Abstract
Invasive aspergillosis is a rare complication in patients with influenza infection. Several cases of invasive pulmonary aspergillosis accompanying influenza infections were reported during the influenza A/H1N1pdm 2009. We encountered a case of acute cerebral aspergillosis in a patient with influenza A/H1N1pdm 2009 infection. A 24-year-old man with uncontrolled diabetes was diagnosed with influenza A/H1N1pdm 2009 infection. Initial evaluation indicated methicillin-sensitive Staphylococcus aureus pneumonia and diabetic ketoacidosis along with influenza. During his hospital course, multiple new rim-enhancing mass lesions not evident in the initial evaluation developed in the fronto-parietal cortical and subcortical white matter and right cerebellum. Pathology and culture results confirmed the presence of Aspergillus fumigatus. Surgical drainage combined with a total of 18 weeks of antifungal therapy resulted in complete resolution of the infection. This case demonstrates that cerebral aspergillosis can present alongside influenza in patients with diabetes or those under intensive care. Clinical suspicion of invasive aspergillosis is required for a definite diagnosis and better prognosis in such cases.
Keywords: Central nervous system, Invasive aspergillosis, Influenza, Brain abscess, Diabetes mellitus
Introduction
Invasive aspergillosis is a well-known opportunistic infection in immunocompromised hosts such as recipients of allogeneic hematopoietic stem cell transplantation and patients with hematologic cancer or prolonged neutropenia. Cytomegalovirus increases the incidence of invasive aspergillosis in such patients [1]. In contrast, seasonal influenza is associated with invasive aspergillosis in some patients without classical risk factors [2]. After the influenza A/H1N1pdm 2009, cases of invasive pulmonary aspergillosis complicating influenza A/H1N1 were reported in patients without such classical risk factors. The number of cases of invasive aspergillosis developing in association with influenza A/H1N1 is increasing [3, 4]. The hard efforts for the diagnosis of influenza lead to large numbers of confirmed patients might facilitate the investigation of this rare infection among influenza patients. Here, we report a case of acute cerebral aspergillosis complicating influenza A/H1N1pdm 2009 infection in a patient with uncontrolled diabetes.
Case Report
In October 2009, a 24-year-old man was admitted to our hospital with decreased mental status. The patient had been diagnosed with type 1 diabetes mellitus 5 years before admission but was not followed-up. He was generally in good health until 5 days before admission, when cough and sore throat occured. He was tested for the influenza A/H1N1pdm 2009 by reverse transcription polymerase chain reaction (RT-PCR), and the result was positive 1 day before admission. Therefore, the patient was administered oseltamivir. The following day, his family reported him exhibiting mental confusion and unusual behavior including walking around naked and being unable to make eye contact during conversation.
Upon examination, the patient's blood pressure was 151/97 mmHg, pulse was 122 beats per minute, temperature was 36.0℃, and respiratory rate was 28 breaths per minute. The patient exhibited labored breathing, and a coarse breathing sound with rales heard in both lung fields. A complete blood count showed a white cell count of 25,370/mm3 with 76.4% neutrophils. His C-reactive protein level was 43.8 mg/dL. Serum chemistry and arterial blood gas analysis showed high anion gap metabolic acidosis (pH, 6.864; pCO2, 19.9 mmHg; HCO3-, 3.5 mEq/L; and anion gap, 28), a glucose level of 532 mg/dL, and a serum creatinine level of 1.6 mg/dL. Chest radiography showed left lower lung field haziness (Fig. 1A). Hemoglobin A1c was 16.2%.
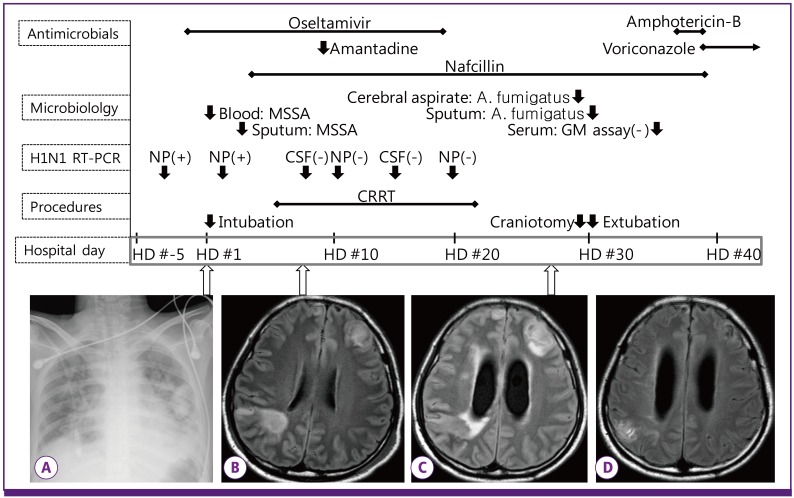
Figure 1.
