Abstract
Non-typhoidal Salmonella species are important foodborne pathogens that can cause gastroenteritis, bacteremia, and subsequent focal infections. Non-typhoidal salmonellosis is problematic, particularly in immunocompromised hosts. Any anatomical site can be affected by this pathogen via hematogenous seeding and may develop local infections. However, cervical lymphadenitis caused by non-typhoidal Salmonella species is rarely reported. Herein, we have reported a case of cervical lymphadenitis caused by group D non-typhoidal Salmonella associated with lymphoma.
Keywords: Lymphadenitis, Salmonella, Lymphoma
Introduction
Non-typhoidal Salmonella species are foodborne pathogens that can cause gastroenteritis, bacteremia, and focal infections. Although the majority of patients with non-typhoidal Salmonella infections have self-limited gastroenteritis, approximately 5% of patients develop subsequent bacteremia. Focal complications of non-typhoidal Salmonella bacteremia are reported in 8.0-16.7% of affected patients [1]. Non-typhoidal Salmonella is of particular concern in immunocompromised individuals, including patients with malignancy, human immunodeficiency virus, or diabetes, and those receiving immunosuppressants [2]. Any anatomical site can be affected by non-typhoidal Salmonella via hematogenous seeding. And, non-typhoidal Salmonella can develop local infections. Cervical lymphadenitis caused by non-typhoidal Salmonella is rarely reported [3]. To our knowledge, thus far, no case of cervical lymphadenitis complicated by non-typhoidal Salmonella infection has been reported in Korea. Herein, we have reported a case of cervical lymphadenitis caused by group D non-typhoidal Salmonella in a patient who was diagnosed with concomitant lymphoma.
Case Report
A 66-year-old woman was admitted to Samsung Medical Center on August 13, 2012, with a 10-day history of a palpable mass in the right supraclavicular area. The lesion had grown in size to 5 cm over 10 days and was complicated with pain, redness, and purulent discharge. Chest computed tomography (CT), which was performed in a other hospital on August 11, had shown a mass extending from the right cervical area to the anterior mediastinum. The patient was transferred to our hospital for further evaluation.
The patient had been treated for hypertension for 10 years. Two years prior to admission, she had undergone partial thyroidectomy for treating papillary thyroid carcinoma. Thyroid cancer had been localized to the right lobe of the thyroid with a size of 0.4 cm. The pathologic findings had shown no evidence of lymph node metastasis of thyroid cancer. The patient was examined periodically by a surgeon. There was no abnormal finding on a thyroid ultrasonography performed in January 2012.
The patient had complained of febrile sensation for 3 days. She had experienced weight loss of approximately 7 kg over the 6 previous months. On physical examination, her body weight was 54.6 kg and height was 153.4 cm. Her vital signs were as follows: blood pressure of 110/73 mmHg, body temperature of 36.6℃, heart rate of 71 beats per minute, and respiration rate of 18 breaths per minute. The size of the palpable mass was approximately 5 cm; it was located in the right supraclavicular area and complicated with redness, tenderness, and discharge.
Her complete blood count revealed the following: white blood cells (WBC), 4,330/mm3 (neutrophils, 68.7%); hemoglobin, 12.1 g/dL; and platelets, 183,000/mm3. Chemistry profiles showed the following: aspartate transaminase (AST), 61 IU/L; alanine aminotransferase (ALT), 40 IU/L; C-reactive protein (CRP), 13.86 mg/dL; procalcitonin, 1.56 ng/mL; and lactate dehydrogenase, 661 IU/L. Thyroid function tests showed the following: triiodothyronine (T3), 42.26 ng/dL; thyroxine (T4), 8.6 ug/dL; thyroid-stimulating hormone (TSH), 0.01 uIU/mL; and free thyroxine, 1.53 ng/dL. The test result for anti-hepatitis B surface (HBs) antibody was positive. Anti-human immunodeficiency virus (HIV) antibody test were also negative. Amoxicillin/clavulanate was given empirically at a dose of 1.0 g/0.2 g, 3 times per day intravenously.
