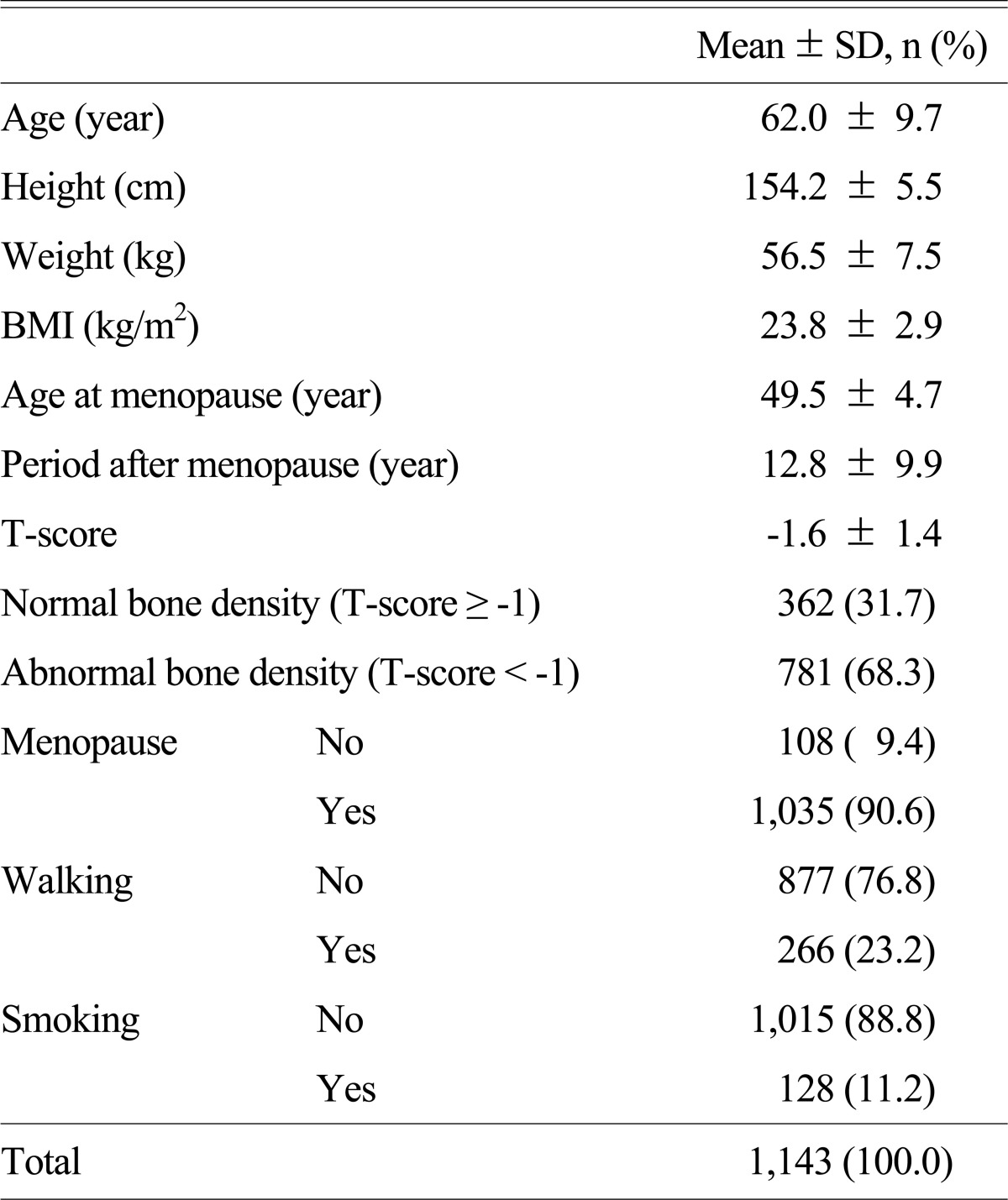
BMI, body mass index
2. The relationship of a BMD and general characteristics
The average age of the normal BMD group and the abnormal BMD group was 56.7 and 64.5, respectively, and there was a statistically significant difference between them (P < .001). The average height of the normal BMD group and the abnormal BMD group was 155.9 cm and 153.4 cm, respectively, also there was a statistically significant difference (P < .001). The average weight of the normal bone mineral density group and the abnormal BMD group was 58.0 kg and 55.8 kg, respectively, and they were significantly different as well (P < .001) (Table 2).