Sequence of clinical and laboratory events. Reverse transcription-polymerase chain reaction for H1N1 virus was performed serially by nasopharyngeal swab (HD #-3, 2, 10, and 19) and CSF (HD #8 and 14). Initial chest radiography (A) showed multiple patchy consolidations. MRI showed a vague rim-enhancing mass lesion on HD #8 (B), and the abscess became evident on HD #27 (C). The lesion disappeared after surgery and 18 weeks of anti-fungal therapy (D).
MSSA, methicillin-sensitive Staphylococcus aureus ; GM, galactomannan; NP, nasopharynx; CSF, cerebrospinal fluid; CRRT, continuous renal replacement therapy; HD, hospital day. HD #1 indicates the day of admission.
Initial fluid resuscitation and insulin therapy were started for diabetic ketoacidosis. The dose of oseltamivir was increased to 150 mg twice per day, and ampicillin, ceftriaxone and levofloxacin were administered as empirical antibiotics. The following day, the diabetic ketoacidosis improved, but his breathing was difficult and his mentality was drowsy. Respiratory oxygenation deteriorated, and mechanical ventilation was started. A nasopharyngeal swab tested positive for influenza A/H1N1pdm 2009 by RT-PCR on hospital day 2. Blood culture from day 1 and sputum culture from day 3 were positive for methicillin-sensitive Staphylococcus aureus(MSSA); therefore, the antibacterial agents were changed to nafcillin, ceftriaxone and levofloxacin still accompanied by oseltamivir. Corticosteroids were not administered. Over the next 4 days, the patient's renal function gradually worsened, resulting in pulmonary congestion and increased body weight. Therefore, continuous renal replacement therapy was started.
The patient's condition began improving with mechanical ventilation and continuous renal replacement therapy, but he did not regain consciousness. On hospital day 8, brain magnetic resonance imaging (MRI) showed diffuse leptomeningeal enhancement and multifocal infarction in the fronto-parietal cortical and subcortical white matter (Fig. 1B). Therefore, lumbar puncture was performed. The opening pressure was 30 cmH2O, and cerebrospinal fluid (CSF) contained a white cell count of 207/mm3 (94% neutrophils), a protein concentration of 120.4 mg/dL, and a glucose concentration of 109 mg/dL (serum glucose: 110 mg/dL). Intravenous acyclovir and oral amantadine were added to his therapeutic regimen to treat possible combined viral encephalitis. The remaining antibacterial agents were changed to nafcillin and meropenem. The PCR results of the CSF were negative for cytomegalovirus, herpes simplex virus, varicella zoster virus, enterovirus, and influenza A/H1N1pdm 2009. The bacterial culture of the CSF was sterile. CSF analysis and MRI results were suggestive of meningitis and arterial infarction. Transthoracic echocardiography was normal. The antiviral agents oseltamivir, acyclovir, and amantadine and antibacterial agents nafcillin and meropenem were continued. On hospital day 10, the patient began regaining consciousness. He could answer questions by nodding but could not move his arms or legs. On hospital day 14, follow-up CSF analysis showed a white cell count of 310/mm3 (64% neutrophils and 2% lymphocytes) and a protein concentration of 316 mg/dL. The patient still had intermittent fever and chilling, but his general condition continued to improve. Mechanical ventilator and continuous renal replacement therapy were eventually ceased. His cognitive function improved but muscle strength in all 4 extremities showed limited improvement; on the basis of a 5-point muscle power scale, the right and left sides were assigned scores of 1 and 3 points, respectively.