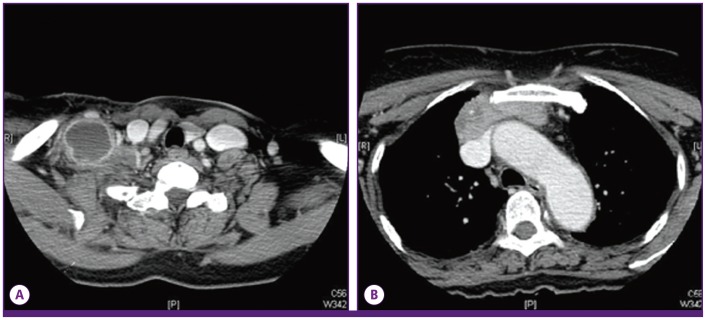
The chest CT showed a mass that was 66 mm in size, extending from the right supraclavicular area through the anterior mediastinum with extensive necrotic lymphadenopathy (Fig. 1). As the chest CT findings suggested probability of thymic carcinoma and right supraclavicular nodal metastasis, 18F-fluorodeoxyglucose positron emission tomography-computed tomography (PET-CT) was performed to assess potential cancer. PET-CT showed a visible mass in the anterior mediastinum that was seen as high 18F-2-fluoro-2-deoxy-glucose (FDG) uptake, the maximum standardized uptake value (SUVmax) of the lesion being 7.3. Further, there was high FDG uptake in the right prevascular lymph node, left mediastinal lymph node, both supraclavicular lymph nodes, and right infraclavicular lymph node; soft tissue swelling was also observed in the right supraclavicular area. These findings suggested a probability of cancer associated with infection (Fig. 2).
Figure 1.
(A) The chest computed tomography scan shows an enlarged lymph node with necrosis in the right supraclavicular area and enhancement of the surrounding area. (B) The chest computed tomography scan shows a mass in the anterior mediastinum with necrosis that runs continuously from the supraclavicular area to the anterior mediastinum.
Figure 2.
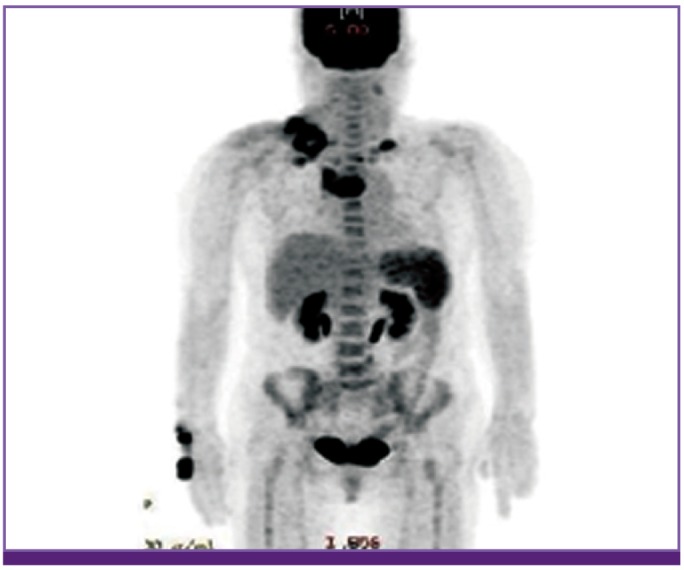
PET-torso image shows increased SUVmax of the lymph nodes in both the supraclavicular areas and anterior mediastinum.
To confirm a diagnosis of infection and cancer, ultrasonography-guided biopsy and culture were performed. Multiple enlarged lymph nodes were observed in both supraclavicular areas. Their size was measured to range from 1.5 cm to 4 cm, and they were found to contain a variety of materials including fluid and debris (Fig. 3). Cultures for bacteria, mycobacteria, and fungi were performed. On hospital day 4, Gram stain results of the lymph node revealed that there were a few gram-negative bacilli. Pathologically, both of the cervical lymph nodes and the mass in the mediastinum showed infarction and granulation tissue with focal viable lymphoid cells and no bacteria observable by light microscopy (Fig. 4). On hospital day 7, tissue samples from the cervical lymph nodes and the mass in the anterior mediastinum were finally confirmed as diffuse large B-cell lymphoma by immunohistochemical staining.
Figure 3.