Table 2.
Comparisons between normal and abnormal bone density
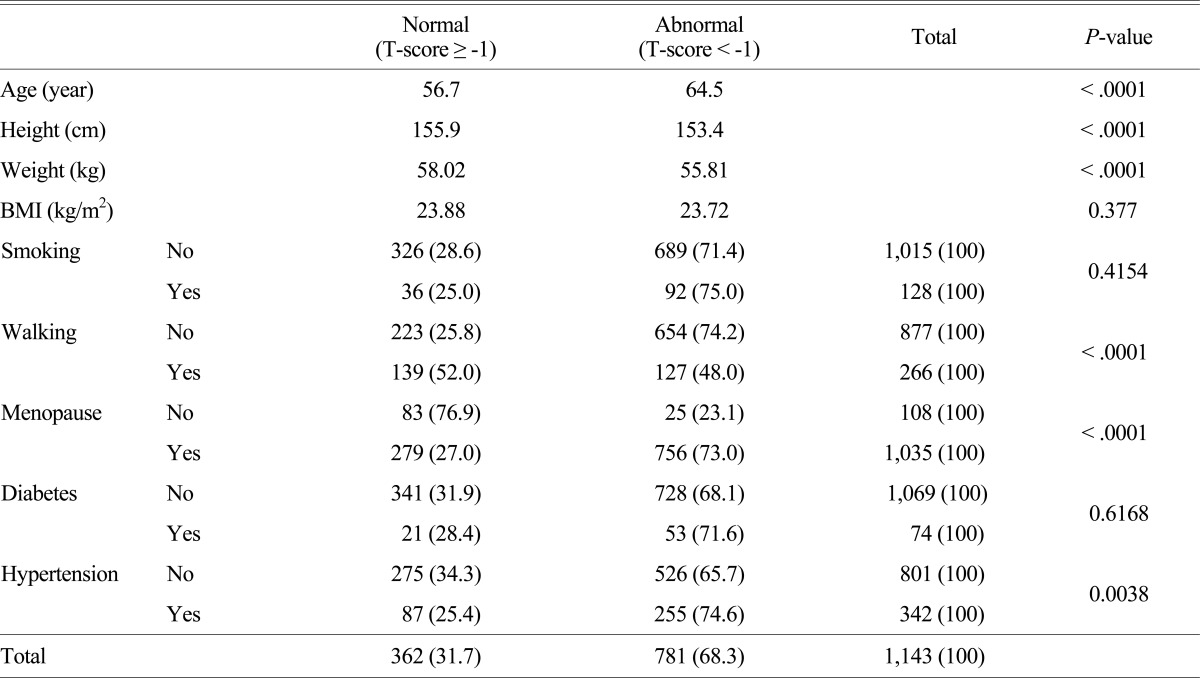
BMI, body mass index
However, the average BMI of the normal BMD group and the abnormal BMD group was 23.9 kg/m2 and 23.7 kg/m2, respectively, but there was no significant difference even though the BMI was higher in the normal BMD (P = 0.38). The subjects with an abnormal BMD were 71.4% in the non-smoking group and 75% in the smoking group but there was no statistically significant difference although the probability having an abnormal BMD was higher in the smoking group than that of in the non-smoking group (P = 0.415). The subjects with an abnormal BMD were 48.0% in the walking exercise group and 74.2% in the non-walking exercise group; this represented that the subjects who didn't have walking exercises had higher probability to have abnormal BMDs and the difference was statistically significant (P < .001). The subjects with an abnormal BMD were 23.1% in the premenopausal group and 73.0% in the postmenopausal group; this indicated that the probability having an abnormal BMD was higher in the postmenopausal group and the difference was statistically significant.
The subjects who had an abnormal BMD in the non-diabetes group and the diabetes group were 68.1% and 71.6%, respectively; this represented that the probability having an abnormal BMD was higher in the diabetes group but the difference was not statistically significant. The subjects who had an abnormal BMD in the non-hypertension group and the hypertension group were 65.7% and 74.6%, respectively; this indicated that the probability having an abnormal BMD was higher in the hypertension group and there was a statistically significant difference.
As the results of correlation, age, history of hypertension (HTNHx), period after menopause (PostMENO), smoking (SMK), exercise (EXE) were negative correlated, but Height, weight, BMI were positive correlated (Table 3).