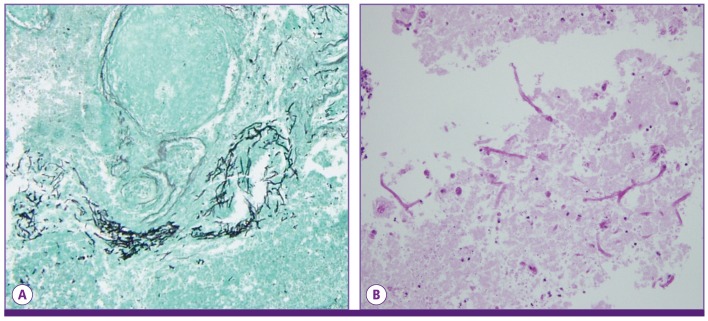
On hospital day 27, the patient exhibited sleeping tendencies, and his motor power decreased further. MRI of the brain was repeated to evaluate the previous cerebral lesions. The results showed newly developed multiple rim-enhancing mass lesions in the cortical and subcortical white matter of fronto-pariental lobe, right cerebellum, and left upper medulla (Fig. 1C). The level of diffusion restriction in the MRI was variable, suggestive of fungal origin. On hospital day 29, surgical drainage of the frontal cerebral abscess was performed. Gomori methenamine-silver stain and periodic acid-Schiff stain of the surgical specimen revealed a few degenerating fungal hyphae (Fig. 2). Culture of the aspirated abscess indicated the presence of A. fumigatus. Serum galactomannan assay result was negative. A single culture of transtracheal aspiration performed one month after admission was positive for A. fumigatus. Chest computed tomography scan showed multifocal bronchiectasis in the right middle lobe and lingular segment of the left upper lobe. Bronchoscopy was not performed.
Figure 2.
Brain abscess pathology. Gomori methenamine-silver (A), and periodic acid-Schiff stains (B) revealed fungal hyphae.
After an initial 2 days of amphotericin B deoxycholate, intravenous voriconazole 240 mg twice per day was started. After 4 weeks of therapy, voriconazole was changed to liposomal amphotericin B with 3 mg/kg/day owing to elevated liver enzymes. After 3 weeks, the antifungal agent was switched to oral voriconazole 200 mg twice per day, which was continued for 11 weeks. Voriconazole was subsequently discontinued because of recurrent Clostridium difficile-associated diarrhea, and the patient was followed-up without antifungal therapy. The patient's medical condition was stabilized with clear mental awareness and wheel chair ambulation. However, right hemiparesis persisted. He was followed-up for 2 years and showed no evidence of recurred aspergillosis by both clinical assessment and brain imaging during this period (Fig. 1D).
Discussion
Invasive aspergillosis is an opportunistic infection that occurs mainly in patients who are immunocompromised with prolonged chemotherapy-induced neutropenia or hematopoietic stem cell transplantation [5]. However, not-severe immunocompromised hosts such as patients with chronic obstructive pulmonary disease receiving long-term corticosteroids, and patients in the intensive care unit (ICU) are also reported to be at risk of the invasive aspergillosis [2, 6-8]. Influenza infection is also thought to be associated with invasive pulmonary aspergillosis. To our knowledge, this is the first report of a culture-proven case of acute cerebral aspergillosis complicating influenza A/H1N1 infection.
It is difficult to elucidate the cause of the cerebral aspergillosis in the present case. The patient had uncontrolled diabetes as evidenced by a hemoglobin A1c level of 16.2%. The influenza triggered a complicated clinical course including diabetic ketoacidosis and MSSA pneumonia/bacteremia. Diabetes mellitus is suggested to contribute to the pathogenesis of invasive aspergillosis, while acute hyperglycemia is suggested to impair the immune function of peripheral leukocytes [2, 9]. The case of invasive pulmonary aspergillosis developing in an insulin-dependent patient with diabetes without other risk factors also supports this possibility [10, 11]. ICU care itself can cause immune suppression. Prolonged use of antimicrobial agents as well as the presence of multiple catheters and mechanical ventilation is known to adversely affect immune function [8]. Critically ill patients with prolonged ICU stay exhibit macrophage deactivation and altered cellular responses [12].