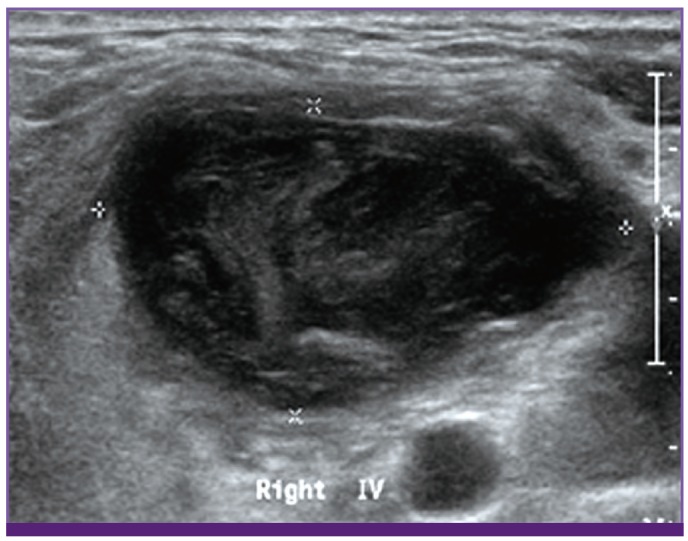
Ultrasonography image shows a lymph node in the right supraclavicular area (diameter is measured to 4 cm and minor axis is 2 cm), filled with debris and fluid.
Figure 4.
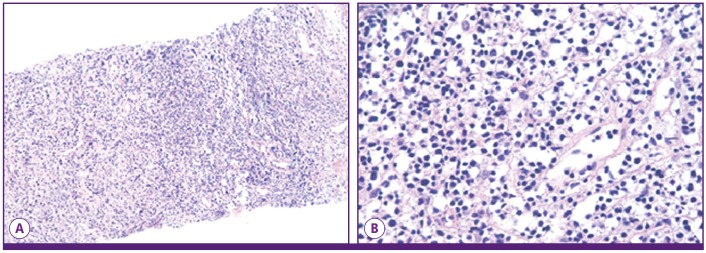
Histopathology of the cervical lymph node shows infarction and granulation tissue with focal lymphoid cells. (A) (hematoxylin and eosin [H&E] stain, ×100), (B) High magnification (hematoxylin and eosin [H&E] stain, ×400).
On the same day, group D non-typhoidal Salmonella was isolated from cultures obtained from the lymph nodes. Growth of cultured organisms that were oxidase negative was observed on MacConkey agar, necessitating the use of an automated microbial analyzing system (Microscan system, gram negative combo panel type 53, Siemens Healthcare, Sacramento, CA, USA) to identify Salmonella to the genus level. The pathogen was confirmed to be group D non-typhoidal Salmonella using polyvalent antisera (Joongkyeom, Kyungki, Korea). Group D non-typhoidal Salmonella isolated from culture was sensitive to most antibiotics except for ampicillin/sulbactam, ticarcillin, and colistin. The results of the drug sensitivity test revealed that the minimal inhibitory concentration (MIC) for the various drugs were as follows: ampicillin/sulbactam, > 16/8 mg/L; amikacin, ≤ 8 mg/L; aztreonam, ≤ 1 mg/L; ceftazidime, ≤ 1 mg/L; colistin, > 4 mg/L; ciprofloxacin, ≤ 0.5 mg/L; cefepime, ≤ 1 mg/L; fosfomycin, ≤ 16 mg/L; gentamicin, ≤ 1 mg/L; imipenem, ≤ 1 mg/L; levofloxacin, ≤ 1 mg/L; meropenem, ≤ 1 mg/L; minocycline, ≤ 4 mg/L; piperacillin/tazobactam, ≤ 8 mg/L; trimethoprim/sulfamethoxazole, ≤ 2/38 mg/L; tigecycline, ≤ 0.5 mg/L; ticarcillin, > 64 mg/L; and tobramycin, ≤ 2 mg/L.
On the basis of antimicrobial susceptibility results, the antibiotic treatment was changed to ciprofloxacin. Oral ciprofloxacin (500 mg twice daily) was administered to the patient for 14 days. The mass in the right supraclavicular area improved after the administration of ciprofloxacin and the initiation of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemotherapy. The PET-CT after 2 months of treatment showed small mild hypermetabolic tumors in the right anterior mediastinum (SUVmax = 2.1) and right supraclavicular lymph node, (SUVmax = 2.4), a marked improvement compared to that in the previous scans. The patient has been receiving chemotherapy without serious complication.