Table 3.
Correlation between T-score and other variables
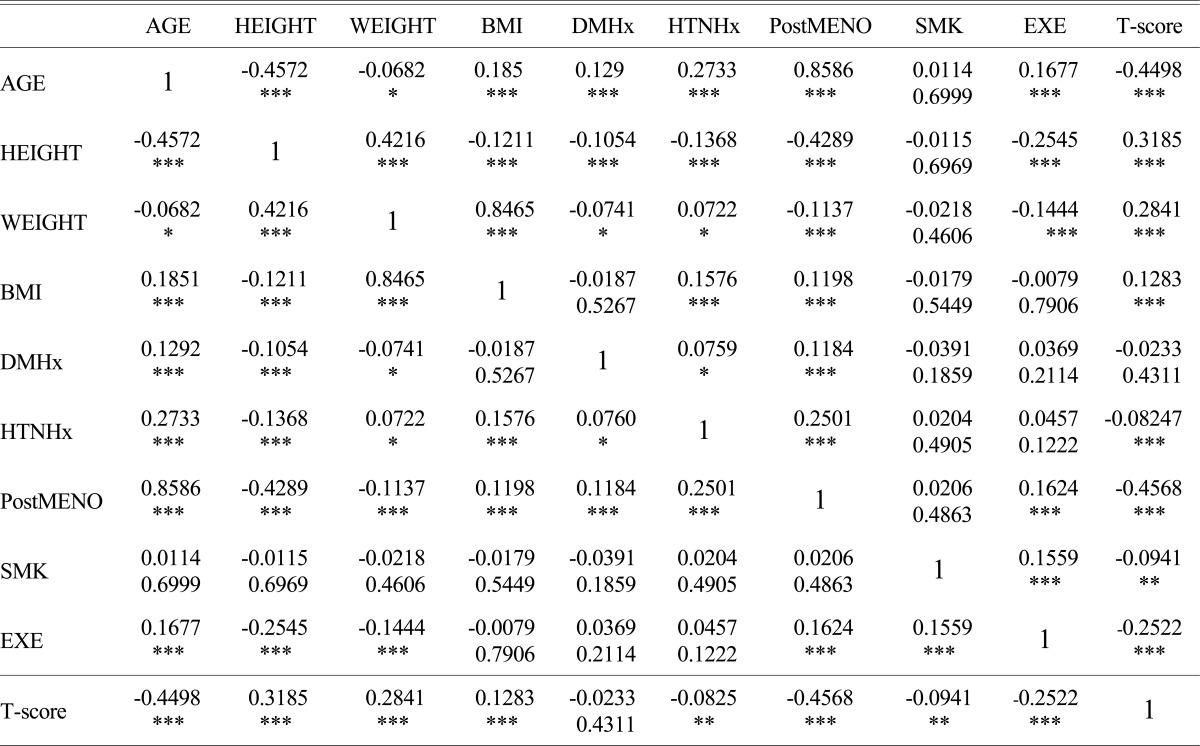
*P < 0.05, **P < 0.01, ***P < 0.001
BMI, body mass index; DMHx, histroy of diabetes; HTNHx, history of hypertension; PostMENO, period after menopause; SMK, smoking; EXE, exercise
3. Logistic regression analysis results between normal and abnormal BMD groups
We investigated how an independent variable, which could be a risk factor in the normal and abnormal BMD groups including age, height, body weight, BMI, a menopausal status, a diabetes status, a hypertension status, a walking exercise status, and a smoking status, affects on the BMD. When multicollinearity was examined among several independent variables, variance inflation factor (VIF) between body weight and BMI was more than 100, indicating high VIF and VIF between the other variables was less than 10, indicating low VIF. Since BMI and body weight were the main variables in the relationship with a BMD, it was classified into Model I, focused on BMI, and Model II, focused on body weight, and then logistic regression analysis was performed including the other independent variables. The appropriateness of Model I and Model II was compared by Akaike's information criterion (AIC) values.