Influenza replicates in respiratory epithelial cells and causes inflammation, congestion, and airway necrosis. Pathology findings from human cases and animal models show extension of these inflammatory conditions into the alveoli, resulting in an increased incidence of diffuse alveolar damage; this was even more evident in patients of the influenza A/H1N1pdm 2009 and H5N1 than those with seasonal influenza [13]. These inflammatory processes may have facilitated the systemic invasion of mold in the present case. In addition, influenza infection causes lymphopenia and functional alteration of peripheral lymphocytes, accompanying transient immunosuppression [14, 15]. This has been demonstrated in cases of disseminated aspergillosis complicating influenza A/H3N2 [16]. In addition, recently identified cases with such complications during the influenza A/H1N1pdm 2009 further indicate that influenza infection is a risk factor of invasive aspergillosis [1, 3, 4, 17-19]. Our patient tested positive for influenza by RT-PCR until hospital day 2 and exhibited a decreasing lymphocyte count, reaching a nadir value of 816/mm3 on hospital day 7.
Invasive aspergillosis most commonly involves the lungs followed by dissemination into multiple organs. Central nervous system involvement and absence in other organs are also reported [20]. Aspergillosis of the central nervous system is typically believed to result from hematogenous spread from the lungs. One report describes cerebral watershed area infarction during the course of possible invasive pulmonary aspergillosis and influenza A/H1N1pdm 2009 [17]. Pathologic examination of the present case revealed the presence of fungal hyphae, and the culture of the aspirated abscess revealed the presence of A. fumigatus. Transtracheal aspiration culture one month after admission also revealed the presence of A. fumigates, suggesting that the lungs may have been the portal of entry.
During the development of a brain abscess, the capsule is formed within 1-2 weeks [21]. Considering the clinical course and imaging follow-up of the present patient, it is evident that his acute cerebral aspergillosis developed shortly after the influenza infection, suggesting that influenza played a greater role in the pathogenesis of cerebral aspergillosis in the present case. Moreover, the late immunologic recovery with glycemic control and improvement of influenza and S. aureus infection may have contributed to the abscess formation.
The present patient was confirmed to have influenza A/H1N1pdm 2009 with MSSA pneumonia by RT-PCR. Since the patient's medical condition improved with antiviral and antibacterial therapy, fungal disease was not initially considered. His neurologic abnormality was also initially evaluated from the perspective of viral or bacterial infection and poor general condition. One month elapsed until the confirmatory diagnosis of cerebral aspergillosis. Only microbiological and histological evidence from the abscess led to specific antifungal therapy. Therefore, clinical suspicion of the potential association between influenza and invasive aspergillosis may enable early diagnosis.
In conclusion, we report a case of influenza A/H1N1pdm 2009 complicated by microbiologically confirmed acute cerebral aspergillosis. The possible risk factors for invasive cerebral aspergillosis were uncontrolled diabetes mellitus and ICU care together with influenza.
References
- 1.Passouant O, Mateu P, Commandini M, Brenkle K, Just B. Pulmonary aspergillosis in non-immunocompromised patient with acute respiratory distress syndrome during A (H1N1) infection. Ann Fr Anesth Reanim. 2011;30:e75–e76. doi: 10.1016/j.annfar.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Stevens DA, Melikian GL. Aspergillosis in the 'nonimmunocompromised' host. Immunol Invest. 2011;40:751–766. doi: 10.3109/08820139.2011.614307. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Vidal C, Barba P, Arnan M, Moreno A, Ruiz-Camps I, Gudiol C, Ayats J, Ortí G, Carratalà J. Invasive aspergillosis complicating pandemic influenza A (H1N1) infection in severely immunocompromised patients. Clin Infect Dis. 2011;53:e16–e19. doi: 10.1093/cid/cir485. [DOI] [PubMed] [Google Scholar]