Discussion
Virtually, any anatomical site may be seeded hematogenously by non-typhoidal salmonellae, and it may develop a local infection, even if the bacteremia is successfully treated [2]. However, lymphadenitis as an extraintestinal manifestation of Salmonella infection is known to be rare [3]. Although Peagues et al. extensively reviewed focal infection caused by Salmonella infection, lymphadenitis was not mentioned in their review [4]. In addition, Chen et al. performed a retrospective study in a Taiwanese hospital and described the kinds of focal infection caused by non-typhoidal Salmonella. Of 129 patients, 51 (39.5%) patients had focal infections, including mycotic aneurysm, pneumonia, empyema, osteomyelitis, hepatic or splenic abscess, arthritis, bacterial peritonitis, and infective endocarditis. However, no case of lymphadenitis was reported among the patients in this study [1]. There have been several reports of lymphadenitis complicated with non-typhoidal Salmonella infection [3, 5-11]. To our knowledge, however, our case is only the second report of lymphadenitis caused by non-typhoidal Salmonella with lymphoma present in the same lesion, and only the third case associated with malignant lesion [3, 12]. Furthermore, in Korea, there have been no reports of lymphadenitis caused by non-typhoidal Salmonella infection prior to this case.
In 1961, 2 boys with non-typhoidal Salmonella infection associated with appendicitis and mesenteric lymphadenitis were reported [8]. Since 1961, there have been reports of mesenteric lymphadenitis, both associated with and independent of appendicitis [6, 7, 13, 14]. There have also been cases of lymphadenitis occurring in other parts of body. Faber et al. described a 66-year-old man with an abscess in the left femoral lymph node caused by Salmonella Typhi [15]. In 1987, 2 cases with Salmonella lymphadenitis were identified. In 1 case, Salmonella Typhi was isolated from a supraclavicular node in a patient with stomach cancer. In the second case observed in a 68-year-old diabetic patient, Salmonella Typhimurium was isolated from an axillary mass that included lymph nodes infiltrated with reticulum cell sarcoma [3, 12]. In 2001, 1 study described cervical lymphadenitis due to non-typhoidal Salmonella (Salmonella Braenderup) in a tumor-infiltrated lymph node occurring in a patient with Hodgkin's lymphoma [3]. Therefore, our case is the third case worldwide of lymphadenitis, caused by Salmonella, in a lymph node infiltrated with malignancy, in adults.
In pediatric patients, there have been 3 reports of cervical lymphadenitis due to Salmonella [10, 11, 16]. The case of Salmonella Enteritidis cervical lymphadenitis was reported in a 12-year-old girl [11]. She was found to have complete IL-12/IL-23Rβ1 deficiency. Further, there are 2 reports of Salmonella cervical lymphadenitis occurring in otherwise healthy pediatric patients. One case was observed in a 10-year-old who developed a granulomatous lymphadenitis of a submandibular lymph node caused by a Salmonella group B following gastroenteritis [10] and the other involved a submandibular suppurative lymphatic abscess caused by Salmonella Typhi in an 8-year-old child [16].
In Korea, there have been multiple reports of patients with focal infections due to Salmonella, primarily osteomyelitis and thyroid abscesses occurring in patients with systemic lupus erythematosus, and pyogenic myositis in patients with multiple myeloma. Vertebral osteomyelitis, septic arthritis of the knee, rhabdomyolysis, splenic abscess, and infected aneurysm of the aorta have all been reported as focal infections occurring in previously healthy patients [17]. In a previous study by Cho et al., 36 patients diagnosed with necrotizing lymphadenitis were reported, of whom one patient was diagnosed with infection due to Salmonella Typhi by repeated blood cultures [18]. The patient was a 20-year-old woman who had persistent fever, headache, myalgia for 1 week and multiple cervical lymphadenopathy for 3 weeks. However, the result of lymph node cultures was not mentioned in this study [18]. Therefore, the diagnosis of lymphadenitis associated with Salmonella Typhi in this study was supported only by blood culture results. On the other hand, our case was confirmed to be bacterial lymphadenitis associated with non-typhoidal Salmonella by lymph node cultures.
We suggest Salmonella lymphadenitis should be included in the differential diagnosis of cervical lymphadenitis. When physicians evaluate the cause of abnormally enlarged lymph nodes, they should be aware of the possibility of Salmonella infection and malignancy in the same lesion. Hence, appropriate biopsy and culture tests are required for accurate diagnosis.