In Model I (BMI), the odd ratio of the relationship with the BMD depending upon age was 1.044 (95% CI = 1.009-1.080), indicating that the risk of an abnormal BMD increased by 5.6% as 1 year older when other variables were controlled. The odd ratio of the relationship with the BMD depending upon a walking exercise status was 2.597 (95% CI = 1.878-3.591), representing that the risk of an abnormal BMD increased by 2.6 times when the walking exercise was not performed. The odd ratio of the relationship with the BMD depending upon a menopause status was 2.663 (95% CI = 1.516-4.679) and this represented that the risk of an abnormal BMD increased by 2.7 times in case of the menopause group. The odd ratio of the relationship between BMI and a BMD, the main variables of interest, was 0.909 (95% CI = 0.862-0.959), indicating that the risk of an abnormal BMD decreased by 9% as BMI increased by 1 kg/m2 (Table 4).
Table 4.
The results of logistic regression for normal and abnormal bone density
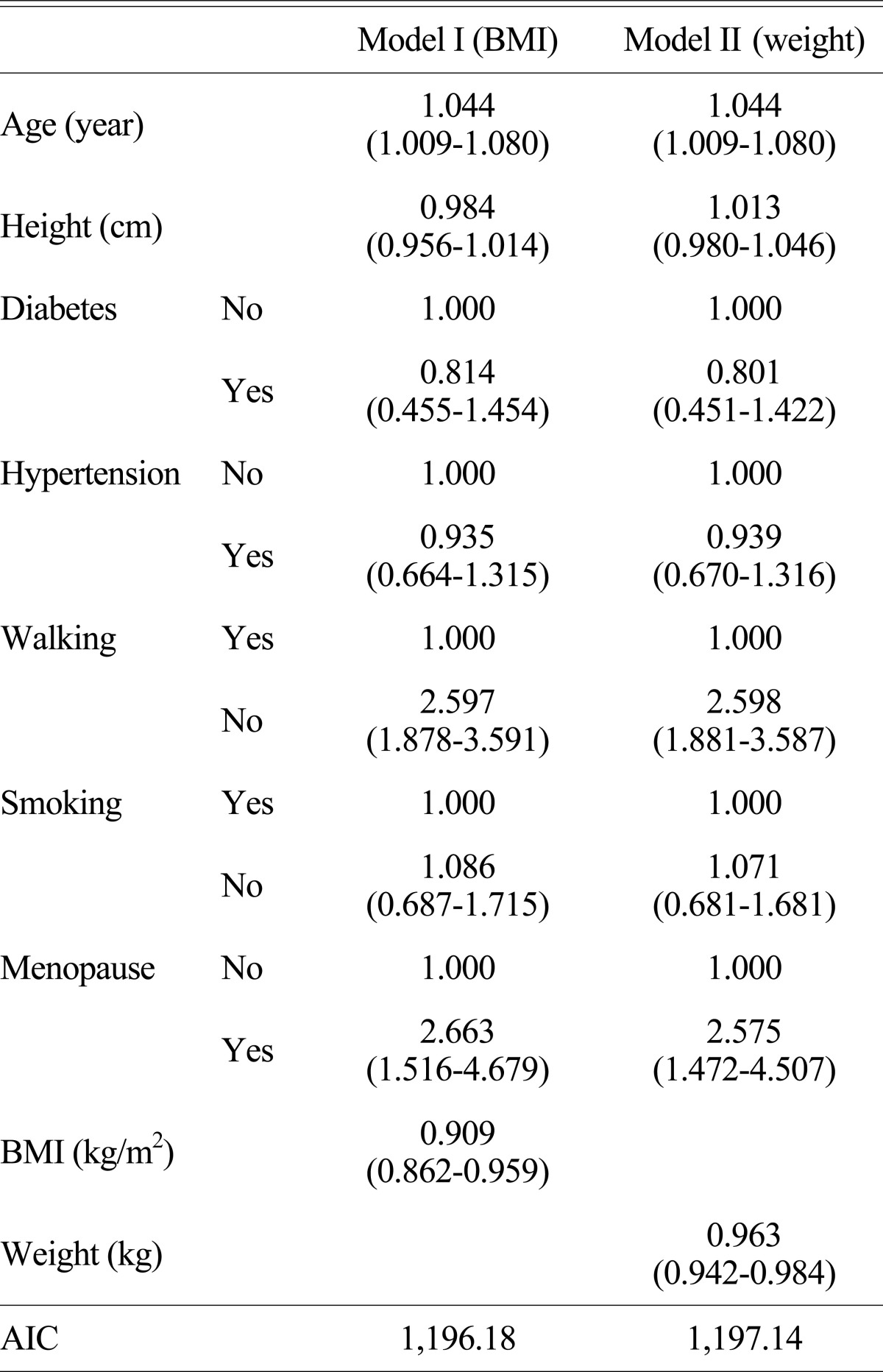
( ), 95% confidence interval
BMI, body mass index; AIC, Akaike's information criterion
In Model II (body weight), the odd ratios of the relationship with the BMD depending upon age, a walking exercise status, and a menopause status were 1.044 (95% CI = 1.009-1.080), 2.598 (95% CI = 1.881-3.587), and 2.575 (95% CI = 1.472-4.507), respectively. The odd ratio of the relationship between BMI and a BMD, the main variables of interest, was 0.963 (95% CI = 0.942-0.984), indicating that the risk of an abnormal BMD decreased by 3.7% as body weight increased by 1 kg.