- 4.Carfagna P, Brandimarte F, Caccese R, Campagna D, Brandimarte C, Venditti M. Occurrence of influenza A(H1N1)v infection and concomitant invasive pulmonary aspergillosis in a patient with chronic obstructive pulmonary disease. Mycoses. 2011;54:549–551. doi: 10.1111/j.1439-0507.2010.01998.x. [DOI] [PubMed] [Google Scholar]
- 5.Kousha M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011;20:156–174. doi: 10.1183/09059180.00001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutkiewicz R, Hage CA. Aspergillus infections in the critically ill. Proc Am Thorac Soc. 2010;7:204–209. doi: 10.1513/pats.200906-050AL. [DOI] [PubMed] [Google Scholar]
- 7.Garnacho-Montero J, Amaya-Villar R, Ortiz-Leyba C, León C, Alvarez-Lerma F, Nolla-Salas J, Iruretagoyena JR, Barcenilla F. Isolation of Aspergillus spp. from the respiratory tract in critically ill patients: risk factors, clinical presentation and outcome. Crit Care. 2005;9:R191–R199. doi: 10.1186/cc3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meersseman W, Vandecasteele SJ, Wilmer A, Verbeken E, Peetermans WE, Van Wijngaerden E. Invasive aspergillosis in critically ill patients without malignancy. Am J Respir Crit Care Med. 2004;170:621–625. doi: 10.1164/rccm.200401-093OC. [DOI] [PubMed] [Google Scholar]
- 9.Kwoun MO, Ling PR, Lydon E, Imrich A, Qu Z, Palombo J, Bistrian BR. Immunologic effects of acute hyperglycemia in nondiabetic rats. JPEN J Parenter Enteral Nutr. 1997;21:91–95. doi: 10.1177/014860719702100291. [DOI] [PubMed] [Google Scholar]
- 10.Janes SM, Barker KF, Mak V, Bell D. Invasive pulmonary aspergillosis in an insulin-dependent diabetic. Respir Med. 1998;92:972–975. doi: 10.1016/s0954-6111(98)90201-3. [DOI] [PubMed] [Google Scholar]
- 11.Grizzanti JN, Knapp A. Diabetic ketoacidosis and invasive aspergillosis. Lung. 1981;159:43–49. doi: 10.1007/BF02713896. [DOI] [PubMed] [Google Scholar]
- 12.Lederer JA, Rodrick ML, Mannick JA. The effects of injury on the adaptive immune response. Shock. 1999;11:153–159. doi: 10.1097/00024382-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Guarner J, Falcón-Escobedo R. Comparison of the pathology caused by H1N1, H5N1, and H3N2 influenza viruses. Arch Med Res. 2009;40:655–661. doi: 10.1016/j.arcmed.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Lewis DE, Gilbert BE, Knight V. Influenza virus infection induces functional alterations in peripheral blood lymphocytes. J Immunol. 1986;137:3777–3781. [PubMed] [Google Scholar]
- 15.Astry CL, Jakab GJ. Influenza virus-induced immune complexes suppress alveolar macrophage phagocytosis. J Virol. 1984;50:287–292. doi: 10.1128/jvi.50.2.287-292.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee FE, Daigle CC, Urban MA, Metlay LA, Treanor JJ, Trawick DR. Fever and progressive respiratory failure in three elderly family members. Chest. 2005;128:1863–1864. 1865–1867. doi: 10.1378/chest.128.3.1863. [DOI] [PubMed] [Google Scholar]
- 17.Adalja AA, Sappington PL, Harris SP, Rimmele T, Kreit JW, Kellum JA, Boujoukos AJ. Isolation of Aspergillus in three 2009 H1N1 influenza patients. Influenza Other Respi Viruses. 2011;5:225–229. doi: 10.1111/j.1750-2659.2011.00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lat A, Bhadelia N, Miko B, Furuya EY, Thompson GR., 3rd Invasive aspergillosis after pandemic (H1N1) 2009. Emerg Infect Dis. 2010;16:971–973. doi: 10.3201/eid1606.100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SH, Kim MN, Lee SO, Choi SH, Kim YS, Woo JH, Lim CM, Koh Y, Hong SB. Fatal pandemic influenza A/H1N1 infection complicated by probable invasive pulmonary aspergillosis. Mycoses. 2012;55:189–192. doi: 10.1111/j.1439-0507.2011.02051.x. [DOI] [PubMed] [Google Scholar]
- 20.Patterson TF, Kirkpatrick WR, White M, Hiemenz JW, Wingard JR, Dupont B, Rinaldi MG, Stevens DA, Graybill JR I3 Aspergillus Study Group. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. Medicine (Baltimore) 2000;79:250–260. doi: 10.1097/00005792-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Britt RH, Enzmann DR. Clinical stages of human brain abscesses on serial CT scans after contrast infusion. Computerized tomographic, neuropathological, and clinical correlations. J Neurosurg. 1983;59:972–989. doi: 10.3171/jns.1983.59.6.0972. [DOI] [PubMed] [Google Scholar]