References
- 1.Chen PL, Chang CM, Wu CJ, Ko NY, Lee NY, Lee HC, Shih HI, Lee CC, Wang RR, Ko WC. Extraintestinal focal infections in adults with nontyphoid Salmonella bacteraemia: predisposing factors and clinical outcome. J Intern Med. 2007;261:91–100. doi: 10.1111/j.1365-2796.2006.01748.x. [DOI] [PubMed] [Google Scholar]
- 2.Hohmann EL. Nontyphoidal salmonellosis. Clin Infect Dis. 2001;32:263–269. doi: 10.1086/318457. [DOI] [PubMed] [Google Scholar]
- 3.Campbell WN. Salmonella lymphadenitis associated with undiagnosed lymphoma. Eur J Clin Microbiol Infect Dis. 2001;20:359–361. doi: 10.1007/s100960100500. [DOI] [PubMed] [Google Scholar]
- 4.Peugues DA, Miller SI. Salmonella species, including Salmonella typhi. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 7th ed. Philadelphia: Elsevier Churchill Livingstone; 2009. chapter 223. [Google Scholar]
- 5.Cornut P, Courtieu AL. Suppurated cervical adenitis caused by Salmonella Typhi murium. Pediatrie. 1960;15:169–173. [PubMed] [Google Scholar]
- 6.García-Corbeira P, Ramos JM, Aguado JM, Soriano F. Six cases in which mesenteric lymphadenitis due to non-typhi Salmonella caused an appendicitis-like syndrome. Clin Infect Dis. 1995;21:231–232. doi: 10.1093/clinids/21.1.231. [DOI] [PubMed] [Google Scholar]
- 7.Guarga A, Urrutia A, Paret A, Rey-Joly C. Mesenteric lymphadenitis caused by Salmonella Enteritidis. Med Clin (Barc) 1984;82:136. [PubMed] [Google Scholar]
- 8.Mautner LS, McIntyre AJ, Ord JV. Isolation of Salmonella organisms from mesenteric lymph nodes. Can Med Assoc J. 1961;85:139–141. [PMC free article] [PubMed] [Google Scholar]
- 9.Meng GR. Acute mesenteric lymphadenitis due to Salmonella enteritidis mimicking appendicitis: case report. Mil Med. 1974;139:277. [PubMed] [Google Scholar]
- 10.Murray JC, Singh RR, Brandt ML, Kearney DL, Ogden AK. Granulomatous submandibular lymphadenitis caused by Salmonella species in a healthy child. Clin Infect Dis. 1994;19:1175–1176. doi: 10.1093/clinids/19.6.1175. [DOI] [PubMed] [Google Scholar]
- 11.van de Vosse E, Ottenhoff TH, de Paus RA, Verhard EM, de Boer T, van Dissel JT, Kuijpers TW. Mycobacterium bovis BCG-itis and cervical lymphadenitis due to Salmonella enteritidis in a patient with complete interleukin-12/-23 receptor beta1 deficiency. Infection. 2010;38:128–130. doi: 10.1007/s15010-009-9222-0. [DOI] [PubMed] [Google Scholar]
- 12.Cohen JI, Bartlett JA, Corey GR. Extra-intestinal manifestations of Salmonella infections. Medicine (Baltimore) 1987;66:349–388. doi: 10.1097/00005792-198709000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Rhee PL, Lee JK, Lee KT, Son HJ, Kim JJ, Koh KC, Paik SW, Lee WJ, Lim HK, Rhee JC. The etiology and clinical characteristics of mesenteric adenitis in Korean adults. J Korean Med Sci. 1997;12:105–110. doi: 10.3346/jkms.1997.12.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Likitnukul S, Wongsawat J, Nunthapisud P. Appendicitis-like syndrome owing to mesenteric adenitis caused by Salmonella typhi. Ann Trop Paediatr. 2002;22:97–99. doi: 10.1179/027249302125000247. [DOI] [PubMed] [Google Scholar]
- 15.Faber LM, Simoons-Smith AM, Razenberg PP. An unusual late manifestation of a Salmonella typhi infection. Am J Gastroenterol. 1990;85:81–83. [PubMed] [Google Scholar]
- 16.Singh NP, Manchanda V, Gomber S, Kothari A, Talwar V. Typhoidal focal suppurative lymphatic abscess. Ann Trop Paediatr. 2002;22:183–186. doi: 10.1179/027249302125000922. [DOI] [PubMed] [Google Scholar]
- 17.Lee S, Lee HN, Yang H, Kang J, Kim N, Sin Y, Lee S. A case of septic arthritis of the knee joint due to Group D non-typhoidal Salmonella. Infect Chemother. 2012;44:303–306. [Google Scholar]
- 18.Cho KJ, Kim CW, Park SH, Lee SK. Necrotizing lymphadenitis-a clinico-pathologic study of 36 cases with immunohistochemical analysis. J Korean Med Sci. 1991;6:55–61. doi: 10.3346/jkms.1991.6.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]