AIC values in Model I (BMI) and Model II (body weight) were 1,196.18 and 1,197.14, respectively, so the Model I had lower AIC value than that of Model II, indicating that Model I (BMI) was more appropriate than Model II (body weight). In other words, BMI would be more appropriate as the risk factor of an abnormal BMD when considering other variables such as age, height, smoking, walking exercise, menopause, diabetes, hypertension.
DISCUSSION
In the relationship between smoking, a walking exercise, and a menopause status and a BMD, the subjects with an abnormal BMD was 71.4% in the non-smoking group and 75.0% in the smoking group; the probability having the abnormal BMD was higher in the smoking group but the difference was not statistically significant with P-value 0.415. Higher proportion of subjects who had an abnormal BMD was shown in the non-walking exercise group compared to the walking exercise group and there was significant difference. Compared to the non-menopause group, the menopause group had higher proportion of the subjects with an abnormal BMD and the difference was statistically significant as well. This confirmed what was suggested previously; menopause, low physical activities, or non-physical activities were the risk factors of osteoporosis. This recommends that regular exercises are required in the prevention of osteoporosis and women, especially, should pay more attentions during the postmenopausal period; osteoporosis examination should be performed on a regular basis and more attentions are required in order to prevent osteoporosis during the postmenopausal period.
As a result of Chi-square test analysis when other variables were not controlled, higher proportion of the subjects who had an abnormal BMD was observed in the non-diabetes group compared to the diabetes group however the difference was not statistically significant. In contrast, the hypertension group had higher proportion of the subjects with an abnormal BMD compared to the non-hypertension group and there was a statistically significant difference.
On the other hand, there was no statistically significant difference in the relationship between hypertensions and diabetes and a BMD in the result of logistic regression analysis as the other variables were controlled. Hypertension is associated with low BMD but there are no studies suggesting that it is the risk factor of osteoporosis. The average BMI in the normal BMD group and the abnormal BMD group was 23.9 kg/m2 and 23.7 kg/m2, respectively; it was lower in the abnormal BMD group but t-test analysis showed that there was no significant difference (P = 0.38). However, the result of logistic regression analysis represented that the relationship of BMI and a BMD in Model I (BMI) was statistically significant when age, height, a diabetes status, a hypertension status, a walking exercise practice status, a smoking status, and a menopause status were controlled.
Height, body weight, and BMI were all positively correlated with dependent variable T-score and each independent variable; height and body weight exhibited the stronger correlation with T-score compared to BMI. In the study analyzed the data of Women's Health Initiative (WHI), Cardiovascular Health Study (CHS), and Epidémiologie de l'Ostéoporose (EPIDOS),[13] the linear regression analysis model including age, height, and BMI as independent variables, were separated from the linear regression analysis model that has age, height, and body weight and then analyzed; in that, a regression coefficient (standardized) of height from the model with BMI was bigger than the model includes body weight, meaning that either a regression coefficient of body weight or BMI is relatively small; particularly, when comparing these two models, it was considered that BMI would not be significant than body weight if the a regression coefficient of height from the regression analysis model is higher than the one from the regression analysis model with body weight. Thus, it was concluded that body weight is a better predictor compared to BMI when it comes to BMD. The study, however, only included age, height, bodyweight (or BMI) as explanatory variables and addressed that body weight is a better predictor for the BMD compared to BMI without considerations of physical exercise, smoking as well as menopause which has an important effect on women. In model II (body weight), body weight and the BMD were statistically significantly associated each other when age, height, a diabetes status, a hypertension status, a walking exercise practice status, a smoking status, and a menopause status were controlled; the AIC value of Model I was lower than Model II, indicating the better model appropriateness.
Given the results above, body weight represented a higher association with BMD compared to BMI and the association between BMD and BMI was more appropriate than that with body weight if other variables including age, height, menopause status, diabetes, hypertension, walking exercise, smoking were taken account.
In conclusion, body weight exhibited a stronger association with a BMD without the considerations of other variables, while BMI was more appropriate to explain the association with a BMD when considering variables such as age, a menopause status, exercise, a current smoking status. Further, as BMI better reflect the obesity degree than the simple body weight, it will be useful, as a risk factor of osteoporosis to explain the relationship between the obesity and the BMD.
There are various risk factors of osteoporosis and risks of fractures due to osteoporosis would be increased when such multiple risk factors are applied altogether rather than single factor; thus, effects of such factors including non-physical activities (or no exercise), smoking, menopause are critical.
In the study, we would like to investigate the particular effects of exercise on osteoporosis. The limitations of the study are that exercises can be subdivided into either aerobic and body weight load exercise or low, medium and high intensity exercise depending upon the types and the intensity of exercises, respectively; further, it would be different effects from exercise depending on the duration of the exercise (e.g., 0.5, 1, 1.5, and 2 hr etc.). Thus, it is necessary to quantify the exercise utilizing the scoring system that is classified with criteria including types, intensity, and duration of exercise and then investigate the association with a BMD. On the other hand, other factors that may affect a BMD (e.g., calcium and caffeine intake) should be considered and further studies are needed regarding these factors.
References
- 1.Peck WA, Burckhardt P, Christiansen C, et al. Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 2.Riggs BL, Melton LJ., 3rd Involutional osteoporosis. N Engl J Med. 1986;314:1676–1686. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- 3.Dempster DW. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. 2011;17 Suppl 6:S164–S169. [PubMed] [Google Scholar]
- 4.National Osteoporosis Foundation. Clinician's Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2010. [Google Scholar]
- 5.Ross PD. Osteoporosis. Frequency, consequences, and risk factors. Arch Intern Med. 1996;156:1399–1411. doi: 10.1001/archinte.156.13.1399. [DOI] [PubMed] [Google Scholar]
- 6.Haczynski J, Jakimiuk A. Vertebral fractures: a hidden problem of osteoporosis. Med Sci Monit. 2001;7:1108–1117. [PubMed] [Google Scholar]
- 7.Akdeniz N, Akpolat V, Kale A, et al. Risk factors for postmenopausal osteoporosis: anthropometric measurements, age, age at menopause and the time elapsed after menopause onset. Gynecol Endocrinol. 2009;25:125–129. doi: 10.1080/09513590802549817. [DOI] [PubMed] [Google Scholar]
- 8.Krall EA, Dawson-Hughes B. Smoking and bone loss among postmenopausal women. J Bone Miner Res. 1991;6:331–338. doi: 10.1002/jbmr.5650060404. [DOI] [PubMed] [Google Scholar]
- 9.Nelson HD, Nevitt MC, Scott JC, et al. Study of Osteoporotic Fractures Research Group. Smoking, alcohol, and neuromuscular and physical function of older women. JAMA. 1994;272:1825–1831. doi: 10.1001/jama.1994.03520230035035. [DOI] [PubMed] [Google Scholar]
- 10.Felson DT, Kiel DP, Anderson JJ, et al. Alcohol consumption and hip fractures: the Framingham Study. Am J Epidemiol. 1988;128:1102–1110. doi: 10.1093/oxfordjournals.aje.a115052. [DOI] [PubMed] [Google Scholar]
- 11.Kanis JA, Delmas P, Burckhardt P, et al. Guidelines for diagnosis and management of osteoporosis. The European Foundation for Osteoporosis and Bone Disease. Osteoporos Int. 1997;7:390–406. doi: 10.1007/BF01623782. [DOI] [PubMed] [Google Scholar]
- 12.Hadzibegovic I, Miskic B, Cosic V, et al. Increased bone mineral density in postmenopausal women with type 2 diabetes mellitus. Ann Saudi Med. 2008;28:102–104. doi: 10.5144/0256-4947.2008.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robbins J, Schott AM, Azari R, et al. Body mass index is not a good predictor of bone density: results from WHI, CHS, and EPIDOS. J Clin Densitom. 2006;9:329–334. doi: 10.1016/j.jocd.2006.02.005. [DOI] [PubMed] [Google Scholar]


